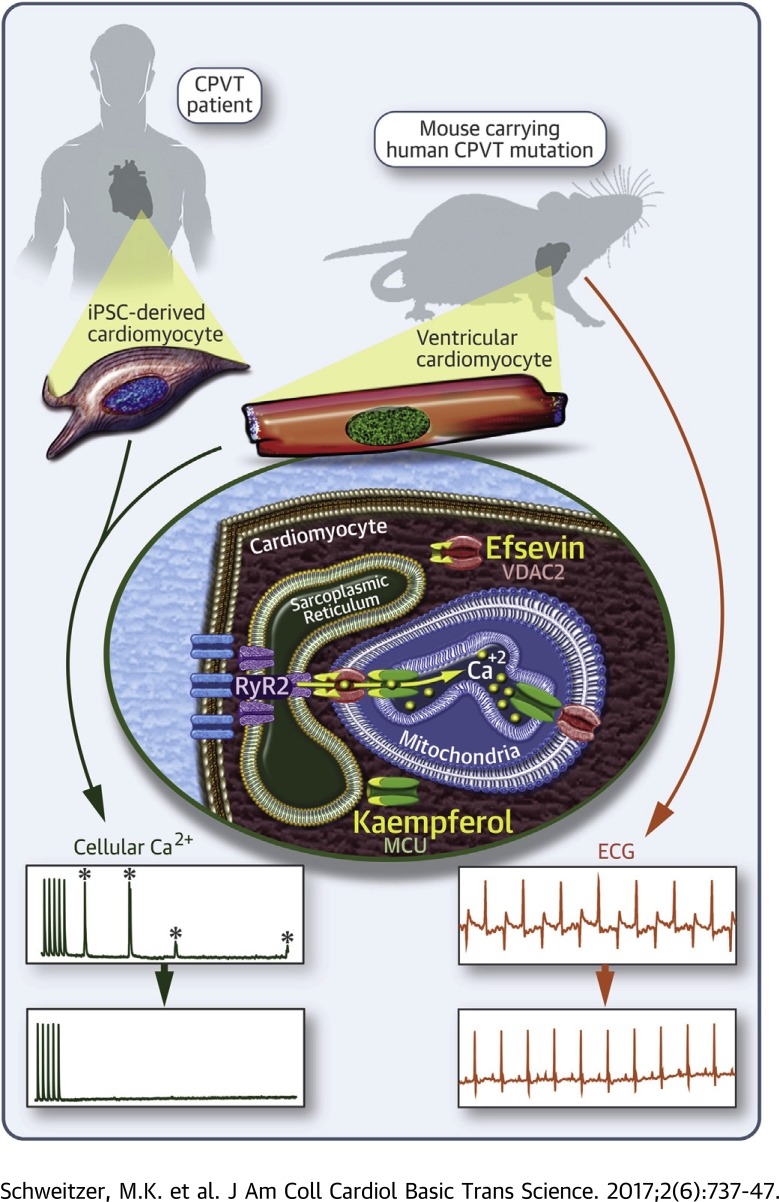
Visual Abstract
Key Words: CPVT, MCU, MiCUp, mitochondria, RyR2, VDAC2
Abbreviations and Acronyms: CPVT, catecholaminergic polymorphic ventricular tachycardia; epi/caff, epinephrine/caffeine; iPSC, induced pluripotent stem cell; ISO, isoproterenol; MCU, mitochondrial calcium uniporter; MiCUp, mitochondrial calcium uptake enhancer; RyR2, ryanodine receptor type 2; SR, sarcoplasmic reticulum; VDAC2, voltage-dependent anion channel type 2; WT, wild type
Highlights
-
•
Fast transfer of Ca2+ from the sarcoplasmic reticulum into mitochondria in cardiomyocytes can be enhanced by the MiCUps efsevin, targeting the VDAC2, and kaempferol, targeting the MCU.
-
•
Enhancing sarcoplasmic reticulum-to-mitochondria Ca2+ transfer with MiCUps suppresses arrhythmogenic Ca2+ events and spontaneous action potentials in cardiomyocytes from a mouse model of CPVT.
-
•
In vivo treatment of CPVT mice with MiCUps reduces episodes of ventricular tachycardia after adrenergic stimulation.
-
•
In induced pluripotent stem cell-derived cardiomyocytes from a CPVT patient, both MiCUps reduce arrhythmogenic Ca2+ events.
-
•
Our data establish fast mitochondrial Ca2+ uptake as a promising candidate structure for pharmacological treatment of human cardiac arrhythmia.
Summary
Cardiovascular disease-related deaths frequently arise from arrhythmias, but treatment options are limited due to perilous side effects of commonly used antiarrhythmic drugs. Cardiac rhythmicity strongly depends on cardiomyocyte Ca2+ handling and prevalent cardiac diseases are causally associated with perturbations in intracellular Ca2+ handling. Therefore, intracellular Ca2+ transporters are lead candidate structures for novel and safer antiarrhythmic therapies. Mitochondria and mitochondrial Ca2+ transport proteins are important regulators of cardiac Ca2+ handling. Here, the authors evaluated the potential of pharmacological activation of mitochondrial Ca2+ uptake for the treatment of cardiac arrhythmia. To this aim, the authors tested substances that enhance mitochondrial Ca2+ uptake for their ability to suppress arrhythmia in a murine model for ryanodine receptor 2 (RyR2)-mediated catecholaminergic polymorphic ventricular tachycardia (CPVT) in vitro and in vivo and in induced pluripotent stem cell-derived cardiomyocytes from a CPVT patient. In freshly isolated cardiomyocytes of RyR2R4496C/WT mice efsevin, a synthetic agonist of the voltage-dependent anion channel 2 (VDAC2) in the outer mitochondrial membrane, prevented the formation of diastolic Ca2+ waves and spontaneous action potentials. The antiarrhythmic effect of efsevin was abolished by blockade of the mitochondrial Ca2+ uniporter (MCU), but could be reproduced using the natural MCU activator kaempferol. Both mitochondrial Ca2+ uptake enhancers (MiCUps), efsevin and kaempferol, significantly reduced episodes of stress-induced ventricular tachycardia in RyR2R4496C/WT mice in vivo and abolished diastolic, arrhythmogenic Ca2+ events in human iPSC-derived cardiomyocytes. These results highlight an immediate potential of enhanced mitochondrial Ca2+ uptake to suppress arrhythmogenic events in experimental models of CPVT and establish MiCUps as promising pharmacological tools for the treatment and prevention of Ca2+-triggered arrhythmias such as CPVT.
