Abstract
The present study aimed to investigate the correlation between apolipoprotein E (APOE) polymorphisms and the intracellular concentration of Ca2+ in astrocytes in the early stages after an injury. The chondroitin sulfate region of three APOE alleles (ε2, ε3 and ε4) was obtained by reverse transcription-polymerase chain reaction (RT-PCR). A recombinant plasmid, pEGFP-N1-APOE, was constructed and identified by sequencing, while astrocytes were isolated from APOE gene-knockout mice and examined using immunocytochemistry. The recombinant plasmid was transfected into the astrocytes using the liposome-mediated method and cell injury models were constructed by a scratch assay. Laser confocal scanning microscopy (LCSM) was used to detect dynamic alterations in intracellular Ca2+ concentration at 12, 24, 48 and 72 h after injury. Compared with the control group, cells transfected with any of the three alleles demonstrated significant increases in the fluorescence intensity of Ca2+ (P<0.05). The fluorescence intensity of Ca2+ was weak at 12 h after injury, with no statistically significant difference detected between any two groups at this time point (P>0.05). However, the fluorescence intensity increased in a time-dependent manner and at 24, 48 and 72 h post injury, the fluorescence intensity of the ε4 allele-containing cells was significantly higher when compared with that of cells harboring the other two alleles (P<0.05). These results indicate that intracellular Ca2+ overloading may contribute to the deterioration of brain cells and poor outcome subsequent to traumatic brain injury in APOE ε4 carriers.
Keywords: apolipoprotein E, polymorphism, Ca2+, astrocyte, laser confocal scanning microscopy
Introduction
Traumatic brain injury (TBI) is the leading cause of mortality and disability among young people and the incidence rate of TBI has steadily risen around the world. Previous studies have demonstrated that genetic susceptibility may serve an important role in the clinical outcome of individuals with TBI (1–4). Apolipoprotein E (APOE) polymorphisms are the most extensively studied genetic factor in neurotrauma research. Several previous studies have identified that APOE gene polymorphisms are associated with the acute condition and outcome of TBI (1–5). The presence of the APOE ε4 allele has been revealed to predispose an individual to clinical deterioration in the acute phase of TBI and is indicative of a poor long-term outcome. However, the underlying mechanism of this association has not been investigated thus far (6,7). Furthermore, calcium is an important secondary messenger within cells. Cytoplasmic calcium, has extensive physiological effects as a cellular messenger following injury. An increased intracellular calcium concentration is an important cause of cell injury. It has recently been demonstrated that Ca2+ overload and disruption of the intracellular Ca2+ homeostasis are the final events in the process of cell death (8–10).
The aim of the current study was to investigate the effects of APOE polymorphism on the early intracellular Ca2+ concentration in astrocytes following scratch injury using laser confocal scanning microscopy (LCSM). In addition, the study explored the underlying molecular mechanism of the effects of APOE polymorphism in a cell injury model.
Materials and methods
Construction of recombinant plasmids
The present study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical College (Guizhou, China) and written informed consent was obtained from all participants. The chondroitin sulfate (CDS) domain of the APOE ε3 allele was amplified by polymerase chain reaction (PCR) exactly according to the previously described method (11). Briefly, total RNA was extracted from human fetal brain tissue obtained from aborted fetuses at the Affiliated Hospital of Zunyi Medical College (Guizhou, China), using an RNA Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). A High Fidelity PrimeSript™ RT-PCR kit (Takara Biotechnology Co., Ltd.) was used with primers designed by Takara Biotechnology, Co., Ltd. to synthesize total DNA. The reverse transcription reaction was amplified by PCR using PrimeSTAR™ HS DNA polymerase (Takara Biotechnology Co., Ltd.) to get APOE ε3. The gene was ligated with PMD-19 vector and then was excised from PMD19-T-APOE ε3 plasmid and inserted into pEGFP-N1 to construct eukaryotic expression vector. The expression of APOE ε3 gene was identified by western-blot (11). The EcoRI and BamHI restriction enzyme cleavage sites were then introduced at the 5′-ends in the upstream and downstream primers used for the amplification of the CDS domain of APOE ε3, by site-directed mutagenesis. All the primers used in the present study were synthesized by Takara Biotechnology Co., Ltd. Furthermore, the CDS domain of ε2 and ε4 alleles was amplified by reverse transcription-PCR (Table I). The target gene was excised by double enzyme cleavage, purified and subcloned into the eukaryotic expression vector pEGFP-N1 (Takara Biotechnology Co., Ltd.). The expression vector was inserted into Escherichia coli JM109 by thermal transformation as previously described (11,12). The transformed E. coli were plated (1×106/well) in six-well plates and incubated overnight at 37°C (12). A co-digestion assay was performed by EcoR1 and Not1 to get 470 kp and 950 bp fragments, and single colonies positive for the target genes (APOE ε2 and ε4) were cultured, and the recombinant plasmids containing the alleles were purified (11,12).
