ABSTRACT
Extracellular vesicles (EVs) are emerging as novel theranostic tools. Limitations related to clinical uses are leading to a new research area on design and manufacture of artificial EVs. Several strategies have been reported in order to produce artificial EVs, but there has not yet been a clear criterion by which to differentiate these novel biomaterials. In this paper, we suggest for the first time a systematic classification of the terms used to build up the artificial EV landscape, based on the preparation method. This could be useful to guide the derivation to clinical trial routes and to clarify the literature. According to our classification, we have reviewed the main strategies reported to date for their preparation, including key points such as: cargo loading, surface targeting strategies, purification steps, generation of membrane fragments for the construction of biomimetic materials, preparation of synthetic membranes inspired in EV composition and subsequent surface decoration.
KEYWORDS: artificial extracellular vesicles, biomimetic materials, nanomedicine, drug-delivery nanocarrier
Extracellular vesicles in nanomedicine: possibilities and limitations
Extracellular vesicles (EVs) represent an important portion of the secretome. An overview of their functions in physiological conditions of EVs was compiled by a recent position paper from the International Society of Extracellular Vesicles (ISEV) [1]. Some of the described properties can be used for therapeutic uses, and their testing has been transformed sometimes into several registered clinical trials [2]. Exosomes are being applied in antitumour immunotherapy [3], as therapeutic agents against infectious diseases [4], unmodified exosomes for immune-modulatory [5] and regenerative therapies [6], and modified ones for targeted drug delivery [7], especially in gene therapy [8]. Although some of the mechanisms behind their properties remain undescribed, some general characteristics of EVs make them advantageous over other therapeutic strategies.
Both the structure of the membrane and the formation route are the origin of the following advantageous aspects: (1) high selective targetability and minimum off-target effect, thanks to a set of molecules involved in targeting, signalling and receptor-mediated uptake, complete with all the co-receptors needed for the internalization process; (2) capacity of extravasation due to a gel-state core derived from the presence of hydrated macromolecules (proteins and nucleic acids) combined with a minimum cytoskeleton that allows deformability while keeping the whole integrity of the vesicle. (3) size distribution; (4) great stability in the blood due to the evasion of the innate immune system; (5) adaptative responses that cause clearance from the blood, with the corresponding decrease in bioavailability.
EVs can be used as a therapeutic agent by themselves or as delivery systems. The great potential of EVs as drug-delivery vehicles has been acknowledged in the literature [7–12]. In most cases, the encapsulated drug acts in collaboration with elements naturally present in the EVs, creating a synergetic effect. In other cases, EVs serve only as vehicles to reach a specific target population, sometimes highly protected from conventional administration routes.
Nevertheless, limitations of EVs as therapeutic agents have also been reported, including the absence of a good definition from a pharmaceutical point of view [13], an incomplete understanding of their role in the development and spread of pathology, the absence of methods for the isolation of homogeneous populations and subpopulations of EVs, and cost-ineffective technology for the availability of sufficient quantities for clinical trials with constant characteristics. Moreover, it has been acknowledged that there is little understanding on how biological barriers are crossed by EVs, and a need for loading methods with scalable properties in clinical translation has been identified [14].
Bio-engineered and mimetic EVs for nanomedicine: classification of artificial EVs
EVs have been modified in the search for broader therapeutic capability. Sometimes, this included the incorporation of new elements for targeted puporses, in vitro or in vivo traceability, or the material to be delivered. In other cases, modification was aimed at the enhancement of colloidal stability, or change in surface charge to increase their uptake rate. These new approaches have generated new terms such as bio-engineered exosomes, artificial exosomes [15], exosome-mimetic nanovesicles [16], exosome-like nanovesicles [17,18] and exosome-based semi-synthetic vesicles [19]. These expressions have been used with different meanings in the literature, but to date, there has not yet been a clear criterion for their classification. One example is the term “exosome-like nanovesicles”. In some works, this concept is used to name artificial vesicles made from cells through different techniques to mimic exosomes [17,18]. However, cell-derived vesicles with morphological and biochemical characteristics similar to exosomes were also named exosome-like nanovesicles by other authors [19,20]. Other authors working with non-animal research models used this term to refer to vesicles with size and flotation density values similar to those of exosomes. For example, Regente et al. [21] described the presence of exosome-like vesicles in sunflower plant fluids, and Prado et al. [22] showed evidence of vesicles quite similar to exosomes during pollen germination.
In order to provide a systematic classification to move around in this new emerging field, we suggest the nomenclature given in Figure 1. This artificial EVs landscape is based on the concept behind the term. In this way, “artificial EVs” will be used as a general concept to designate all vesicles, modified or manufactured (from natural or synthetic sources), with the aim to mimic EVs (mainly exosomes) for therapeutic uses. Behind this term, two categories of artificial EVs can be discerned: “semi-synthetic EVs” and “fully synthetic” or “EVs mimetic vesicles”, corresponding to modified natural EVs (pre- or post-isolation) and artificial structures, lab-made or generated from cultured cells.
Figure 1.
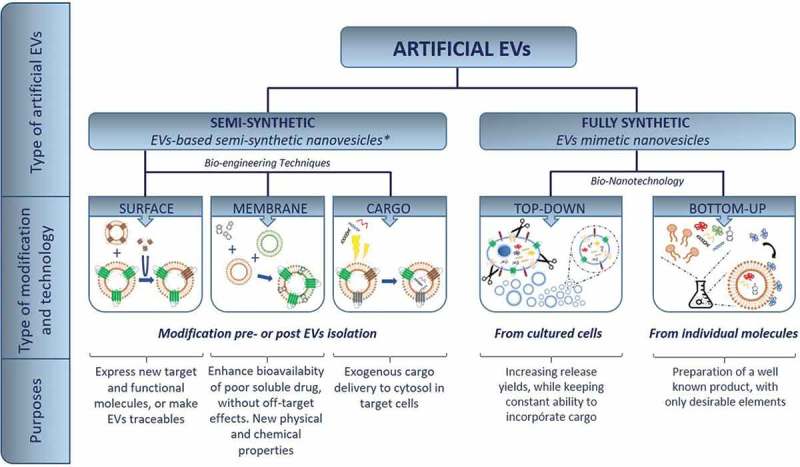
Artificial EV landscape: explored routes to date for the preparation of artificial EVs for specific purposes. *EBSSNs [19].
The former generate semi-synthetic products, as they start from a natural substrate, which can be subsequently modified before or after their isolation. The modification affects the structures of the outer surface of the vesicles, the membrane or the cargo that travels within, and could also include hydrophobic molecules at the membrane.
The term fully synthetic, on the other hand, stresses the artificiality of the product. We have recently briefly commented their potential in therapeutics [23]. These techniques can be classified on the basis of their manufacturing route: those starting from larger substrates (cells) that are reduced to units for the creation of small size vesicles (top-down nanotechnology) or those taking individual molecules (lipids, proteins, etc.) that self-assemble in higher-order structures with tunable composition (bottom-up nanotechnology). Top-down products differ from natural EVs in terms of micro-structure and biochemical composition, since they are formed from cell fragments: the characteristic membrane microdomains [24] (lipid rafts) and associated pools of surface markers (especially tetraspanins) are absent, and the minimal cytoskeleton is not present.
Impact of the artificial EV classification in the design of new therapeutic agents based on EVs
The preparation route chosen is important for the final purpose of artificial EVs, but it could be critical for the clinical trials and subsequent commercialization. The extensive manipulation of EVs during the bio-engineering methods is the reason for their classification as advanced therapy medicinal products (ATMPs) [2], with a particular regulatory framewok. Following the same criteria, fully artificial EVs produced from cultured cells (top-down) should also be incorporated into this category.