Cardiovascular diseases remain the leading cause of death worldwide (1). Approximately one-half of all cardiovascular deaths are attributed to severe cardiac arrhythmia (2). Cardiac rhythmicity critically depends on precisely regulated Ca2+ oscillations in cardiomyocytes (3), and cardiac arrhythmia is frequently associated with perturbations of cellular Ca2+ handling 4, 5. Most currently used antiarrhythmic drugs target receptors and channels in the cell membrane (sarcolemma) to block the propagation of arrhythmic events along the myocardium but do not restore defective intracellular Ca2+ handling. Due to their modulatory effects on the cardiac action potential, these drugs often show pro-arrhythmic side effects. Therefore, intracellular Ca2+ transporters are candidate structures for novel and safer antiarrhythmic therapies.
Mitochondria occupy approximately 30% of the cardiomyocyte volume 6, 7 and closely interact with the sarcoplasmic reticulum (SR) to absorb Ca2+, which is released from the SR into the cytosol through cardiac ryanodine receptors (RyR2) 8, 9. For that, mitochondria are equipped with a regulated network of Ca2+ transporters. While the mitochondrial calcium uniporter (MCU) has been identified as the main route for Ca2+ import in the inner mitochondrial membrane 10, 11, 12, the voltage-dependent anion channel 2 (VDAC2) in the outer mitochondrial membrane has only recently been shown to be a major part of the Ca2+ transport route from the SR into mitochondria 13, 14, 15. In addition to the 2 pore-forming channels, the mitochondrial Ca2+ uptake complex is regulated by several auxiliary subunits such as the essential MCU regulator EMRE or the mitochondrial Ca2+ uptake proteins MICU1 and -2 16, 17. While it is generally accepted that a slow and moderate rise of mitochondrial Ca2+ triggers enhanced energy production under higher workload (18), the immediate role of mitochondria in shaping cellular Ca2+ signals on a beat-to-beat level remains a matter of debate 19, 20. Mitochondria have been demonstrated to be regulators of cardiac rhythmicity 21, 22, but their potential to serve as therapeutic targets has not been evaluated so far. The synthetic compound efsevin has been newly identified to activate VDAC2 and to enhance mitochondrial Ca2+ uptake (15). Using efsevin, we demonstrated that cardiac rhythmicity in zebrafish embryos can be critically modulated by enhancing mitochondrial Ca2+ uptake (15).
This study evaluated the translational potential of pharmacological activation of mitochondrial Ca2+ uptake for the prevention and treatment of cardiac arrhythmia. To this end, we used 2 models of catecholaminergic polymorphic ventricular tachycardia (CPVT), exemplifying a Ca2+-triggered arrhythmia. CPVT manifests in early adolescence and is characterized by episodes of life-threatening ventricular tachycardia upon catecholaminergic stimulation after physical exercise or emotional stress. The RyR2R4496C/WT mouse harbors an R4496C mutation in the SR Ca2+ release channel RyR2, homologous to the human R4497C mutation, which is associated with CPVT (23) and displays a CPVT phenotype (24). We show that activating mitochondrial Ca2+ uptake suppresses arrhythmia in RyR2R4496C/WT cardiomyocytes in vitro and in RyRR4496C/WT mice in vivo. Finally, we demonstrate that activating mitochondrial Ca2+ uptake is likewise efficient in blocking arrhythmogenesis in induced pluripotent stem cell (iPSC)-derived cardiomyocytes from a CPVT patient heterozygous for a different RyR2 mutation (RyR2S406L).
Methods
Isolation of cardiomyocytes
Isolation of ventricular cardiomyocytes from heterozygous knock-in RyR2R4496C/WT mice (24) was performed using a Langendorff perfusion-based enzymatic digestion protocol (25) with minor modifications. Only excitable, rod-shaped, quiescent cells were used for experiments.
Human iPSC-based model
iPSCs from a skin biopsy from a CPVT patient carrying the RyR2S406L/WT mutation and from a healthy donor were differentiated into spontaneously beating explants 26, 27 and enzymatically dissociated into single cardiomyocytes.
Ca2+ imaging
Ca2+ transients, spontaneous diastolic Ca2+ waves, and Ca2+ sparks were measured in cardiomyocytes loaded with Fluo-4 acetoxymethyl ester (AM) (Thermo Fisher Scientific, Darmstadt, Germany), using confocal microscopy in line scan mode. Cells were paced by extracellular electrodes at 0.5 Hz.
Electrophysiology
Action potentials were recorded in current clamp mode, using the perforated patch-clamp technique. Cells were paced by repetitive, depolarizing intracellular current injections at 0.5 Hz, followed by a 60-s pause to detect potentially pro-arrhythmic events during this diastolic phase.
cAMP accumulation assay
For evaluation of intracellular cAMP levels, cardiomyocytes were labeled with 3H-labeled adenine to measure accumulation of [3H]cAMP over 15 min.
In vivo arrhythmia testing
Drugs were administered to RyR2R4496C/WT mice 8 to 12 weeks of age through osmotic minipumps, and subsequently, electrocardiography recordings were performed under light isoflurane anesthesia, to monitor ventricular tachycardia after bolus injection of epinephrine/caffeine (epi/caff). All animal procedures were performed in accordance with national and European ethical regulations (directive 2010/63/EU) and approved by the responsible government agency (BMWFW-66.010/0012-WF/V/3b/2015).
Mitochondrial Ca2+ uptake
Mitochondrial Ca2+ uptake in response to 10 mM caffeine to open RyR2 was measured in Rhod-2 AM-loaded permeabilized HL-1 cardiomyocytes (28) on a fluorescence 96-well plate reader. The Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (1 mM) was used to restrict Ca2+ to the low micrometer range around RyR clusters 8, 29.
Statistical analysis
Data are mean ± standard error of the mean. Normality of data was determined by Shapiro-Wilk test, and respective tests for statistical significance were conducted as indicated. Post hoc tests were Dunn’s test for Kruskal-Wallis tests and Tukey’s multiple comparisons test for ANOVA. Significance for contingency tables was calculated using Fisher’s exact test.
Please see the detailed Methods in the Supplemental Appendix.
