Abstract
Introduction and Objective
Nephrocalcinosis (NC) is commonly present in primary hyperparathyroidism (HPT), distal renal tubular acidosis (dRTA), and medullary sponge kidney disease (MSKD) but has not been studied in patients with CaP stones who do not have systemic disease.
Methods
We studied patients undergoing percutaneous nephrolithotomy (PNL) who had CaP or CaOx stones and did not have HPT, dRTA, or MSKD. On post-op (PO) day 1, all patients underwent a non-contrast CT scan. If there were no residual calcifications, the patient was categorized as not having NC. If there were residual calcifications, the patient underwent a secondary PNL. If the calcifications were found to be stones then the patient was categorized as not having NC. If the calcifications were not stones then the patient was categorized as having NC. Patients were grouped based on the type of stones they formed: hydroxyapatite (HASF), brushite (BRSF), and idiopathic calcium oxalate (ICSF). The extent of NC was quantified: 0=absence of NC and 3=extensive NC. Patients with residual calcifications on PO day 1 NCCT scan who did not undergo secondary PNL were excluded. The presence or absence of NC was correlated with metabolic studies.
Results
67 patients were studied (14 HASF, 19 BRSF, and 34 ICSF). NC was present in 10/14 (71.4%), 11/19 (57.9%) and 6/34 (17.6%) for the HASF, BRSF, and ICSF groups respectively (Chi-square (X2): p=0.01) (Table 1). The extent of NC per group was 1.98, 1.32, and 0.18 for HASF, BRSF, and ICSF respectively (p=<0.001). The presence of NC was positively correlated with urine calcium excretion (287.39±112.49 v. 223.68±100.67, p=0.03).
Conclusions
Patients without systemic disease who form hydroxyapatite and brushite stones commonly have coexistent NC. NC can occur in calcium oxalate stone formers but the quantity and frequency of NC in this group is dramatically less.
Keywords: Nephrocalcinosis, calcium phosphate, brushite, hydroxyapatite, oxalate, PCNL
Introduction
The term nephrocalcinosis (NC) is commonly used to describe renal parenchymal calcifications. NC was originally defined by Albright in 1934 as diffuse renal calcifications in patients with primary hyperparathyroidism (HPT)1. The pathological definition of NC is the presence of calcium crystal deposits within renal tissue. From this perspective, virtually all calcium stone formers have NC either in the form of interstitial deposits of HA, so called Randall’s plaque, over which CaOx stones form (ICSF) or as in collecting duct (CD) and Bellini duct (BD) plugs of calcium phosphate (CaP) in CaP and brushite (BR) stone formers (HASF, BRSF). These crystals can be calcium oxalate (CaOx) or calcium phosphate (CaP). The more clinically relevant definition is the radiographic definition of NC which is the presence of diffuse, fine renal parenchymal calcifications detectable radiolographically2. The most common causes of NC are primary HPT, medullary sponge kidney disease (MSKD), and Type 1 or distal renal tubular acidosis (dRTA). The majority of calcifications associated with the above mentioned conditions are easily seen on standard radiographs. HPT, dRTA, and MSKD typically cause both NC and nephrolithiasis. Distinguishing between these two entities clinically and radiographically can be a challenge. Fortunately, improvements in endourologic technology and techniques now allow urologists to distinguish between NC and nephrolithiasis3–4. As mentioned above, NC is associated with a number of systemic diseases. However, NC can also be present when systemic disease is not evident. To our knowledge, no study has looked at the incidence/prevalence of NC in patients with CaP and CaOx nephrolithiasis who do not have systemic disease.
