Abstract
Acanthopanax senticosus, also known as Siberian ginseng, is widely distributed throughout northern Asia and used in traditional Chinese medicine; it has been reported to prevent a number of diseases. However, the association between the antitumour and immunostimulatory activities of polysaccharide extracted from A. senticosus (ASPS) remains to be elucidated. The aim of the present study was to investigate the anti-tumour and immunomodulatory effects of polysaccharide extracted from ASPS on Crocker sarcoma S180, hepatic carcinoma H22 and uterine cervical carcinoma U14 tumour cell lines implanted in mice. High performance liquid chromatography, gas chromatography and infrared spectroscopy were used to analyse the monosaccharide composition of ASPS. The monosaccharide composition of ASPS (Arabic candy: Xylose: Glucose: Mannose) was 7.1:22.3:7.6:1.0. On day 0, female Kunming mice, were injected subcutaneously with 1×108 tumour cells in 0.2 ml. The inoculated mice were subsequently divided into five groups (10 mice/group) as follows: Model group, treated with normal saline; positive control group, treated with 30 mg/kg cyclophosphamide (CTX); and three treatment groups, treated with 200, 100 or 50 mg/kg ASPS. Non-inoculated mice were divided into the normal group, which was treated with normal saline, and the negative control group, which was treated with 200 mg/kg ASPS (n=10/group). CTX and ASPS were administered intragastrically once daily for 10 days. All mice were sacrificed on day 11. ASPS was observed to have an inhibitory effect on the growth of S180, H22 and U14 cells in solid and ascites tumour-bearing mice. Serum interleukin (IL)-2 and IL-12 levels were significantly increased in S180 solid tumour-bearing mice treated with 200 or 100 mg/kg ASPS compared with mice in the normal, control and model groups (P<0.05), whereas serum IL-2 and IL-12 levels were significantly decreased in the cyclophosphamide treatment group compared with the normal, control and model groups (P<0.05). No significant difference in serum levels of tumour necrosis factor-α level was observed between any groups. In S180 and U14 solid tumour-bearing mice, no significant differences in serum levels of interferon (INF)-γ level in were observed between groups; however, in H22 solid tumour-bearing mice, treatment with ASPS significantly increased serum INF-γ compared with the positive control group (P<0.05). The results may provide a basis for the potential application of ASPS in clinical treatment for cancer.
Keywords: Acanthopanax senticosus, polysaccharide, cytokine, tumour, immunity
Introduction
Malignant cancer is a serious disease that has a negative impact on human health worldwide (1–3). Clinical treatment typically comprises chemically synthesized medicines; however, these are very expensive and often have serious side effects (4–6). Recently, several studies have reported that some agents that are currently used to promote immunity may also inhibit tumour growth (7–9). Research into the activity of natural products and their potential for as cancer treatments is therefore of great medical importance (10,11).
Acanthopanax senticosus, also known as Siberian ginseng, is a perennial xylophyta species in the family of Araliaceae, prefers warm and wet habitats. It is widely distributed in the mountainous broad-leaved forests, mixed forests and forest edges of eastern Hokkaido, Korean Peninsula, northern China and Siberia. A. senticosus has been used in traditional Chinese medicine as an adaptogen due to its pharmacological effects, including anti-bacterial, anti-cancer, anti-inflammatory, anti-gout, anti-hepatitis, anti-hyperglycemic, anti-leishmanicidal, anti-oxidant, anti-pyretic effects; other effects include choleretic, hemostatic, immunostimulatory, hypocholesterolemic and radioprotectant effects (12–15). It has been shown to protect against oxidative damage and exhibits anti-diabetic activity (16,17). In China, A. senticosus is used as a nutritional supplement and a sedative (18). A number of studies have demonstrated that A. senticosus has significant therapeutic effects on severe neurosis, fatigue, cardiovascular disease (19,20). A. senticosus can also improve the immune function (21). Various compounds from A. senticosus, including acanthosides, eleutherosides, senticoside, triterpenic saponin, flavon, vitamins, minerals and polysaccharides, have been reported to have diverse biological activities (22,23).
ASPS, as an extract, has been shown to have potent immunomodulatory activity, it is typically concentrated (24,25). ASPS has been demonstrated to improve lymphocyte proliferation (26), induce cytokine actions (27), enhance Toll-like receptor-mediated activation of B cells and improve macrophages phagocytosis (28). However, the association between the antitumour and immunostimulatory activities of ASPS remains to be elucidated. The aim of the present study was to investigate the antitumour effect of ASPS on S180, H22 and U14 solid and ascites tumour-bearing mice. The immunomodulatory effect of ASPS was also analysed to obtain additional information regarding its underlying mechanisms. ASPS, its composition, proportion of monosaccharides and anti-tumour activity are reported here for the first time.
Materials and methods
Materials
A specimen of A. senticosus was foraged by Dr Qinglong Meng from the Liaodong planting base of Chinese Medicinal Materials (Qingyuan, China) and Professor Yueying Ren identified the specimen. The specimen was stored in the Cultivation and Breeding of Medicinal Plants Laboratory in State Administration of Traditional Chinese Medicine (Changchun, China) for experimental applications. The specimen was crushed to powder then passed through mesh sieves, the pass rate of the 20 mesh pharmacopoeia sieve was ≥90% and the 80 mesh pharmacopoeia sieve was ≤10%. The pharmacopoeia sieves were provided by Xinxiang Zhuohang Precision Mesh Filter Co., Ltd. (Henan, China). The specimen then underwent 60°C thermostatic drying for 6 h by blowing air in a thermostatic oven (model, DHG-92438-2; Shanghai Fuma Experimental Equipment Co., Ltd., Shanghai, China). The dried powder was extracted with supercritical CO2 and placed in distilled water with 0.01–0.05% of mixed enzymes (protease: Cellulose: pectinase in the ratio 3:1.5:0.5). Continuous upstream extraction was performed using distilled water (material: Liquid, 1:18; extraction temperature, 75°C; extraction time, 2 h). The extracted liquid was filtered, adsorbed with non-polar macroporous resin, washed with 11.2 ml distilled water and filtered through a 0.22 µm membrane. The solid content was concentrated to 20% by 45°C thermostatic drying for 4 h in a thermostatic oven. The ASPS extraction yield was 10.76%.