Table I.
Amplification primer sequences of APOE alleles ε2 and ε4.
| Template | Primer sequence (5′→3′) | Annealing temperature (°C) | Cycle times | Product |
|---|---|---|---|---|
| APOEε3 | Forward: TGGAGGACGTGCGCGGCCGCCTGGTGCAG | 64 | 30 | APOE ε2 |
| Reverse: TGTCCGCGCCCAGCCGGGCCTG | ||||
| APOEε3 | Forward: TGACCTGCAGAAGTGCCTGGCAGTGTAC | 61 | 30 | APOE ε4 |
| Reverse: TCGGCATCGCGGAGGAGCCGCTTA |
APOE, apolipoprotein E.
Cell transfection
Astrocytes were isolated from four 2-day-old APOE-gene-knockout suckling mice (Department of Zoology, Peking University, Beijing, China) using the method described by McCarthy and de Vellis (13). Then cells were washed with 0.1 mol/l PBS for 2 min three times and then incubated for 10 min at 37°C with 3% deionized H2O2 to block endogenous peroxidase. The cells were washed with PBS and incubated with anti-glia fibrillary acidic protein (GFAP) antibodies (1:100; cat. no. Q0287R; Bioengineering Co., Ltd., Shanghai, China) in a wet box overnight at 4°C. The cells were subsequently washed with PBS for 2 min three times and then incubated with poly-horseradish peroxidase anti-rabbit immunoglobulin G (1:4,000; cat. no. C030213; Bioengineering Co., Ltd.). Following identification, the astrocytes were transfected using Lipofectamine™ 2000 (Bioengineering Co., Ltd.), with the recombinant plasmids pEGFP-N1-APOE in order to obtain three groups of astrocytes with humanized APOE ε2, ε3 and ε4. Following transfection with pEGFP-N1-APOE plasmids for 24 h the astrocytes were selected with 200 µg/ml G418 (Ameresco, Inc., Framingham, MA, USA). Fresh medium containing 200 µg/ml G418 was replenished every other day for 15 days. The positive clones of pEGFP-N1-APOE cells were cultured and proliferated in Roswell Park Memorial Institute 1640 medium with 10% fetal bovine serum (Boster Biological Technology, Pleasanton, CA, USA) and 200 µg/ml G418, at 37°C under 5% CO2 for screening stable cell lines expressing APOE.
The recombinant plasmids, pEGFP-N1-APOE, were sent to Takara Biotechnology Co., Ltd. (Dalian, China) for sequencing. The sequences were compared against the sequence provided in the NCBI Databank (cat. no. ID:348) to confirm the successful formation of the recombinant plasmids.
The present study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (14). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (Chongqing, China).
