ABSTRACT
Stellate ganglion blockage (SGB) is a method used for treating Raynaud’s phenomenon (RP). This study primarily aimed to determine whether the perfusion index (PI) can be used an alternative to Horner’s signs in evaluating the efficacy of SGB in patients diagnosed with RP. In a total of 40 patients, aged 18–65 years and diagnosed with primary RP, SGB was applied for 5 days on the same side with the 2-finger method, using 6 mL of 5% levobupivacaine at the 7th cervical vertebra level. The PI values were recorded from the distal end of the 2nd finger of the upper extremity on the side applied with the block at baseline and at 5, 15, 30, 60 and 120 min. The onset time of Horner findings was recorded. The PI values and visual analogue scale (VAS) pain scores were recorded pre-treatment and after 2 weeks.When the PI values of the 40 patients were examined, a 62.7% increase was observed from baseline to the first session at 5 min (p < 0.05). When all sessions were evaluated, a statistically significant increase was determined in the PI values measured at 5, 15, 30, 60 and 120 min compared with the baseline PI values. There was a statistically significant decrease in the post-treatment VAS pain scores and a statistically significant increase in the post-treatment PI values (p < 0.05). By eliminating peripheral vasospasm with the application of SGB in patients with RP, the distal artery blood flow and PI are increased. PI measurement is a more objective method and therefore could be used as an alternative to Horner findings in evaluating the success of SGB. PI is a non-invasive and simple measurement and also an earlier indicator in evaluating the success of SGB than Horner’s signs.
KEYWORDS: Raynaud’s phenomenon, perfusion index, stellate ganglion blockage; pain; Horner's sign
1. Introduction
Raynaud’s phenomenon (RP) is characterised by a temporary reduction in blood circulation associated with vasospasm in the small arteries of the hands and feet that is triggered by cold and emotional stress [1]. Patients complain of pain together with pallor and/or cyanosis in the distal two-thirds of the fingers. These symptoms may be classified as a primary disorder (primary RP) or as secondary to another disease such as a collagen tissue disease (secondary RP) [2,3]. Primary Raynaud is a benign disease that predominantly affects younger women and is transient without serious sequelae. In contrast, secondary Raynaud is generally one of the symptoms of systemic disease and is, in addition to findings of the basic disease, associated with ischemic lesions. Primary Raynaud is mainly diagnosed according to clinical presentation. In secondary Raynaud, additional investigating techniques including imaging studies and laboratory tests for the detection of underlying disease are necessary [4]. Stellate ganglion blockage (SGB) is used for treating painful disorders of the upper extremity, head, neck and chest as well as for Raynaud’s patients with a vasospastic disorder [5]. Vasodilation occurs with the elimination of the sympathetic effect following the block. The efficacy of SGB is traditionally evaluated with the emergence of Horner findings. Horner signs comprise unilateral miosis, ptosis and facial anhydrosis [6]. The time to emergence of these clinical findings may differ depending on the patient or may be unclear [7]. The perfusion index (PI), which is the ratio between pulsatile and non-pulsatile signals, is calculated automatically and non-invasively as a measurement of pulse oximetry [8]. Here, we investigated whether the change in the PI could be used for evaluating block efficacy as an alternative to the clinical signs that emerge after SGB is applied. Treatment success was also evaluated by comparing the PI values measured 2 weeks after treatment with the pre-treatment PI values.
2. Method
The study was approved by the Local Ethics Committee, and informed consent was obtained from all study participants. The study included a total of 40 patients, aged 18–65 years and diagnosed with primary RP, who were treated with SGB in the Algology Clinic of the Anaesthesiology and Reanimation Department of Cumhuriyet University Research and Application Hospital between June 2013 and December 2013. Patients were excluded if they had additional systemic problems, were using medication and/or had any concomitant disease that could affect blood flow in the upper extremity, had an infection in the area to be measured or if they rejected the method or were not co-operative.