Compositional analysis
Sephadex-G75 gel filtration (column size, 16×500 mm; internal diameter, 15 mm) was performed using a high performance liquid chromatography (HPLC) system (ÄKTAFPLC with Fraction Collector Frac-920; all GE Healthcare, Chicago, IL, USA) to purify active molecules from the ASPS extract. The molecules were separated using an Ultrahydrogel™ 500 column (7.8×300 mm), which was provided by Waters GmbH (Eschborn, Germany), on the HPLC system using a 0.9% NaCl water solution and distilled water. The column was operated at 35°C. The samples were of 2 mg ASPS were diluted in 1 ml 0.9% NaCl water solution and a total of 20 µl was injected onto the column. The column was operated at a maximum of 1.6 MPa with a flow rate of 0.5 ml/min. Gas chromatography was then used to analyse the composition of ASPS. Firstly, the samples were hydrated with ethanol (Nanjing Chemical Reagent Co., Ltd., Nanjing, China) for 12 h at 22°C and silylanised with silylate (Shandong Baiqian Chemical Co., Ltd., Shangdong, China) for 30 min at 22°C. Secondly, the samples were analysed with aVarian 7890 gas chromatography spectrometer. The gasification temperature was 300°C. The samples were then separated in high purity nitrogen in a SE-54 column (15 m ×0.2 mm ×0.25 µm) at 120°C for 2 min, increasing by 8°C/min until the column reached 250°C, then 250°C for 30 min. An infrared spectrometer (Perkin Elmer, Inc., Waltham, MA, USA) was used to record the infrared spectrum of ASPS.
Cell lines
The tumour cell lines Crocker sarcoma S180, hepatic carcinoma H22 and uterine cervical carcinoma U14 were provided by a drug clinical trial from Jilin Province Cancer Hospital (Changchun, China) and cultured fro 7 days at 37°C in the Cultivation and Breeding of Medicinal Plants Laboratory.
Mice
A total of 360 female Kunming mice, 6–7 weeks old and weighing 18–22 g, were provided by the Changchun Institute of Biological Products (Changchun, China; license number: SCXKJi 2013-0001). The mice were maintained in clean plastic cages in the laboratory of the College of Chinese Medicinal Materials at Jilin Agricultural University (Changchun, China). The temperature was controlled at 22±2°C with a 12 h light/dark cycle and relative humidity of 50–60%. Standard rodent chow and water were freely accessible. All mice were maintained for an acclimatization period of ~7 days under normal laboratory conditions. All mice were treated according to the National Regulations on the Usage and Welfare of Animals (29) and study protocols were approved by the Animal Ethical and Welfare Committee of the College of Chinese Medicinal Materials, Jilin Agricultural University prior to the experiments (approval no. 2013-006).
Inhibition rate, immune organ index and cytokine levels of solid tumour-bearing mice
On day 0, each mouse was injected subcutaneously with 1×108 tumour cells in 0.2 ml normal saline. A total of 50 Kunming mice used for each cell line and the same number of tumour cells were injected regardless of the cell line used. The inoculated mice were subsequently randomly divided into five groups (10 mice/group) as follows: Model group, treated with normal saline; positive control group, treated with cyclophosphamide (CTX; 30 mg/kg); and treatment groups, administered with 200, 100 or 50 mg/kg ASPS. Non-inoculated mice were divided into the normal group, treated with normal saline, and the negative control group, treated with 200 mg/kg ASPS (n=10/group). CTX and ASPS were administered intragastrically once daily for 10 days. All mice were sacrificed on day 11. Mice were weighed every 2 days throughout the treatment period and, following sacrifice, the tumours, spleens and thymuses were harvested and weighed. The tumour growth inhibition rates and immune organ indices were calculated using the following equations: Inhibition rate (%) = [(Mean tumour weight in the model group-mean tumour weight in the treatment group) / mean tumour weight in the model group] ×100. Immune organ index = Organ weight (mg) / body weight (g) (30,31). Blood was harvested from mice in each group and centrifuged at 1,409 × g at room temperature for 10–15 min. The serum levels of tumour necrosis factor (TNF)-α (cat. no. MTA00B), interleukin (IL)-2 (cat. no. M2000), IL-12 (cat. no. M1270) and interferon (INF)-γ (cat. no. MIF00) were determined using commercial ELISA kits according to the manufacturer's protocols (R&D Systems, Inc., Minneapolis, MN, USA).
Increased life span (ILS) of ascites tumour-bearing mice
A total of 50 female Kunming mice were inoculated, divided into groups and treated as above. For ascites tumour-bearing mice, there were no non-inoculated mice ILS was calculated using the following equation: ILS %=[(Mean survival days of treated group-mean survival days of model group)/mean survival days of model group] ×100 (31).