On the other hand, bottom-up artificial mimetic EVs are more difficult to assign to one or another category. To date, a set of proteins have been fixed on lipidic vesicles with an undefined purpose. While there is some evidence that the EV membrane is important for the uptake process [1], the role of the artificial membranes is not yet clear. Comparative studies of the effects over target cell lines with conventional liposomes and exosome-mimetic nanovesicles would be very useful to clarify this. The work in this field is reviewed in the section “Bottom-up methodologies: artificial membranes decorated with functional proteins to mimic EVs functions.”
A critical evaluation of the fully synthetic EVs concept would imply providing an answer to questions such as: “What we are trying to mimic from EVs?”; “Is the biochemical composition, the morphology or the whole entity?”; “Is it worth mimicking a specific function?” This is still a challenge in the field, since some of these questions are being answered at the same time for natural EVs. The best approach would involve an extensive biochemical characterization of natural EVs and a detailed description of their functions. Regarding functionality, other populations and not only the target cell lines should be assayed. This could provide information about possible side effects. In the same way, multiple parameters should be registered as output factors, not just the process targetted by the EV-based treatment. Proteins, nucleic acids and lipid composition from specific types of exosomes are registered in specialized databases such as ExoCarta [25], EVpedia [26] and Vesiclepedia [27]. But this is not enough: a database with assays performed with EVs reporting treated cell lines, EV type as therapeutical agents, type of assay (in vitro or in vivo) and experimental conditions could be of great interest to the scientific community. The combination of both sources of information would be the perfect scenario for the design of future artificial EV-based therapies.
In any case, both types of artificial EVs should meet product specifications related to “purity, identity, quantity, potency and sterility” in concordance with the pharmaceutical market regulations [28]. Once more, there are several important differences in the definition of these parameters depending on the semi- or fully synthetic character of EVs. These key points will be considered and discussed in the following sections.
Semi-synthetic exosomes: biotechnological modification of naturally released exosomes
The simplest idea to manufacture EVs would be to use the natural mechanisms for the formation of vesicles, that is, the cellular machinery itself. It is known that the composition of the EVs at all levels responds to a high degree of control at very selective cellular mechanisms [29]. Therefore, the composition of the EVs could be controlled by intentional alteration of the cellular environment.
This method would lead to the creation of EVs with a composition profile adapted to a specific purpose. The technological methods used for bioengineering EVs are explained in the following sections. Two key aspects are the selection of producer cells (and their in vitro harvesting conditions) and the EV isolation/enrichment procedures. Both choices will condition subsequent uses.
Selection of EV cellular origin
Cell lines could potentially release EVs vesicles, but there are great differences in release rate and biochemical composition and their susceptibility to modifications [30]. It is also accepted that before translation to clinical use of EVs, limitations regarding biocompatibility, economic viability, harvesting methods and immunotolerance must be overcome. A summary of cell lines used for the production of EVs for clinical purposes, especially drug delivery, can be found in the literature [31]. Dendritic cells and cancer cell lines, such as melanoma, are the most commonly used lines for EV production.
Mesenchymal stem cells (MSCs) are one of the most promising sources of EVs, especially exosomes [32]. Yeo et al. [33]. defended their use for mass production of exosomes with future therapeutic purposes based on some important facts related to their advantages over other cell lines. Mainly, MSCs are easy to obtain from all human tissues (even those considered as medical waste), and they have a high ex vivo expansion capacity compatible with immortalization without compromising their therapeutic efficacy. These two facts are essential to establish a scalable and long-term source of well-characterized EVs, particularly exosomes [14,28]. In addition, their immunomodulatory effect gives them and their derived EVs important features in autologous and allogenic therapeutic applications.
Dendritic cells (DCs) have important roles in immunity (both innate and adaptative). Some authors have paid attention to this cell line in order to enhance the production of clinical grade DC-derived exosomes for immunotherapy [33]. Properties of DCs have even been enhanced with different nanoparticles [34]. Exosomes from DCs modified to express indoleamine 2,3-dioxygenase (IDO), a tryptophan-degrading enzyme that is important for immune regulation and tolerance maintenance, have been used in the treatment of rheumatoid arthritis [35]. In another study, DC-derived exosomes modified to express FasL on the surface (ligand involved in apoptosis induction) were tested as inflammatory and autoimmune therapy [36]. Other recent works are related to the role of tumour-released exosomes to load antigens into DCs for the therapy of malignant mesothelioma [37].
To overcome the problem of low release rate, some authors lowered the micro-environmental pH, mimicking the natural cancer mechanism [38]. By culturing HEK293 cell lines at different pHs, these authors found that low pH values were best to isolate high amounts of exosomes. In spite of the impact of these results, it would be necessary to test similar effects in non-cancerous cell lines and to determine how the change in the harvesting conditions affects EV composition (membrane components and cargo).
Not only human cells have been explored as a source of EVs: exosomes isolated from bovine milk and loaded with different drugs were a promising strategy for mass production of therapeutic EVs [39]. They can be easily isolated by differential centrifugation. The biocompatibility of milk-derived exosomes was checked by clinical biochemical analysis in an animal model by oral gavage during 6 h (short-term toxicity) and 15 days (medium-term toxicity).
In recent years, some attention has been drawn to non-animal (especially plant) EVs and their potential use in therapy [40]. In particular, fruit-derived exosomes (lemon [41] and grapes [42]) have been isolated, characterized and tested as beneficial products. Perhaps this new source of EVs could be used in the near future for the development of EBSSNs following modifications to express the desired targeting molecules against specific cell lines. Evidence about immunotolerance should also be provided in order to avoid any interference in the results.
In any case, studies involving the encapsulation of the same drug into different cell lines-derived EVs would be desirable in order to clarify whether the beneficial effect is due to the drug or the combination of drug/type of carrier.
Obtaining a good substrate for modification: isolation procedures
Since the final destination of artificial EVs would be the administration for therapeutic purposes, the highest level of standards would be required in order to preserve patient safety [27,43]. A key point in artificial EVs development is the enrichment from different biological samples, from cell-culture supernatants to several body fluids. There are different reviews [43–45] and technique-comparative papers [46–49] about isolation procedures. They involve ultracentrifugation, filtration, immunoaffinity isolation, polymeric precipitation and microfluidics techniques, with different degrees of purity for the final product [50]. In this review, we have focussed on scalability, reproducibility and synthetic EV potential damage or physical modification.
Since the efficient function of EVs depends on their size distribution, aggregation and size changes after isolation are important. Lane et al. [47] studied these parameters in four isolation methods: two aggregation kits, a density-based method and ultracentrifugation. These authors found that some methods kept a constant vesicle size, but large differences were observed regarding yield of isolation (the two sedimentation methods gave recovery values two orders of magnitude higher than the other methods). Another reported obstacle is the co-purification of material with similar physical characteristics to EVs [42,51,52].
Scalability is also important, since the batch size is correlated with the homogeneity of the final product; sometimes small batches are more prone to being susceptible to bias during the process, but on other occasions, higher batches yield more heterogeneous populations due to microenvironments during procedures.
The scale-up step with ultracentrifugation (UC) is limited by the number of rotor positions and the maximum sample volume. On the other hand, methods such as size exclusion chromatography (SEC) are easy to scale up by using large columns, but with the associated longer separation time. Pressure application to reduce processing time can disturb EVs [43]. Other methods, such as immunoaffinity isolations, are only used for small amounts of original sample because of the high price of the reagents required. Finally, microfluidic methods [45,53] are promising, with the possibility of being coupled to online analysis [54].
Reproducibility is crucial when the product is going to be used in the clinical field. Comparative results of UC are difficult to obtain due to the high number of models available in the market, and this could affect the quality of the isolated product [43]. The use of a fixed instrument would keep low variability between batches [47].