Although patients with calcium nephrolithiasis in the absence of systemic disease have historically been grouped together, our work over the past decade has documented that such patients represent a heterogeneous group, especially when viewed in the context of the histopathologic findings noted from papillary and cortical biopsies taken during percutaneous stone removal (PNL). We have identified two broad pathways for the initiation of calcium nephrolithiasis5. The first and largest group, idiopathic calcium oxalate stone formers (ICSF) entails CaOx overgrowth on interstitial deposits of hydroxyapatite named Randall’s plaque for their describer6,7 (Figure 1A). The second group, calcium phosphate stone formers (IPSF) is strongly associated with intratubular crystal deposits, a feature noticeably absent from ICSF8–9 (Figure 1B). IPSF comprise two subsets, those which contain some brushite (BRSF) and those that are predominantly hydroxyapatite or calcium phosphate (HASF) with brushite being absent. HASF and BRSF, while both containing intratubular deposits, differ sufficiently in their renal pathology and crystal deposits to establish themselves as distinct and separate clinical phenotypes9–10 (Table 1). The primary objective of this study was to contrast the frequency and extent of nephrocalcinosis in these three groups of calcium stone formers who were carefully studied endoscopically to distinguish between stones and tissue deposits. The uniquely different patients with medullary sponge kidney are not addressed here11.
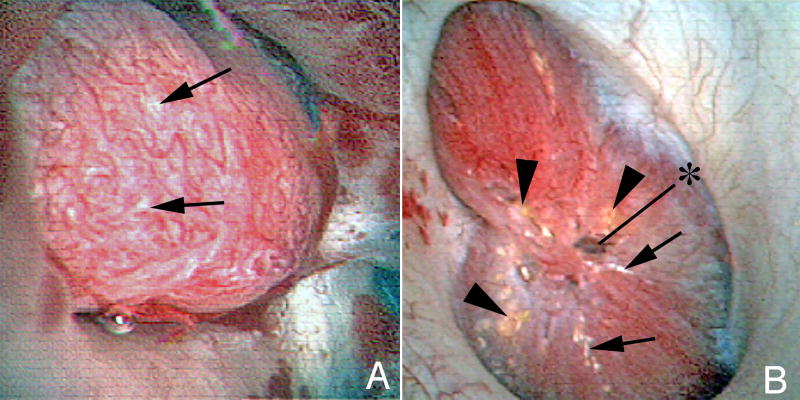
Figure 1.
A: A papillae from an ICSF patient showing a small amount of Randall’s plaque (arrows). The papillae is otherwise normal appearing.
B: A papillae from a BRSF patient. The papillae is severely diseased. It is flattened. In the center of the papillae is a grossly dilated Bellini duct (asterisk). In addition to Randall’s plaque (arrows), Bellini duct deposits are seen (arrowheads).
Table 1.
Clinical features of calcium stone phenotypes
| Idiopathic Calcium Oxalate Stone Formers (ICSF) |
Brushite Stone Formers (BRSF) |
Hydroxyapatite Stone Formers (HASF) |
|
|---|---|---|---|
| RP abundance | +++ | +++ | + |
| Stone hardness | ++ | ++++ | + |
| pH | NL | ↑ | ↑ |
| M/F ratio | 3/2 | 3/2 | 1/5 |
| Stone on RP | ++++ | + | + |
| Papillary injury | Rare | Severe | Moderate |
| BR in stones | No | Yes | No |
Materials and Methods
Patients who underwent percutaneous nephrolithotomy (PNL) for calcium urolithiasis who underwent digital video mapping, as well as biopsy of the renal cortex and papilla as part of our NIH supported stone pathogenesis project, were entered in this study (NIDDK#P01DK56788). The clinical and histopathologic findings in this cohort of patients is the subject of another report9. The study was approved by the Indiana University Institutional Review Board (#1010002261).