Test of acute toxicity
A total of 40 Kunming mice (20 male and 20 female; 6–7 weeks old; 20–22 g) were randomly divided into two groups: Administration and blank (n=20 in each). All mice were fasted for l2 h prior to experimentation, but were not deprived of water. Mice in the administration group were treated with 10 g/kg ASPS once daily for 14 days. Mice in the blank group were administered with 0.2 ml normal saline daily. Observations of the limb flexibility, feeding, diarrhoea, death, hair finish (whether the hair is dry and falling out), and stool and urine secretions were recorded. All mice were sacrificed on day 15. The major organs, including the heart, liver, spleen, lungs and kidneys of the mice, were tested for toxicity following the sacrifice of the mice.
Statistical analysis
Data are presented as the mean ± standard deviation. Differences between groups were analysed using the Least-Significant Difference post-test. Statistical analyses were conducted using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Composition of ASPS
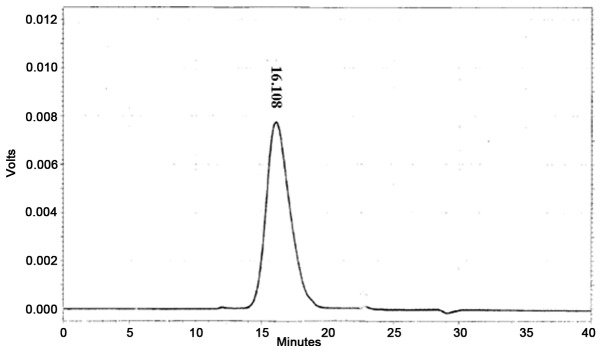
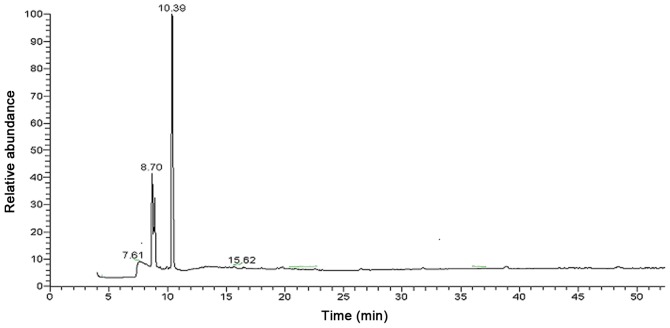
The single, narrow, symmetrical peak of the analysed ASPS sample revealed its homogeneous composition (Fig. 1). The molecular weight of ASPS was 10 kDa and its monosaccharide composition was demonstrated to be (Arabinose: Xylose: Glucose: Mannose)=7.1:22.3:7.6:1.0 (Fig. 2).
Figure 1.
Chromatogram of Acanthopanax senticosus polysaccharide.
Figure 2.
Gas chromatogram of the monosaccharide composition of Acanthopanax senticosus polysaccharide.
Tumour growth inhibitory effect of ASPS in solid tumour-bearing mice
Treatment with high, medium and low dose ASPS had a significant inhibitory effect on tumour growth in mice inoculated with S180 (Table I), H22 (Table II) and U14 (Table III) compared with the model group (all P<0.01). The greatest inhibitory effect in S180 and U14 tumour-bearing mice was achieved with the medium dose (100 mg/kg; Tables I and III), whereas the high dose (200 mg/kg) had the greatest effect in H22 tumour-bearing mice (Table II).
Table I.
Inhibitory effect of ASPS in S180 solid tumour-bearing mice.
| Weight (g) | |||||
|---|---|---|---|---|---|
| Group | Dose (mg/kg) and agent | Pre-treatment | Post-treatment | Tumour weight (g) | Inhibition rate (%) |
| Model | 0 | 22.09±1.34 | 30.45±2.24 | 2.69±0.45 | |
| Positive control | 30 CTX | 22.17±1.28 | 27.31±2.16a | 0.78±0.29a | 71.00 |
| High dose | 200 ASPS | 21.98±1.19 | 32.73±1.93b | 1.63±0.32a,b | 39.41 |
| Medium dose | 100 ASPS | 22.04±1.17 | 31.06±1.37b | 1.49±0.45a,b | 44.61 |
| Low dose | 50 ASPS | 21.97±1.25 | 32.69±2.04b | 1.59±0.41a,b | 40.89 |
P<0.01 vs. model group
P<0.01 vs. positive control group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
Table II.
Inhibitory effect of ASPS in H22 solid tumour-bearing mice.
| Weight (g) | |||||
|---|---|---|---|---|---|
| Group | Dose (mg/kg) and agent | Pre-treatment | Post-treatment | Tumour weight (g) | Inhibition rate (%) |
| Model | 0 | 21.99±1.56 | 29.03±1.98 | 3.08±0.74 | |
| Positive | 30 CTX | 21.95±1.38 | 26.73±2.07a | 0.99±0.36a | 67.86 |
| High dose | 200 ASPS | 22.01±1.62 | 30.95±2.33b | 1.71±0.41a,b | 44.48 |
| Medium dose | 100 ASPS | 21.97±1.48 | 31.29±2.67b | 1.75±0.51a,b | 43.19 |
| Low dose | 50 ASPS | 22.02±1.51 | 30.82±2.18b | 1.87±0.65a,b | 39.29 |
P<0.01 vs. model group
P<0.01 vs. positive control group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
Table III.