Patients were evaluated before the procedure. All blocks were applied by the same person using the same technique. For each patient, age, height, weight, body mass index (BMI) and duration of disease were recorded. Before the procedure, the 100-mm visual analogue scale (VAS)-pain score was fully explained to the patients, and the VAS pain scores between 0 and 10 (0 = no pain and 100 = worst possible pain) were recorded to evaluate pain status before the procedure. The pre-block measurements were taken after the patient had rested for 15 min in a supine position in a room at 24°C. Before the measurement, a vascular route was opened in the patient with a 20G intravenous catheter on the side where the block was not to be applied. Fluid replacement was provided with 0.9% NaCl solution at a volume of 10 mL/kg. The patient was positioned supine, straight, with no cushion support and arms in adduction. The patient’s head was moved into slight extension and was held in extension during the intervention. The oximetry sensor (M-LNCS adult adhesive sensors connected to Masimo SET® Radical™ pulse oximeters; Masimo Corp, Irvine, CA, USA) was attached to the 2nd finger of the upper extremity on the side where the procedure was to be applied. The sensor was connected to a Rad-87TM Pulse Co-Oximeter Monitor (Masimo). Baseline PI, pulse and SpO2 values were recorded from the monitor. The injection technique used for the block was at the 7th cervical vertebra (C7) level determined with the 2-finger method, wherein one finger is placed on the jugular notch of the sternum, and a second finger is placed laterally over the clavicle to join the first finger. Next, two fingers of the other hand are placed with one where this finger finishes, and the other is placed vertically towards the neck. The point where the fingers of the second hand finish is defined as the point of injection. This point is located over the transverse notch at C7 and along the medial edge of the sternocleidomastoid muscle. The area was aseptically cleaned and draped. After the person who was to apply the block had taken position on the side where the block was to be applied, intradermal local anaesthesia (0.5 mL of 2% prilocaine) was administered at the marked point. By moving the sternocleidomastoid muscle inferiorally and laterally with the first and second fingers of the left hand, the carotid sheath was laterally pulled. After palpating the carotid artery pulsation with the lateral fingers, the skin was vertically entered at the marked point with a 20G needle tip injector, containing 6 mL of 5% levobupivacaine, held in the right hand, and the needle was slowly advanced until the transverse notch of the C7 vertebra was felt.
The aspiration test was applied by putting the needle in contact with the transverse notch back up to 0.5 cm, and 2 mL local anaesthetic was given. After waiting for 15–20 s to determine whether an anaphylactic or toxic reaction was observed, the remaining local anaesthetic solution was administered (total 6 mL of 5% levobupivacaine). The time to emergence of Horner findings and signs of facial redness and dryness were recorded, and block success with Horner findings was evaluated; non-Horner blocks were considered unsuccessful. After the procedure, the PI, SpO2 and pulse values were recorded using the same method at 5, 15, 30, 60 and 120 min. The block was applied once a day for 5 days on the same side, and the measurements were repeated. After the five sessions of block treatment were completed, all patients attended a follow-up examination after 2 weeks, and, after the same physical conditions were provided, the PI, SpO2 and pulse values as well as the VAS scores were recorded.
2.1. Statistical analysis
The sample size of 28 patients per group was necessary to detect a difference in PI, as previously observed by Yamazaki et al. [7] who showed that between groups, 5 min after SGB, the alpha error level was set at 0.05 and 80% power to detect effect size. The study data were analysed using SPSS Statistics version 14.0 software. When the data met parametric test assumptions, the Kolmogorov–Smirnov test, Tukey test and the paired t test were used, and when these data did not meet parametric test assumptions, the Mann–Whitney U-test and the Wilcoxon test were used. These data are shown as mean ± standard deviation. A value of p < 0.05 was accepted as statistically significant.
3. Results
The study included a total of 27 women and 13 men. Demographic data of the patients are shown in Table 1. SGB was applied a total of 200 times in five sessions to 40 patients. In 188 (94%) of the procedures, Horner findings and signs such as facial redness and dryness were observed, and the block application was recorded as successful.