Construction of cell scratch wound model
Cell strains with stable expression of each of the APOE alleles were cultured in LCSM-exclusive culture dishes at 37°C for 60 min, following the procedure described in an earlier study (15). Subsequently, astrocyte cell layers in each culture dish were scratched with the plastic tip of a fine micropipette. The scratch length was 10 mm and the width was 1 mm, while the scratches were spaced 2–3 cm apart. A total of 8 scratch lines (4 transverse scratch lines and 4 longitudinal scratch lines) were made in each culture dish. The scratch layout in all the dishes was kept consistent to the extent possible. Non-scratch groups for each of the alleles were established as pre-wound and post-wound controls.
Determination of intracellular Ca2+ levels
A working solution (4.4 µmol/l) of Fluo-3/acetoxymethyl ester dry powder (DingGuo Biotech Co., Ltd., Beijing, China) in anhydrous dimethyl sulfoxide was prepared, according to the method described in a previous study (16). Briefly, at 12, 24, 48 and 72 h after introducing the scratches, astrocytes in the dishes were rinsed with Hank's solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), treated with ~1 ml Fluo-3 working solution and placed in an incubator at 37°C, ensuring that the cells were protected from light, in order to load the cells with fluorescent probe for 45 min. The cells were then rinsed with phosphate-buffered saline to remove free dye and placed on the specimen stage of the microscope (Leica Microsystems GmbH, Wetzlar, Germany). Fluorescence was then measured using an excitation wavelength of 488 nm and an emission wavelength of 520 nm. The laser power and scanning parameters were kept constant throughout the experiment as previously described (17). Visual fields with even fluorescence intensity were selected using Leica LCSM matching analytical software (version 2.0; Leica Microsystems GmbH, Wetzlar, Germany), and 5 cells were randomly selected for measuring the intensity. The relative fluorescence intensity was considered to represent the relative concentration of Ca2+.
Statistical analysis
Ca2+ fluorescence intensity is expressed as the mean ± standard deviation. Statistical analysis was conducted using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Comparison between two groups was performed using Student's t-test, while comparison between multiple groups was examined using one-way analysis of variance and a Student-Newman-Keuls post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Sequencing of recombinant plasmids
The recombinant plasmids, pEGFP-N1-APOE, were sent to Takara Biotechnology Co., Ltd. for sequencing. The sequences were compared against the sequence provided in the NCBI Databank (cat. no. ID:348) and successful construction of the recombinant plasmids, (pEGFP-N1-APOEε3, pEGFP-N1-APOEε2 and pEGFP-N1-APOEε4) as well as that it complied with the requirements of the subsequent experimental procedures, was verified. By sequencing, we can see that APOEε2 and APOEε4 differ from APOEε3 by single amino acid substitutions at position 112 or 158, which corresponds with the gene bank data (Fig. 1A-C).
Figure 1.
Sequencing maps of (A) pEGFP-N1-APOE ε3, (B) pEGFP-N1-APOE ε2 and (C) pEGFP-N1-APOE ε4. APOE, apolipoprotein E.
Construction of cell wound model
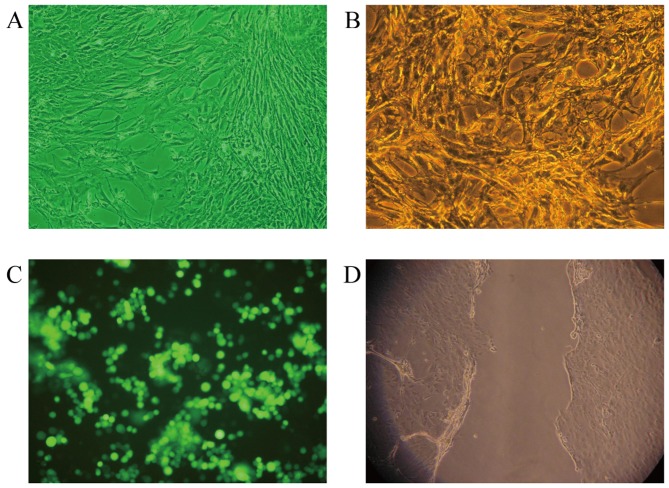
The purity of the isolated primary astrocytes was 95% and the yield was 3–6×106/ml. The cell morphology was observed under an inverted microscope. It was observed that the cells fused with each other and their cell processes were distinct. The cells intersected and formed a cobblestone mass around the bottom of the flask and a small number of cells began to grow on the astrocyte layer (Fig. 2A). Immunofluorescent labeling of the astrocyte specific marker, GFAP, confirmed the identity of the cells (Fig. 2B). Astrocytes were then transfected with recombinant plasmids, and successful transfection was confirmed by the appearance of green fluorescein at 24 h after transfection, as observed under a fluorescence microscope (Fig. 2C). Successful establishment of the cell scratch wound model was also confirmed by fluorescence microscopy (Fig. 2D).