Inhibitory effect of ASPS in U14 solid tumour-bearing mice.
| Weight (g) | |||||
|---|---|---|---|---|---|
| Group | Dose (mg/kg) and agent | Pre-treatment | Post-treatment | Tumour weight (g) | Inhibition rate (%) |
| Model | 0 | 21.82±1.76 | 29.65±2.05 | 2.89±0.64 | |
| Positive control | 30 CTX | 21.98±1.91 | 26.47±2.19a | 0.93±0.41a | 67.82 |
| High dose | 200 ASPS | 22.08±1.62 | 29.87±2.40b | 1.65±0.46a,b | 42.91 |
| Medium dose | 100 ASPS | 22.03±1.81 | 30.53±2.81b | 1.61±0.58a,b | 44.29 |
| Low dose | 50 ASPS | 21.94±1.71 | 30.12±2.17 | 1.82±0.53a,b | 37.02 |
P<0.01 vs. model group
P<0.01 vs. positive control group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
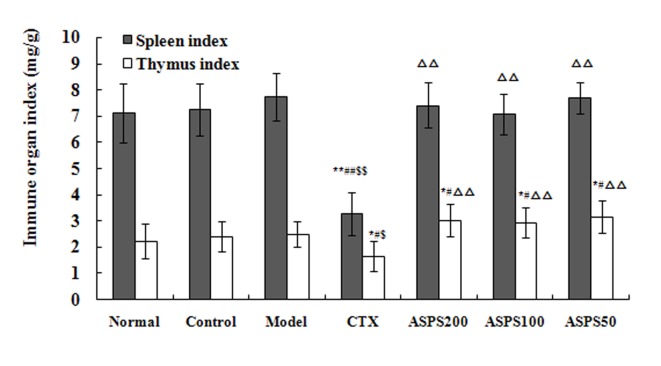
Effect of ASPS on immune organ index in solid tumour-bearing mice
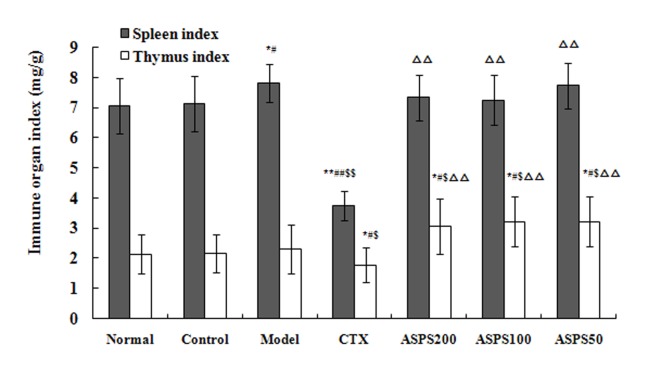
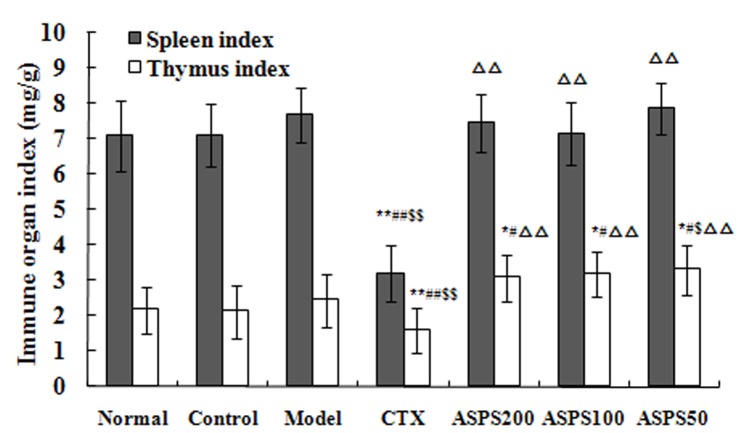
Treatment with high, medium and low doses of ASPS increased the immune organ indices in S180, H22 and U14 solid tumour-bearing mice. Spleen and thymus indices in the CTX group were significantly decreased compared with the normal, control and model groups for all cell lines (P<0.01; Figs. 3–5). Spleen and thymus indices in the high, medium and low ASPS treatment groups were significantly increased compared with the positive control group for all cell lines (P<0.01; Figs. 3–5).
Figure 3.
Immune organ indices of ASPS in S180 solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; #P<0.05 and ##P<0.01 vs. control group; $P<0.05 and $$P<0.01 vs. model group; ∆∆P<0.01 vs. CTX group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Figure 5.
Immune organ indices of ASPS in U14 solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; #P<0.05 and ##P<0.01 vs. control group; $P<0.05 and $$P<0.01 vs. model group; ∆∆P<0.01 vs. CTX group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Effect of ASPS on cytokines levels in solid tumour-bearing mice
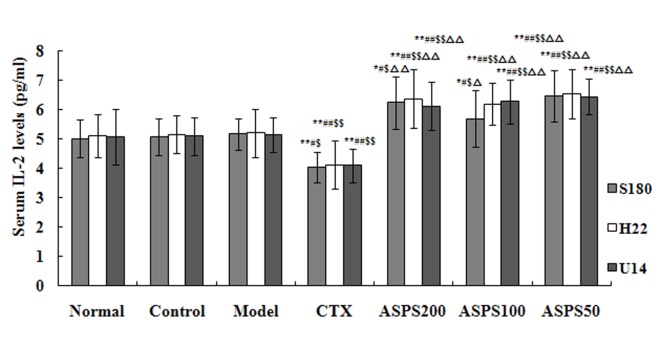
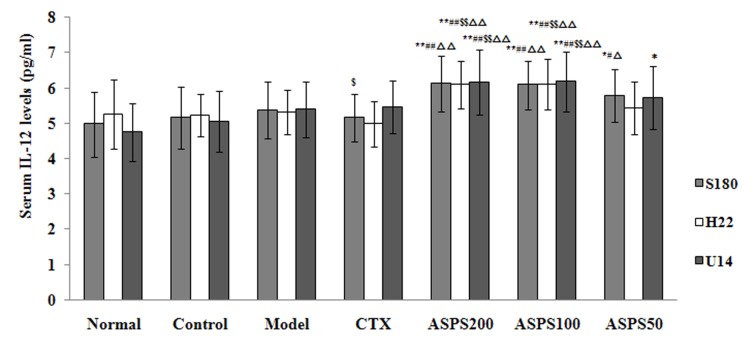
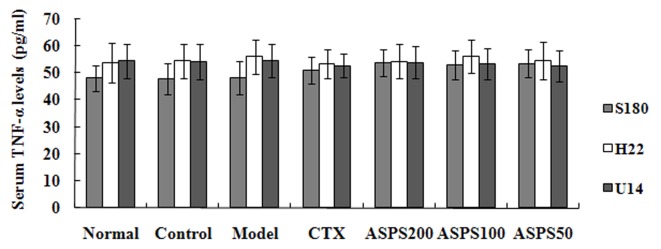
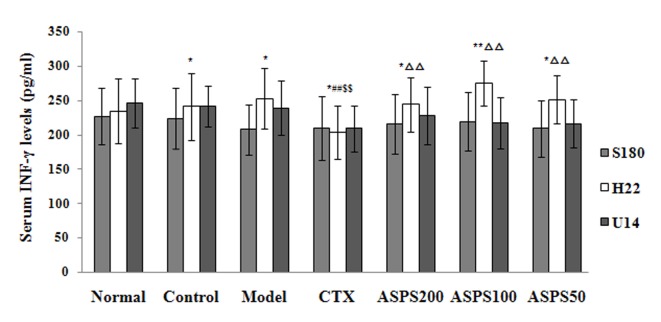
Treatment with high, medium and low doses of ASPS significantly increased serum IL-2 levels in S180, H22 and U14 solid tumour-bearing mice compared with the normal, control, CTX and model groups (S180, P<0.05, P<0.05 and P<0.01, respectively; H22 and U14, all P<0.01; Fig. 6). IL-2 levels were significantly decreased in the CTX group compared with the normal, control and model groups (S180, P<0.01, P<0.05 and P<0.05, respectively; H22 and U14, all P<0.01; Fig. 6). High and medium doses of ASPS significantly increased serum IL-12 levels in H22 and U14 solid tumour-bearing mice compared with the normal, control, CTX and model groups (P<0.01; Fig. 7); furthermore, IL-12 levels were significantly increased with high or medium-dose ASPS treatment in S180 tumour-bearing mice compared with the control, CTX and normal groups (P<0.01; Fig. 7). No significant differences in serum TNF-α levels were observed between groups, irrespective of treatment (Fig. 8). No significant differences in serum INF-γ levels were observed in S180 and U14 solid tumour-bearing mice in different treatment groups (Fig. 9). However, treatment with high (P<0.05), medium (P<0.01) and low (P<0.05) doses of ASPS significantly increased INF-γ expression in H22 solid tumour-bearing mice compared with the positive control group (Fig. 9).