Welton et al. [55] reported that ready-to-use SEC columns could overcome some problems related to homemade poured columns [52], thereby avoiding variations from column to column, and facilitating robust protocols to be used routinely. The main problem associated with this method is dilution of EVs in the final sample and the subsequent need for concentration using precipitating agents or UC. This increases retention time and the possibilities of co-precipitation of other molecules with the same size and physical properties.
Considerable effort has been made in the field of EV isolation methods, which are still limiting the expansion of this field. There is not yet a perfect and universal method, and it is also accepted that selection of the isolation method could impact downstream steps [56].
Strategies for biochemical modification
Pre-isolation modification using own cellular machinery
The advantages and disadvantages of the methods applied to incorporate proteins of nucleic acids are shown in Tables 2 and 3. Based on the study of exosome biogenesis-related mechanisms, several methods have been developed [50,57–62]. The following criteria have been identified for their classification (see Table 1):
(I) location of exosomal functional entities, such as transmembrane proteins and the use of their natural tropism to co-localized the exogenous element;
(II) strategies using molecular mechanisms for the introduction of exogenous molecules into EVs for cytosolic delivery;
(III) increasing the amount of molecules into the cellular plasma to be encapsulated by passive mechanism during MVB formation.
Table 2.
Pre-isolation methods for cargo incorporation into EVs.
| Cargo incorporation previous to the release of exosomes | |||||
|---|---|---|---|---|---|
| Method | EV modified component | Category of modification (according with Table 1) | Advantages | Disadvantages | Molecules incorporated |
| Genetic fusion of cargo gene with an exosomal protein gene | Surface | Class I | Efficient exposure of targeting moiety on the surface of EVs By selecting the EVs protein to be fusioned with, different expression rate can be achieved |
Only successfully explored with exosomal membrane proteins | Peptides, small proteins such as GE11 peptide [69], HLA [63], OVA [65], HER2 [66], and RVG [67] |
| Exosome surface display technology | Surface | Class I | Suitable to induce expression of protein in both, extravesicular and intravesicular sides at the same time | Not tested with high-molecular-weight proteins | Fluorescent proteins [62] such as GFP and RFP |
| RNA zipcodes | Cargo | Class II | Alternative to electroporation of miRNA | Applicable only to mRNA and miRNA Influence of mRNA size not tested |
mRNA [70] and miRNA [71] modified with zipcodes |
| EXPLORE platform | Cargo | Class II | Excellent platform to load proteins to be delivered to the cytosol of the target cell Expected better results than commercial solutions available |
Limited loading capacity due to the presence of fluorescence reporter proteins in future work, this protein can be omitted | mCherry, Bax, SrIκB and Cre recombinase proteins [61] |
| TAMEL platform | Cargo | Class II | By selection of one component of the platform, the EV-enriched protein loading efficiency can be controlled | Highly cost-effective method Required highly experimented personal EE values depending on RNA molecule size |
RNA [59] with variable length |
| Overexpression of RNA cargo into producing cells | Cargo | Class III | Used in all types of exosomes Applicable to all types of RNA |
Nonspecific loading mechanism Low efficiency, especially for mRNA |
RNA and proteins by expression of RNA into cell producer cytosol |
| Fusion with liposomes | Surface and/or cargo | Class III | High efficiency Both hydrophilic and hydrophobic compounds can be loaded |
Cellular uptake rate can be decreased Better efficiencies for hydrophobic molecules |
Hydrophobic and hydrophilic compounds, such as DiI and calcein respectively [72] Photosensitizer drugs, such as ZnPc [72] |
Table 3.
Post-isolation methods for cargo incorporation into EVs.
| Cargo incorporation after the release of exosomes | |||||
|---|---|---|---|---|---|
| Method | EVs Modified component | Category of modification (according with Table 1) | Advantages | Disadvantages | Molecules incorporated |
| Co-incubation with exosomes | Cargo | Class IV | The simplest method Inexpensive Compatible with the addition of a small amount of organic solvent for the enhancement of hydrophobic drug dissolution |
More suitable for hydrophobic molecules | Low- and medium-molecular-weight hydrophobic molecules such as curcumin [74], placlitaxel [95], cucurbitacin I [75], celastrol [76] and different porphyrins [58] Enzymes, such as catalase [80] |
| Electroporation | Cargo | Class Va | Used in all types of exosomes Able to incorporate large compounds, such as 5 nm NPs |
Applicable only for hydrophilic compounds RNA type-dependence effectiveness Slight differences in EE depending on the cellular origin (cell line) of the exosomes Induce aggregation of siRNA, with valuable reduction in EE. Exosome aggregation trend during electroporation process |
RNA, especially siRNA [67] Different drugs such as placlitaxel [60], porphyrins [58] SPIONs [79] |
| Extrusion | Cargo | Class Va | Simple method | Induces changes in EVs which reduce delivery efficiency | Small molecules such as Porphyrins with different hydrophobicity [58] Enzymes, such as catalase [80] |
| Saponin-assisted loading | Cargo | Class Va | Similar loading efficiency to electroporation, but without the associated problems Saponin can enhance in some cases the efficiency of co-incubation |
Low efficiency for some large molecules, but better than simple incubation | |
| Hypotonic dialysis | Cargo | Class Va | Not tested with large molecules | ||
| Sonication | Cargo | Class Va | Enhance simple incubation through decreasing bilayer rigidity | Not tested with hydrophilic molecules Not tested with different EV populations |
Small molecules such as placlitaxel [95] or Doxorubicin Enzymes, such as Catalase [80] |
| Click chemistry | Surface | Class Vb | Keep constant morphology or functionality of EV properties Applicable to any molecule previously modified |
A two-step procedure with subsequent purification steps to remove unbound molecules and activate agents | Fluorescent dyes such as azide-Flour 545 [81] Potentiality to any type of molecule susceptible to being modified by azide groups |
| Fusion with liposomes | Surface and/or membrane | Lipids with different chemical nature [85] (zwitterionic, cationic, anionic, PEGliated, etc.) | |||
Table 1.
Classification of techniques for the production of artificial EVs, mainly exosomes, according to type of final product (semi- or fully synthetic) and the principle of the obtention mechanism.
| Semi-synthetic exosome production: modification of vesicles naturally produced by cells | |
|---|---|
| Pre-isolation modification | |
| Class I | Co-localization of cargo and exosomal carrier moiety thanks to the natural tropism of the second |
| Class II | Use of sequences (i.e. nucleic acid-based sequences) for the exosomal biogenesis pathway signalling |
| Class III | Take advantage of passive loading via increments of their presence, by genetic overexpression or active loading of producer cells |
| Post-isolation modification | |
| Class IV or passive methods | Methods that use passive adsorption of molecules into external surface of EVs, owing to their hydrophobicity nature |
| Class V or active methods | V.a (Physical methods), based on the creation of transitional alteration in the integrity of EVs that allows cargo to enter the vesicles by concentration gradient or by passive incorporation during subsequent restoring of initial status post-stimuli V.b (Chemical methods), based on induced chemical reactions between EVs and cargo with or without previous introduction of functionalization agents into vesicles |
| Creation of artificial mimetic structures of the natural exosomes | |
| Type I or top-down bio-nanotechnology | Starting from larger substrates (cells) that are reduced to units for the creation of vesicles with reduced size |
| Type II or bottom-up bio-nanotechnology | Starting with individual molecules (lipids, proteins, etc.) that are assembled in a controlled way for generating complex structures of higher order |
Class I methods (Table 2) involve the design of chimeric constructions of proteins by genetic engineering. In this case, the fusion between the gene of a protein to be incorporated and the gene of an exosomal-localized protein can be used for the expression of the former on the outer surface of exosomes. This has been referred to in the literature as Exosome Display technology [63], and it enables the manipulation of the protein content of exosomes and the subsequent tailoring of activities. The potential of the method was successfully demonstrated by the production of specific antibodies against human leukocyte antigen (HLA), a low immunogenic antigen [64].