Figure 2.
(A) Primary culture of astrocytes in APOE−/− mice (magnification, ×200). (B) Astrocyte glia fibrillary acidic protein immunostaining (magnification, ×200). (C) Expression of pEGFP-N1-APOE was observed under a fluorescence microscope. (D) At 12 h after the cell scratch, cells were observed under an inverted microscope (magnification, ×200). APOE, apolipoprotein E.
Alterations in the intensity of Ca2+ fluorescence
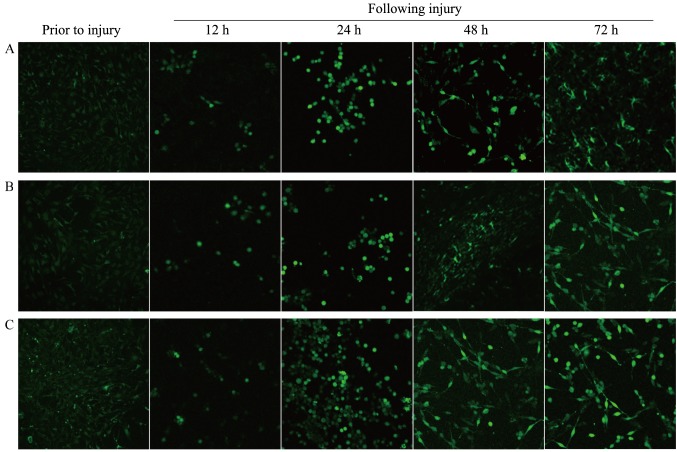
Prior to the scratch wounding, the three groups of ε2, ε3 and ε4 type astrocytes exhibited weak fluorescence intensities. The difference in the fluorescence intensities between any two types of cells was not statistically significant (P>0.05; Table II). However, subsequent to scratch, the Ca2+ fluorescence intensity of each type of astrocytes increased gradually until 72 h after the scratch (Fig. 3). The Ca2+ fluorescence intensity of each type of astrocytes at each time point following the scratch was significantly higher compared with that prior to the scratch (P<0.05; Table II).
Table II.
Fluorescence intensity of Ca2+ of three groups before and after injury (n=5; mean ± standard deviation).
| Following injury | |||||
|---|---|---|---|---|---|
| Group | Prior to injury | 12 h | 24 h | 48 h | 72 h |
| APOE ε2 | 34.70±12.04 | 80.28±24.62a | 88.47±23.82a | 106.04±31.37a | 129.72±38.24a |
| APOE ε3 | 30.58±13.61 | 72.75±20.57a | 78.29±35.20a | 87.33±34.80a | 98.16±30.90a |
| APOE ε4 | 40.39±8.41 | 90.68±29.71a | 152.29±46.63b,c | 178.82±32.67b,c | 208.00±35.49b,c |
P<0.05 vs. prior to injury
P<0.05 vs. the ε2 group
P<0.05 vs. the ε3 group. APOE, apolipoprotein E.
Figure 3.
Under confocal laser scanning microscope, the fluorescence intensity of Ca2+ in the (A) APOE ε2, (B) APOE ε3 and (C) APOE ε4 type astrocytes at different time points subsequent to injury was investigated (magnification, ×200). APOE, apolipoprotein E.
