Abstract
Renal cell carcinoma (RCC) is a common tumor of the urinary system. Previously, miR-191-5p has been reported to be associated with various types of cancer; however, its specific functions in RCC have not been investigated to date. In the present study, the expression of miR-191-5p in the 786-O and ACHN cell lines was detected in vitro by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The results of RT-qPCR revealed that miR-191-5p was significantly downregulated in the two cell lines compared with the 293T cell line. miR-191-5p was also significantly downregulated in RCC tissue compared with paired normal tissue. In addition, the effects of miR-191-5p on cell proliferation, migration, invasion and apoptosis were examined by CCK-8, MTT, wound scratch, Transwell and flow cytometry assays. Downregulation of miR-191-5p was observed to promote cell proliferation, migration and invasion, as well as to repress the cell apoptosis of 786-O and ACHN cells. Therefore, the current study suggests that miR-191-5p functions as a tumor suppressor in RCC. Further studies are required to uncover the underlying signaling pathway of miR-191-5p and its potential role as a biomarker for early detection and prognosis prediction, and as a therapeutic target of RCC.
Keywords: microRNA, microRNA-191-5p, renal cell carcinoma, suppressor
Introduction
Renal cell carcinoma (RCC) accounts for ~2% of all cancer diagnoses and cancer-associated mortalities, with ~295,000 new kidney cancer cases are diagnosed and ~134,000 mortalities recorded worldwide annually (1). RCC is classified into three major subtypes, including clear cell, papillary and chromophobe RCC. Clear cell RCC is the most common subtype accounting for approximately 75% of all RCC cases (2). According to the American Joint Committee on Cancer staging of RCC (3), the 5-year survival rate of patients with stage I RCC is ~98%, while that of patients with stage III is ~5% (4). Thus, early detection and treatment is important. However, ~33% of patients have metastatic disease at the time of diagnosis, and 30–40% of patients relapse subsequent to the initial nephrectomy (5,6). Therefore, there is also a pressing need for effective diagnosis and treatment.
microRNAs (miRNAs or miRs) are short non-coding single stranded RNAs of 20–22 nucleotides in length that regulate the gene expression at the post-transcriptional level (7). Emerging evidence revealed that miRNAs are aberrantly expressed in numerous types of human cancer, as well as serve important roles in the initiation, development and metastasis of cancer (8–10). miRNAs also function as oncogenes or tumor suppressors in various cancer types (11). Previous studies have demonstrated that certain miRNAs were associated with RCC carcinogenesis, upregulated miRNAs act as tumor activator whereas downregulated miRNAs act as tumor suppressors (12–14). miR-191-5p is known to be dysregulated in several tumors, including intrahepatic cholangiocarcinoma (15), breast cancer (16) and colorectal cancer (17). However, the role of miR-191-5p in RCC remains largely unknown.
Therefore, the aims of the current study were to detect the expression of miR-191-5p in the ACHN and 786-O cell lines, as well as to reveal the function of miR-191-5p on the cell proliferation, invasion, migration and apoptosis in RCC.
Materials and methods
Sample collection
A total of 24 pairs of RCC and adjacent normal renal tissues were collected from Peking University Shenzhen Hospital (Shenzhen, China). The characteristics of the patients from whom tissues were collected are presented in Table I. The only inclusion criterion was that the patient had RCC, there were no exclusion criteria. Written informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Research Ethics Committee of Peking University Shenzhen Hospital (Shenzhen, China). Tissues were dissected, immersed in RNAlater (Qiagen GmbH, Hilden, Germany) for 30 min and stored at −80°C until use. Each pair of tissues consisted of RCC tissues along with adjacent normal tissues at 2 cm distant from the visible RCC lesions. Primary tumor stage and Fuhrman nuclear grading system were used to classify the specimens (18,19).
Table I.
Clinicopathological features of patients with renal cell carcinoma.
| Characteristic | Value |
|---|---|
| Mean age (range), years | 44 (25–62) |
| Males, n | 9 |
| Females, n | 15 |
| Histological type, n | |
| Clear cell | 21 |
| Papillary | 3 |
| pT-stage, n | |
| T1 | 19 |
| T2 | 3 |
| T3+T4 | 2 |
| Fuhrmann grade, n | |
| I | 3 |
| II | 17 |
| III | 2 |
| IV | 2 |
| AJCC clinical stage, n | |
| I | 8 |
| II | 14 |
| III + IV | 2 |
pT, primary tumor; AJCC, American joint committee on cancer.