Lactadherin C1C2 domain was also used for similar purposes by Zeelemberg et al. [65] and Hartman et al. [66] to induce expression of chicken egg ovalbumin (OVA) peptide and the human epidermal growth factor receptor 2 antigen (HER2), respectively. Álvarez-Erviti et al. [67] described a method of inducing surface expression of the central nervous system-specific rabies viral glycoprotein (RVG) peptide on exosomes isolated from immature dendritic cells derived from mice. Complementarily, these brain-targeted exosomes were loaded with siRNA for the first time by electroporation. The delivery of GAPDH-siRNA specifically to neurons, microglia and oligodendrocytes in the brain resulted in a specific gene knockdown. This was considered the first example of EV-based genetic therapy. One of the most important facts of this work was the successful treatment of a highly protected tissue, the brain, in spite of the existence of brain–blood-barrier selectivity.
Tian et al. [68] used lysosome-associated membrane glycoprotein 2b to target electroporated doxorubicin-loaded exosomes (20% of loading efficacy) produced in dendritic cells. Ohno et al. [69] used platelet-derived growth factor receptor transmembrane domain to anchor GE11 peptide, a ligand of the epidermal growth factor receptor (EGFR). This construction was transfected to Human Embryonic Kidney cell line 293 (HEK293) using pDisplay vector and FuGENE HD transfection reagent. As a model cargo, siRNA let-7 was selected for its ability to alter cell-cycle progression and reduce cell division in cancer cells. This siRNA was introduced into EV producer cells by the lipofection method. Modified exosomes (15–21% of total released exosomes) were isolated by centrifugation and intravenously injected into an animal model with induced breast cancer. GE11 peptide as the targeting moiety was selected by the elevated expression of his receptor (EGFR) in tumours of epithelial origin.
More recently, Stickney et al. [62] developed another genetic engineering method for surface expression of proteins in human cells, called surface display technology. In this case, tetraspanin CD63 was used as a scaffold for the presentation in both extravesicular and intravesicular orientations.
Class II methods include a heterogeneous group of strategies that have in common the use of specific molecular interactions between two elements and can be used to transport the complex into the exosomes.
One example of this strategy is the interaction between specific sequences in RNA molecules and proteins that are present in the route of exosomes formation [16]. Highly observed sequence motifs into RNA types studied in EVs, called EXOmotifs, were found in mRNA [70] and miRNA [71]. Exosomes for Protein Loading via Optically Reversible protein–protein interactions (EXPLORs) and Targeted and Molecular EV Loading (TAMEL) are technologies based on the molecular interaction between certain types of proteins or between RNA special structures and specific proteins. Proteins of interest can also be loaded into the inner compartment of exosomes for their direct delivery to the cytosol of the target cell as an alternative for therapeutic target locations. EXPLORs [61] has recently presented for that purpose. The system integrates two elements: one is produced by the genetic fusion of the photoreceptor cryptochrome 2 (CRY2) to the protein to be loaded, and the other is made by the fusion of a truncated version of the CRY-interacting basic-helix-loop-helix 1 (CIBN) to tetraspanin CD9. Both elements can be transitorily attached by exposure to blue light, which induces the interaction between CRY and CIBN, and the interaction can be stopped once the blue light is not present.
TAMEL is another genetic engineering tool that has recently been published [59] for the active cargo of RNA. This platform is a fusion between an engineered EV-loading protein and the RNA to be loaded. Engineered EV-loading protein is also a fused product between an EV-enriched protein and an RNA-binding domain. This construction is transfected into EV producer cells to make his function. Different loading degrees can be obtained by selecting the EV-enriched protein. This is related to the natural expression of different proteins into EVs.
Class III methods includes the simplest method: passive loading into vesicles through their biogenesis. There can be two different approximations to this objective. First, the overexpression of RNA cargo in the producer cells. The major disadvantage of this method is the lack of selectivity in the loading process, since it is gradient-driven (the higher the concentration in the cytoplasm, the higher its possibility of being trapped into exosomes during invagination of MVB formation). On the other hand, the great advantage of the method is that by translation of mRNA into receptor cell cytoplasm, codified proteins can also be passively loaded into EVs.
The second approximation explores the active loading of the producer cells, i.e. by nanocarriers such as fusogenic liposomes. This strategy is based on physico-chemical properties that govern the type of mechanism (the fusiogenic properties of the two elements that take place in the method, cells and liposomes). Second, they modify the whole cell and not only the exosomes.
As an alternative method for the incorporation of exogenous molecules (specially designed for hydrophobic compound) into EVs, Lee et al. [72] presented the use of membrane fusogenic liposomes (MFLs). By the treatment of cells with MFLs loaded with a hydrophobic compound (DiI) and a hydrophilic molecule (calcein), these authors obtained EVs modified with both molecules. Only slight differences in the efficiency of incorporation into EVs were found, since a hydrophobic compound would remain in the plasma membrane after liposome fusion and in the subsequent formation of EVs membrane through their routes of biogenesis. In contrast, a hydrophilic molecule would be released into the cytosol. Intercellular transport of both molecules mediated by EVs was successfully observed in vitro in a multicellular tumour spheroid model.
A similar method was used to modify the composition of EVs, with a special focus on the modification of the properties of the EV membrane [57]. Dyes, fluorescent lipids with different lengths and saturation grade of acyl chains, and chemotherapeutics were loaded into cells by means of EVs.
These authors also carried out a membrane surface modification, with the possibility of conjugation with molecules for targeting purposes. They first prepared MFLs containing azide-modified lipids which were fused with cells. By simple incubation with dibenzocyclooctyne (DBCO)-modified peptides, a covalent bond was created due to the fast and selective reaction between DBCO and the azide group [73]. This strategy will allow new possibilities of surface ligand decoration on EVs for targeting purposes (such as peptides, aptens or antibodies) or for the introduction of molecules with therapeutic properties via interaction with selective receptors. By the combination of a different headgroup modified lipids, several different ligands could be incorporated into EV outer membranes, including receptors and co-receptors.
Physical and chemical post-isolation modifications
These are the methods that require an external force (chemical or physical) to incorporate new molecules on previously isolated exosomes (Table 3).
Passive methods. Incubation of EVs and cargo
The simplest way to incorporate any cargo into cell culture or body fluid isolated EVs is the co-incubation of both elements. This strategy was explored by Sun et al. [74], who found that curcumin exosome-loaded exhibited a better stability and higher bioavailability in serum in an animal model. For these therapeutic-modified exosomes, an improvement in in vivo anti-inflammatory and septic shock was observed.
In another study [75], two anti-inflammatory compounds were loaded into exosomes and microparticles, and they were administrated intranasally, opening up new therapeutic possibilities. The effects of solvents and drug release kinetics from loaded exosomes by dialysis have been studied [39,76].
Active methods. Physical methods: electroporation and other temporary membrane disruptive methods
The most commonly used method for cargo incorporation into EVs after their release is electroporation [77]. This technique involves the temporary permeabilization of membranes through the creation of pores due to the application of high-voltage electricity. Some authors have pointed out that this method is not suitable for siRNA cargo into EVs due to technical problems, and an overestimated encapsulation into EVs could be observed. It has been reported that electroporation induces siRNA aggregation and co-pelletization with EVs during purification by ultracentrifugation, without any dependence on electroporation buffer composition [78]. They also postulated that slight differences could be found between different EVs regarding their cellular origin (e.g. primary cells).