Table 1.
Demographic data.
| N = 40 | |
|---|---|
| Sex Male-Female | 13–27 (32.5–67.5%) |
| Age (years) | 19–55 (29.3) |
| Weight (kg) | 49–86 (66.4) |
| Height (cm) | 154–184 (166.6) |
| Disease duration (years) | 1–12 (4.6) |
| BMI (kg/m2) | 18.4–32.1 (23.9) |
Data shown are total number, percentage, or range (mean).
BMI: Body mass index
When the procedures of the 1st session were evaluated, a significant increase in the PI values was measured at 5 min (2.36 ± 0.30) compared to baseline PI values (1.45 ± 0.15) (p < 0.05). The increase in the PI values measured from baseline to 5 min was 67%, and the increase from baseline to 15 min was 97.2%. When all sessions were compared, a statistically significant difference was observed between the baseline PI values and PI values measured at all time points (p < 0.05). The PI values measured during all sessions are shown in Table 2.
Table 2.
PI measurement values at each session.
| 1st session | 2nd session | 3rd session | 4th session | 5th session | |
|---|---|---|---|---|---|
| Baseline | 1.45 ± 0.15 | 1.56 ± 1.01 | 1.59 ± 1.42 | 1.70 ± 1.09 | 1.70 ± 1.06 |
| 5 min | 2.36 ± 0.30 | 2.24 ± 1.03 | 2.76 ± 2.25 | 2.81 ± 1.65 | 3.13 ± 1.88 |
| 15 min | 2.86 ± 0.27 | 2.83 ± 2.00 | 2.78 ± 1.70 | 3.15 ± 1.55 | 3.18 ± 1.64 |
| 30 min | 2.70 ± 0.21 | 2.55 ± 1.33 | 2.50 ± 1.69 | 2.69 ± 1.42 | 3.11 ± 1.63 |
| 60 min | 2.76 ± 0.24 | 2.24 ± 1.08 | 2.51 ± 1.33 | 2.35 ± 1.01 | 2.69 ± 1.69 |
| 120 min | 2.41 ± 0.19 | 2.27 ± 1.17 | 2.71 ± 1.81 | 2.55 ± 1.48 | 2.62 ± 1.48 |
| F = 10.98 | F = 7.34 | F = 6.12 | F = 13.01 | F = 10.47 | |
| p = 0.001* | p = 0.001* | p = 0.001* | p = 0.001* | p = 0.001* | |
| p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
* Significant at P value of 0.05
In 188 applications evaluated as successful, the mean time of Horner onset was 5.84 ± 1.55 min (range, 3–10 min). In 107 applications, the time to observation of Horner signs was longer than 5 min. In patients where Horner signs had not yet developed, the PI values at 5 min after the block increased 61.7% compared to baseline values (p < 0.05). The PI values were calculated as mean 1.06 ± 0.78 at baseline and mean 1.28 ± 0.73 at 5 min in the 12 procedures for which Horner signs did not occur.
No statistically significant difference was observed in SpO2 and pulse values measured at different time points in each session (p > 0.05). There was a statistically significant difference of 56.6% in the PI values measured at 2 weeks after treatment (2.24 ± 1.25) compared to pre-treatment PI values (1.43 ± 0.98) (p < 0.05). Moreover, there was a statistically significant decrease in the VAS scores measured at 2 weeks after treatment (3.00 ± 1.15) compared to pre-treatment values (3.80 ± 1.57) (p < 0.05). The PI, SpO2 and pulse values as well as the VAS scores of the patients before and after treatment are shown in Table 3.
Table 3.