Comparison of the fluorescence intensities among the different types of astrocytes at the three time points revealed that the fluorescence intensities in ε4 type astrocytes at 24, 48 and 72 h were significantly stronger in comparison with those in the ε2 and ε3 type astrocytes at the corresponding time points (P<0.05). However, there were no statistically significant differences between the intensities in the different types of astrocytes at 12 h, while the difference in the fluorescence intensities between the ε2 and ε3 type astrocytes were not statistically significant at any point (P>0.05; Table II).
Discussion
APOE is an important apolipoprotein, synthesized in the liver, brain, spleen and kidney. It is distributed extensively over the entire body via the blood circulation, and serves an important role in the transport and metabolism of plasma cholesterol and triglycerides (18). APOE has three alleles, including APOE ε2, APOE ε3 and APOE ε4, which have base substitutions at two sites in the DNA sequence (19). The brain is the second leading organ that synthesizes APOE, mainly by the astrocytes and oligodendrocytes (20). The most important function of APOE in the central nervous system is to protect and repair the neural tissues (21,22). In recent years, APOE polymorphism has been associated with central nervous system diseases, particularly Alzheimer's disease and brain trauma (23).
Clinical studies have identified that, among Caucasians, patients with APOE ε4 presented decreased tolerance to brain injury and were likely to have a poor prognosis following brain injury (24,25). Compared with patients of the non-ε4 genotypes, those with the ε4 genotype exhibited longer duration of coma and high in-hospital mortality rate (26,27). The authors of the present study previously investigated the relevance of APOE polymorphism and TBI among the Asian population in the Chinese mainland, and observed that the presence of APOE ε4 was associated with exacerbation of the acuteness (<7 days) and poor prognosis following a TBI (27–30). Furthermore, our group also investigated the association between APOE gene polymorphism and the occurrence of any acute alterations in the neural electrophysiology subsequent to TBI (31). It was observed that the ε4 genotype was a risk factor for acute changes in the electroencephalogram following mild to moderate brain injuries (31). Based on these results, preliminary experimental investigation of the mechanism underlying the effects of APOE from the perspective of ion channels was conducted in a previous study. The results revealed that, following scratching, the number of ε4 type neurons/gliocytes, which are considered as the early prognostic cells, was evidently higher compared with that of the ε2 and ε3 type (32). Furthermore, APOE ε4 cells have been demonstrated to exhibit a greater inhibition of delayed rectifier potassium channels in neurons following scratching (32). The observations of these aforementioned studies suggested that, the effects of APOE on the pathophysiological alterations occurring following brain injury are subtype-specific, and the molecular mechanism of these effects possibly involves ion channels.
It has been demonstrated that Ca2+ overload and disruption of Ca2+ homeostasis in neurons are the final events in the death of neurons (9). The shear stress and tension associated with traumatic injury may stretch the cell membranes of injured neurons, increase the permeability of the membranes and result in an increased influx of Ca2+ (33). A series of secondary neurophysiological alterations in the microenvironment around the injury area, particularly the excess production of excitatory amino acids, may activate the ionotropic N-methyl-D-aspartate receptor (NMDAR), leading to further increase in the influx of Ca2+ (34). Increased Ca2+ subsequently triggers a cascade of events. It increases the activities of L-type voltage gated calcium channels and results in the release of Ca2+ from the endoplasmic reticulum, further increasing the intracellular Ca2+ levels. Interactions of several other factors finally lead to Ca2+ overload in neurons and axons through several channels via a cycle, and ultimately result in cell death around the injury area (29). However, the impact of APOE on the intracellular calcium concentration in neurons following an injury has not been reported thus far.