Another relevant problem concerning electroporation is exosome aggregation and subsequent decrease in functionality. To avoid these problems, Hood et al. [79] electroporated exosomes from mouse B16-F10 melanoma cells by incorporating 5 nm superparamagnetic iron oxide nanoparticles (SPIONs) as model exogenous cargo. Other authors compared the loading efficiency of different porphyrins with different hydrophobicities into EVs with various origins by passive loading (co-incubation), and by active loading, such as electroporation, extrusion, saponin-assisted drug loading and hypotonic dialysis [58]. The best results were obtained for hydrophobic compounds and for electroporation. Interestingly, zeta potential (ζ) related to the chemical composition of EV membranes seems to play a role in loading efficiency, since higher ζ values led to higher EE. The chemical properties of cargo are also relevant, since their charge will condition the final outcome. Electroporation did not induce drug precipitation.
In contrast, extrusion over polycarbonate membranes altered the morphology of vesicles and, subsequently, their delivery efficacy. Other authors used the sonication of EVs in the presence of drug solutions [60].The loading efficacy was found to follow the order: incubation at RT < electroporation ≪ mild sonication. A similar trend was observed for size changes after the loading procedure. On the other hand, surface charge and protein profile were similar after loading, evidencing no alteration in exosome stability. These authors explained the results concerning sonication as a decrease in bilayer rigidity after sonication, which allowed a better incorporation of the hydrophobic drug. Therefore, mild sonication should be considered as an enhancement of co-incubation. Additionally, loaded exosomes were stable over large periods of time at different temperatures.
A similar comparative study was carried out with the enzyme catalase [80]. For the preparation of exosomes modified with this oxidative stress-protecting agent used for the treatment of Parkinson’s disease, these authors selected four methods: incubation at RT in the presence/absence of saponin, freeze/thaw, sonication and extrusion. Sonication yielded the higher EE (26.1%) and the more stable product. In contrast, this method also produced the higher increment in size, from 105 nm naïve exosomes to 183.7 nm in catalase-loaded exosomes. Associated with size increment, AFM observation also revealed a change in morphology, with a final non-spherical shape.
Despite these promising results regarding the encapsulation of different molecules into exosomes, standardization in systematic conditions followed by the study of several cell lines is still necessary to strongly support the use of these methods as routine practice in the clinical field.
Chemical methods: click chemistry mediated functionalization and other targeted drug-delivery strategies
The chemical copper-catalysed reaction between an alkyne and an azide that forms a triazole linkage (click chemistry) has been used for the surface functionalization of exosomes [81]. These were first chemically modified by the incorporation of alkyne groups into amine groups from proteins by carbodiimide chemistry [82]. These authors conjugated azide-Fluor 545 (a fluorescent compound) to activated EVs. Since no differences in morphological and functional properties were found, it was concluded that modification by click chemistry does not alter exosome characteristics and allows the incorporation of exogenous molecules to the surface of EVs.
Finally, there is another type of cargo modification that has been applied into artificial vesicular systems (liposomes) with potential applicability to EVs. This method is based on the ability of some peptides to be incorporated into lipidic membranes causing disruption [83,84]. By fusion of the peptide D1-7 to an adhesion molecule expressed in cells, targeted lipidic carriers with therapeutic cargo were produced and successfully tested in vitro and in vivo. Another interesting application of this strategy is its ability to insert peptidic cargo into live cell membranes, giving possibilities of imaging live cells and modifying cell surfaces. This last property could be explored for the modification of plasma membrane in EV producer cells.
The modification of EV membranes results in changes in surface charge, fusiogenic properties, immunogenicity decrease and colloidal stability [85]. Engineered hybrid exosomes were prepared by membrane fusion with liposomes formulated with different types of lipids (i.e. zwitterionic, anionic, cationic and PEGlilated). Fusion properties with cell-culture-derived exosomes were studied according to the chemical nature of liposome lipids [86]. It was found that zwitterioninc and anionic lipids did not alter the uptake rate, while the introduction of cationic lipid greatly decreased the phenomena, and PEGilated lipid increased it by twofold. Therefore, it can be concluded that functional properties could be tuned by modifying the membrane composition.
Top-down and bottom-up methods for the development of full synthetic EVs
Production of artificial EVs by generation of plasma membrane fragments: a top-down-inspired methodology
Different approaches based on top-down nanotechnology have been developed for the production of EVs mimetic nanovesicles using cells as precursors of plasma membrane fragments. Those strategies rely on the principle of self-assembly of lipids and lipid membranes into spherical structures and the encapsulation of surrounding material into the aqueous cavity of generated nanovesicles. Current methods include extrusion over membrane filters [16,17,87], hydrophilic microchannels [88] or cell slicing by SixNy blades [18] (see Table 4).
Table 4.
Summary of the published work about the generation of mimetic EVs nanovesicles by top-down bio-nanotechnology (cell source and type of cargo are encapsulated, and main characteristics are given).
| Generation technique | Precursor cell type | Type of material encapsulated | Nanovesicles characteristics | Reference |
|---|---|---|---|---|
| Manual extrusion over polycarbonate membrane filters | Monocytes and macrophages | Exogenous, chemotherapeutic drugs | Mean size and distribution similar to that of exosomes Exosomal protein profile similar to that of natural exosomes EE of chemotherapeutics dependent on the original amount used during extrusion High rate of drug release 100 times more that of nanovesicles than exosomes from the same number of cells Results reproducible with different cell types |
[16] |
| Pressurization and extrusion over hydrophilic parallel microchannels in a microfluidic device | Murine embryonic stem cells | Endogenous, proteins and RNA | Average size in the exosome range Similar intracellular and membrane protein and total RNA profile to the original cells and exosomes Same ability to deliver RNA content as exosomes |
[88] |
| Centrifugal force and extrusion over a filter with micro-size pores into a polycarbonate holder structure | Murine embryonic stem cells | Endogenous, proteins and RNA | NVs size and morphology similar to exosomes Cargo of RNA, intracellular proteins and plasma membrane proteins similar in types to exosomes Small RNA profile differs in quantity, especially in miRNA with respect to exosomes Intravesicle contain twice the concentration of natural exosomes 250 times more vesicles than naturally secreted exosomes |
[87] |
| Slicing living cell membrane with silicon nitride blades in a microfluidic device | Murine embryonic stem cells | Exogenous, polystyrene latex beads | Generated NVs in the size range of exosomes Nanovesicle production 100 times more productive than natural exosome 30% of EE for 22 nm nanoparticles as model of exogenous material encapsulation NVs can deliver exogenous encapsulated material |
[18] |
Extrusion over polycarbonate membrane filters
Jang et al. [16] used a serial extrusion through polycarbonate membrane filters with decreasing pore sizes (10 μm, 5 μm and 1 μm) in a mini-extruder, similar to those commonly used for the preparation of liposomes. Human monocytes were chosen as precursors for membrane fragments. The yield production of NVs was 100-fold in comparison with the production of exosomes by using the same number of cells. Morphological studies of these NVs by crio-TEM and NTA showed many similarities with the exosomes, round shape and a peak diameter around 130 nm. Even the exosomal protein marker profile (CD63, Tsg101, moesin and beta-actin) checked by Western blot was identical for the NVs and exosomes. The chemotherapeutic drugs, doxorubicin, 5-FU, gemcitabine and carboplatin were added to the buffer where cells were resuspended. The encapsulation efficiency in the final purified NVs was found to be dependent on the initial amount of added drug.
Looking for a scaled-up process using extrusion as the generation procedure of NVs, Jo et al. [87] developed a device that uses centrifugal force to extrude cells over polycarbonate filters (10 μm and 5 μm pore sizes). The device has a central piece where filters are located and connected to two syringes where the sample is dispensed by the centrifugal force. Uniformly sized 100 nm NVs with a yield 250-fold higher than that of exosomes from the same number of cells was achieved. Analysis of the filters by TEM showed that many cells remained trapped in the structure.