Cell culture
The 786-O and ACHN RCC cell lines, as well as 293T human embryonic kidney cells, were obtained from the American Type Culture Collection (Manassas, VA, USA). 786-O cells were cultured in a 37°C humidified incubator containing 5% CO2 with RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), while ACHN and 293T cells were cultured with Dulbecco's modified Eagle medium (Gibco; Thermo Fisher Scientific, Inc.) at the same temperature and humidity. Media were supplemented with 10% fetal bovine serum and 1% antibiotics (100 µl/ml penicillin and 100 mg/ml streptomycin sulfates; Pen Strep) and 1% glutamine (all from Gibco; Thermo Fisher Scientific, Inc.).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues by TRIzol (Life Sciences; Thermo Fisher Scientific, Inc.) and purified with the RNeasy Maxi kit (Qiagen GmbH) according to the manufacturer's protocol. Next, the RNA concentration in the sample was detected with a NanoDrop 2000/2000c spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA synthesis was performed at 7°C for 60 min, 95°C for 5 min, the products were stored at 4°C. RT was conducted using 1 µg total RNA of each sample according to the procedure described in the miScript Reverse Transcription kit (Qiagen GmbH). Subsequently, qPCR was performed to detect the expression of miR-191-5p with the miScript SYBR Green PCR kit (Qiagen GmbH) on the Roche Lightcycler 480 Real-Time PCR system (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's protocols. The primers for miR-191-5p and U6 (serving as the internal control) used in qPCR assay are listed in Table II, they were produced by Invitrogen (Thermo Fisher Scientific, Inc.). The qPCR thermal cycling conditions were as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The expression of miR-191-5p was calculated according to the 2−ΔΔCq method (20).
Table II.
Sequences of primers and microRNAs.
| Primer or miRs | Sequence |
|---|---|
| miR-191-5p | Forward: 5′-CAACGGAATCCCAAAAGCAGCTG-3′ |
| Reverse: As provided by the miScript SYBR Green kit | |
| U6 | Forward: 5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse: 5′-ACGCTTCACGAATTTGCGT-3′ | |
| miR-191-5p mimics | Forward: 5′-CAACGGAAUCCCAAAAGCAGCUG-3′ |
| Reverse: 5′-GCUGCUUUUGGGAUUCCGUUGUU-3′ | |
| NC mimics | Forward: 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Reverse: 5′-ACGUGACACGUUCGGAGAATT-3′ | |
| miR-191-5p inhibitor | 5′-CAGCUGCUUUUGGGAUUCCGUUG-3′ |
| NC inhibitor | 5′-CAGUACUUUUGUGUAGUACAA-3′ |
miR, microRNA; NC, negative control.
Cell transfection
In order to upregulate the expression level of miR-191-5p in 786-O and ACHN cells, transfection was performed with synthesized miR-191-5p mimics (GenePharma, Inc., Shanghai, China). For downregulation of the expression, cells were transfected with a synthesized miR-191-5p inhibitor (GenePharma, Inc.). Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) mixed in the Opti-MEMR I Reduced Serum Medium (Gibco Thermo Fisher Scientific, Inc.) was used for transfection, following the manufacturer's instructions. The expression level of miR-191-5p was monitored by RT-qPCR to determine whether upregulation or downregulation was successful. The sequences of the miRNAs and negative control (NC) mimics and inhibitors used are listed in Table II.
Wound scratch assay
The effect of miR-191-5p the migration of 786-O and ACHN cells was investigated by a wound scratch assay. Briefly, approximately 3×105 cells were seeded into each well of a 12-well plate, and transfected with 40 pmol miR-191-5p mimics, miR-191-5p inhibitor, NC mimics or NC inhibitor after 24 h using Lipofectamine 2000. The cell monolayer was scraped in a straight line using a micropipette tip and washed with phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific, Inc.) to remove any cell debris after 6 h of transfection. Subsequently, the scraped monolayer was incubated at 37°C in a humidified chamber containing 5% CO2. A digital camera system (Olympus Corporation, Tokyo, Japan) was used to capture images of the scratches at 0 and 12 h after the scratch through a Leica DMIRB inverted fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Transwell assay
The effect of miR-191-5p on the migration and invasion of 786-O and ACHN cells was measured by conducting a transwell assay. Transwell chamber inserts (BD Biosciences, Franklin Lakes, NJ, USA) with (for invasion) or without Matrigel (for migration) were used in the assay according to the manufacturer's protocol. Briefly, 200 µl serum-free medium containing ~1×104 transfected cells was plated in the upper compartment of the chamber. Complete medium as the source of chemo-attractants was added into the lower chamber. The migration time was 36 h in the two cell lines, while the invasion time was 48 and 60 h in 786-O and ACHN cells, respectively. Cells that had migrated to or invaded the lower surface of the inserts were then stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and were counted under the microscope at magnification, ×100 (Leica Microsystems GmbH).
MTT assay
The cell proliferation analysis was conducted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, ~5,000 cells were seeded into each well of a 96-well plate, and then 5 pmol miR-191-5p mimics, miR-191-5p inhibitor, NC mimics or NC inhibitor was used for transfection. At 4 days after transfection, 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added into the medium, which was replaced by 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) after 4 h. The experimental wells were shaken by a reciprocating decolorization shaking table (TSB-108; Qilinbeier, Jiangsu, China) for 10 min in the dark, and then the absorbance was read at 595 nm on an ELISA microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was performed to detect the cell proliferation ability of 786-O and ACHN cells in vitro. Approximately 5,000 cells were seeded into each well of a 96-well plate. Following incubation for 24 h, the cells were transfected with 5 pmol miR-191-5p mimics, inhibitor, NC mimics or NC inhibitor. At 0, 24, 48 and 72 h after transfection, 10 µl CCK-8 (Beyotime Institute of Biotechnology, Haimen, China) was added to each well and incubated for 30 min. Subsequently, the absorbance values of the experimental wells were detected at 490 nm on a ELISA microplate reader (model 680; Bio-Rad Laboratories, Inc.).