Astrocytes are the main cells producing APOE in the central nervous system. In the past, astrocytes were considered to mainly serve a nutritional and supporting role for neurons (34–36). Accumulating evidence in more recent studies has demonstrated that astrocytes serve an important role in maintaining the normal physiological activities of the nervous system, and are involved in brain development and in the neuropathological processes (31). In the present study, astrocytes transfected with three humanized APOE alleles were used as experimental models to investigate the alterations in intracellular Ca2+ in the initial 72 h after the introduction of a scratch injury in cells. The present study also aimed to investigate the potential molecular mechanism underlying the effects of APOE polymorphism. The results demonstrated that Ca2+ in astrocytes increased progressively until 72 h after scratching, while the Ca2+ fluorescence intensity of all astrocyte types at any time point following the scratch was significantly different from that prior to the scratch. It was also observed that the early changes in the intracellular Ca2+ concentration in astrocytes after the scratch were APOE-allele-specific. The Ca2+ concentration in ε4 type cells was significantly higher at 24 h after the scratch as compared with that in ε2 and ε3 type cells. An earlier study (29) reported that, subsequent to scratching, the early apoptosis rate in neurons/gliocytes transfected with APOE increased in a time-dependent manner. Similarly, the apoptosis rate of ε4 type neurons/gliocytes in the current study was significantly higher in comparison with that of the ε2 and ε3 type neurons/gliocytes at 24, 48 and 72 h after the scratch. However, in the previous study there was no significant difference in the apoptosis rates among the three types of cells at 12 h after scratch (29). The results of the present and previous studies indicated that early apoptosis in neurons/gliocytes following scratching corresponded temporally with changes in the early intracellular Ca2+ concentrations in astrocytes subsequent to the scratch. Therefore, it is concluded that trauma results in Ca2+ overload in astrocytes and early apoptosis of neurons. This pathological process is APOE-subtype-specific, and the ε4 subtype is clearly more potent compared with the other two genotypes.
The signaling and/or receptor pathway through which APOE influences the intracellular Ca2+ aggregation in wounded astrocytes remains unknown. The mechanism underlying the subtype specificity is also unclear. Certain studies have postulated that APOE may interfere with the Ca2+ influx mediated by NMDAR by activating extracellular signal-regulated kinase 1/2, an extracellular signal-adjusting kinase, in the neurons (37). Further investigations identified that APOE receptors and NMDAR may interact under certain conditions by forming a polyprotein complex (38). Future research needs to focus on the possible influence of the APOE gene on NMDAR in a subtype-specific way following brain injury and its role in altering the functioning of Ca2+ channels, which results in different Ca2+ levels in neurons around the injury area and different degrees of cell death, ultimately affecting the prognosis of cerebral trauma patients.
In conclusion, the present study demonstrated that APOE ε4 may worsen the outcomes of TBI by secondary brain injury mediated by the calcium overload signaling pathway. Moreover, the results of the present study also provided evidence that there may be the potential to develop precise medications for TBI according to individual genotypes. Further studies are required to explore the therapeutic aspect of the mechanism and to identify a means to increase the protection by APOE ε3 and decrease the impairment by APOE ε4.
Acknowledgements
The authors would like to thanks the following agencies for their support: The Guizhou Science and Technology Department (grant no. 2013-2332), and the National Nature Science Foundation of China (grant no. 81560227). The service provided by the Molecular and Biology Laboratory of Zunyi Medical College is also acknowledged.