The same device previously cited was employed by Jeon et al. [17] to produce exosome-mimetic NVs from murine embryonic stem cells for the treatment of mice isolated skin fibroblasts. These authors wanted to explore the potential of mimetic exosomes to induce proliferation and recovery after injury in an in vitro model. Genomic and proteical profiles similar to original cells were assessed by PCR and Western blotting of specific markers, and it was confirmed that successful delivery of genetic material by NVs was reached.
Pressurization, extrusion and slicing over hydrophilic parallel microchannels in a microfluidic device
Jo et al. [87] produced exosome-mimetic nanovesicles by extruding cells over hydrophilic microchannels, with the aim of delivering endogenous RNA across the plasma membrane with high efficiency and low toxicity. These authors developed a microfluidic device made of PDMS by soft lithography. This device had an array of 37 parallel microchannels, with a common inlet and outlet connection for the pumping with a syringe pump and the collection of NVs, respectively. The higher amount of nanovesicles generated by extrusion over hydrophilic channels with a similar size to exosomes was obtained with a length of 200 μm and a width of 5 μm. These results showed that an appropriate total shear force induced by the channel has to be reached in order to produce NVs with acceptable results. This force is responsible for NV generation due to elongation of the plasma membrane on the microchannel surface. When the elongated membrane reaches a certain value, it breaks into small portions that directly form nanovesicles, thanks to the self-assembly property of lipids in aqueous media.
With the appropriate channel morphology, these authors produced 100 nm nanovesicles similar in composition (proteinical and nucleic acid profile) to naturally produced exosomes. The analysis of these NVs [88] revealed that the formation of exosome-like NVs through hydrophilic channels produced a delivery system of endogenous material with identical results to those with exosomes.
More recently, Yoon et al. [18] reported the production of exosome-mimetic nanovesicles by the slicing of cells through SixNy blades aligned to the flow direction over hydrophilic microchannels. These authors combined the induced shear stress formation on NVs with the fragmentation of plasma membrane by the blades to obtain fragments for the generation of exosome-like nanovesicles and the co-encapsulation of exogenous material (polystyrene latex beads as a model substance). It was found that NV diameter increased as the width channel increased. This is because channel morphology is proportional to the Reynolds number (Re). In this particular case, Re is proportional to the hydraulic diameter and, therefore, to the inertial force, which directly increases with the channel width. In other words, NVs travelling through wide channels have a higher inertial force when they reach the blades, generating larger sliced fragments that produce larger NVs. These have a similar composition to that of parental cells and naturally released exosomes by those cell lines.
One of the most interesting works in the literature [18] describes the encapsulation of 22 nm fluorescent polystyrene latex beads as an exogenous simulated material, adding these nanoparticles to the media where cells were diluted before slicing. With a final corrected EE of 30%, these NVs containing exogenous material were given to fibroblasts in an in vitro experiment. After a period of time, red dots corresponding to fluorescent beads were detected in the cytoplasm of fibroblast by confocal microscopy. The delivery efficiency of encapsulated beads into exosome-like NVs was higher than that of bead-aggregated NVs, revealing that exogenous material delivery with these NVs was possible, but the efficiency was still lower than that achieved with parental cell-component generated NVs.
Bottom-up methodologies: artificial membranes decorated with functional proteins to mimic EV functions
The third option for obtaining artificial EVs is their construction in a fully synthetic way by assembling individual molecules (lipids, proteins and cargo) into complex structures, such as a bilayer structure resembling EV membranes functionalized with proteins for mimicking EV functions. This could be achieved by assuming that not all components in natural exosomes are essential for specific and efficient delivery [13], including the transport of a message through direct contact with target cell receptors. Another assumption that encourages researchers to explore this route is that, from a structural and biochemical point of view, exosomes are liposomes with attached proteins. Therefore, this type of vesicular system could be an ideal substrate to develop exosome-mimetic structures. The main functional components of exosomes to be incorporated in mimetic materials have been reviewed [13]. The three main components of exosomes reported were lipids, membrane proteins and therapeutic cargo.
One of the main advantages of fully artificial EVs over previous strategies is the production of pure and well-defined biomaterial. In addition, production strategies of artificial EVs based on liposomes are more sustainble and easier to scale up [11,89]. This fact is quite important for preclinical and clinical studies and in order to manufacture products ready to be sent to the market [2].
Liposome preparation techniques have been extensively reviewed [89–101], but not all the methods yield vesicles suitable for becoming an artificial exosome. It could be considered that only small unilamellar vesicles (SUVs) are ideal precursors due to their similarities to natural exosomes (size range and membrane disposition).
Methodologies for SUV preparation (Table 5) can be classified according to different criteria [92]. For example, number of steps to reach SUV. Another classification is based on the physical principle applied to prepare vesicles: mechanical processes, organic solvent replacement, detergent removal and other techniques as microfluidic-based methods. Reverse-phase evaporation, ethanol injection method, ether injection method (EtIM), thin-film hydration method (TFH), homogenization techniques, French press cell extrusion, microfluidization, extrusion over membranes, ultrasound and membrane contactors are some of the techniques developed for SUV preparation.
Table 5.
Advantages and disadvantage of most frequently used methods for small unilamellar vesicles (SUVs) preparation.
| Method | One-step method for SUVs preparation | Physical method applied for preparation | Advantages | Disadvantages |
|---|---|---|---|---|
| Ether injection method | Yes | Organic solvent replacement | Scale-up adapted High hydrophobic compound encapsulation No mechanical degradation of compounds |
Not suitable for thermosensitive compounds Solvent not suitable for some biocompounds |
| Ethanol injection method | Yes | Organic solvent replacement | Scale-up adapted Non-dangerous substances are handled High hydrophobic compound encapsulation No mechanical degradation of compounds |
Ultrasounds are needed when concentrated samples are produced Low encapsulation efficacies of low-molecular-weight hydrophilic compounds Not suitable for thermosensitive compounds |
| Reverse-phase evaporation | No | Emulsification/organic solvent replacement | Widely used Suitable for mass production |
Frequently used solvents are not suitable for some biocompounds |
| Thin-film hydration method | No | Mechanical processes | Applied for any type of amphiphilic molecules High encapsulation of Hydrophilic compounds compared to other methods |
Difficult to scale up production Timely and cost-ineffective due to necessary downsizing techniques |
| Downsizing Techniques French press cell extrusion Microfluidization Extrusion over membranes |
/ | Mechanical processes | Good reproducibility Adapted to scale-up requirements |
Product loss associated with clogging of membrane by concentrated samples |
| Ultrasounds | Yes | Mechanical processes | Simple methodology Possibility of being scaled up |
Degradation of biological compounds Scale-up unadapted |
All these techniques rely on the self-assembly of amphiphilic molecules, such as lipids, in ordered structures due to their physicochemical behaviour in aqueous media [93,94]. This principle is the basis of bottom-up nanotechnology. Vesicles with a size range similar to that of natural EVs could be obtained [95] when operational variables were oprtimized by design of experiments. In addition, a wide spectrum of molecules with biological activity, independently of their physicochemical nature (hydrophilic or hydrophobic, low molecular weight or macromolecules), can be incorporated into liposomes, during or after their formation [96].