Results
Efsevin reduces arrhythmogenic Ca2+ waves in RyR2R4496C/WT cardiomyocytes
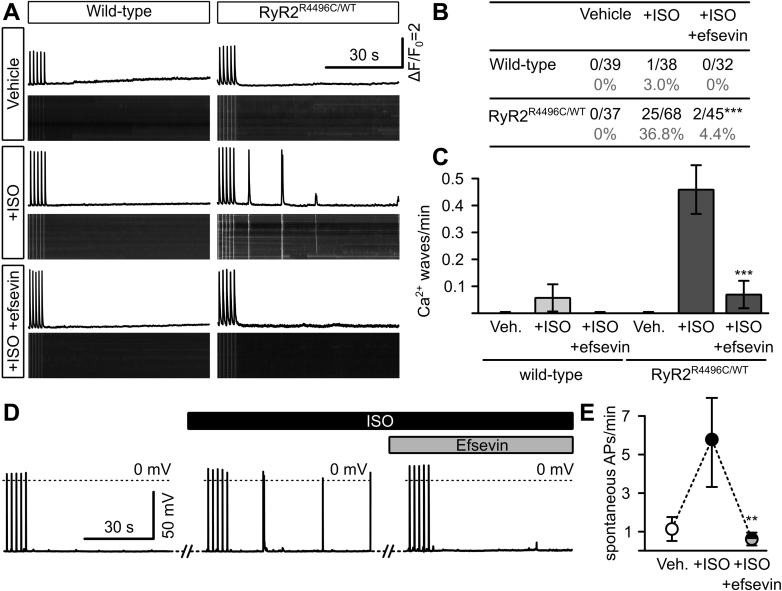
The triggers for arrhythmia originating from imbalanced cellular Ca2+ homeostasis, such as CPVT, are intracellular Ca2+ waves during diastole, which arise from an increased SR Ca2+ leak through RyR2 (30). We therefore recorded diastolic Ca2+ waves in freshly isolated ventricular cardiomyocytes from RyR2R4496C/WT mice and their wild-type (WT) littermates. Under control conditions (vehicle), neither WT cardiomyocytes nor RyR2R4496C/WT cells showed spontaneous Ca2+ waves within 90 s after preceding electrical stimulation at 0.5 Hz. However, unlike WT cells, RyR2R4496C/WT cardiomyocytes displayed pronounced spontaneous, diastolic Ca2+ waves after stimulation with the catecholamine isoproterenol (ISO) (Figures 1A to 1C). Strikingly, application of 15 μM efsevin significantly reduced the number of cells displaying such Ca2+ waves and the average number of Ca2+ waves per minute in RyR2R4496C/WT cardiomyocytes to levels of unstimulated RyR2R4496C/WT cells and WT cells. Notably, in contrast to previous data from unstimulated cells (15), efsevin did not exert any significant effects on the amplitude and kinetics of electrically evoked Ca2+ transients under ISO stimulation (Supplemental Figure 1).
Figure 1.
Efsevin Reduces Diastolic Ca2+ Waves and Spontaneous Action Potentials in RyR2R4496C/WT Cardiomyocytes
(A) Confocal line scans across the long axis of freshly isolated cardiomyocytes from wild-type and RyR2R4496C/WT mice loaded with Fluo-4 AM to measure intracellular Ca2+. Images show line scans, and graphs depict fluorescence intensity plots. Cells were stimulated at 0.5 Hz for 30 s to reach steady state conditions (last 5 Ca2+ transients are shown) before pulsing was stopped and the diastolic phase was recorded for 90 s to screen for spontaneous diastolic Ca2+ waves. (B) While wild-type cells never showed a significant frequency of spontaneous diastolic Ca2+ waves, 25 of 68 RyR2R4496C/WT cardiomyocytes showed waves, which could be significantly reduced to 2 of 45 cells (p < 0.001, Fisher’s exact test) by addition of 15 μM efsevin. (C) Average number of Ca2+ waves per minute was significantly enhanced from 0 in vehicle-treated RyR2R4496C/WT cardiomyocytes to 0.46 ± 0.09 waves/min in cells treated with 1 µM ISO (p < 0.001, Kruskal-Wallis test). Addition of 15 µM efsevin reduced the number of waves/minute to 0.07 ± 0.05 to levels indistinguishable from untreated cells (p = 0.578). (D) Patch clamp recordings from RyR2R4496C/WT cardiomyocytes showed an increase in spontaneous action potentials after superfusion with ISO, which were eliminated by addition of 15 μM efsevin. (E) Quantitative analysis of patch clamp recordings revealed a significant increase of spontaneous diastolic action potentials (APs) from 1.12 ± 0.62 APs/min under vehicle (Veh.) to 5.76 ± 2.45 APs/min after superfusion with ISO (p = 0.018, Friedman test, n = 15) and a significant reduction to 0.58 ± 0.34 APs/min when simultaneously treated with ISO and efsevin (p = 0.011). ∗∗p < 0.01; ∗∗∗p < 0.001. ISO = isoproterenol.
Efsevin reduces spontaneous action potentials in RyR2R4496C/WT cardiomyocytes
Cardiac arrhythmia is triggered when Ca2+ waves activate the sarcolemmal sodium–calcium exchanger, leading to a transient depolarizing sodium inward current and finally spontaneous action potentials (31), which can propagate along the myocardium. Hence, we recorded spontaneous action potentials in patch-clamped RyR2R4496C/WT cardiomyocytes. We observed a significant increase in the frequency of spontaneous action potentials after cells were superfused with 1 µM ISO. Subsequent treatment with 15 μM efsevin effectively reduced these spontaneous depolarizations to baseline levels (Figures 1D and 1E). Notably, efsevin did not exert any effects on the resting membrane potential and amplitude of electrically evoked action potentials under ISO stimulation but caused a significant change of the repolarization phase (Supplemental Figure 1), namely a prolongation of the action potential duration at 50% repolarization (APD50) but not at 90% repolarization (APD90). To evaluate whether this effect seen on the fast, inactivating mouse action potential (32) could lead to QT prolongation in human cells, we recorded action potentials from human iPSC-derived cardiomyocytes in the presence of efsevin and found no changes in APD50 and APD90 compared to vehicle-treated cells (Supplemental Figures 2A and 2B). Furthermore, efsevin did not inhibit hERG channel activity in a heterologous expression system at relevant concentrations (Supplemental Figure 2C).
The Antiarrhythmic effect of efsevin is mediated by mitochondrial Ca2+ uptake
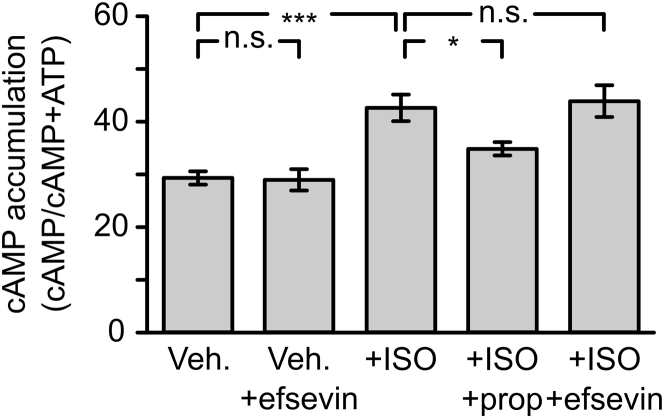
We next investigated the mechanism of efsevin’s antiarrhythmic effect. To exclude the possibility that efsevin directly blocks catecholaminergic stimulation by ISO, we measured cAMP accumulation in efsevin-treated RyR2R4496C/WT cardiomyocytes. Stimulation by ISO induced a significant increase in cellular cAMP, which was blocked by the beta-adrenoreceptor blocker propranolol. Addition of efsevin alone did not increase or decrease cellular cAMP concentrations, and addition of efsevin to stimulated cells had no effect on the ISO-induced cAMP increase, indicating that efsevin does not influence beta-adrenergic signaling in cardiomyocytes (Figure 2).