The IPSF were separated into two groups, HASF and BRSF. A cut point of 50% HA as an average of all analyzed stone material was utilized to distinguish between HASF and ICSF. All calculi were analyzed by micro-CT, a non-destructive technique followed by FTIR spectroscopy. Patients with any struvite were excluded. In addition to a medical history and physical examination, all patients had blood and 24 hour urine studies to exclude systemic diseases including dRTA and primary HPT. On post-operative (PO) day 1, all patients underwent a non-contrast CT examining for residual calcifications. If there were no residual calcifications, the patient was categorized as not having NC. If there were residual calcifications, the patient underwent a thorough second look nephroscopy to determine if the residual calcifications were NC or nephrolithiasis. Patients with subcapsular, perinephric, and nephrostomy tract calcifications were excluded. Some details about the technical aspects of the second look nephroscopy are important. Although we use digital flexible instruments for mapping at the primary PNL procedure, these instruments are not used during the second look nephroscopy procedures. Digital instruments have limited maneuverability due to the metal cap at the tip of the endoscope which limits the deflectability of the scope. During second look nephroscopy procedures, both Storz and Pentax flexible nephroscopes are utilized. Flexible ureteroscopes are also used when there is a difficult to locate calyx or when the infundibulum to a given calyx is not accommodating. In addition, if a calcification is visible on fluoroscopy but not able to be identified endoscopically, a puncture is performed down on to the calcification utilizing an 18-gauge needle and a zip or glidewire is passed into the more central renal collecting system. The wire is then pulled out through and through and is followed to lead to the occult calyx of interest. When this so called non-dilated puncture technique is utilized, a skin incision is not required, no dilation of the access is required, and a nephrostomy tube is not employed afterwards. If on secondary nephrolithotomy the calcifications were found to be stones then the patient was categorized as not having NC. However, if calcifications identified on CT were not identified endoscopically then the patient was categorized as having NC. Patients were grouped based on the type of stones that they formed: HASF, BRSF, and ICSF. The extent of NC was also quantified: 0=absence of NC, 1=minimal NC, 2=moderate NC, and 3=extensive NC (Figure 2). Patients with residual calcifications on their PO day 1 CT scan who did not undergo a second look nephroscopy were excluded as we were unable to determine if the residual calcifications were stones or NC.
Figure 2.
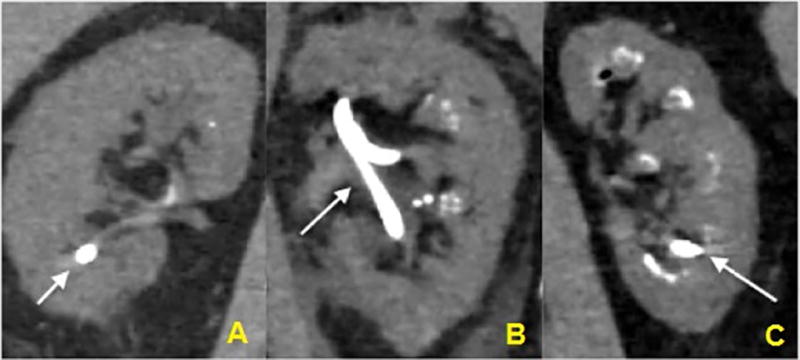
Extent of nephrocalcinosis: A, minimal; B, moderate; C, extensive. Arrows depict nephrostomy tubes.
All CT scans were done at Methodist Hospital and were interpreted by a single radiologist (TH) who was blinded to the endoscopic findings.
All analyses were performed using SPSS Statistical Software, release 19.0 (SPSS Inc., Chicago, IL, USA). In all tests, a two-tailed significance level of 0.05 was used.
Results
We had a total of 54 patients (11 HASF, 15 BRSF, and 28 ICSF) and 67 renal units (14 HASF, 19 BRSF, and 34 ICSF). Table 2 displays each patient in our study categorized by stone type, presence or absence of NC, and location and quantity of NC, if present. NC was present in the majority of HASF 10/14 (71.4%) and BRSF 11/19 (57.9%), and a minority of ICSF 6/34 (17.6%) patients respectively (Chi-square (X2): p=0.01). All patients in the HASF group who had NC had grades 2 or 3. There were no patients in the HASF group who had grade 1 NC. Similarly, the majority of the BRSF who had NC had grade 3, as well. In contrast, all patients in the ICSF group who had NC had grade 1. No patient in the ICSF group who had NC had a grade 2 or 3. The mean grade of NC was 1.98, 1.32, and 0.18 for the HASF, BRSF, and ICSF groups respectively (p<=0.001) (Table 3).
Table 2.