Figure 6.
Serum IL-2 levels of ASPS in solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; #P<0.05 and ##P<0.01 vs. control group; $P<0.05 and $$P<0.01 vs. model group; ∆P<0.01 and ∆∆P<0.01 vs. CTX group. IL, interleukin; ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Figure 7.
Serum IL-12 levels of ASPS in solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; #P<0.05 and ##P<0.01 vs. control group; $P<0.05 and $$P<0.01 vs. model group; ∆P<0.01 and ∆∆P<0.01 vs. CTX group. IL, interleukin; ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Figure 8.
Serum TNF-α levels of ASPS in solid tumour-bearing mice. TNF, tumour necrosis factor; ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Figure 9.
Serum INF-γ levels of ASPS in solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; ##P<0.01 vs. control group; $$P<0.01 vs. model group; ∆∆P<0.01 vs. CTX group. INF, interferon; ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
Antitumour effect of ASPS in ascites tumour-bearing mice
Treatment with medium (P<0.01) and low (P<0.05) doses of ASPS had inhibitory effects on the growth of S180 in ascites tumour-bearing mice compared with the model group (Table IV). Treatment with high (P<0.01), medium (P<0.01) and low (H22, P<0.05 and U14, P<0.01) doses of ASPS had an inhibitory effect on the growth of H22 and U14in ascites tumour-bearing mice (Tables V and VI). The greatest inhibitory effect in S180 tumour-bearing mice was observed with the ASPS medium dose (100 mg/kg; Table IV). The greatest inhibitory effect in H22 and U14 tumour-bearing mice was observed with the ASPS high dose (200 mg/kg; Tables V and VI).
Table IV.
Antitumour effect of ASPS in S180 ascites tumour-bearing mice.
| Group | Dose (mg/kg) and agent | Survival time (days) | ILS (%) |
|---|---|---|---|
| Model | 0 | 13.67±2.65 | |
| Positive control | 30 CTX | 18.78±2.68b | 37.38 |
| High dose | 200 ASPS | 15.44±2.70c | 12.95 |
| Medium dose | 100 ASPS | 17.22±2.58b | 25.97 |
| Low dose | 50 ASPS | 16.33±2.12a,c | 19.46 |
P<0.05
P<0.01 vs. model group
P<0.01 vs. positive control group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
Table V.
Antitumour effect of ASPS in H22 ascites tumour-bearing mice.
| Group | Dose (mg/kg) and agent | Survival time (days) | Increased life span (%) |
|---|---|---|---|
| Model | 0 | 11.67±2.65 | |
| Positive control | 30 CTX | 16.89±2.09b | 44.73 |
| High dose | 200 ASPS | 15.21±2.39b | 30.25 |
| Medium dose | 100 ASPS | 14.67±2.55b | 25.71 |
| Low dose | 50 ASPS | 13.89±2.57a | 19.02 |
P<0.05
P<0.01 vs. model group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
Table VI.