Flow cytometry assay for apoptosis detection
In order to detect the apoptotic rates of 786-O and ACHN cells in vitro, flow cytometry assay was performed. Briefly, ~3×105 cells were seeded into each well of a 6-well plate and then transfected with 200 pmol miR-191-5p mimics, inhibitor, NC mimics or NC inhibitor. After 48 h, the cells were collected and washed with cold PBS, and then resuspended with 100 µl 1X binding buffer. Next, 5 µl Annexin V-fluorescein isothiocyanate and 5 µl propidium iodide (Invitrogen; Thermo Fisher Scientific, Inc.) were added to each cell suspension. After 15 min of staining in the dark at room temperature, 400 µl binding buffer was added to each tube. The data were collected and analyzed on an EPICS XL-4 flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Statistical analysis
Paired t-tests were used to compare the expression levels of miR-191-5p in the matched tumor and normal tissues, or in the different cells. Student's t-test was used to analyze assays for characterizing phenotypes of cells. All the statistical analyses were conducted using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-191-5p is downregulated in RCC clinical specimens and cell lines
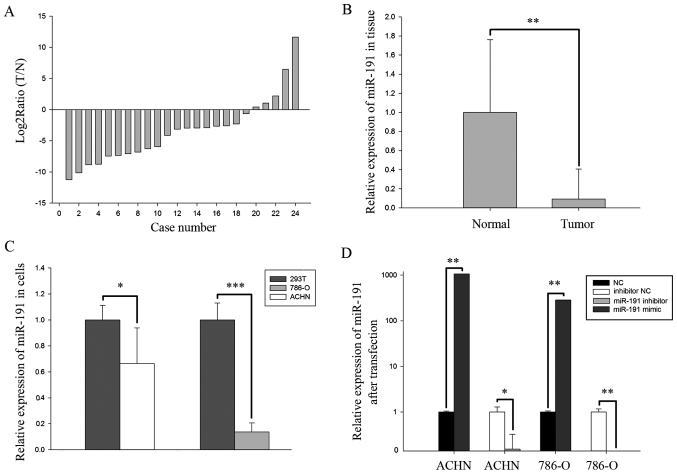
The ratio of miR-191-5p expression in 24 paired clinical specimens was presented in Fig. 1A as the log2ratio of tumor over adjacent normal tissues. The expression of miR-191-5p in RCC tissues was significantly downregulated compared with that in adjacent normal tissues (P<0.01; Fig. 1B). In addition, the expression of miR-191-5p in the 293T normal human embryonic kidney cell line was significantly upregulated compared with that in ACHN (P<0.05) and 786-O (P<0.001) RCC cell lines, which was in accordance with the expression pattern of miR-191-5p in the clinical specimens (Fig. 1C).
Figure 1.
(A) Log2 T/N ratio of miR-191-5p, and (B) relative expression of miR-191-5p in 24 paired clinical specimens of RCC and adjacent normal tissues. (C) Relative expression levels of miR-191-5p in the normal 293T cells and two RCC cell lines (ACHN and 786-O). (D) Relative expression levels of miR-191-5p in ACHN and 786-O cell lines after transfection with mimics or inhibitors. *P<0.05, **P<0.01 and ***P<0.001. RCC, renal cell carcinoma; miR, microRNA; NC, negative control; T, RCC tissues; N, normal tissues.
Transfection efficiency validation
miR-191-5p or NC mimics, and miR-191-5p or NC inhibitors were transfected into 786-O and ACHN cells and the transfection efficiency was evaluated by RT-qPCR. As observed in Fig. 1D, the expression levels of miR-191-5p were downregulated by 84.57% in ACHN cells (P<0.05) and 98.75% in 786-O cells (P<0.01) following transfection with the miR-191-5p inhibitor as compared with NC inhibitor, while the expression levels of miR-191-5p were 1,062.56 times higher in ACHN cells (P<0.01) and 283.39 times higher in 786-O cells (P<0.01) following transfection with miR-191-5p mimics compared with the corresponding NC group.
Upregulation of miR-191-5p suppresses RCC cell proliferation, while downregulation promotes RCC cell proliferation
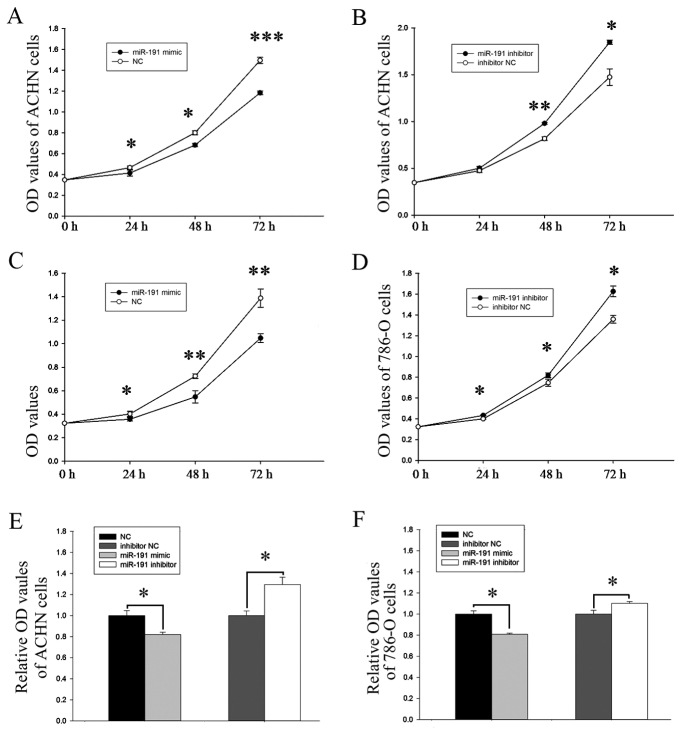
A CCK-8 assay was used to observe whether differential expression of miR-191-5p affected the proliferative ability of the RCC cells. The proliferative ability of the miR-191-5p mimic group in the ACHN cells was significantly decreased by 11.16% (P<0.05), 14.83% (P<0.05) and 20.83% (P<0.001) at 24, 48 and 72 h after transfection, respectively, compared with the NC group (Fig. 2A). By contrast, cell proliferation in the miR-191-5p inhibitor group was increased by 5.55% (P>0.05), 19.92% (P<0.01) and 25.24% (P<0.05) at 24, 48 and 72 h after transfection, respectively (Fig. 2B). Similarly, in the 786-O cells, the proliferation rates of the miR-191-5p mimic group were significantly decreased by 11.13, 24.34 and 24.49% at 24, 48 and 72 h after transfection, respectively (P<0.05, P<0.01 and P<0.01; Fig. 2C), while the rates of proliferation increase in the miR-191-5p inhibitor-transfected cells were 8.26, 9.91 and 19.70% at 24, 48 and 72 h, respectively (P<0.05; Fig. 2D).
Figure 2.
Analysis of proliferative ability using cell counting kit-8 assay in ACHN cells transfected with miR-191-5p (A) mimic and (B) inhibitor, and in 786-O cells transfected with miR-191-5p (C) mimic and (D) inhibitor. Analysis of viability in (E) ACHN and (F) 786-O cells using MTT assay. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control; OD, optical density.
Upregulation of miR-191-5p suppresses RCC cell viability, while downregulation promotes RCC cell viability
The viability of miR-191-5p was also assessed using MTT assay. As shown in Fig. 2E, the viability of the miR-191-5p mimic group was decreased by 17.97% in the ACHN cells compared with the corresponding NC group (P<0.05), while the viability of the miR-191-5p inhibitor group was promoted by 29.41% (P<0.05). Similarly, in 786-O cells, the viability of the miR-191-5p mimic group was decreased by 19.12% (P<0.05), while that of the miR-191-5p inhibitor group was promoted by 10.18% (P<0.05; Fig. 2F).
Upregulation of miR-191-5p suppresses RCC cell mobility, while downregulation of miR-191-5p promotes RCC cell mobility
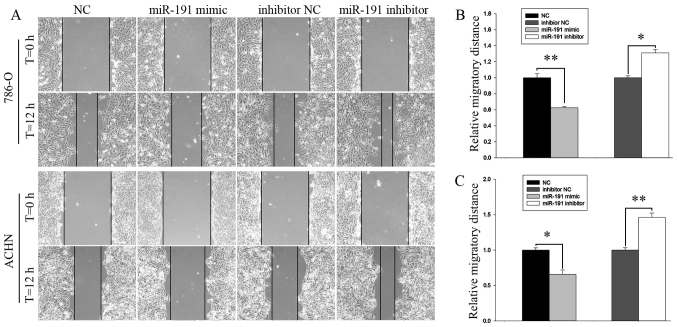
Cell mobility was examined by a wound scratch and transwell assays. The results of the wound scratch assay suggested that the migratory distance of 786-O cells transfected with miR-191-5p mimic was decreased by 37.55% (P<0.01; Fig. 3A and B) and by 34.28% (P<0.05; Fig. 3A and C) in ACHN cells, as compared with the corresponding NC group. By contrast, the increase in the migration rate following transfection with miR-191-5p inhibitor were 31.00% for 786-O cells (P<0.05; Fig. 3A and B) and 46.15% for ACHN cells (P<0.01; Fig. 3A and C).
Figure 3.
(A) Wound scratch assay demonstrating the migratory abilities of 786-O and ACHN cells. Analysis of migratory distances in (B) 786-O and (C) ACHN cells. *P<0.05 and **P<0.01. miR, microRNA; NC, negative control; T, time.
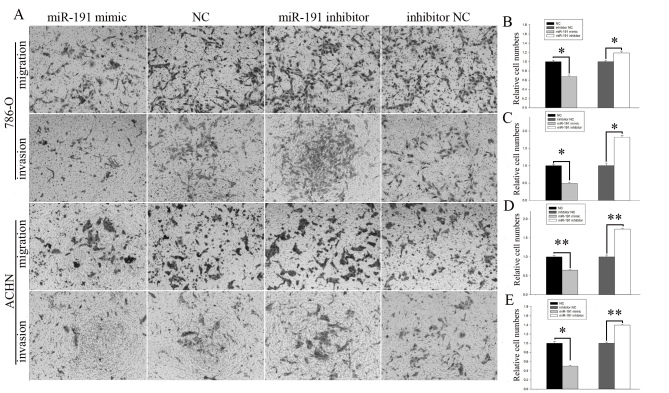
The results of the transwell assay (Fig. 4A) indicated that the migration rates in the miR-191-5p mimic group were significantly inhibited by 32.35% compared with the normal control (P<0.05; Fig. 4B) and the invasion rates were significantly reduced by 50.93% in 786-O cells compared with the normal controls (P<0.05; Fig. 4C). The migration rates in the miR-191-5p mimic group were significantly inhibited by 35.36% compared with the normal control (P<0.01; Fig. 4D) and the invasion rates were significantly inhibited by 49.77% in the ACHN cells compared with the normal control group (P<0.05; Fig. 4E). The migratory ability of the miR-191-5p inhibitor group was significantly increased by 19.02% compared with the normal control inhibitor group (P<0.05; Fig. 4B) and the invasive ability of the miR-191-5p inhibitor group was significantly increased by 81.83% in 786-O cells compared with the normal control inhibitor group (P<0.05; Fig. 4C). Additionally, the migratory ability of the miR-191-5p inhibitor group was significantly increased by 73.16% compared with the normal control inhibitor group (P<0.01; Fig. 4D) and the invasive ability of the miR-191-5p inhibitor group was significantly promoted by 39.74% in ACHN cells compared with the normal control inhibitor group (P<0.01; Fig. 4E).
Figure 4.
(A) Images of transwell assay of 786-O and ACHN cells. The (B) migration and (C) invasion of 786-O cells, and the (D) migration and (E) invasion of ACHN cells are demonstrated. *P<0.05 and **P<0.01. miR, microRNA; NC, negative control.
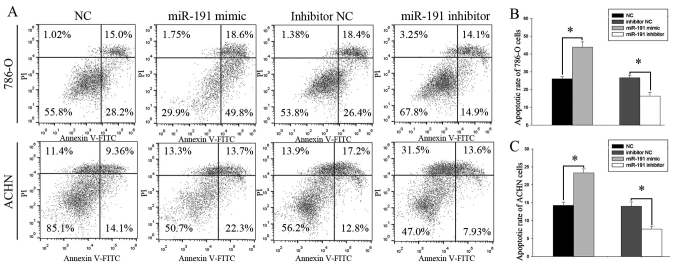
Upregulation of miR-191-5p induces cell apoptosis, whereas downregulation reduces cell apoptosis
The results of flow cytometry (Fig. 5A) revealed that the early apoptotic rate was significantly increased in the miR-191-5p mimic-transfected 786-O cells in comparison with the NC-transfected cells (43.86±2.97 and 26.03±1.36%; P<0.05; Fig. 5B). Similarly, the apoptotic rate was also markedly enhanced in the miR-191-5p mimic-transfected ACHN cells compared with the NC-transfected cells (23.33±1.13 vs. 14.26±0.89%; P<0.05, Fig. 5C). Thus, miR-191-5p upregulation resulted in enhanced apoptosis in the two cell lines examined. In addition, the early apoptosis rate of 786-O cells transfected with NC inhibitor or miR-191-5p inhibitor was 26.66±1.41 vs. 16.33±2.14% (P<0.05; Fig. 5B), while the ACHN cells transfected with NC inhibitor or miR-191-5p inhibitor was 14.03±1.20 vs. 7.66±0.68% (P<0.05; Fig. 5C), indicating that downregulation of this miR reduced the cell apoptosis.
Figure 5.
(A) Apoptosis investigated using flow cytometry assay. Analysis of early apoptotic rate in (B) 786-O and (C) ACHN cells. *P<0.05. miR, microRNA; PI, propidium iodide; FITC, fluorescein isothiocyanate; NC, negative control.
Discussion
The implication of miRNAs in tumorigenesis was first recognized when miRNA genes were observed to be specifically deleted in patients with leukemia (21). Subsequently, numerous studies demonstrated that miRNAs serve important roles in various carcinomas (22–26). In addition, Polioudakis et al (27) reported that miRNAs are located at 50% of all fragile regions or sites presenting copy number alterations in cancer.
The function of miR-191-5p differs among different tumors. According to the study of Tian et al (28), miR-191-5p inhibits tumor necrosis factor-α-induced apoptosis in endometrial carcinoma cells by targeting death-associated protein kinase 1 (DAPK1). Additionally, Zhang et al (17) demonstrated that miR-191-5p promotes the tumorigenesis of colorectal cancer by targeting CCAAT-enhancer-binding protein β. Di Leva et al (29) also reported that expression of the miR-191/425 cluster reduced the proliferation and impaired tumorigenesis in breast cancer cells. However, Nagpal et al (30) reported that miR-191-5p functions as an estrogen inducible promoter in breast cancer by targeting SATB homeobox 1. Another study by Nagpal et al (31) confirmed that miR-191-5p promotes migration in breast cancer through complex regulation of transforming growth factor-β-signaling in a hypoxic microenvironment. miR-191-5p was further demonstrated to promote osteosarcoma cell proliferation by targeting checkpoint kinase 2, according to the study by Huang et al (32). It was also observed that miR-191-5p functions as a tumor promoter by modulating the tet methylcytosine dioxygenase 1-p53 pathway in intrahepatic cholangiocarcinoma (15). Recently, miR-191-5p has been revealed to promote pancreatic cancer progression by targeting ubiquitin specific peptidase 10 (33). Shi et al (34) reported that miR-191-5p exhibits a promotive effect by targeting N-deacetylase and N-sulfotransferase 1 in MGC803 human gastric cancer cells. In a lung cancer research, Xu et al (35) demonstrated that miR-191-5p positively modulated the epithelial-mesenchymal transition and cancer stem cell-like properties and functioned as an onco-miR in transfected cells. In addition, miR-191-5p overexpression has been observed in oral squamous cell carcinoma, although it remains unclear how it contributes to the development of this tumor. Furthermore, miR-191-5p is associated with poor prognosis in pancreatic cancer (36), chromophobe RCC (37), acute myeloid leukemia (38), glioblastoma (39) and malignant melanoma (40). In contrast to these previous studies, the present study observed that miR-191-5p was downregulated in RCC tissues and cells. In addition, miR-191-5p was observed to promote the proliferation, migration and invasion, as well as to inhibit the apoptosis, of 786-O and ACHN cells.
miR-191-5p is also known to serve an important role in non-neoplastic diseases. Previous studies have demonstrated that miR-191-5p may modulate the malignant transformation of endometriosis by targeting DAPK1 and TIMP metallopeptidase inhibitor 3 (28,41).
According to a large number of studies, miR-191-5p has great potential for clinical use as a novel biomarker and as a therapeutic agent in cancer. Elyakim et al (42) reported that miR-191-5p inhibition has great potential in the treatment of hepatocellular carcinoma patients. In addition, miR-191-5p is a potential target in treating radiation-resistant lung cancer according to the study by Liu and Huang (43). Several studies also reported that miR-191-5p may be of value as a prognostic and predictive biomarker in certain tumors, including breast cancer (44), osteosarcoma (45), and head and neck squamous cell carcinoma (46). Furthermore, miR-191-5p was suggested to be a potentially non-neoplastic biomarker to diagnose traumatic brain injury and predict the patient prognosis (47).
In conclusion, in the present study, it was demonstrated that miR-191-5p was downregulated in 786-O and ACHN cells compared with the adjacent normal RCC cells. Downregulation of miR-191-5p promoted the proliferation, migration and invasion, as well as suppressed the apoptosis, of 786-O and ACHN cells. Further studies are required to uncover the underlying signaling pathway of miR-191-5p and to examine the potential role of miR-191-5p as a biomarker for early detection and prognosis prediction, and as a therapeutic target in RCC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81101922), Science and Technology Development Fund Project of Shenzhen (nos. JCYJ20150403091443329 and JCYJ20170307111334308), the fund of ‘San-ming’ Project of Medicine in Shenzhen (grant no. SZSM201612066) and the fund of Guangdong Key Medical Subject.
References
- 1.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritz HK, Lindgren D, Ljungberg B, Axelson H, Dahlbäck B. The miR(21/10b) ratio as a prognostic marker in clear cell renal cell carcinoma. Eur J Cancer. 2014;50:1758–1765. doi: 10.1016/j.ejca.2014.03.281. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 4.Tusong H, Maolakuerban N, Guan J, Rexiati M, Wang WG, Azhati B, Nuerrula Y, Wang YJ. Functional analysis of serum microRNAs miR-21 and miR-106a in renal cell carcinoma. Cancer Biomark. 2017;18:79–85. doi: 10.3233/CBM-160676. [DOI] [PubMed] [Google Scholar]
- 5.Khella HWZ, Daniel N, Youssef L, Scorilas A, Nofech-Mozes R, Mirham L, Krylov SN, Liandeau E, Krizova A, Finelli A, et al. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J Clin Pathol. 2017;70:854–859. doi: 10.1136/jclinpath-2017-204341. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Wang X, Ruan A, Han W, Zhao Y, Lu X, Xiao P, Shi H, Wang R, Chen L, et al. miR-141 is a key regulator of renal cell carcinoma proliferation and metastasis by controlling EphA2 expression. Clin Cancer Res. 2014;20:2617–2630. doi: 10.1158/1078-0432.CCR-13-3224. [DOI] [PubMed] [Google Scholar]
- 7.Aguiari G. MicroRNAs in clear cell renal cell carcinoma: Biological functions and applications. J Kidney Cancer VHL. 2015;2:140–152. doi: 10.15586/jkcvhl.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10:396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 9.Jin L, Li Y, He T, Hu J, Liu J, Chen M, Zhang Z, Gui Y, Mao X, Yang S, Lai Y. miR-15a-5p acts as an oncogene in renal cell carcinoma. Mol Med Rep. 2017;15:1379–1386. doi: 10.3892/mmr.2017.6197. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Li Y, Chen D, Jin L, Su Z, Liu J, Duan H, Li X, Qi Z, Shi M, et al. miR-30a-5p in the tumorigenesis of renal cell carcinoma: A tumor suppressive microRNA. Mol Med Rep. 2016;13:4085–4094. doi: 10.3892/mmr.2016.5024. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Qin L, Zhu Z, Wang X, Liu Y, Fan Y, Zhong S, Wang X, Zhang X, Xia L, et al. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin α5. Oncotarget. 2016;7:27445–27457. doi: 10.18632/oncotarget.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 13.Shenouda SK, Alahari SK. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 14.Guil S, Esteller M. DNA methylomes, histone codes and miRNAs: Tying it all together. Int J Biochem Cell Biol. 2009;41:87–95. doi: 10.1016/j.biocel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhou ZQ, Yang ZR, Tong DN, Guan J, Shi BJ, Nie J, Ding XT, Li B, Zhou GW, Zhang ZY. MicroRNA-191 acts as a tumor promoter by modulating the TET1-p53 pathway in intrahepatic cholangiocarcinoma. Hepatology. 2017;66:136–151. doi: 10.1002/hep.29116. [DOI] [PubMed] [Google Scholar]
- 16.Mar-Aguilar F, Luna-Aguirre CM, Moreno-Rocha JC, Araiza-Chávez J, Trevino V, Rodríguez-Padilla C, Reséndez-Pérez D. Differential expression of miR-21, miR-125b and miR-191 in breast cancer tissue. Asia Pac J Clin Oncol. 2013;9:53–59. doi: 10.1111/j.1743-7563.2012.01548.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XF, Li KK, Gao L, Li SZ, Chen K, Zhang JB, Wang D, Tu RF, Zhang JX, Tao KX, et al. miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPbeta. Oncotarget. 2015;6:4144–4158. doi: 10.18632/oncotarget.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Carducci MA, Fishman M, Hancock SL, Hauke RJ, Hudes GR, Kantoff P, Kuzel TM, Lange PH, Levine EG, et al. Kidney Cancer. Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005;3:84–93. [PubMed] [Google Scholar]
- 19.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Dyrskjøt L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL, Andersen CL, Zieger K, et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 22.Kabir TD, Ganda C, Brown RM, Beveridge DJ, Richardson KL, Chaturvedi V, Candy P, Epis M, Wintle L, Kalinowski F, et al. A miR-7/GAS6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma. Hepatology. 2017 doi: 10.1002/hep.29478. [DOI] [PubMed] [Google Scholar]
- 23.Lynam-Lennon N, Heavey S, Sommerville G, Bibby BA, Ffrench B, Quinn J, Gasch C, O'Leary JJ, Gallagher MF, Reynolds JV, Maher SG. MicroRNA-17 is downregulated in esophageal adenocarcinoma cancer stem-like cells and promotes a radioresistant phenotype. Oncotarget. 2017;8:11400–11413. doi: 10.18632/oncotarget.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herr I, Sähr H, Zhao Z, Yin L, Omlor G, Lehner B, Fellenberg J. MiR-127 and miR-376a act as tumor suppressors by in vivo targeting of COA1 and PDIA6 in giant cell tumor of bone. Cancer Lett. 2017;409:49–55. doi: 10.1016/j.canlet.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Yan J, Zhang Y, She Q, Li X, Peng L, Wang X, Liu S, Shen X, Zhang W, Dong Y, et al. Long Noncoding RNA H19/miR-675 axis promotes gastric cancer via FADD/Caspase 8/Caspase 3 signaling pathway. Cell Physiol Biochem. 2017;42:2364–2376. doi: 10.1159/000480028. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang X, Xiong H, Gurbani D, Li L, Liu Y, Liu A. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141. doi: 10.1186/s12943-017-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polioudakis D, Abell NS, Iyer VR. MiR-191 regulates primary human fibroblast proliferation and directly targets multiple oncogenes. PLoS One. 2015;10:e0126535. doi: 10.1371/journal.pone.0126535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian X, Xu L, Wang P. MiR-191 inhibits TNF-α induced apoptosis of ovarian endometriosis and endometrioid carcinoma cells by targeting DAPK1. Int J Clin Exp Pathol. 2015;8:4933–4942. [PMC free article] [PubMed] [Google Scholar]
- 29.Di Leva G, Piovan C, Gasparini P, Ngankeu A, Taccioli C, Briskin D, Cheung DG, Bolon B, Anderlucci L, Alder H, et al. Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 2013;9:e1003311. doi: 10.1371/journal.pgen.1003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagpal N, Ahmad HM, Molparia B, Kulshreshtha R. MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis. 2013;34:1889–1899. doi: 10.1093/carcin/bgt107. [DOI] [PubMed] [Google Scholar]
- 31.Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, Kulshreshtha R. HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFβ-signaling in hypoxic microenvironment. Sci Rep. 2015;5:9650. doi: 10.1038/srep09650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YZ, Zhang J, Shao HY, Chen JP, Zhao HY. MicroRNA-191 promotes osteosarcoma cells proliferation by targeting checkpoint kinase 2. Tumour Biol. 2015;36:6095–6101. doi: 10.1007/s13277-015-3290-9. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu J, Gao FH. MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol. 2014;35:12157–12163. doi: 10.1007/s13277-014-2521-9. [DOI] [PubMed] [Google Scholar]
- 34.Shi X, Su S, Long J, Mei B, Chen Y. MicroRNA-191 targets N-deacetylase/N-sulfotransferase 1 and promotes cell growth in human gastric carcinoma cell line MGC803. Acta Biochim Biophys Sin (Shanghai) 2011;43:849–856. doi: 10.1093/abbs/gmr084. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu X, Zhao Y, Luo F, Wang B, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54(Suppl 1):E148–E161. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- 36.Song Z, Ren H, Gao S, Zhao X, Zhang H, Hao J. The clinical significance and regulation mechanism of hypoxia-inducible factor-1 and miR-191 expression in pancreatic cancer. Tumour Biol. 2014;35:11319–11328. doi: 10.1007/s13277-014-2452-5. [DOI] [PubMed] [Google Scholar]
- 37.Ge YZ, Xin H, Lu TZ, Xu Z, Yu P, Zhao YC, Li MH, Zhao Y, Zhong B, Xu X, et al. MicroRNA expression profiles predict clinical phenotypes and prognosis in chromophobe renal cell carcinoma. Sci Rep. 2015;5:10328. doi: 10.1038/srep10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruvolo PP, Ruvolo VR, Jacamo R, Burks JK, Zeng Z, Duvvuri SR, Zhou L, Qiu Y, Coombes KR, Zhang N, et al. The protein phosphatase 2A regulatory subunit B55α is a modulator of signaling and microRNA expression in acute myeloid leukemia cells. Biochim Biophys Acta. 2014;1843:1969–1977. doi: 10.1016/j.bbamcr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tivnan A, Heilinger T, Ramsey JM, O'Connor G, Pokorny JL, Sarkaria JN, Stringer BW, Day BW, Boyd AW, Kim EL, et al. Anti-GD2-ch14.18/CHO coated nanoparticles mediate glioblastoma (GBM)-specific delivery of the aromatase inhibitor, Letrozole, reducing proliferation, migration and chemoresistance in patient-derived GBM tumor cells. Oncotarget. 2017;8:16605–16620. doi: 10.18632/oncotarget.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caramuta S, Egyházi S, Rodolfo M, Witten D, Hansson J, Larsson C, Lui WO. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J Invest Dermatol. 2010;130:2062–2070. doi: 10.1038/jid.2010.63. [DOI] [PubMed] [Google Scholar]
- 41.Dong M, Yang P, Hua F. MiR-191 modulates malignant transformation of endometriosis through regulating TIMP3. Med Sci Monit. 2015;21:915–920. doi: 10.12659/MSM.893872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elyakim E, Sitbon E, Faerman A, Tabak S, Montia E, Belanis L, Dov A, Marcusson EG, Bennett CF, Chajut A, et al. hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010;70:8077–8087. doi: 10.1158/0008-5472.CAN-10-1313. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Huang S. Inhibition of miR-191 contributes to radiation-resistance of two lung cancer cell lines by altering autophagy activity. Cancer Cell Int. 2015;15:16. doi: 10.1186/s12935-015-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tajbakhsh A, Mokhtari-Zaer A, Rezaee M, Afzaljavan F, Rivandi M, Hassanian SM, Ferns GA, Pasdar A, Avan A. Therapeutic potentials of BDNF/TrkB in breast cancer; current status and perspectives. J Cell Biochem. 2017;118:2502–2515. doi: 10.1002/jcb.25943. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Ji F, Dai Z, Xie Y, Yuan D. Increased expression of microRNA-191 as a potential serum biomarker for diagnosis and prognosis in human osteosarcoma. Cancer Biomark. 2015;15:543–550. doi: 10.3233/CBM-150493. [DOI] [PubMed] [Google Scholar]
- 46.Salazar C, Nagadia R, Pandit P, Cooper-White J, Banerjee N, Dimitrova N, Coman WB, Punyadeera C. A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell Oncol (Dordr) 2014;37:331–338. doi: 10.1007/s13402-014-0188-2. [DOI] [PubMed] [Google Scholar]
- 47.Yang T, Song J, Bu X, Wang C, Wu J, Cai J, Wan S, Fan C, Zhang C, Wang J. Elevated serum miR-93, miR-191, and miR-499 are noninvasive biomarkers for the presence and progression of traumatic brain injury. J Neurochem. 2016;137:122–129. doi: 10.1111/jnc.13534. [DOI] [PubMed] [Google Scholar]