References
- 1.Jiang Y, Sun X, Xia Y, Liu H, Cao Y, Gu Y. Assoocaition between apolipoprotein E gene polymorphism and brain injury. Chin J Traumatol. 2005;21:520–523. (In Chinese) [Google Scholar]
- 2.Jiang Y, Sun X, Xia Y, Liu H, Cao Y, Gu Y. Study on the relationship between apolipoprotein E gene polymorphism and the development of traumatic brain injury. Chin J Neuropsychiatric Dis. 2006;32:431–435. (In Chinese) [Google Scholar]
- 3.Jiang Y, Sun X, Xia Y, Tang W, Cao Y, Gu Y. Effect of APOE polymorphisms on early response to traumatic brain injury. Neurosci Lett. 2006;408:155–158. doi: 10.1016/j.neulet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Sun X, Gui L, Xia Y, Tang W, Cao Y, Gu Y. Correlation between APOE-491AA promoter in epsilon4 carriers and clinical deterioration in early stage of traumatic brain injury. J Neurotrama. 2007;24:1802–1810. doi: 10.1089/neu.2007.0299. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Arrastia R, Baxter VK. Genetic factor in outcome after traumatic brain injury: What the human genome project can teach us about brain trauma. J Head Trauma Rehabil. 2006;21:361–374. doi: 10.1097/00001199-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Mosconi L, Murray J, Davies M, Williams S, Pirraglia E, Spector N, Tsui WH, Li Y, Butler T, Osorio RS, et al. Nutrient intake and brain biomarkers of Alzheimer's disease in at-risk cognitively normal individuals: A cross-sectional neuroimaging pilot study. BMJ Open. 2014;4:e004850. doi: 10.1136/bmjopen-2014-004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong J, Cheng C, Liu H, Huang Z, Wu Y, Teng Z, He J, Zhang H, Wu J, Cao F, et al. Bexarotene protects against traumatic brain injury in mice partially through apolipoprotein E. Neuroscience. 2017;343:434–448. doi: 10.1016/j.neuroscience.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Lee DG, Park J, Lee HS, Lee SR, Lee DS. Iron overload-induced calcium signals modulate mitochondrial fragmentation in HT-22 hippocampal neuron cells. Toxicology. 2016;365:17–24. doi: 10.1016/j.tox.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Rodríguez M, García-Durillo M, Villalobos C, Núñez L. In vitro aging promotes endoplasmic reticulum (ER)-mitochondria Ca2+ cross talk and loss of store-operated Ca2+ entry (SOCE) in rat hippocampal neurons. Biochim Biophys Acta. 2016;1863:2637–2649. doi: 10.1016/j.bbamcr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhong XZ, Zou Y, Sun X, Dong G, Cao Q, Pandey A, Rainey JK, Zhu X, Dong XP. Inhibition of transient receptor potential channel mucolipin-1 (TRPML1) by lysosomal adenosine involved in severe combined immunodeficiency diseases. J Biol Chem. 2017;292:3445–3455. doi: 10.1074/jbc.M116.743963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo L, Jiang Y, Sun X, Zheng L. Cloning of human APOE ε3 gene and construction of its eukaryotic expression vector. J Chongqing Med Univ. 2008;33:784–787. (In Chinese) [Google Scholar]
- 12.Wu H, Fan Z, Jiang X, Chen J, Chen GQ. Enhanced production of polyhydroxybutyrate by multiple dividing E. coli. Microb Cell Fact. 2016;15:128. doi: 10.1186/s12934-016-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglia cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayne K, Ramachandra GS, Rivera EA, Wang J. The evolution of animal walfare and the 3Rs in Brazil, Chian, and India. J Am Assoc Lab Anim Sci. 2015;54:181–191. [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo JY, Hwang CH, Hong HN. A model of glial scarring analogous to the environment of a traumatically injured spinal cord using kainate. Ann Rehabil Med. 2016;40:757–768. doi: 10.5535/arm.2016.40.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai S, Jiang Y, Wang Y, Wu X, Ren J, Lee MS, Lee S, Huang L. Modulation on brain gray matter activity and white matter integrity by APOE ε4 risk gene in cognitively intact elderly: A multimodal neuroimaging study. Behav Brain Res. 2017;322:100–109. doi: 10.1016/j.bbr.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Chen R, Li Y, Zhuang H, Chen J, Wang L. Autofluorescence spectroscopy and imaging of platymonas subcordiformis irradiated by diode laser based on LSCM. Scanning. 2008;30:443–447. doi: 10.1002/sca.20127. [DOI] [PubMed] [Google Scholar]