Antitumour effect of ASPS in U14 ascites tumour-bearing mice.
| Group | Dose (mg/kg) and agent | Survival time (days) | Increased life span (%) |
|---|---|---|---|
| Model | 0 | 12.22±3.15 | |
| Positive control | 30 CTX | 16.89±3.33b | 38.22 |
| High dose | 200 ASPS | 15.44±2.07b | 26.35 |
| Medium dose | 100 ASPS | 14.67±2.92b | 20.05 |
| Low dose | 50 ASPS | 14.11±2.62a | 15.47 |
P<0.05
P<0.01 vs. model group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide.
Test of acute toxicity
All mice in the oral administration groups survived until the end of the 14-day observation period. Mice were sacrificed on day 15 and no visible lesions were observed in the vital organs. These results suggest that mice are able to tolerate >10 g/kg ASPS without adverse effects. According to the standard classification of acute toxicity of chemical substances (32), ASPS is a non-toxic material. No abnormalities in eating, drinking, stool, urine, disposition, mobility (data not shown) or body weight (Table VII) were observed in any groups.
Table VII.
Body weight changes of mice following oral administration of 10 g/kg Acanthopanax senticosus polysaccharide for 14 days.
| Body weight (g) | ||||||
|---|---|---|---|---|---|---|
| Group | Sex | Day 0 | Day 2 | Day 6 | Day 10 | Day 14 |
| Administration | Male | 19.2±0.94 | 23.6±1.01 | 29.1±1.62 | 32.6±2.04 | 35.0±2.72 |
| Female | 18.7±0.96 | 22.1±1.39 | 26.1±1.45 | 27.5±2.13 | 28.9±2.17 | |
| Blank | Male | 19.4±0.97 | 23.7±1.01 | 29.1±1.37 | 32.6±1.85 | 33.4±2.33 |
| Female | 18.5±0.82 | 21.75±1.23 | 25.5±1.53 | 27.0±2.27 | 27.2±2.66 | |
Discussion
Biological response modifiers have previously been used as a tool for inhibiting tumour growth and metastasis (33,34). In the tumour microenvironment, tumour cells often secrete immunosuppressive factors that alter host immune function and suppress immune response cells (35). The dysregulation of immunity and abnormal immune effector cells may lead to reduced anti-tumour activity (36); therefore, reversing the apoptotic pathway in tumour-induced immune cells has emerged as a method of tumour therapy (37). In the present study, the inhibitory effects of ASPS on tumour growth and its immunomodulatory activity were demonstrated to be independent of dosage. The medium dosage (100 mg/kg) of ASPS had the greatest inhibitory effect on tumour growth in mice. Previous studies have reported that the key determinant of polysaccharide regulatory function is the level in the body rather than the administered dose (38–40). These results suggest that ASPS may regulate immunity levels during normal autoimmune processes.
The immune system is a network comprising cells and organs that protect the body against external attacks. The degeneration and atrophy of immune organs will therefore negatively affect the function of the whole immune system. For example, the spleen filters and serves as a reservoir for blood; if the spleen is damaged or removed, the individual will be more susceptible to infection (41). In the present study, ASPS treatment had a positive effect on thymus and spleen indices in tumour-bearing mice.
Cellular immunity is important for host tumour immunity and cytokines serve an important role in the development of the immune response. IL-2 is a prominent immune factor that is secreted from helper T lymphocytes, natural killer cells, lymphokine activated killer cells and macrophages, all of which are involved in antitumour immunological mechanisms (42). Inflammatory cytokines, including TNF-α, are secreted by macrophages and serve a role in the activation of T cells and tumour cell recognition (43). IL-12 is secreted by phagocytic antigen-presenting cells, including macrophages and dendritic cells, and is considered to be one of the most essential cytokines in antitumour immunity, serving as a multifunctional cytokine in the early stages of the immune response (44,45). Previous animal studies have demonstrated that IL-12 has potent antitumour and antimetastasis activities, and its effect is most likely mediated via IFN-γ (45,46).
In the present study, ASPS was demonstrated to have an inhibitory effect on the growth of S180, H22 and U14 cells in both solid and ascites tumour-bearing mice, potentially via increasing serum IL-2, IL-12 and INF-γ levels. The results may provide a basis for the potential applications of ASPS in clinical treatment for cancer. There were a number of limitations in the present study, including a lack of pharmacodynamic tests for each independent component. During the treatment of tumour-bearing mice, each active component may exert different functions. In future studies, it will be necessary to assess the pharmacodynamic effects of each component of ASPS. In addition, although ASPS is an active component extracted from a natural pharmaceutical, its potential adverse effects require further investigation.
Figure 4.
Immune organ indices of ASPS in H22 solid tumour-bearing mice. *P<0.05 and **P<0.01 vs. normal group; #P<0.05 and ##P<0.01 vs. control group; $P<0.05 and $$P<0.01 vs. model group; ∆∆P<0.01 vs. CTX group. ASPS, Acanthopanax senticosus polysaccharide; CTX, cyclophosphamide; ASPS200, 200 mg/kg ASPS; ASPS100, 100 mg/kg ASPS; ASPS50, 50 mg/kg ASPS.