Figure 2.
Efsevin Does Not Affect Beta-Adrenergic Stimulation in RyR2R4496C/WT Cardiomyocytes
cAMP accumulation in freshly isolated RyR2R4496C/WT cardiomyocytes was measured in response to different stimuli. Efsevin had no effect on cellular cAMP levels (p > 0.999, Kruskal-Wallis test), whereas ISO induced a significant increase in cellular cAMP (p < 0.001). This increase could be blocked using the beta-adrenoreceptor blocker propranolol (prop) (p = 0.036), although efsevin had no effect on the ISO-induced increase in cellular cAMP (p = 0.999), indicating that efsevin neither activates nor blocks the beta-adrenergic system in cardiomyocytes. ∗p < 0.05; ∗∗∗p < 0.001. Abbreviations as in Figure 1.
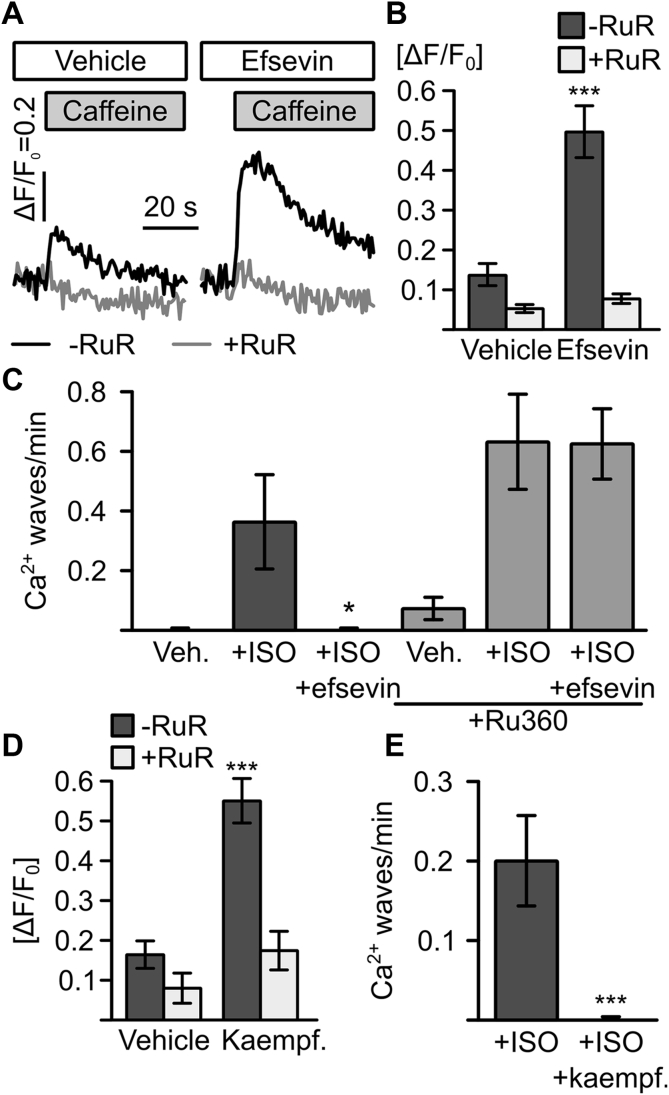
Efsevin significantly enhanced transfer of Ca2+ from the SR into mitochondria in a Ca2+ uptake assay in permeabilized cultured HL-1 cardiomyocytes. Mitochondrial Ca2+ was measured after addition of 10 mM caffeine to open RyR2s in the presence of the Ca2+ chelator BAPTA to restrict Ca2+ released from RyRs to the low micrometer range around RyR clusters 8, 29 (Figure 3A). The efsevin-sensitive Ca2+ transfer between the SR and mitochondria was blocked by addition of the MCU blocker ruthenium red. To test whether the enhanced SR–mitochondria Ca2+ transfer was directly responsible for the reduction of diastolic Ca2+ wave frequency in RyR2R4496C/WT cardiomyocytes, we assessed whether blocking of mitochondrial Ca2+ uptake abolished the antiarrhythmic effect of efsevin. We measured catecholamine-induced Ca2+ waves in RyR2R4496C/WT myocytes under simultaneous blockade of mitochondrial Ca2+ uptake, using the MCU inhibitor Ru360 (Figure 3B). We observed a moderately higher Ca2+ wave frequency under all conditions, consistent with the idea that mitochondrial Ca2+ uptake prevents Ca2+ wave formation. Most strikingly, the ability of efsevin to suppress the ISO-induced increase in Ca2+ wave frequency was abolished in the presence of Ru360, indicating that the suppression of diastolic Ca2+ waves by efsevin is solely mediated by enhanced mitochondrial Ca2+ uptake.
Figure 3.