Functionalization of liposomes with biomolecules is possible, owing to the different headgroup-modified lipids that are available [97]. Headgroup modification usually includes a molecule of polyethylene glycol as a spacer between the functional group and the polar region of the lipid. This avoids the sterical hindrance caused by the proximity of biomolecules and liposome surface. The chemical modification includes the introduction of different types of functional groups, such as biotin, amine, maleimide, carboxylic acid, folate, cyanur, DBCO, azide and succinyl groups. These groups determine the crosslinking strategy [98–101] which should not compromise the biological function. Bioconjugation should ideally be carried out under mild conditions, aqueous media and chemoselectivity, and with a high yield.
Successful conjugation of peptides/proteins with liposomes can be checked using conventional molecular biology techniques such as dot-blot [15], SDS-PAGE [102] or even flow cytometry [102]. A preliminary purification step is required in order to remove unconjugated biomolecules. For this purpose, the authors have used classical separation methods, such as ultracentrifugation [102] or gel filtration [15,103] (SEC) with high exclusion limit resins (Sepharose CL-2B, 4B mainly). Dialysis, however, is not used due to the high molecular weight of biomolecules selected for mimicking exosomes.
Undecorated liposomes have also been used in the EV research field as EV models for comparing isolation efficacy and physical integrity [47], detection by flow cytometry [104] and EV refractive index study [105]. However, their use as a scaffold for artificial EV development could offer new possibilities in basic research about EVs and theranostic applications. To date, there have been few examples of this approximation for the development of mimetic exosomes, and no comparative results are available owing to the great differences between the methods used. A summary of the main experimental work on mimicking exosomes by bottom-up nanotechnology is given in Table 6 and briefly commented on below.
Table 6.
Summary of published work about the development of mimetic EVs nanovesicles by bottom-up bio-nanotechnology, showing formulation of the vesicles, molecules for the surface functionalization and main physical characteristic (size).
| Formulation | Preparation method | Conjugation strategy | Size | Protein for functionalization | Reference |
|---|---|---|---|---|---|
| PC:SM:Cho:DOGS-NTA (55:30:10:5) weight ratio For fluorescent labelling, 0.25% mole/mole DSPE-RhodB |
Thin-film hydration method (KCl 100 mM, HEPES 10 mM pH 7.0, EDTA 0.1 mM; KHE buffer) Filtered and degassed + extrusion over 200 nm membranes |
Ni2+-NTA headgroup functionalized lipid + histidine-tagged recombinant peptides 37°C, 30 min |
150–200 nm | APO2L/TRAIL-His10 | [102] |
| PC:Cho:DSPE-PEG:DSPE-PEG-MAL (1:0.5:0.04:0.01) Molar ratio For fluorescent labelling, 1% of PC amount of DSPE-RhodB |
Thin-film hydration method (Hepes 25mM, NaCl 140mM; pH 7.4) Filtered and degassed + extrusion over 100 nm membranes |
Maleimide headgroup functionalized lipid + Traut’s reagent protein activation 1h RT 20/1 ratio |
100 nm | MHC class I peptide complexes and FAB regions against T-cell receptors (adhesion, early and late activation and survival) | [15] |
| Micro-emulsion phase PC:CpEL (7:3, w/w) Micelle phase In 10:1 v/v DE:A DOPE:DC-Cho (4:1, w/w) In 1:2 v/v EtOH:DW |
Micro-emulsion and micelle combining method + sonication step for 3min | Carboxilic group from ChoS and amine group from protein EDC/NHS 4°C for 12 h |
82 nm | Monoclonal antibody against DEC205 antigen expressed on dendritic cells | [103] |
The most frequent preparation technique for SUVs as templates for EVs mimicking is the TFH method combined with extrusion over polycarbonate membranes and with [102] or without [15] previous freeze–thaw cycles. Martínez-Lostao et al. [102] had a formulation that included lipids and stoichiometry inspired in natural exosomes. The introduction of only 5% (w/w) of an iminodiacetic acid derivative or DOGS-NTA allowed the binding of APO2L/TRAIL-His10 to liposomes in a single step. Its bioactivity was a higher activity than that of the soluble ligand. Moreover, a treatment based on these synthetic exosomes achieved 60% of disease improvement in a rheumatoid arthritis-induced animal model. In another study, liposome-bound Apo2L/TRAIL overcame the resistance to the soluble ligand exhibited by chemorresistive tumour cell mutants [106]. The mechanism of action of LUV-TRAIL in haematologic cells [107] was also studied using mimetic structures of exosomes.
Another approximation to artificial EVs (exosomes) for therapeutic purposes was carried out by De la Peña et al. [15] using a reported formulation [108,109]. The main components were phosphatidylcholine and cholesterol, and headgroup-modified lipids such as DSPE-PEG DSPE-PEG-MAL. In order to make traceable NVs, both in vivo and in vitro, a fluorescent lipid was included in the formulation, and magnetic nanoparticles (SPIONs) were encapsulated during a thin-film hydration step. After optimization of chemical-activated ligands binding, mimetic SNVs simulating DCs derived exosomes were successfully tested as new tools in basic and clinical immunology. A T-cell expansion rate higher than that with previously reported experiments using conventional methods was achieved.
An innovative methodology for the production of protein encapsulated nanoliposomes was also reported [103]. This produced 82 ± 4 nm antibody-coated liposomes with approximately 93% EE of BSA. The preparation route that combined a micro-emulsion contained the protein to be encapsulated, with micelles, in order to create a lipid bilayer formed through layer-by-layer assembly. In this work, the authors selected a Box–Behnken experimental design to optimize (maximize) the EE by adjusting some formulation parameters. The final optimized formulation is summarized in Table 6. In this particular case, researchers selected mimetic exosomes for the potential transmission of antigen to DCs by a controlled target delivery using a conjugated monoclonal antibody anti-DEC205, a highly expressed receptor on the surface of DCs. The introduction of cholesterol succinate in the outer layer of the liposomes allowed the bioconjugation of the Ab by EDC/NHS chemistry.
Despite the promising results and the advantages of these methods for the development of liposome-based artificial EVs, there are several limiting aspects that hinder the transfer to the clinical field. While technological progress has allowed the design, development and production of nanomedicines with high pharmaceutical grade, their clinical impact has been smaller than expected due to a lack of sufficient information about in vivo interactions and fates inside the human body [11]. Specific challenges [13] are related to the functionalization of vesicle surface with a combination of functional proteins at the same time because actual methodologies are time-consuming and because of the incorporation of nucleic acids with acceptable efficiencies. The dependence of vesicle formulation on parameters such as fusion properties and stability could be another limit of special relevance to immunotolerance. Finally, the knowledge about key components in exosomes is not yet complete, since they may vary from one cell line to another. They could even be health-state-dependent and sensitive to harvesting conditions.
Despite attempts to mimic the exosome natural lipid composition, there is a need for actually checking whether the formulation is active and involved in the expected functions or is just a passive element involved only in the scaffolding of true functional elements. Comparison of the efficiency of the intended integrated component in differently formulated vesicles could be an interesting experiment to elucidate the role of membrane components. Parameters of uptake route and incorporation efficiency could also be measured with different cell lines, in order to assess the role of the target cell. Other compounds as an alternative to lipids, with a high grade of biocompatibility, could also be used for the formulation of artificial exosome bilayers. One option is to use non-ionic surfactants [110] for the preparation of niosome-based artificial exosomes. These compounds offer several advantages [111] over lipids, such as price, versatility and sustainability. On the other hand, their chemical structure offers enhanced stability from both a chemical and physical point of view. Niosomes with a size range close to that of EVs can also be produced [95].
In recent years, microfluidics has been playing an important role in the development of enhanced vesicular systems, enabling robust and highly controlled preparation routes of vesicles [112] and allowing rapid characterization of products [113]. Another important aspect in the development of exosome-based therapy, regarding any preparation route, is the creation of reduced systems for the study of traffic and delivery into in vivo microenvironments [19]. Again, microfluidic-based systems are opening up new possibilities by the development of organ-on-a-chip platforms that enable the study of these processes in an innovative and highly efficient way [114].