References
- 1.Koto K, Murata H, Sawai Y, Ashihara E, Horii M, Kubo T. Cytotoxic effects of zoledronic acid-loaded hydroxyapatite and bone cement in malignant tumors. Oncol Lett. 2017;14:1648–1656. doi: 10.3892/ol.2017.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson DC, Meyers RL. Malignant tumors of the liver in children. Semin Pediatr Surg. 2016;25:265–275. doi: 10.1053/j.sempedsurg.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Mamyrbayev A, Djarkenov T, Dosbayev A, Dusembayeva N, Shpakov A, Umarova G, Drobchenko Y, Kunurkulzhayev T, Zhaylybaev M, Isayeva G. The incidence of malignant tumors in environmentally disadvantaged regions of Kazakhstan. Asian Pac J Cancer Prev. 2016;17:5203–5209. doi: 10.22034/APJCP.2016.17.12.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Wu Y, Luo SH, Yang H, Li L, Zhou S, Shen R, Lin H. Efficacy and toxicity of low-dose versus conventional-dose chemotherapy for malignant tumors: A meta-analysis of 6 randomized controlled trials. Asian Pac J Cancer Prev. 2017;18:479–484. doi: 10.22034/APJCP.2017.18.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pramanik R, Agarwala S, Gupta YK, Thulkar S, Vishnubhatla S, Batra A, Dhawan D, Bakhshi S. Metronomic chemotherapy vs best supportive care in progressive pediatric solid malignant tumors: A randomized clinical trial. Jama Oncol. 2017;3:1222–1227. doi: 10.1001/jamaoncol.2017.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura K, Kawai Y, Kiguchi T, Okamoto M, Kaneko M, Maemondo M, Gemba K, Fujimaki K, Kirito K, Goto T, et al. Efficacy and safety of febuxostat for prevention of tumor lysis syndrome in patients with malignant tumors receiving chemotherapy: A phase III, randomized, multi-center trial comparing febuxostat and allopurinol. Int J Clin Oncol. 2016;21:996–1003. doi: 10.1007/s10147-016-0971-3. [DOI] [PubMed] [Google Scholar]
- 7.Kroemer G, Galluzzi L. Autophagy-dependent danger signaling and adaptive immunity to poorly immunogenic tumors. Oncotarget. 2017;8:5686–5691. doi: 10.18632/oncotarget.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17-a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 9.Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J Immunol. 2016;197:2016–2026. doi: 10.4049/jimmunol.1502376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gechev TS, Hille J, Woerdenbag HJ, Benina M, Mehterov N, Toneva V, Fernie AR, Mueller-Roeber B. Natural products from resurrection plants: Potential for medical applications. Biotechnol Adv. 2014;32:1091–1101. doi: 10.1016/j.biotechadv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7:119–136. doi: 10.1016/j.apsb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi JM, Kim MS, Seo SW, Lee KN, Yook CS, Kim HM. Acanthopanax senticosus root inhibits mast cell-dependent anaphylaxis. Clin Chim Acta. 2001;312:163–168. doi: 10.1016/S0009-8981(01)00613-1. [DOI] [PubMed] [Google Scholar]
- 13.Lin QY, Jin LJ, Ma YS, Shi M, Xu YP. Acanthopanax senticosus inhibits nitric oxide production in murine macrophages in vitro and in vivo. Phytother Res. 2007;21:879–883. doi: 10.1002/ptr.2171. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Lee SG, Kang SK, Chung SH. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch Pharm Res. 2006;29:768–776. doi: 10.1007/BF02974078. [DOI] [PubMed] [Google Scholar]
- 15.Yoon TJ, Yoo YC, Lee SW, Skin KS, Choi WH, Hwang SH, Ha ES, Jo SK, Kim SH, Park WM. Anti-metastatic activity of Acanthopanax senticosus extract and its possile immunological mechanism of action. J Ethnopharmacol. 2004;93:247–253. doi: 10.1016/j.jep.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Lin QY, Jin LJ, Cao ZH, Xu YP. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophages. J Ethnopharmacol. 2008;118:231–236. doi: 10.1016/j.jep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22:228–237. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- 18.Tian ZH, Ma CH, Huang Y, Zhou YH, Cui HF, Sun MJ. Optimization of extraction and purification processes for anti-fatigue and sedative ingredients from Acanthopanacis Senticosi Radix Et Rhizoma Seu Caulis. Zhongguo Shiyanfangjixue Zazhi. 2013;19:38–42. (In Chinese) [Google Scholar]
- 19.Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, Bode AM, Bowden GT, Lee HJ, Dong Z. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68:6021–6029. doi: 10.1158/0008-5472.CAN-08-0899. [DOI] [PubMed] [Google Scholar]
- 20.Tohda C, Ichimura M, Bai Y, Tanaka K, Zhu S, Komatsu K. Inhibitory effects of Eleutherococcus senticosus extracts on amyloid beta(25–35)-induced neuritic atrophy and synaptic loss. J Pharmacol Sci. 2008;107:329–339. doi: 10.1254/jphs.08046FP. [DOI] [PubMed] [Google Scholar]
- 21.Smalinskiene A, Savickiene N, Zitkevicius V, Pangonyte D, Sadauskiene I, Kasauskas A, Ivanov L, Lesauskaite V, Savickas A, Rodovičius H. Effect of Acanthopanax senticosus on the accumulation of cadmium and on the immune response of spleen cells. J Toxicol Environ Health A. 2014;77:1311–1318. doi: 10.1080/15287394.2014.924453. [DOI] [PubMed] [Google Scholar]
- 22.Davydov M, Krikorian AD. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J Ethnopharmacol. 2000;72:345–393. doi: 10.1016/S0378-8741(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Shin DS, Oh KB, Skin KH. Antibacterial compounds from leaves of Acanthopanax senticosus. Arch Pharm Res. 2003;26:40–42. doi: 10.1007/BF03179929. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Sun B, Zhang Z, Chen J, Hao Q, Sun Y, Yang Y, Wang Z, Pei J. Effects of Acanthopanax senticosus polysaccharide on the proliferation, apoptosis and cell cycle in human HepG2 cells. Pharmazie. 2016;71:201–204. [PubMed] [Google Scholar]