- 18.Teng Z, Guo Z, Zhong J, Cheng C, Huang Z, Wu Y, Tang S, Luo C, Peng X, Wu H, et al. ApoE influences the blood-brain barrier through the NF-κB/MMP-9 pathway after traumatic brain injury. Sci Rep. 2017:6649. doi: 10.1038/s41598-017-06932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun GZ, He YC, Ma XK, Li ST, Chen DJ, Gao M, Qiu SF, Yin JX, Shi J, Wu J. Hippocampal synaptic and neural network deficits in young mice carrying the human APOE4 gene. CNS Neurosci Ther. 2017;23:748–758. doi: 10.1111/cns.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo M, Kim Y. Cortical functional connections and fluid intelligence in adolescent APOE ε4 carriers. Dement Geriatr Cogn Disord. 2017;44:153–159. doi: 10.1159/000479276. [DOI] [PubMed] [Google Scholar]
- 21.Mahley RW, Huang Y. Apolipoprotein E: From atheroscierosis to Alzheimer's disease and beyond. Curr Opin Lipidol. 1999;10:207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Poirer J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17:525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MW, Crenshaw D, Welsh-Bohmer KA, Burns DK, Roses AD. New genetic approaches to AD: Lessons from APOE-Tomm40 phylogenetics. Curr Neuurol Neurosci Rep. 2016;16:48. doi: 10.1007/s11910-016-0643-8. [DOI] [PubMed] [Google Scholar]
- 24.Liberman JN, Stewart WF, Wesnes K, Troncoso J. Apolipoprotein E epsilon 4 and short-term recovery from predominantly mild brain injury. Neurology. 2002;58:1038–1044. doi: 10.1212/WNL.58.7.1038. [DOI] [PubMed] [Google Scholar]
- 25.McCarron MO, Weir CJ, Muir KW, Hoffmann KL, Graffagnino C, Nicoll JA, Lees KR, Alberts MJ. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol Scand. 2003;107:106–109. doi: 10.1034/j.1600-0404.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 26.Liaquat I, Dunn LT, Nicoll JA, Teasdale GM, Norrie JD. Effect of apolipoprotein E genotype on hematoma volume after trauma. J Neurosurg. 2002;96:90–96. doi: 10.3171/jns.2002.96.1.0090. [DOI] [PubMed] [Google Scholar]
- 27.Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: A prospective cohort study. Brain. 2005;128:2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- 28.Ariza M, Pueyo R, Matarín Mdel M, Junqué C, Mataró M, Clemente I, Moral P, Poca MA, Garnacho A, Sahuquillo J. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:1191–1193. doi: 10.1136/jnnp.2005.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isoniemi H, Tenovuo O, Portin R, Himanen L, Kairisto V. Outcome of traumatic brain injury after three decades-relationship to ApoE genotype. J Neurotrauma. 2006;23:1600–1608. doi: 10.1089/neu.2006.23.1600. [DOI] [PubMed] [Google Scholar]
- 30.Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, Babaey S, Yehuda B, Groswasser Z. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/WNL.52.2.244. [DOI] [PubMed] [Google Scholar]
- 31.He XZ, Sun XC, Dan W, Liu FY, Jiang Y, Ruan J. Association between apolipoprotein E gene polymorphism and EEG changes during acute phase of mild to moderate brain injury. Chin J Traumatol. 2008;24:619–623. (In Chinese) [Google Scholar]
- 32.Pfenniger A, Meens MJ, Pedrigi RM, Foglia B, Sutter E, Pelli G, Rochemont V, Petrova TV, Krams R, Kwak BR. Shear stress-induced atherosclerotic plaque composition in ApoE(−/-) mice is modulated by connexin37. Atherosclerosis. 2015;243:1–10. doi: 10.1016/j.atherosclerosis.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 33.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benarroch EE. Neuron-astrocyte interactions: Partnership for normal function and disease in the central nervous system; Mayo Clin Proc; 2005; pp. 1326–1338. [DOI] [PubMed] [Google Scholar]
- 35.Horiuchi M, Tomooka Y. An attempt to generate neurons from an astrocyte progenitor cell line FBD-104. Neurosci Res. 2005;53:104–115. doi: 10.1016/j.neures.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 37.Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005;93:145–155. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, Ehlers MD, Rebeck GW. Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J Biol Chem. 2006;281:3425–3431. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]