It is expected that microfluidic synthesis of nanovesicles will open the path for new artificial EV routes, with the required control of size and EE, and minimal consumption of reagents.
Other recently explored drug-delivery systems have developed platelet-mimetic nanoparticles by also using bottom-up technology. These authors have produced unilamellar polymeric nanoparticles functionalized with immunomodulatory and adhesion antigens, and they have tested them as another approach to disease-targeted delivery [115].
Conclusions and future perspectives
Knowledge about all the biological aspects related with EVs, especially exosomes, has opened up new frontiers in the clinical field. After an explosion of publications in recent years about the role of EVs in physiological and pathological conditions, novel opportunities for the development of enhanced therapeutic biomaterials have arisen. These observations could help in the production of new materials inspired by natural vesicles, without the classical inconveniences associated with up-to-date synthetic alternatives (liposomes, polymersomes, inorganic nanoparticles, etc.). EV-based therapies include tissue regeneration or immunomodulation, but drug delivery is one of the most promising applications. Production, isolation, modification and purification at a large-scale clinical grade are the main limitations of EVs becoming a true clinically settled therapeutic agent.
These limitations have promoted the development of mimetic materials inspired by EVs, the so-called artificial EVs. In this article, we have introduced a systematic classification of the types of artificial EVs according to their preparation routes. Two well-defined strategies have been developed: semi-synthetic or fully synthetic products. The first strategy uses natural exosomes as precursors that are modified at the moment of their biogenesis (pre-isolation modifications), whereas the second strategy modifies the vesicles after their release by cells and their isolation from cultured media or biological fluids. Genetic engineering-based modifications, active loading platforms, specific signalling sequences for selective sorting or precursor cell modifications with nanomaterials are some of the methods developed for exosome-based semi-synthetic nanovesicle production.
Fully synthetic vesicles with EVs mimetic properties can be produced by bio-nanotechnology. Top-down techniques that produce vesicles made of membrane fragments obtained from the extrusion or slicing of cells, or bottom-up techniques that take advantage of supra-molecular chemistry (mainly self-assembly) to produce vesicles from individual molecules, represent the technology developed for that purpose.
Despite the great potential of artificial EVs, some limitations to their development as therapeutic tools have been identified. There is no perfect technique, and, depending on the final purpose of artificial EVs, combinations of procedures could offer new insights in the field. Systematic studies with different cellular origins and target cell lines would expand and consolidate the applications of artificial exosomes. Comparative work including the encapsulation into different artificial vesicles would be interesting in order to identify effects due to the carrier.
Multidisciplinary teams with complementary actions in the fields of applied biology, pharmacology, chemical engineering, material sciences and medicine would allow the definitive consolidation of these therapeutic biomaterials in clinical routines.
Acknowledgements
This work was supported by the Ministerio de Economía y Competitividad (MINECO, Spain), under the Grant CTQ2013-47396-R. This study was also financed by the Consejería de Economía y Empleo del Principado de Asturias (Plan de Ciencia, Tecnología e Innovación 2013-2017), under the Grant GRUPIN14-022. Support from the European Regional Development Fund is gratefully acknowledged.
Funding Statement
This work was supported by the Consejería de Economía y Empleo del Principado de Asturias [GRUPIN14-022]; Ministerio de Economía y Competitividad (MINECO, Spain) [CTQ2013-47396-R].
Highlights
A new systematic classification of artificial EVs is provided.
Bio-engineering modification of naturally released exosomes is summarized.
Relevant examples in pre- or post-released modifications are presented.
Bio-nanotechnological methods for fully artificial EVs generation are compelled.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Tran TH, Mattheolabakis G, Aldawsari H, et al. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160:46–18. [DOI] [PubMed] [Google Scholar]
- [4]. Aline F, Bout D, Amigorena S, et al. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;74:4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Dalal J, Gandy K, Domen J.. Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr Res. 2012;71:445–451. [DOI] [PubMed] [Google Scholar]
- [6]. Lamichhane TN, Sokic S, Schardt JS, et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357–367. [DOI] [PubMed] [Google Scholar]
- [9]. van Dommelen SM, Vader P, Lakhal S, et al. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 2012;161:635–644. [DOI] [PubMed] [Google Scholar]
- [10]. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. van der Meel R, Fens MHAM, Vader P, et al. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. [DOI] [PubMed] [Google Scholar]
- [12]. El Andaloussi S, Lakhal S, Mäger I, et al. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. [DOI] [PubMed] [Google Scholar]
- [13]. Kooijmans SAA, Vader P, van Dommelen SM, et al. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lakhal S, Wood MJA. Exosome nanotechnology: an emerging paradigm shift in drug delivery. Bioessays. 2011;33:737–741. [DOI] [PubMed] [Google Scholar]
- [15]. De La Peña H, Madrigal JA, Rusakiewicz S, et al. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods. 2009;344:121–132. [DOI] [PubMed] [Google Scholar]
- [16]. Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. [DOI] [PubMed] [Google Scholar]
- [17]. Jeong D, Jo W, Yoon J, et al. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials. 2014;35:9302–9310. [DOI] [PubMed] [Google Scholar]
- [18]. Yoon J, Jo W, Jeong D, et al. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59:12–20. [DOI] [PubMed] [Google Scholar]
- [19]. Forterre A, Jalabert A, Berger E, et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: A new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9:e84153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Bryniarski K, Ptak W, Jayakumar A, et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013;132:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Regente M, Corti-Monzón G, Maldonado AM, et al. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363–3366. [DOI] [PubMed] [Google Scholar]
- [22]. Prado N, de Dios Alché J, Casado-Vela J, et al. Nanovesicles are secreted during pollen germination and pollen tube growth: A possible role in fertilization. Mol Plant. 2014;7:573–577. [DOI] [PubMed] [Google Scholar]
- [23]. García-Manrique P, Gutiérrez G, Blanco-López MC. Fully Artificial Exosomes: towards New Theranostic Biomaterials. Trends Biotechnol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- [24]. Laulagnier K, Motta C, Hamdi S, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1:18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kim DK, Lee J, Simpson RJ, et al. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin Cell Dev Biol. 2015;40:4–7. [DOI] [PubMed] [Google Scholar]
- [27]. Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Gimona M, Pachler K, Laner-Plamberger S, et al. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci. 2017;18:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, et al. Sorting it out: regulation of exosome loading. Sem Cancer Biol. 2014;28:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Yeo RWY, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. [DOI] [PubMed] [Google Scholar]
- [31]. Johnsen KB, Gudbergsson JM, Skov MN, et al. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta Reviews Cancer. 2014;1846:75–87. [DOI] [PubMed] [Google Scholar]
- [32]. Lai RC, Yeo RWY, Tan KH, et al. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–551. [DOI] [PubMed] [Google Scholar]
- [33]. Lamparski HG, Metha-Damani A, Yao JY, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. [DOI] [PubMed] [Google Scholar]
- [34]. Klippstein R, Pozo D. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomed Nanotechnol Biol Med. 2010;6:523–529. [DOI] [PubMed] [Google Scholar]
- [35]. Bianco NR, Kim SH, Ruffner MA, et al. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase–positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheumatol. 2009;60:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Hee Kim S, Bianco N, Menon R, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. [DOI] [PubMed] [Google Scholar]
- [37]. Mahaweni N, Lambers M, Dekkers J, et al. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J Extracell Vesicles. 2013;2:22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Ban JJ, Lee M, Im W, et al. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461:76–79. [DOI] [PubMed] [Google Scholar]
- [39]. Munagala R, Aqil F, Jeyabalan J, et al. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Zhang M, