Abstract
The aim of the present study was to investigate whether oridonin is able to increase the effects of lentinan (LNT) in HepG2 human hepatoblastoma cells by MTT, flow cytometry, reverse transcription-quantitative polymerase chain reaction and western blot analysis. The in vitro results demonstrated that 20 µg/ml of oridonin was a nontoxic concentration for L02 normal liver cells and HepG2 liver cancer cells. Furthermore, treatment with 0–200 µg/ml LNT was only able to decrease the viability of HepG2 liver cancer cells. The growth inhibitory rate of the LNT-L (100 µg/ml) treatment group was 20.7% and the rate of the LNT-H (200 µg/ml) treatment group was 54.8%. Notably, the growth inhibitory rate of the oridonin + LNT-H group was 84.3%. The highest percentage of apoptotic cells was observed in the oridonin + LNT-H group (20 µg/ml oridonin and 200 µg/ml LNT). The percentage of apoptotic cells in the oridonin + LNT-H group was significantly different from the percentage of apoptotic cells in the LNT-H (26.1%) and the LNT-L (16.8%) groups. Treatment with LNT produced an increase in caspase-3, caspase-9, Bcl-2-like protein 4, p53, p21, nuclear factor κB inhibitor-α mRNA and protein expression and a decrease in B-cell lymphoma 2 and nuclear factor-κB expression in HepG2 cells compared with untreated control cells. Treatment with a combination of oridonin and LNT-H induced a further increase in expression with the biggest differences in expression observed between the oridonin + LNT-H group and control. It was observed that treatment with oridonin was able to increase the anticancer effects of LNT in HepG2 cells. Therefore, oridonin may be used to sensitize cells to LNT.
Keywords: oridonin, lentinan, HepG2 human hepatoblastoma cells, growth inhibition, expression
Introduction
Lentinan (LNT) is a type of medicinal polysaccharide isolated from shiitake with various activities including, immune regulation, anti-tumor, anti-virus and anti-infection effects. LNT has high efficacy and limited side effects (1). LNT has good curative effects on gastric, colon, breast and lung cancer, which is able to prolong the survival time of patients with tumor (2). LNT is often used as an immune enhancer in clinical application, which can enhance curative effects or reduce side effects in combination with other drugs (2). Murata et al (3) reported that a combination of cisplatin and lentinan significantly increased the anti-cancer activity in the treatment of colon cancer. Drandarska et al (4) demonstrated that treatment with LNT is able to increase the activation of Bacillus Calmette-Guérin (BCG)-induced pulmonary macrophages in guinea pigs and reduce systemic adverse reactions of BCG vaccine.
Oridonin is an ent-kaurene diterpenoid compound mainly isolated from R. rubescen (5). Previous studies have reported that oridonin is able to promote tumor cell apoptosis (3,6). Apoptotic rate has been hypothesized to have an effect on the sensitivity of tumors to radiation (7). A previous study also indicated that the sensitization effect of oridonin may enhance the efficacy of radiotherapy in liver cancer cell lines (8).
An ideal cancer therapeutic would be a drug that can induce differentiation and apoptosis of tumor cells. Previous clinical studies have focused on Chinese medicine preparations based on anti-proliferative effects, while in recent years the focus has shifted to the development of preparations which can induce differentiation and apoptosis of cancer cells (9,10). Drugs that induce apoptosis can selectively target cancer cells and therefore normal cells are unaffected by treatment (9).
A number of active chemical substances in natural plants have strong apoptotic-inducing effects on cancer cells (8–10), and studies have demonstrated that lentinan and oridonin are active substances with cancer cell apoptosis-inducing effects (3,8).
Substances that can induce apoptosis in cancer cells, which are extracted from plants have a low level of toxicity and can be safely used. These substances are also able to relieve pain in the process of treatment. However, the efficacy of numerous cancer inhibitor components in plants is much lower compared with synthetic drugs (11). Therefore, a combination of different natural substances can increase the inhibitory and therapeutic effects (11).
Finding a reasonable combination is an important aspect of the research in the anti-cancer activities of natural products. By studying how oridonin is able to increase the anticancer effect of lentinan in vitro, the present study aimed to verify the effects of a novel combination of anti-cancer substances. By using MTT assay, flow cytometry, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting, the present study investigated the effect of oridonin treatment on growth inhibition hepatoblastoma cells in vitro. The study also investigated the effect of lentinan treatment on the apoptosis of hepatoblastoma cells, in order to accumulate further data that may enable future animal experiments and even clinical application.
Materials and methods
Cell lines and treatments
Human normal liver L02 cell lines and human hepatoblastoma HepG2 cells were purchased from the Conservation Genetics CAS Kunming Cell Bank (Kunming, China). Oridonin and lentinan were purchased from Shanghai Shamrock Imp and Exp Trading Co., Ltd. (Shanghai, China). Lentinan-Low (LNL-L, 100 µg/ml) and Lentinan-High (LNL-H, 200 µg/ml) treatment groups were generated for the two cell lines.
Cell culture
The normal human liver L02 cell lines (control group) and human hepatoblastoma HepG2 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and cultured in an incubator under humidified atmosphere of 5% CO2 at 37°C. The medium was changed 2–3 times a week and sub-cultured for 6–7 days. Subsequently, the cells were seeded in a 96-well culture plate at a density of 1×104/ml with 180 µl per well, and cultured for 24 h under humidified atmosphere of 5% CO2 at 37°C.
MTT assay
The L02 (control group) and HepG2 cells were incubated with Oridonin (20 µg/ml) and Lentinan (100 µg/ml) for 24 h at room temperature, respectively. After 24 h, 20 µl MTT solution (5 mg/ml; Ameresco Inc., Framingham, MA, USA) was added to each well and incubated at 37°C for 4 h, following which the culture medium was replaced with 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The absorbance was measured at 540 nm using a microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following formula was used to calculate the inhibitory rate: Percentage cell viability=[(Absorbance of untreated cells-absorbance of treated cells)/absorbance of untreated cells] ×100 (12).
Flow cytometry analysis of cell cycle distribution and apoptosis
HepG2 cells were seeded at a density of 50×104 cells/60-mm dish and incubated overnight at 37°C. Oridonin was added to a final concentration of 40 µM and cells were incubated for 24 h at 37°C. The cells were treated as follows: i) 20 µg/ml oridonin + 200 µg/ml lentinan; ii) PBS (negative control); iii) 100 µg/ml lentinan; and iv) 200 µg/ml lentinan. Detached and adherent cells were collected and centrifuged at 1,500 × g for 5 min at 4°C. Pellets were rinsed with ice-cold PBS and fixed with 70% ethanol at 4°C overnight. The density of HepG2 cells was adjusted to 5×105 cells/ml, and the cells were washed with PBS three times. Cells were subsequently stained with staining buffer (PBS containing 20 µg/ml of propidium iodide, 100 µg/ml RNase A and 0.1% Triton X-100; BD Biosciences, Franklin Lakes, NJ, USA) for 15 min at 4°C in the dark. The cells were subsequently labeled with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (Annexin V-FITC apoptosis detection kit; BD Biosciences; cat. no. 556547). Samples were analyzed using a flow cytometer (BD Biosciences) and Cell Quest acquisition software (version 2.9; BD Biosciences) (13).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
Cells from different treatment groups (20 µg/ml oridonin + 200 µg/ml lentinan; PBS as negative control; 100 µg/ml lentinan; and 200 µg/ml lentinan) were collected, and total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was reverse transcribed into cDNA using a cDNA reverse transcription kit (cat. no., 1708840; Bio-Rad Laboratories, Inc.) according to manufacturer's protocol. The resultant DNA (10 µl) was subjected to a 25 µl PCR conducted in an iCycler thermal cycler (Bio-Rad Laboratories, Inc.) using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.). The thermocycling conditions that were used were as follows: Initial denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec, with a final extension of 72°C for 5 min. β-actin was used as the internal reference gene. The relative expression levels were calculated using the 2−ΔΔCq method (14) and gene expression was normalized to β-actin. The primers used in the present study were as follows: B-cell lymphoma (Bcl)-2 forward, 5′-CAAAGGTGGATCAGATTCAAG-3′; Bcl-2 reverse, 5′-GGTGAGCATTATCACCCAGAA-3′; Bcl-2-associated protein X (Bax) forward, 5′-TGGCAGCAGTGACAGCAGCG-3′; Bax reverse, 5′-TACGGAGGTGGAGTGGGTGT-3′; caspase-3 forward, 5′-AAAGTTTTCAATGACCAAGC-3′; caspase-3 reverse, 5′-TCTGACGAATCTCCTCCAC-3′; caspase-9 forward, 5′-AGTCTATTTTATTATGGGCTCG-3′; caspase-9 reverse, 5′-TGGATGTTTATGTCACCTTTTC-3′; p21 forward, 5′-ATGGAGAACACTGAAAACTC-3′; p21 reverse, 5′-TGTGAGCATGGAAACAATAC-3′; p53 forward, 5′-ACTCCCATTCTTCCACCTTTG-3′; p53 reverse, 5′-CCCTGTTGCTGTAGCCATATT-3′; nuclear factor κB (NF-κB) forward, 5′-GCTATTCAGGCTGTGCTGTC-3′; NF-κB reverse, 5′-GGTAGTCGGTGAGATCTCGG-3′; nuclear factor κB inhibitor α (IκB-α) forward, 5′-CCAACTATTGCTTCAGCTCCA-3′ IκB-α reverse, 5′-GTGTCCAGGCTCCAAATGT-3′; β-actin forward, 5′-AGCCTTCTCCATGGTCGTGA-3′; and β-actin reverse, 5′-CGGAGTCAACGGATTTGGTC-3′. Primers were synthesized by Invitrogen; Thermo Fisher Scientific, Inc.
Western blot analysis
HepG2 cells treated with oridonin and/or lentinan as aforementioned were homogenized and lysed with radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.; 100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate and protease inhibitor). Lysates were sonicated for 5 sec on ice and centrifuged at 6,000 × g for 5 min at 4°C. Supernatants were collected and the protein concentration was detected using a Bio-Rad protein assay kit (cat. no. 500–0002; Bio-Rad Laboratories, Inc.). A total of 20 µg protein/well was loaded to SDS-PAGE (10% gel; GE Healthcare Life Sciences, Shanghai, China) and transferred onto polyvinylidene difluoride membranes, which were activated by methanol. The membrane was blocked using 10% skimmed milk at room temperature for 1 h. Subsequently, the membranes were incubated with the primary antibodies against caspase-3 (1:1,000; cat. no. ab13847), caspase-9 (1:1,000; cat. no. ab18571), Bcl-2 (1:1,000; cat. no. ab194583), Bax (1:1,000; cat. no. ab32503), p38 (1:1,000; cat. no. ab31828), p53 (1:1,000; cat. no. ab1431), NF-κB (1:1,000; cat. no. ab32360), IκB-αα (1:1,000; cat. no. ab7217) and β-actin (1:5,000; cat. no. ab8226), all purchased from Abcam (Cambridge, UK), overnight at 4°C. The following day, the membranes were washed with TBST for 10 min, prior to incubation with the horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:1,000; cat. no. ab6728; Abcam) for 1 h at room temperature and washed three times with TBST (10 min per wash). The membranes were visualized using ECL chemiluminescence agent, and β-actin was used as a control for normalization. Immunoreactivity was determined using enhanced chemiluminescent reagent (Thermo Fisher Scientific, Inc.) using an ImageQuant Las4000 digital imager (Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical evaluation was performed using Student's t-test or one-way analysis of variance followed by Student-Newman-Keuls test using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of oridonin and lentinan treatment on L02 and HepG2 cells
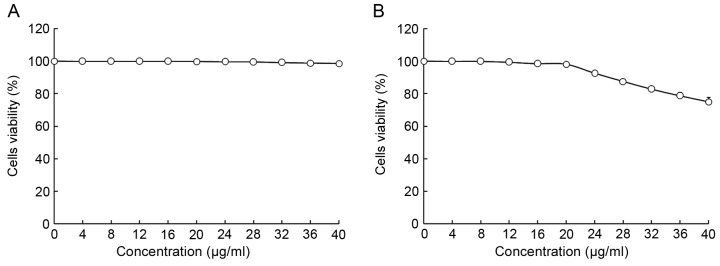
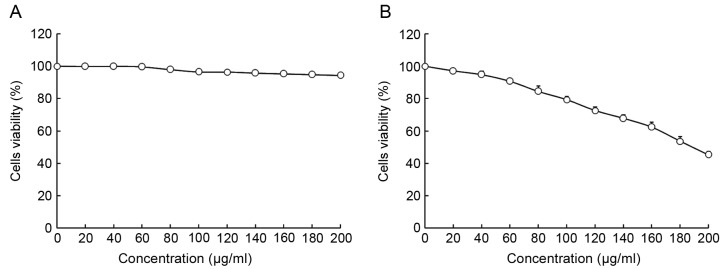
The viability of HepG2 and L02 cells following treatment with oridonin or LNT was determined using MTT assay (Figs. 1 and 2). Treatment with 0–20 µl/ml oridonin did not result in a decrease in viability in L02 or HepG2 cells (Fig. 1A and B). It was observed that LNT treatment was able to decrease the viability of HepG2 cells at an increased concentration. Treatment with 0–200 µl/ml LNT was able to decrease the viability of HepG2 cancer cells. However, treatment with the same concentration of LNT did not result in a decrease in viability in L02 cells.
Figure 1.
Effect of oridonin treatment on the viability of human normal liver cells (A) L02 and (B) HepG2 human hepatoblastoma cells as determined by MTT assay.
Figure 2.
Effect of lentinan treatment on the viability of human normal liver cells (A) L02 and (B) HepG2 human hepatoblastoma cells as determined by MTT assay.
Based on these results, 20 µl/ml oridonin was selected for subsequent experiments to investigate whether oridonin treatment is able to increase the anti-cancer effect of LNT. Concentrations of 100 and 200 µl/ml were selected to verify the anticancer effects of LNT (Fig. 2).
The growth inhibition values of HepG2 human hepatoblastoma cells by oridonin and lentinan are presented in Table I. The highest OD540 value was observed in the HepG2 cancer cells; oridonin + LNT-H (20 µg/ml oridonin + 200 µg/ml LNT), LNT-H (200 µg/ml LNT) and LNT-L (100 µg/ml LNT) treatments were able to decrease the OD540 value compared with the cells in the control group. Treatment of HepG2 cells with a combination of 20 µg/ml oridonin and 200 µg/ml LNT increased the growth inhibitory rate compared with treatment with 200 µg/ml LNT alone (Table I).
Table I.
Growth inhibition of HepG2 human hepatoblastoma cells by oridonin and lentinan as assessed by MTT assay.
| Treatment | OD540 value | Inhibitory rate (%) |
|---|---|---|
| Oridonin + LNT-H | 0.076±0.006a | 84.3±2.5a |
| Control | 0.484±0.005a | – |
| LNT-L | 0.384±0.010a | 20.7±1.9a |
| LNT-H | 0.219±0.011a | 54.8±2.2a |
P<0.05 vs. control, according to Duncan's multiple range test. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml lentinan; OD, optical density.
DNA content of sub-G1 HepG2 cells
Following treatment of HepG2 cancer cells with LNT, the percentage of apoptotic cells (percentage of sub-G1DNA content) was increased compared with the control cells (2.3±0.4%; Fig. 3). The percentage of apoptotic HepG2 cells treated with a high concentration of LNT (LNT-H, 200 µg/ml) was 28.1±1.9%, and the percentage of cells treated with a low concentration of LNT (LNT-L, 100 µg/ml) was 16.8±1.8%. A nontoxic concentration (20 µg/ml) of oridonin was able to increase the percentage of apoptoticHepG2 cells that were also treated with LNT-H (oridonin + LNT-H vs. LNT-H, 43.7±2.8 vs. 28.1±1.9%; Fig. 3).
Figure 3.
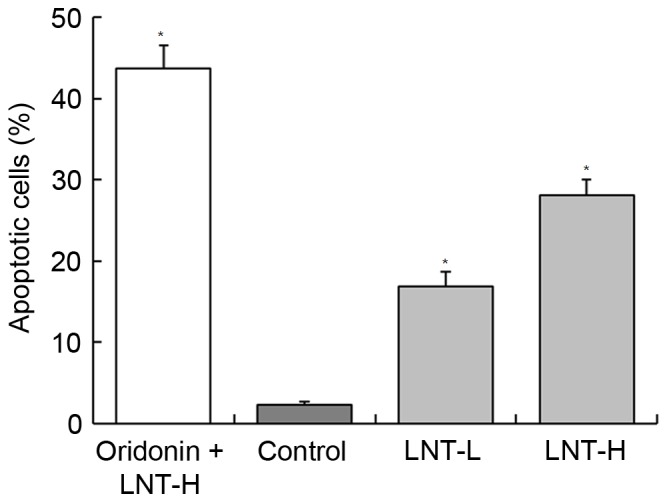
Flow cytometric analysis of the percentage of apoptotic HepG2 human hepatoblastoma cells. The DNA content of the sub-G1 phase was evaluated. a-d*Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan's multiple range test. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml; LNT, lentinan.
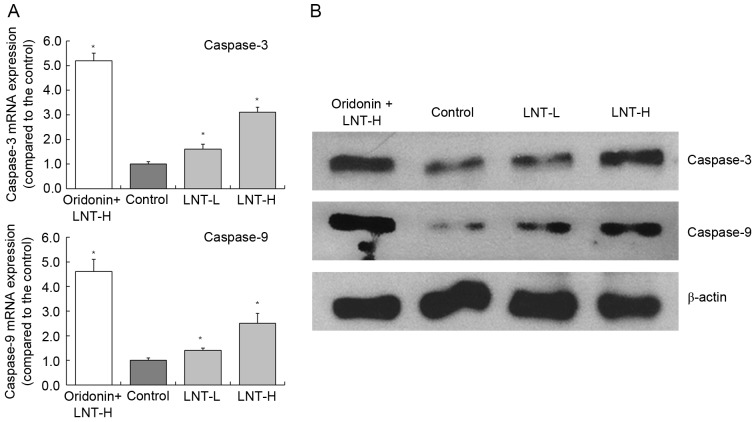
mRNA and protein expression of caspase-3 and −9 in HepG2 cells
The highest levels of caspase-3 and −9 mRNA expression were observed in HepG2 cells treated with oridonin and LNT-H, with a 3.10 and 2.51-fold increase compared with the control, respectively (Fig. 4). The oridonin + LNT-H treated HepG2 cells also exhibited higher caspase-3 and caspase-9 protein expression compared with the cells in the other groups (control, LNT-L and LNT-H).
Figure 4.
Expression of caspase-3 and caspase-9 in HepG2 human hepatoblastoma cells by reverse transcription-quantitative polymerase chain reaction and western blot analysis. (A) mRNA and (B) protein expression. *P<0.05 vs. control. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml lentinan; LNT, lentinan.
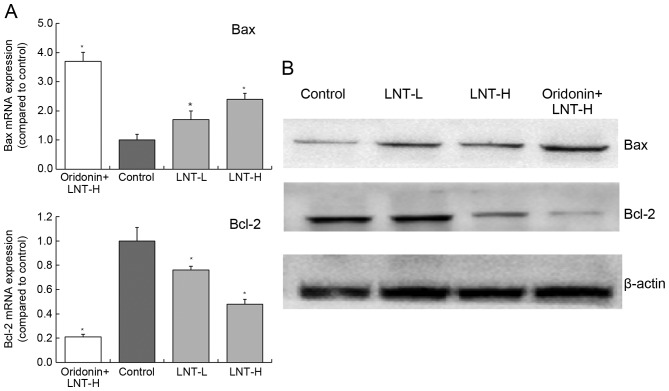
Gene and protein expression of Bax and Bcl-2 in HepG2 cells
The levels of Bax mRNA expression in cells treated with oridonin + LNT-H, LNT-H and LNT-L demonstrated a3.71-, 1.70- and 2.42-fold increase compared with the control (Fig. 5). The levels of Bcl-2 mRNA expression in cells treated with oridonin + LNT-H, LNT-H and LNT-L demonstrated a0.21-, 0.48- and 0.76-fold decrease compared with the control. The highest protein expression of Bax was observed in the oridonin + LNT-H treatment group, and the lowest Bcl-2 protein expression was also observed in the oridonin + LNT-H group (Fig. 5).
Figure 5.
Expression of Bax and Bcl-2 in HepG2 human hepatoblastoma cells by (A) reverse transcription-quantitative polymerase chain reaction and (B) western blot analysis. *P<0.05 vs. control. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml lentinan; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-like protein 4; LNT, lentinan.
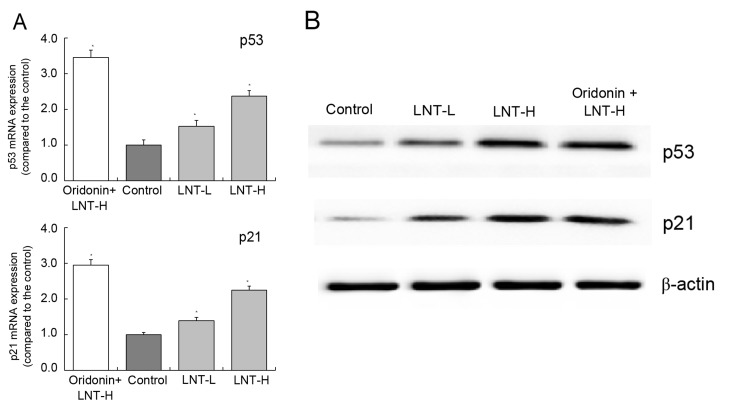
mRNA and protein expression of p53 and p21 in HepG2 cells
The highest levels of p53 and p21 mRNA and protein expression were observed in the oridonin + LNT-H group. The levels of p53 and p21 were observed to be higher in the oridonin + LNT-H group compared with the LNT-L group (Fig. 6A and B). The levels of p53 and p21 mRNA expression in the oridonin + LNT-H group were 2.37 and 2.25 times higher compared with the control, respectively (Fig. 6A).
Figure 6.
Expression of p53 and p21 in HepG2 human hepatoblastoma cells by (A) reverse transcription-quantitative polymerase chain reaction and (B) western blot analysis. *P<0.05 vs. control. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml lentinan; LNT, lentinan.
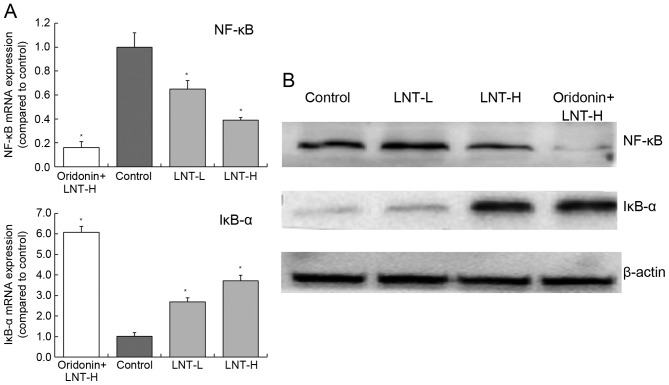
mRNA and protein expression of NF-κB and IκB-α in HepG2 cells
Treatment of cells with oridonin and LNT-H was able to reduce NF-κB expression and increase IκB-α expression compared with the control (Fig. 7). The levels of NF-κB mRNA was decreased 0.39-fold and the levels of IκB-α mRNA were increased 3.72-fold in cells treated with oridonin and LNT-H, compared with control cells (Fig. 7A). There was a similar trend in the expression of NF-κB and IκB-α proteins as the mRNA expression of these proteins (Fig. 7B).
Figure 7.
Expression of NF-κB and IκB-α in HepG2 human hepatoblastoma cells by (A) reverse transcription-quantitative polymerase chain reaction and (B) western blot analysis. *P<0.05 vs. control. Oridonin + LNT-H, 20 µg/ml oridonin + 200 µg/ml lentinan; LNT-L, 100 µg/ml lentinan; LNT-H, 200 µg/ml lentinan; LNT, lentinan; NF-κB, nuclear factor κB; IκB-α, nuclear factor κB inhibitor α.
Discussion
Liver cancer cell apoptosis has an important role in the development of liver cancer. It is now known that there are a variety of cell signals mediated by receptors involved at the initiation of liver cancer cell apoptosis. There are also a number of proteases involved in apoptosis signal conduction as well as multiple genes involved in the regulation of liver cancer cell apoptosis. In previous years, it has been demonstrated that caspase-3 protease contributes a major function in apoptosis signaling transduction (15,16). The mechanism of caspase-3 protease activation is highly complex and regulated by various factors, thus there are different activation pathways. The activation of caspase-3 protease activation is closely associated with liver cancer cell apoptosis. Among the different proteases, caspase-3 has a key role in apoptosis; it is the core protease that causes caspase cascade reactions leading to apoptosis. Caspase-3 is activated in apoptosis of liver cancer cell and is induced by a variety of factors (17,18). Therefore, by inhibiting the activation and activity of caspase-3, this may inhibit the apoptosis of liver cancer cells (18).
Studies have reported have reported that Bcl-2 family members exert an important regulatory role in the process of caspase-3 activation (18,19). Anti-apoptotic member of Bcl-2 family, Bcl-xL inhibits oligomers of apoptotic protease-activating factor 1 (Apaf-1), which results in the loss-of-function of Apaf-1 molecules and inhibits Apaf-1-dependent activation of caspase-9 (20). Anti-apoptotic members of the Bcl-2 family, which is primarily present in the outer membrane of the mitochondria, are able to prevent the release of cytochrome c from the mitochondria, which therefore inhibits the activation of pro-caspase-9 (21).
All pro-apoptosis Bcl-2 family members can form miscellaneous dimers with Bcl-2, Bcl-xL, A1 and Mcl-1 in the BH3 domain, which demonstrates that pro-apoptosis Bcl-2 family members, at least in part, function by interacting with anti-apoptosis Bcl-2 family members. Pro-apoptosis Bcl-2 family members are also able to induce the activation of caspases (22). Studies have also demonstrated that p53 is able to cause apoptosis through the activation of caspases (23,24). Fuchs et al (25) reported that fas-deficient cells, which induced the expression of wild type p53, are able to activatecaspase-3 expression and characteristic changes in apoptosis, indicating that p53-dependent apoptosis can directly activate caspases, dependent on the involvement of Fas (25). Apoptosis inhibitory factor, Bcl-2, regulates apoptosis by forming dimer by itself or different dimers with Bax protein. An increase in the Bcl-2/Bax ratio results in inhibition of apoptosis, yet if the ratio decreases apoptosis is promoted. The mechanism of action of Bcl-xL is similar to that of Bcl-2 (26). Silencing Bcl-2 gene is also able to induce the activation of p53-dependent apoptotic signaling pathway. In p53 wild-type cancer cells, following the activation of p53, Bax expression increases if the concentration of ultraviolet radiation treatment increases. However, the expression of Bcl-2 and Bcl-xL decreases with the increase of inhibitor concentration. These findings indicated that in the process of killing cancer cells by inhibitors, p53 induces apoptosis primarily through the Bax/Bcl-2 and Bax/Bcl-xL signaling pathways (27). Another study has indicated that in cancer cells, ultraviolet radiation may change the expression of p21 through p53, therefore inducing cell cycle arrest. The increase in the expression of p21 and p53 is one of the markers that indicate that ultraviolet radiation is able to induce cancer cell apoptosis (28).
NF-κB is a type of nuclear transcription regulatory factor, which is present in the majority of cells. When the cell is not stimulated, NF-κB and its inhibitor IκB exist in cytoplasm in an activated form (29). However, when cells are stimulated by cellular damage or viruses, IκB is phosphorylated and degraded. This results in the translocation of NF-κB to the nucleus and consequently activation of NF-κB. Following activation, NF-κB can promote transcription of cytokines, chemokines and adhesion factors (30). In previous years, numerous studies have revealed that NF-κB can control proliferation, regulate cell cycle and apoptosis, affect differentiation, promote tumor metastasis and have a close association with the occurrence and development of tumors (30,31).
In the present study, MTT, flow cytometry, RT-qPCR and western blot analysis were performed. Treatment with LNT induced a decrease in cell viability in HepG2 cancer cells in a dose-dependent manner, and oridonin treatment promoted the anticancer effects of LNT in vitro. The results from the present study provide evidence that oridonin may be used to sensitize cells to LNT in vitro.
References
- 1.Kupfahl C, Geginat G, Hof H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int Immunopharmacol. 2006;6:686–696. doi: 10.1016/j.intimp.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Hou XJ, Chen W. Optimization of extraction process of crude polysaccharides from wild edible BaChu mushroom by response surface methodology. Carbohyd Polym. 2008;72:67–74. doi: 10.1016/j.carbpol.2007.07.034. [DOI] [Google Scholar]
- 3.Murata T, Hatayama I, Kakizaki I, Satoh K, Sato K, Tsuchida S. Lentinan enhances sensitivity of mouse colon 26 tumor to cis-diamminedichloroplatinum (II) and decreases glutathione transferase expression. Jpn J Cancer Res. 1996;87:1171–1178. doi: 10.1111/j.1349-7006.1996.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drandarska I, Kussovski V, Nikolaeva S, Markova N. Combined immunomodulating effects of BCG and Lentinan after intranasal application in guinea pigs. Int Immunopharmacol. 2005;5:795–803. doi: 10.1016/j.intimp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang RL. Dong ling caozhiliaoyuanfaxingganai 31 li linchuang guan cha. Ai Zheng. 1984;3:50. (In Chinese) [Google Scholar]
- 6.Zhang JF, Chen GH, Lu MQ, Liu JJ. Anti proliferation effects of oridonin on hepatocellular carcinoma BEL-7402 cells and its mechanism. Chinese Traditional Patent Med. 2006;28:1325–1329. [Google Scholar]
- 7.Huang HL, Weng HY, Wang LQ, Yu CH, Huang QJ, Zhao PP, Wen JZ, Zhou H, Qu LH. Triggering Fbw7-mediated proteasomal degradation of c-Myc by oridonin induces cell growth inhibition and apoptosis. Mol Cancer Ther. 2012;11:1155–1165. doi: 10.1158/1535-7163.MCT-12-0066. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Yu HS, Xue HW. Radio sensitization effect of oridonin on HepG2 in vitro. Med J Qi lu. 2007;22:339–342. (In Chinese) [Google Scholar]
- 9.Mullard A. Pioneering apoptosis-targeted cancer drug poised for FDA approval. Nat Rev Drug Discov. 2016;15:147–149. doi: 10.1038/nrd.2016.23. [DOI] [PubMed] [Google Scholar]
- 10.Denisenko TV, Sorokina IV, Gogvadze V, Zhivotovsky B. Mitotic catastrophe and cancer drug resistance: A link that must to be broken. Drug Resist Updat. 2016;24:1–12. doi: 10.1016/j.drup.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor SE. Plant biochemistry. Fighting cancer while saving the mayapple. Science. 2015;349:1167–1168. doi: 10.1126/science.aad1801. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Wang Q, Li GJ, Chen F, Qian Y, Wang R. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J Funct Food. 2014;7:590–598. doi: 10.1016/j.jff.2013.12.026. [DOI] [Google Scholar]
- 13.Zhao X, Qian Y, Zhou YL, Wang R, Wang Q, Li GJ. Pu-erh tea has in vitro anticancer activity in TCA8113 cells and preventive effects on buccal mucosa cancer in U14 cells injected mice in vivo. Nutr Cancer. 2014;66:1059–1069. doi: 10.1080/01635581.2014.916317. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food. 2013;16:9–19. doi: 10.1089/jmf.2012.2316. [DOI] [PubMed] [Google Scholar]
- 16.Wong RS. Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9:738–742. [PubMed] [Google Scholar]
- 18.Rodríguez-Berriguete G, Galvis L, Fraile B, de Bethencourt FR, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. Immunoreactivity to caspase-3, caspase-7, caspase-8, and caspase-9 forms is frequently lost in human prostate tumors. Hum Pathol. 2012;43:229–237. doi: 10.1016/j.humpath.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 20.Swanton E, Savory P, Cosulich S, Clarke P, Woodman P. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene. 1999;18:1781–1787. doi: 10.1038/sj.onc.1202490. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Jeon YK, You DH, Nam MJ. Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2 family in human hepatic cancer cells. Food Chem Toxicol. 2013;60:542–549. doi: 10.1016/j.fct.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu SY, Xiao DJ, Luan YZ, Wang YS, Wang L, Shi K. Regulatory mechanism of cell cycle block and apoptosis in p53 mutated gastric cancer cells during cisplatin stress. J Shandong Univ (Health Sci) 2008;46:478–484. (In Chinese) [Google Scholar]
- 24.Moulin M, Carpentier S, Levade T, Arrigo AP. Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis. 2007;12:1703–1720. doi: 10.1007/s10495-007-0096-2. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs EJ, McKenna KA, Bedi A. p53-dependent DNA damage-induced apoptosis requires Fas/APO-1-independent activation of CPP32beta. Cancer Res. 1997;57:2550–2554. [PubMed] [Google Scholar]
- 26.Klostergaard J, Leroux ME, Auzenne E, Khodadadian M, Spohn W, Wu JY, Donato NJ. Hyperthermia engages the intrinsic apoptotic pathway by enhancing upstream caspase activation to overcome apoptotic resistance in MCF-7 breast adenocarcinoma cells. J Cell Biochem. 2006;98:356–369. doi: 10.1002/jcb.20729. [DOI] [PubMed] [Google Scholar]
- 27.Woo SM, Choi YK, Kim AJ, Cho SG, Ko SG. p53 causes butein-mediated apoptosis of chronic myeloid leukemia cells. Mol Med Rep. 2016;13:1091–1096. doi: 10.3892/mmr.2015.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollmann G, Linden R, Giangrande A, Allodi S. Increased p53 and decreased p21 accompany apoptosis induced by ultraviolet radiation in the nervous system of a crustacean. Aquat Toxicol. 2016;173:1–8. doi: 10.1016/j.aquatox.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter SL, Centenera MM, Tilley WD, Selth LA, Butler LM. IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor. BMC Cancer. 2016;16:141. doi: 10.1186/s12885-016-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seubwai W, Vaeteewoottacharn K, Kraiklang R, Umezawa K, Okada S, Wongkham S. Inhibition of NF-κB activity enhances sensitivity to anticancer drugs in cholangiocarcinoma cells. Oncol Res. 2016;23:21–28. doi: 10.3727/096504015X14424348426071. [DOI] [PMC free article] [PubMed] [Google Scholar]