Findings of nephrocalcinosis in different kinds of stone formers.
| Right | Quantity | Location | Left | Quantity | Location | |
|---|---|---|---|---|---|---|
| Hydroxyapatite | ||||||
| 1 | No | |||||
| 2 | Excluded; no secondary | |||||
| 3 | Yes | Moderate | CM;diffuse | Yes | Moderate | CM;diffuse |
| 4 | No | |||||
| 5 | Yes | Moderate | CM;diffuse | |||
| 6 | Yes | Min-Mod Punctate | Papillary; diffuse | |||
| 7 | Yes | Min-Mod Punctate | CM;mid-upper pole | Yes | Min-Mod Punctate | CM:mid-upper pole |
| 8 | Yes | Moderate Punctate | CM;diffuse | Yes | Moderate Punctate | CM;diffuse |
| 9 | No | |||||
| 10 | No | |||||
| 11 | Yes | Moderate | CM;diffuse | Yes | Moderate | CM;diffuse |
| Brushite | ||||||
| 1 | Excluded; no secondary | |||||
| 2 | Yes | Single; Punctate | Cortical; midpole | |||
| 3 | Yes | Single; Punctate | CM;upper pole | No | ||
| 4 | Yes | Moderate | CM;diffuse | Yes | Moderate | CM;diffuse |
| 5 | Yes | 3; punctate | CM; midpole | |||
| 6 | No | |||||
| 7 | Yes | Moderate | CM/papillary; diffuse | |||
| 8 | No | |||||
| 9 | No | |||||
| 10 | Yes | Moderate | CM/diffuse | Yes | Moderate | CM/diffuse |
| 11 | Yes | Moderate | CM/papillary; diffuse | Yes | Moderate | CM/papillary; diffuse |
| 12 | No | |||||
| 13 | No | No | ||||
| 14 | Yes | Minimal Punctate | CM;upper and mid pole | No | ||
| 15 | Excluded; no secondary | |||||
| Calcium oxalate | ||||||
| 1 | Excluded; no secondary | |||||
| 2 | No | |||||
| 3 | No | |||||
| 4 | No | Yes | Single; punctate | papillary | ||
| 5 | Yes | Single; punctate | papillary | No | ||
| 6 | No | No | ||||
| 7 | Excluded; no secondary | |||||
| 8 | Excluded; no secondary | |||||
| 9 | No | No | ||||
| 10 | No | |||||
| 11 | No | |||||
| 12 | Yes | Single; punctate | papillary | No | ||
| 13 | No | |||||
| 14 | Excluded; no secondary | |||||
| 15 | No | |||||
| 16 | No | |||||
| 17 | No | |||||
| 18 | No | No | ||||
| 19 | No | |||||
| 20 | Yes | 5; punctate | CM;diffuse | |||
| 21 | No | |||||
| 22 | No | |||||
| 23 | No | |||||
| 24 | No | Yes | Single; punctate | CM; upper pole | ||
| 25 | No | Yes | Single; punctate | Papillary; mid pole | ||
| 26 | No | |||||
| 27 | No | No | ||||
| 28 | No | No | ||||
CM: corticomedullary
Table 3.
Nephrocalcinosis scores in different kinds of stone formers.
| Nephrocalcinosis Extent | Total | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Mean | ||
| Hydroxyapatite | 4 | 0 | 3 | 7 | 1.98 | 14 |
| Brushite | 8 | 4 | 0 | 7 | 1.32 | 19 |
| ICSF | 28 | 6 | 0 | 0 | 0.18 | 34 |
Urinary calcium excretion and calcium phosphate supersaturation were significantly higher in those patients exhibiting NC (Table 4). There was no correlation with the percent HA or BR and the presence of NC.
Table 4.