Efsevin Acts Through Mitochondrial Ca2+ Uptake
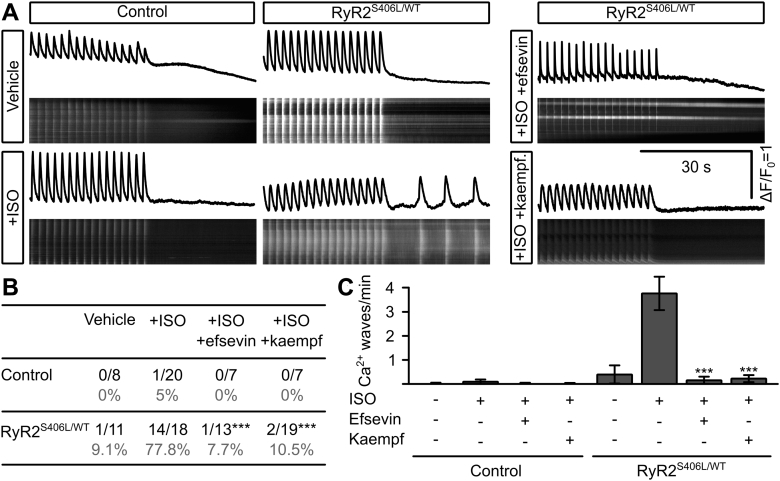
(A) Representative recordings of mitochondrial Ca2+ in permeabilized HL-1 cardiomyocytes. Superfusion with caffeine induced a rapid uptake of Ca2+ into mitochondria (black trace), which was enhanced by 15 μM efsevin and blocked by 10 μM RuR (gray trace). (B) Quantification of peak mitochondrial fluorescence showed a significant increase in mitochondrial Ca2+ uptake in cells treated with efsevin from ΔF/F0 = 0.14 ± 0.03 (n = 10) to 0.50 ± 0.07 (n = 19, p < 0.001, ANOVA). (C) 15 µM efsevin reduces spontaneous propagating waves in RyR2R4496C/WT cardiomyocytes under ISO influence from 0.36 ± 0.16 waves/min (n = 15) to 0 (black bars) (n = 21, p = 0.035, Kruskal-Wallis test). In the presence of 8 µM of the mitochondrial Ca2+ uptake blocker Ru360, vehicle-treated cells showed 0.07 ± 0.04 waves/min (n = 29), which increased to 0.63 ± 0.16 under ISO influence (n = 21, p < 0.001) but could not be blocked with efsevin as shown by an indistinguishable value of 0.62 ± 0.12 waves/min under ISO influence together with efsevin (gray bars) (n = 62, p = 0.354 compared to that with ISO). (D) 10 µM of the MCU activator kaempferol significantly increased mitochondrial Ca2+ uptake after SR Ca2+ release from ΔF/F0 = 0.16 ± 0.04 (n = 9) to 0.55 ± 0.05 (n = 10, p < 0.001, ANOVA) Ca2+ uptake was inhibited by 50 μM RuR (gray bars). (E) Treatment of RyR2R4496C/WT cardiomyocytes with 10 μM kaempferol completely eliminated ISO-induced spontaneous diastolic Ca2+ waves from 0.19 ± 0.04 waves/min (n = 49) to 0 (n = 45; p < 0.001, Mann-Whitney U test). *p < 0.05; ***p < 0.001. ΔF/F0 = change in fluorescence over baseline fluorescence; MCU = mitochondrial Ca2+ uniporter; RuR = ruthenium red; SR = sarcoplasmic reticulum.
We next tested if enhancing mitochondrial Ca2+ uptake was a general pharmacological approach to suppress arrhythmogenic events or if it is limited to a specific effect of efsevin on VDAC2. To this purpose, we used another activator of mitochondrial Ca2+ uptake, the natural plant flavonoid kaempferol, which was reported to directly activate MCU in the inner mitochondrial membrane 33, 34. Indeed, kaempferol increased SR–mitochondria Ca2+ transfer, comparable to efsevin (Figure 3D). To evaluate the antiarrhythmic potential of kaempferol, we measured diastolic Ca2+ waves in kaempferol-treated RyR2R4496C/WT cardiomyocytes under ISO to induce catecholaminergic stimulation. Strikingly, 10 μM kaempferol completely eliminated ISO-induced arrhythmogenic Ca2+ waves in RyR2R4496C/WT cardiomyocytes (Figure 3E).
It was previously reported that enhanced mitochondrial Ca2+ uptake in cardiomyocytes restricts diffusion of Ca2+ inside the cytosol (13) and thereby prevents propagation of cytosolic Ca2+ signals under conditions of Ca2+ overload (15). Because an enhanced RyR-mediated Ca2+ leak was reported to be the mechanism responsible for arrhythmogenesis in CPVT 35, 36, 37, we recorded Ca2+ sparks from RyR2R4496C/WT cardiomyocytes (Figure 4). We observed an increase in Ca2+ spark frequency and amplitude after treatment with ISO, consistent with previous work (36), thus explaining the enhanced Ca2+ wave frequency under catecholaminergic stimulation. Strikingly, the SR leak in cells treated with ISO together with efsevin was reduced compared to that in cells treated with ISO alone, as indicated by a decrease in Ca2+ spark frequency and amplitude. Also, we found removal of cytosolic Ca2+ was accelerated under efsevin stimulation, leading to a reduction of full width and full duration of Ca2+ sparks. Together, these effects explain the suppressive effect of efsevin on propagating Ca2+ waves.
Figure 4.
Efsevin Reduces Intracellular Ca2+ Spark Frequency, Amplitude, and Cytosolic Ca2+ Clearance Leading to a Restriction of Individual Ca2+ Sparks
(A) Representative line scan confocal images from RyR2R4496C/WT cardiomyocytes show spontaneous Ca2+ sparks. (B) Treatment with ISO induced a higher spark frequency and amplitude and accelerated cytosolic Ca2+ removal (taudecay) leading to narrower (full width) and shorter (full duration) sparks. Administration of efsevin reduced Ca2+ spark frequency (p = 0.049, Kruskal Wallis test) and amplitude (p < 0.001, Kruskal Wallis test) and limited spatial (p < 0.001, Kruskal Wallis test) and temporal (p = 0.002, Kruskal Wallis test) expansion of Ca2+ sparks. *p < 0.05; **p < 0.01; ***p < 0.001. Abbreviations as in Figure 1.
MiCUps reduce episodes of stress-induced ventricular tachycardia in vivo
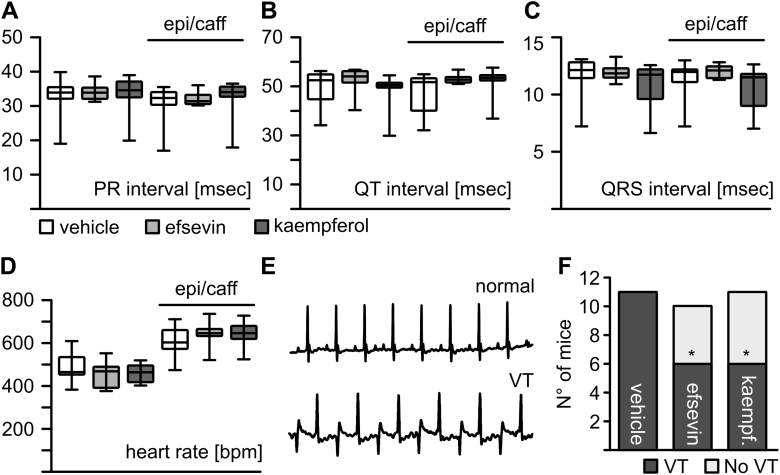
To assess the potency of both of the mitochondrial Ca2+ uptake enhancers (MiCUps), efsevin and kaempferol, to suppress arrhythmia in vivo, we administered efsevin and kaempferol to RyR2R4496C/WT mice, using implantable osmotic minipumps. All mice recovered well from surgery, and their behavior was grossly normal. Treated mice showed no signs of discomfort, stress, or abnormal behavior. After 3 days, efsevin and kaempferol showed no effect on electrocardiography (ECG) parameters such as the interval between atrial and ventricular depolarization (PR), the interval between ventricular depolarization and subsequent repolarization (QT), or conduction of ventricular depolarization (QRS) and basal heart rate (Figures 5A and 5B). To activate their adrenergic response, we injected mice with a bolus of 2 mg/kg epinephrine and 120 mg/kg caffeine (epi/caff). The epi/caff injection induced a significant increase in heart rate in all 3 groups, but no differences were observed between vehicle-treated mice and mice treated with MiCUps. Most strikingly, both of the MiCUps significantly reduced episodes of bidirectional ventricular tachycardia under catecholaminergic stimulation. Injection of epi/caff provoked bidirectional ventricular tachycardia in all vehicle-treated control animals (n = 11) but only in 6 of 10 mice treated with efsevin and 6 of 11 mice treated with kaempferol (Figures 5D and 5E). Comparable results were obtained from mice treated with efsevin for 8 days (Supplemental Figure 3).