- 25.Fu J, Fu J, Yuan J, Zhang N, Gao B, Fu G, Tu Y, Zhang Y. Anti-diabetic activities of Acanthopanax senticosus polysaccharide (ASP) in combination with metformin. Int J Biol Macromol. 2012;50:619–623. doi: 10.1016/j.ijbiomac.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, Liu Z, Zhao J, Chen R, Meng F, Zhang M, Ge W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosus. Food Chem. 2011;127:434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- 27.Steinmann GG, Esperester A, Joller P. Immunopharmacological in vitro effects of Eleutheroeoceus senticosus extracts. Arzneimittelforschung. 2001;5l:1–83. doi: 10.1055/s-0031-1300006. [DOI] [PubMed] [Google Scholar]
- 28.Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, Yoon WK, Park SK, Kim HM. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int lmmunopharmacol. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu K, Tang XJ. Ethics and welfare in laboratory animal breeding and transportation. Zhongguo Bijiaoyixue Zazhi. 2011;Z1:165–169. (In Chinese) [Google Scholar]
- 30.Xu SY, Bian RL, Chen X. Methodology of pharmacology experiment. People's Medical Publishing House; Beijing: 1991. pp. 1423–1430. [Google Scholar]
- 31.Li YK, Wang QM, Zhou JH. Methodology of pharmacology experiment in China. Shanghai Scientific and Technical Publishers; Shanghai: 1991. pp. 512–513. [Google Scholar]
- 32.World Health Assembly, corp-author. Evaluation of the effects of chemicals on health. World Health Organization; Geneva: 1977. [Google Scholar]
- 33.Suto T, Fukuda S, Moriya N, Watanabe Y, Sasaki D, Yoshida Y, Sakata Y. Clinical study of biological response modifiers as maintenance therapy for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33:145–148. doi: 10.1007/BF00686688. [DOI] [PubMed] [Google Scholar]
- 34.Yoo YC, Saiki I, Sato K, Azuma I. MDP-Lys(L18), a lipophilic derivative of muramyl dipeptide, inhibits the metastasis of haematogenous and non-haematogenous tumours in mice. Vaccine. 1994;12:175–180. doi: 10.1016/0264-410X(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 35.Ahn YH, Hong SO, Kim JH, Noh KH, Song KH, Lee YH, Jeon JH, Kim DW, Seo JH, Kim TW. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-β receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity. Clin Exp Immunol. 2015;181:164–178. doi: 10.1111/cei.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu S, Plautz GE, Krauss JC, Chang AE. Tumor immunology. J Am Assoc. 1997;278:111–141. doi: 10.1001/jama.278.22.1972. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya A, Mandal D, Lahiry L, Sa G, Das T. Black tea protects immunocytes from tumor-induced apoptosis by changing Bcl-2/Bax ration. Cancer Lett. 2004;209:147–154. doi: 10.1016/j.canlet.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Besedovsky H, Sorkin E. Network of immune-neuroendocrine interactions. Clin Exp Immunol. 1977;27:1–12. [PMC free article] [PubMed] [Google Scholar]
- 39.Han J, Bian L, Liu X, Zhang F, Zhang Y, Yu N. Effects of Acanthopanax senticosus polysaccharide supplementation on growth performance, immunity, blood Parameters and expression of pro-inflammatory cytokines genes in challenged weaned piglets. Asian-Australas J Anim Sci. 2014;27:1035–1043. doi: 10.5713/ajas.2013.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bang JS, Chung YH, Chung SJ, Lee HS, Song EH, Shin YK, Lee YJ, Kim HC, Nam Y, Jeong JH. Clinical effect of a polysaccharide-rich extract of Acanthopanax senticosus on alcohol hangover. Pharmazie. 2015;70:269–273. [PubMed] [Google Scholar]
- 41.Martić AJ, Ivanković S, Antica M, Hiršl N, Jukić T, Jurin M. Transplanted tumor growth and the incidence of T-lymphocyte populations in the spleen of newcastle virus-treated mice. Cancer Biother Radiopharm. 2015;30:182–186. doi: 10.1089/cbr.2014.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuno H, Yanoma S, Nishimura G, Hattori S, Ito T, Okudera K, Tsukuda M. Therapeutic efficiency of IL-2 gene transduced tumor vaccine for head and neck carcinoma. Cancer Lett. 2000;152:175–185. doi: 10.1016/S0304-3835(00)00336-0. [DOI] [PubMed] [Google Scholar]
- 43.Tanigawa K, Craig RA, Stoolman LM, Chang AE. Effects of tumor necrosis factor-alpha on the in vitro maturation of tumor-reactive effector T cells. J Immunother. 2000;23:528–535. doi: 10.1097/00002371-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa M, Yu WG, Umehara K, Iwasaki M, Wijesuriya R, Tsujimura T, Kubo T, Fujiwara H, Hamaoka T. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58:2426–2432. [PubMed] [Google Scholar]
- 45.Lasek W, Feleszko W, Golab J, Stokłosa T, Marczak M, Dabrowska A, Malejczyk M, Jakobisiak M. Antitumor effects of the combination immunotherapy with interleukin-12 and tumor necrosis factor alpha in mice. Cancer Immunol Immunother. 1997;45:100–108. doi: 10.1007/s002620050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, Lee FT, Hanai N, Smyth FE, Burgess AW, Old LJ, Scott AM. Cytokine enhancement of in vitro antibody-dependent cellular cytotoxicity mediated by chimeric anti-GD3 monoclonal antibody KM871. Cancer Immun. 2002;2:13. [PubMed] [Google Scholar]