Metabolic data on patients who did or did not have nephrocalcinosis
| Nephrocalcinosis Absent(33) |
Nephrocalcinosis Present (22) |
P- Value |
|
|---|---|---|---|
| Urine volume (L/day) | 1.96 ± 0.77 | 1.92 ± 0.65 | 0.82 |
| Urine pH | 6.09 ± 0.40 | 6.22 ± 0.27 | 0.21 |
| Urine calcium (mg/day) | 223.68 ± 100.67 | 287.39 ± 112.49 | 0.03 |
| Urine citrate (mg/day) | 512.86 ± 277.07 | 435.02 ± 296.63 | 0.33 |
| Urine sulfate (mg/day) | 44.49 ± 16.34 | 39.88 ± 17.81 | 0.33 |
| Urine ammonia (mmol/day) | 35.71 ± 16.45 | 26.55 ± 8.85 | 0.03 |
| Urine oxalate (mg/day) | 42.98 ± 17.10 | 36.09 ± 13.56 | 0.12 |
| Supersaturation calcium oxalate | 7.923 ± 3.55 | 7.95 ± 2.90 | 0.97 |
| Supersaturation calcium phosphate | 1.26 ± 0.734 | 1.73 ± 0.61 | 0.02 |
| Serum creatinine (mg/dL) | 1.04 ± 0.212 | 0.99 ± 0.27 | 0.44 |
| Serum bicarbonate (mmol/L) | 27.13 ± 2.09 | 26.34 ± 2.12 | 0.15 |
| Serum calcium (mg/dL) | 9.22 ± 0.61 | 9.51 ± 0.62 | 0.07 |
Nephrocalcinosis absent 40
Missing metabolic studies 7
Nephrocalcinosis present 27
Missing metabolic studies 5
Discussion
As evidenced by the original definition proposed by Dr. Albright, NC has become highly associated with the etiologies of primary HPT, dRTA, and medullary sponge kidney. NC is not thought to be common in stone formers without systemic disease. However, no one has ever looked at the incidence/prevalence of NC in a cohort of patients with calcium nephrolithiasis who do not have systemic disease. The main reason for this is that in the past we did not have the ability to make the distinction between NC and nephrolithiasis. With the dramatic improvements in endourologic technology, we are now able to distinguish between these two entities.
The radiologist is usually one of the first to suggest the presence of NC. However, studies have shown that the radiologist’s ability to detect NC is often not accurate12,13. The most sensitive radiological tool to detect NC is computed tomography and standard x-rays are used to follow the course of NC14. Although ultrasound is the modality of choice for assessing pediatric NC it is rarely used in the adult population because it is subjective and lacks specificity13,14. The diagnosis of NC can be suggested on ultrasound, abdominal x-ray and computed tomography however none possesses diagnostic capacity to distinguish between NC and stone4. Additionally, it is our experience that even when the radiologist suggests a diagnosis of NC, on numerous occasions we have found stones to be present on endoscopic evaluation and treatment15.
In the past, all calcium stone formers have been considered as a single group. However, our previous work has demonstrated that a significant difference exists between CaP stone formers and ICSF. Moreover, there is also a distinct difference between HASF and BRSF. This paper further substantiates the underlying difference between these three calcium stone forming groups.
Our main finding is that NC is common even in the absence of systemic disease. More specifically, in calcium stone forming patients who do not have primary HPT, dRTA or MSKD, a significant number of these patients will have NC. Previously, absent these conditions, NC has not been thought to be a common occurrence, however, there are no formal studies addressing the issue. The data presented herein are the first to document the incidence/prevalence of NC in patients with calcium urolithiasis who do not have systemic disease or MSKD and has been made possible by the dramatic advances in endourologic technology and techniques of the current era of nephrolithiasis surgery.
Our observation that NC can occur and indeed is common in the absence of systemic disease is noteworthy given the increasing incidence of HASF and BRSF16,17. Viewed from another perspective, based on these data, the majority of patients with predominantly calcium phosphate stone may be expected to have concomitant NC in the absence of systemic disease. The implications of our findings are important emphasizing the advantages of endourologic approaches such as PCNL and URS over ESWL which cannot discriminate NL from NC.
The characteristics of NC in ICSF patients differs significantly from HASF and BRSF both in the frequency and amount of NC present as would be expected given our understanding of the pathophysiology of ICSF. While it is possible that the NC found in ICSF could be explained by stones which were overlooked during the secondary PCNL, we think this is unlikely given the extensive use of multiple flexible instruments and non-dilated punctures during the procedures. The amount of NC when present in ICSF was dramatically less than observed in the HASF and BRSF.
In concordance with our earlier work, the presence of NC is another factor that can be used to distinguish the three calcium forming groups: HASF, BRSF, and ICSF. As we have demonstrated here, NC is prevalent in the majority of HASF and BRSF patients but rare in the ICSF group. These findings are entirely consistent with the differing pathways for stone formation for CaP and CaOx stones: intra-tubular deposits are greater in the HASF and BRSF groups and non-existent in the ICSF group. Additionally, urinary pH, supersaturation of CaP, and calcium excretion were significantly greater in the HASF and BRSF groups compared to the ICSF groups in this cohort of patients9.
Conclusion
The only accurate method to distinguish between NC and nephrolithiasis is endoscopically. Using endoscopic techniques, we have demonstrated that patients without systemic disease who form HA and BR stones commonly have coexistent NC. NC can occur in ICSF, as well. However, the quantity and frequency of NC in this group of patients is dramatically less than in patients who form calcium phosphate stones.
Acknowledgments
Supported in part by an NIH grant PO1DK56788.
List of abbreviations
- NC
Nephrocalcinosis
- HPT
Hyperparathyroidism
- dRTA
Distal renal tubular acidosis
- MSKD
Medullary sponge kidney disease
- PNL
Percutaneous nephrolithotomy
- HASF
Hydroxyapatite stone former
- BRSF
Brushite stone former
- ICSF
Idiopathic calcium oxalate stone former
- CaOx
Calcium oxalate
- CaP
Calcium phosphate
References
- 1.Albright FBP, Cope O, Bloomberg E. Studies on the physiology of the parathyroid glands. IV. Renal complications of hyperparathyroidism. Am J Med Sci. 1934;187(1):49–64. [Google Scholar]
- 2.Wrong O. Nephrocalcinosis. In: Davison AM, Cameron JS, Brunfield J, et al., editors. Oxford Textbook of Clinical Nephrology. Oxford University Press; Oxford: 2005. p. 1375. [Google Scholar]
- 3.Humphreys MR, Miller NL, Williams JC, Jr, et al. A new world revealed: early experience with digital ureteroscopy. J Urol. 2008 Mar;179(3):970–975. doi: 10.1016/j.juro.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 4.Miller NL, Humphreys MR, Coe FL, et al. Nephrocalcinosis: re-defined in the era of endourology. Urol Res. 2010 Dec;38:421–427. doi: 10.1007/s00240-010-0328-8. [NIHMS515482] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AP, Worcester EM, Coe FL, et al. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl 1):S19–S32. doi: 10.1007/s00240-014-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;108:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. The Journal of Clinical Investigation. 2003 Mar;111(5):607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Intl. 2005 Feb;67(2):576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 9.Evan AP, Lingeman JE, Worcester EM, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxyapatite, brushite, or calcium oxalate stones. Anat Rec. 2014 Apr;297(4):731–748. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krambeck AE, Handa SE, Evan AP, et al. Profile of the brushite stone former. J Urol. 2010 Oct;184:1367–1371. doi: 10.1016/j.juro.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evan AP, Worcester EM, Williams JC, Jr, et al. Biopsy proven medullary sponge kidney: clinical findings, histopathology, and role of osteogenesis in stone and plaque formation. Anatomical Record. doi: 10.1002/ar.23105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheidde L, Ajzen SA, Tamer Langen CH, et al. A critical appraisal of the radiological evaluation of nephrocalcinosis. Nephron Clin Pract. 2007;106(3):c119–124. doi: 10.1159/000102999. [DOI] [PubMed] [Google Scholar]
- 13.Dick PT, Shuckett BM, Tang B, et al. Observer reliability in grading nephrocalcinosis on ultrasound examinations in children. Pediatr Radiol. 1999 Jan;29(1):68–72. doi: 10.1007/s002470050539. [DOI] [PubMed] [Google Scholar]
- 14.Manz F, Jaschke W, van Kaick G, et al. Nephrocalcinosis in radiographs, computed tomography, sonography and histology. Pediatr Radiol. 1980;9(1):19–26. doi: 10.1007/BF00973964. [DOI] [PubMed] [Google Scholar]
- 15.Gdor Y, Faddegon S, Krambeck AE, et al. Multi-institutional assessment of ureteroscopic laser papillotomy for chronic flank pain associated with papillary calcifications. J Urol. 2011 Jan;185:192–197. doi: 10.1016/j.juro.2010.08.081. [DOI] [PubMed] [Google Scholar]
- 16.Mandel N, Mandel I, Fryjoff K, et al. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026–2029. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 17.Parks JH, Worcester EM, Coe FL, et al. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Intl. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]