Figure 5.
MiCUps Reduce Episodes of Ventricular Tachycardia in RyR2R4496C/WT Mice
Three-lead surface ECGs from RyR2R4496C/WT mice receiving efsevin or kaempferol through an osmotic minipump are shown. After a baseline ECG was recorded, mice were injected with epi/caff as an adrenergic stimulus. Basic parameters of the ECG like PR (A), QT (B), and QRS (C) intervals were comparable between the vehicle-treated group and both of the MiCUp-treated groups at baseline and after epi/caff challenge (Kruskal-Wallis test). (D) Heart rate increased upon administration of epi/caff in all 3 groups but remained unaffected by the administration of MiCUps (Kruskal-Wallis test). (E) Examples of a normal ECG recording and a recording showing bidirectional ventricular tachycardia. (F) Episodes of bidirectional ventricular tachycardia were recorded in 11 of 11 vehicle-treated mice but were significantly reduced to 6 of 10 in mice with efsevin and 6 of 11 in mice with kaempferol (p = 0.035, Fisher’s exact test). *p < 0.05. ECG = electrocardiography; epi/caff = epinephrine/caffeine; MiCUp = mitochondrial calcium uptake enhancer.
MiCUps reduce arrhythmogenic Ca2+ waves in iPSC-derived cardiomyocytes from a CPVT patient
In order to evaluate the translational potential of MiCUps for the treatment of CPVT, we used a human cell-based arrhythmia model for CPVT. Human iPSC-derived cardiomyocytes from a CPVT patient heterozygous for the RyR2S406L mutation associated with CPVT (26) were used to record arrhythmogenic Ca2+ waves, whereas cells obtained from a healthy 32-year-old female without history of cardiac disease served as control. In accordance with the CPVT phenotype, where patients show a normal ECG pattern under baseline conditions, only few untreated cells displayed Ca2+ waves (Figure 6), whereas beta-adrenergic stimulation induced by ISO led to a significant increase in diastolic Ca2+ waves in RyR2S406L/WT cells but did not induce Ca2+ waves in control cells. Treatment of RyR2S406L cells with either efsevin or kaempferol significantly reduced the number of cells displaying Ca2+ waves and the average frequency of Ca2+ waves per minute to baseline levels.
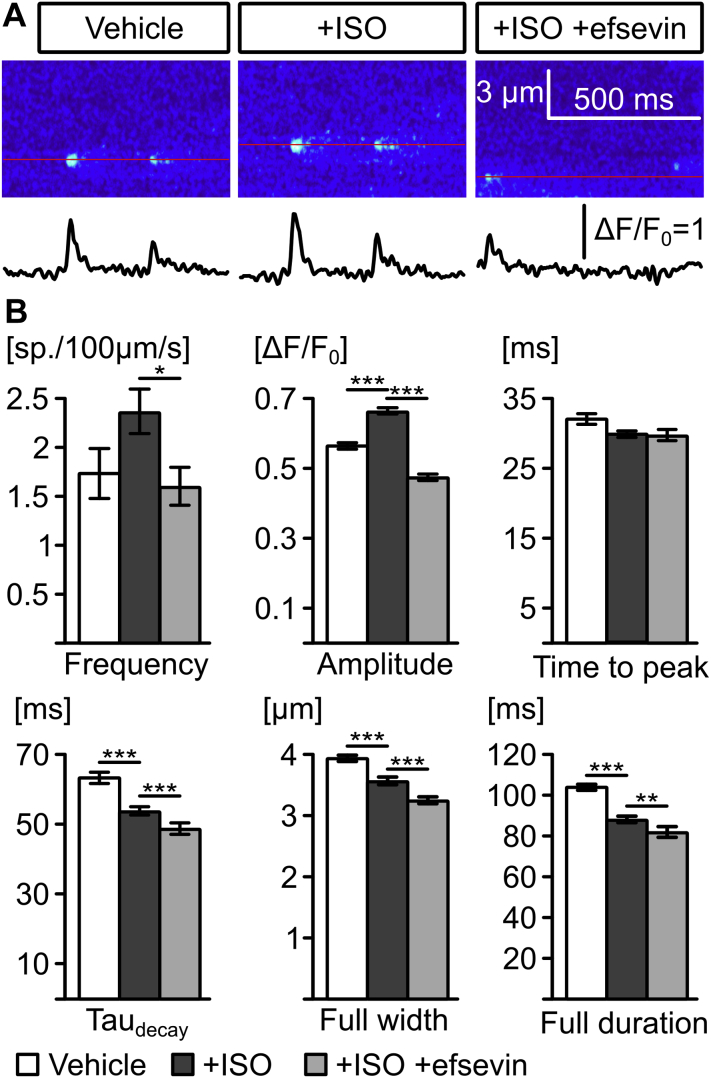
Figure 6.
MiCUps Reduce Spontaneous Ca2+ Waves in Human iPSC-derived Cardiomyocytes
Confocal line scans of iPSC-derived human cardiomyocytes from a CPVT patient (RyR2S406L/WT) and a healthy individual are shown (control). (A) Confocal line scans across iPSC-derived human cardiomyocytes loaded with Fluo-4 AM to measure intracellular Ca2+ and intensity plots show that stimulation with ISO does not induce spontaneous, diastolic Ca2+ waves in control cells but induced waves in RyR2S406L/WT cardiomyocytes. These waves can be blocked with either 15 μM efsevin (upper right panel) or 10 μM kaempferol (lower right panel). (B) Quantitative analysis revealed a significant reduction in the number of RyR2S406L/WT cells showing ISO-induced Ca2+ waves upon treatment with MiCUps, to a level comparable to that of unstimulated cells (asterisks denote significance compared to RyR2S406L/WT +ISO by Fisher’s exact test). (C) Quantification of waves/min reveal a significant reduction from 3.78 ± 0.72 waves/min in RyR2S406L/WT cardiomyocytes treated with ISO to levels indistinguishable from control after treatment with efsevin (0.15 ± 0.15; p < 0.001 compared to ISO: p = 0.884 compared to vehicle, Kruskal-Wallis test) or kaempferol, respectively (0.21 ± 0.14, p < 0.001 compared to ISO; p = 0.980 compared to vehicle, Kruskal-Wallis test). ***p < 0.001. CPVT = catecholaminergic polymorphic ventricular tachycardia; iPSC = induced pluripotent stem cell; other abbreviations as in Figure 1.