Pre- and post-treatment values.
| Pre-treatment | 2 weeks post-treatment | Result | |
|---|---|---|---|
| PI | 1.43 ± 0.98* | 2.24 ± 1.25 | t = 3.74 p = 0.001 |
| SpO2 | 97.75 ± 1.16 | 97.29 ± 1.32 | t = 1.37 p = 0.171 |
| VAS | 3.80 ± 1.57* | 3.00 ± 1.15 | p = 0.001 |
| Pulse | 92.27 ± 10.54 | 90.20 ± 13.11 | t = 0.76 p = 0.441 |
* Significant at P value of 0.05
We observed hoarseness in three patients after SGB in this study. Other complications such as haematoma, dyspnoea, hypotension and arrhythmia were not observed.
4. Discussion
With the removal of the sympathetic effect following SGB in patients with primary RP, peripheral blood flow increases in the upper extremity [9], and PI values begin to increase in proportion to the increase in blood flow. This study demonstrated that PI, as a non-invasive and simple measurement, showed successful results in evaluating block success.
RP occurs with repeated, reversible constriction of the small vessels in the distal ends of the hands and feet [10]. Increased sympathetic activity is important in the table that emerges. Treatment is effective in controlling the increasing sympathetic activity [11]. SGB is a method used for treating Raynaud’s patients as it increases blood flow in the upper extremity. In evaluating whether the block is successful, clinical signs such as Horner findings, hypohydrosis and facial flushing are used, giving subjective results according to the observer. These findings, which are based on clinical observation, may be unclear in some patients. In the evaluation of stellate sympathetic block, in addition to Horner findings, many different methods have been used based on the measurement of increased temperature that occurs in areas affected by the ganglion [12,13]. In this study, SGB was applied five times to 40 patients. When all sessions of the patients were separately evaluated, a statistically significant increase was observed in the post-treatment PI values measured at 5, 15, 30, 60 and 120 min compared with the pre-treatment PI values (p < 0.05).
In our study, the injection technique used for the block was at the C7 level determined with the 2-finger method. Ultrasound-guided SGB is a safer procedure compared to other techniques. It visualises vascular structures (inferior thyroidal, cervical, vertebral and carotid arteries) and soft tissue structures (thyroid, oesophagus and nerve roots). Accordingly, the risk of vascular and soft tissue injury may be minimal [14]. Choi et al. [15] observed Horner signs in all 20 patients who had undergone ultrasound-guided SGB. Nevertheless, complications may occur with ultrasound-guided blocks. Rastogui et al. [16] reported cardiac arrest following ultrasound-guided SGB.
The PI, which is measured with pulse oximetry in a specific measurement area, is the ratio between pulsatile and non-pulsatile signals [8]. Increasing values indicate an increase in peripheral circulation in the area with the sensor. The efficacy of SGB can be evaluated with increasing blood flow after the block. The results of this study showed that PI can be successfully used in evaluating SGB. In addition, PI monitorisation, which can be measured with pulse oximetry, is a non-invasive method that can be easily applied.
In recent studies, the importance of PI measurement has been assessed in evaluating the efficacy of both neuroaxial and peripheral nerve blocks. The basic principle is vasodilation induced by sympathectomy. PI measurement is an alternative method providing rapid results rather than techniques such as increased skin temperature and the pinprick test in the evaluation of increased regional blood circulation following nerve blocks. Kuş et al. [17] applied infraclavicular brachial plexus block under ultrasound guidance to 46 patients undergoing elective upper extremity surgery. After 10 min, the PI increased by mean 120% compared to the baseline values. Based on this result, they reported that PI could be used to evaluate infraclavicular block success. Ginosar et al. [18] placed a lumbar epidural catheter in 40 patients and reported that the PI measurement gave earlier, more sensitive and clearer information in the evaluation of epidural-induced sympathectomy compared to that of skin temperature and mean arterial pressure. In this study, 188 applications were accepted as successful based on the presence of signs such as Horner signs. When the onset times of Horner findings in the applications were examined, the earliest was at 3 min, the latest was at 10 min and the mean onset time was 5.84 ± 1.55 min. In 107 of the applications, the time to onset of Horner signs was >5 min. An increase of 61.7% was observed in the PI value at 5 min compared to the baseline PI value in patients who did not develop Horner signs within 5 min after SGB. When evaluating the success of the block in light of these results, it can be said that PI measurement provides earlier data. It is not a tool to compare the PI and Horner findings. Waiting for the time to emergence of Horner findings is not sufficient to evaluate SGB. Horner findings are visually evaluated by the clinician. Some clinicians may say that Horner findings are complete, whereas other clinicians may have not fully observed it. The data obtained in this study are consistent with the findings of both Kuş et al. and Ginosar et al.
Oximeters capable of measuring PI have similar cost ranges as other oximeters. Moreover, the time difference between the two techniques can be minimal or even unnoticed. The data obtained by PI measurement are objective, but Horner findings are based entirely on observational findings because the evaluation of whether Horner findings such as myosis, ptosis and facial anhidrosis are precisely formed may differ according to the clinician.
In a retrospective study of 30 patients, Yamazaki et al. observed that SGB treatment applied with 6 mL of 1% mepivacaine was accepted as effective in 21 patients based on the presence of Horner findings. In these 21 patients, an increase of 61.4% was observed in the PI values measured in the ipsilateral ear lobe at 5 min compared to baseline PI values, and an increase of 60.5% was observed in the upper extremity. In two patients with successful block and PI increase, the efficacy was confirmed by measuring blood flow with a laser Doppler flowmeter [7]. Similar results were obtained in this study. When only the first session of the patients was evaluated, an increase of 62.7% was observed in the PI values at 5 min after SGB compared to the baseline PI values (p < 0.05). When all sessions were evaluated, there was a statistically significant difference between the baseline PI values and PI values measured at all time points (p < 0.05) (Figure 1).
Figure 1.
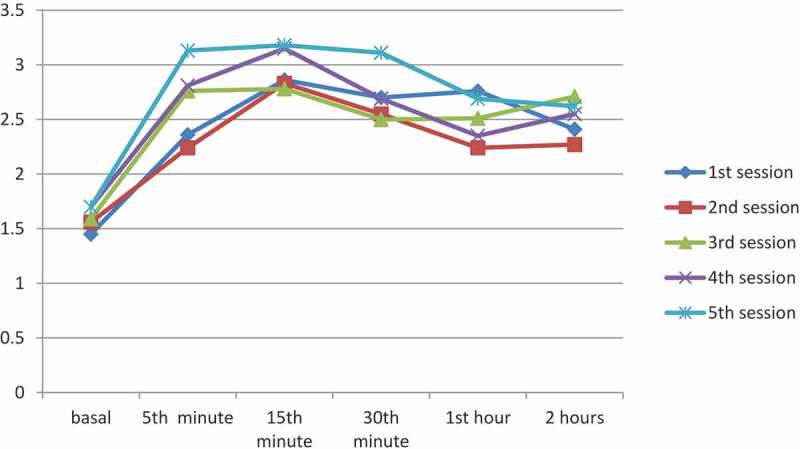
The change in PI values at different time points during the treatment sessions (y axis).
To evaluate the clinical status of Raynaud’s patients and the effectiveness of the applied treatment, symptomatic relief and pain levels are evaluated. Capillaroscopy, thermography, laser Doppler and angiography methods can be used to evaluate peripheral blood flow [19]. In this study, five sessions of SGB treatment were applied over 5 days, and PI values recorded before treatment were compared with those recorded 2 weeks after treatment. A statistically significant difference was observed between the post-treatment PI value (2.24 ± 1.25) and the pre-treatment PI value (1.43 ± 0.98).
Hypoxia associated with recurring vasospasms in RP causes pain. In this study, the VAS scores measured 2 weeks after treatment decreased compared with those measured before treatment (p = 0.001) (Table 3).
To the best of our knowledge, no other study has reported the use of PI monitorisation in SGB treatment in patients diagnosed with RP. In conclusion, the use of PI in regional anaesthesia can increase the success of SGB as it is a rapid, non-invasive and simple method providing measurable data earlier compared to Horner signs and similar clinical signs. However, further studies are required to verify these results and to ensure that PI can be used in treatment follow-up of patients with primary RP.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Lambova SN, Müller-Ladner U.. New lines in therapy of Raynaud’s phenomenon. Rheumatol Int. (2009);29:355–5. [DOI] [PubMed] [Google Scholar]
- [2]. Landry GJ. Current medical and surgical management of Raynaud’s syndrome. J Vasc Surg. 2013;57(6):1710–1716. [DOI] [PubMed] [Google Scholar]
- [3]. Pope JE. The diagnosis and treatment of Raynaud’s phenomenon. Drugs. 2007;67(4):517–525. [DOI] [PubMed] [Google Scholar]
- [4]. Fardoun MM, Nassif J, Issa K, et al. Raynaud’s phenomenon: a brief review of the underlying mechanisms. Front Pharmacol. 2016;7:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Shiokawa Y, Morimoto M, Kamamoto H, et al. Usefulness of perfusion index in evaluation of stellate ganglion block. Acta Medica Kinki Univ. 2009;34(2):83–86. [Google Scholar]
- [6]. Vilallonga R, Fort JM, Mazarro A, et al. Postthyroidectomy Horner’s syndrome Case Rep Med. 2012. (2012);316984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Yamazaki H, Nishiyama J, Suzuki T. Use of perfusion index from pulse oximetry to determine efficacy of stellate ganglion block. Local Reg Anesth. 2012;5:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Lima A, Jansen TC, van Bommel J, et al. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37(3):934–938. [DOI] [PubMed] [Google Scholar]
- [9]. Omote K, Kawamata M, Namiki A. Adverse effects of stellate ganglion block on Raynaud’s phenomenon associated with progressive systemic sclerosis. Anesth Analg. 1993;77(5):1057–1060. [DOI] [PubMed] [Google Scholar]
- [10]. Khouri C, Blaise S, Carpentier P, et al. Drug‐induced Raynaud’s phenomenon: beyond β‐adrenoceptor blockers. Br J Clin Pharmacol. 2016;82(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Hughes M, Herrick AL. Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2016;30(1):112–132. [DOI] [PubMed] [Google Scholar]
- [12]. Malmqvist E-Å, Bengtsson M, Sörensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth Pain Med. 1992;17(6):340–347. [PubMed] [Google Scholar]
- [13]. Murakawa K, Noma K, Ishida K, et al. Changes of tympanic temperature by stellate ganglion block. Masui. 1995;44(6):824–827. [PubMed] [Google Scholar]
- [14]. Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep. 2014;18(6):424. [DOI] [PubMed] [Google Scholar]
- [15]. Choi EM, Kim EM, Chung MH, et al. Effects of ultrasound-guided stellate ganglion block on acute pain after arthroscopic shoulder surgery. Pain Physician. 2015;18(3):E379–E388. [PubMed] [Google Scholar]
- [16]. Rastogi S, Tripathi S. Cardiac arrest following stellate ganglion block performed under ultrasound guidance. Anaesthesia. 2010;65(10):1042. [DOI] [PubMed] [Google Scholar]
- [17]. Kus A, Gurkan Y, Gormus SK, et al. Usefulness of perfusion index to detect the effect of brachial plexus block. J Clin Monit Comput. 2013;27(3):325–328. [DOI] [PubMed] [Google Scholar]
- [18]. Ginosar Y, Weiniger CF, Meroz Y, et al. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol Scand. 2009;53(8):1018–1026. [DOI] [PubMed] [Google Scholar]
- [19]. Dinsdale G, Herrick AL. Vascular diagnostics for Raynaud’s phenomenon. J Vasc Diagn. 2014;2:127–139. [Google Scholar]