Discussion
Mitochondria as drug targets
Mitochondria occupy approximately 30% of the cardiomyocyte volume 6, 7. Although their crucial roles in ATP synthesis, regulation of respiratory rate, and apoptosis are well understood, mitochondrial contributions to cardiac Ca2+ handling are still under debate. While it is generally accepted that a gradual and moderate rise in mitochondrial Ca2+ enhances energy production (18), the role of a low-affinity/high-conductance, fast mitochondrial Ca2+ uptake remains unclear. Different experimental approaches suggest an immediate role for this uptake in the regulation of cardiomyocyte bioenergetics (38), contraction (39), and rhythmicity 15, 21, but the potential of this rapid mitochondrial Ca2+ uptake mechanism to serve as a drug target in cardiovascular disease has not been sufficiently evaluated. It was proposed that inhibition of MCU by Ru360 ameliorates myocardial damage after ischemia reperfusion injury in rats, presumably by inhibiting depolarization of mitochondria and following opening of the mitochondrial permeability transition pore (40). However, in healthy myocardium, the role of fast mitochondrial Ca2+ uptake remains elusive. Regarding arrhythmia, protective (22) as well as pro-arrhythmic (41) effects of activated mitochondria were discussed. We have recently identified the novel compound efsevin, which binds to the outer mitochondrial membrane VDAC2, enhances mitochondrial Ca2+ uptake, and restores rhythmic cardiac contractions in a zebrafish model for Ca2+-induced cardiac arrhythmia (15). Here we show that enhancing mitochondrial Ca2+ uptake by using MiCUps efficiently suppresses arrhythmia in a murine and a human model of CPVT in vitro and in vivo. Our results establish pharmacological activation of rapid mitochondrial Ca2+ uptake as a novel preventive and therapeutic strategy against CPVT. We have previously shown that efsevin suppresses arrhythmia in cellular models for Ca2+ overload induced by high extracellular Ca2+ (15). Thus, due to their potent role of suppressing arrhythmogenic Ca2+ waves in both Ca2+ overload (15) and CPVT, it is conceivable that MiCUps may also be applied in other more common forms of Ca2+-induced cardiac arrhythmias. These include arrhythmias in the setting of heart failure, which are triggered by cellular Ca2+ overload or atrial fibrillation, also linked to imbalances in cardiomyocyte Ca2+ handling (5). This study serves as a proof-of-principle, holding great promise for additional indications.
Optimized MiCUps
Our work shows that enhancing mitochondrial Ca2+ uptake efficiently reduces arrhythmia in experimental models of CPVT. In order to develop MiCUps toward human therapeutics, several steps of optimization must be taken. Candidate compounds need to be optimized to achieve a high affinity MiCUp with low side effects and suitable pharmacokinetics for application in human subjects. We show that the antiarrhythmic effect can be achieved by activation of at least 2 distinct target proteins within the mitochondrial Ca2+ uptake complex: efsevin, targeting VDAC2 in the outer mitochondrial membrane, and kaempferol, targeting MCU in the inner membrane. Suppression of arrhythmia is thus attributable to enhanced mitochondrial Ca2+ uptake and is independent of the molecular target protein within the fast mitochondrial Ca2+ uptake complex. Our work thus establishes the entire protein complex as a pharmacological target structure and allows for future optimization of this therapeutic concept through novel compounds and targets. Apart from VDAC2 and MCU, the auxiliary MCU regulators MICU1 and -2, MCUb, EMRE, and MCUR1 16, 17 may serve as future candidate targets.
Side effects
Mitochondrial Ca2+ uptake proteins such as VDAC2 and MCU are ubiquitously expressed. Regarding a therapeutic application, it is thus important to evaluate potential side effects of a MiCUp-based therapy. It is important to note that we did not observe any adverse effects of MiCUps in mice treated with efsevin or kaempferol for 3 to 8 consecutive days. Furthermore, kaempferol was previously used in animal experiments, and no adverse effects, even after up to 1 year of treatment or at high doses, were observed 42, 43, 44. However, further long-term experiments and large animal studies are needed to further evaluate safety of a MiCUp-based therapy. Although we did not observe changes in cytosolic Ca2+ in our experiments, long-term effects of enhanced mitochondrial Ca2+ uptake on a potential redistribution of cellular Ca2+ in the heart and other organs must be evaluated. Furthermore, because an enhanced mitochondrial Ca2+ uptake was observed to activate mitochondrial metabolism and reactive oxygen species production, special focus should be directed toward side effects related to changes in cellular bioenergetics.
Common side effects of actual antiarrhythmic drugs like Na+, K+, and Ca2+ channel blockers include changes in cardiac electrophysiology like deceleration of cardiac de- or repolarization, the latter often expressed as a prolonged QT interval. We observed an effect of efsevin on the repolarization phase of the action potential in mice (Supplemental Figure 1), namely a prolongation of APD50 but not APD90. However, whereas repolarization in mice is carried mainly by fast potassium currents (Ito, IK, slow), the human action potential displays a pronounced plateau phase, and phase 3 repolarization is carried predominantly by the delayed K+ currents IKr and Iks (32). To rule out the possibility that the observed prolongation of APD50 by efsevin in mice could be relevant for human therapy, we showed that efsevin does not influence action potential duration in human iPSC-derived cardiomyocytes and does not block hERG activity. It is thus conceivable that efsevin has a direct impact on the fast repolarizing currents in mice but does not influence the human action potential. Most importantly, however, we did not observe effects of MiCUp administration on ECG parameters like PR, QT, and QRS interval and heart rate in mice treated with MiCUps. Because MiCUps target intracellular structures to suppress the generation of ectopic depolarizations and do not influence the cardiac action potential, they might be less prone to severe side effects like, for example, the typical pro-arrhythmic effects observed with class I or III antiarrhythmic drugs. However, the murine repertoire of ion channels governing the cardiac action potential of mice varies from the human one, and additional experiments in other mammalian species are needed to solve this issue.
Conclusions
Common antiarrhythmic drugs aim at inhibiting expansion of ectopic activity and display perilous side effects. Because major arrhythmias are often associated with imbalanced intracellular Ca2+ homeostasis 3, 4, 5, intracellular Ca2+ transporters are attractive candidates for newer and safer therapies. Here we show that enhancing mitochondrial Ca2+ uptake by pharmacological agonists of the mitochondrial Ca2+ uptake proteins VDAC2 and MCU efficiently suppresses arrhythmia in a murine and a human model for CPVT. Our data establish MiCUps as attractive compounds for a novel preventive and therapeutic strategy to treat Ca2+-triggered cardiac arrhythmias.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Mitochondria regulate cardiac rhythmicity. Pharmacological activation of mitochondrial Ca2+ uptake by MiCUps suppresses arrhythmogenesis in murine and human iPSC-based models for catecholaminergic polymorphic ventricular tachycardia.
TRANSLATIONAL OUTLOOK 1: Optimization of compounds and a careful investigation on pharmacokinetics and drug metabolism are needed to develop a potential MiCUp-based human therapy.
TRANSLATIONAL OUTLOOK 2: Additional experiments using models of other Ca2+-triggered arrhythmias could further expand the application range of MiCUps.
Acknowledgements
The authors thank Brigitte Mayerhofer for technical assistance and Petra Eigner, Monika Mittermeier, and Clarinda Hofer and the staff of the Institute for Biomedical Research of the Medical University of Graz for animal husbandry.
Footnotes
Supported by Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (SCHR 1471/1-1 to Dr. Schredelseker; TRR152 to Drs. Gudermann, Lipp, Moretti, and Laugwitz), the Friedrich-Baur-Stiftung, Munich, Germany (to Dr. Schredelseker), and U.S. National Institutes of Health (R01GM071779 to Dr. Kwon). Dr. Sedej is supported by the Austrian Science Fund FWF, Vienna, Austria (P27637-B28). Dr. Priori has received research support from Boston Scientific; and is a member of the advisory boards for General Electric and Audentes. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2016 update. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007;40:S118–S122. doi: 10.1016/j.jelectrocard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Bers D.M. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–17. [PubMed] [Google Scholar]
- 4.Choi B.-R., Burton F., Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiser M., Lederer W.J., Schotten U. Alterations of atrial Ca2+ handling as cause and consequence of atrial fibrillation. Cardiovasc Res. 2011;89:722–733. doi: 10.1093/cvr/cvq389. [DOI] [PubMed] [Google Scholar]
- 6.Barth E., Stämmler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.-D., Kim C.H., Rah Bb.-J., Chung H.-I., Shim T.-S. Quantitative study on the relation between structural and functional properties of the hearts from three different mammals. Anat Rec. 1994;238:199–206. doi: 10.1002/ar.1092380206. [DOI] [PubMed] [Google Scholar]
- 8.Szalai G., Csordás G., Hantash B.M., Thomas A.P., Hajnóczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 9.Dorn G.W., Maack C. SR and mitochondria: calcium cross-talk between kissing cousins. J Mol Cell Cardiol. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 11.Baughman J.M., Perocchi F., Girgis H.S. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subedi K.P., Kim J.-C., Kang M., Son M.-J., Kim Y.-S., Woo S.-H. Voltage-dependent anion channel 2 modulates resting Ca2+ sparks, but not action potential-induced Ca2+ signaling in cardiac myocytes. Cell Calcium. 2011;49:136–143. doi: 10.1016/j.ceca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Min C.K., Yeom D.R., Lee K.-E. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem J. 2012;447:371–379. doi: 10.1042/BJ20120705. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H., Schredelseker J., Huang J. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife. 2015;4 doi: 10.7554/eLife.04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhun B.S., Mishra J., Monaco S. The mitochondrial Ca2+ uniporter: regulation by auxiliary subunits and signal transduction pathways. Am J Physiol Cell Physiol. 2016;311:C67–C80. doi: 10.1152/ajpcell.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchi S., Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandes R., Bers D.M. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997;80:82–87. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Williams G.S.B., Boyman L., Chikando A.C., Khairallah R.J., Lederer W.J. Mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Rourke B., Blatter L.A. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seguchi H., Ritter M., Shizukuishi M. Propagation of Ca2+ release in cardiac myocytes: role of mitochondria. Cell Calcium. 2005;38:1–9. doi: 10.1016/j.ceca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z., Gordan R., Wen H., Fefelova N., Zang W.-J., Xie L.-H. Modulation of intracellular calcium waves and triggered activities by mitochondrial ca flux in mouse cardiomyocytes. PLoS One. 2013;8:e80574. doi: 10.1371/journal.pone.0080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priori S.G., Napolitano C., Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 24.Cerrone M., Colombi B., Santoro M. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell T.D., Rodrigo M.C., Simpson P.C. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 26.Jung C.B., Moretti A., Mederos y Schnitzler M. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti A., Bellin M., Welling A. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 28.Claycomb W.C., Lanson N.A., Stallworth B.S. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma V.K., Ramesh V., Franzini-Armstrong C., Sheu S.S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 30.Paavola J., Viitasalo M., Laitinen-Forsblom P.J. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur Heart J. 2007;28:1135–1142. doi: 10.1093/eurheartj/ehl543. [DOI] [PubMed] [Google Scholar]
- 31.Allen D.G., Eisner D.A., Orchard C.H. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nerbonne J.M., Nichols C.G., Schwarz T.L., Escande D. Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here? Circ Res. 2001;89:944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- 33.Montero M., Lobatón C.D., Hernández-Sanmiguel E. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vay L., Hernández-Sanmiguel E., Santo-Domingo J. Modulation of Ca2+ release and Ca2+ oscillations in HeLa cells and fibroblasts by mitochondrial Ca2+ uniporter stimulation. J Physiol. 2007;580:39–49. doi: 10.1113/jphysiol.2006.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang D., Xiao B., Zhang L., Chen S.R.W. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ Res. 2002;91:218–225. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Velasco M., Rueda A., Rizzi N. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedej S., Heinzel F.R., Walther S. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 38.Tomar D., Dong Z., Shanmughapriya S. MCUR1 Is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drago I., De Stefani D., Rizzuto R., Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Rivas G., de J., Carvajal K., Correa F., Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie W., Santulli G., Reiken S.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. doi: 10.1038/srep11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H., Bao J., Wei Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol Rep. 2014;33:868–874. doi: 10.3892/or.2014.3662. [DOI] [PubMed] [Google Scholar]
- 43.Montero M., de la Fuente S., Fonteriz R.I., Moreno A., Alvarez J. Effects of long-term feeding of the polyphenols resveratrol and kaempferol in obese mice. PLoS One. 2014;9:e112825. doi: 10.1371/journal.pone.0112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih T.-Y., Young T.-H., Lee H.-S., Hsieh C.-B., Hu O.Y.-P. Protective effects of kaempferol on isoniazid- and rifampicin-induced hepatotoxicity. AAPS J. 2013;15:753–762. doi: 10.1208/s12248-013-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.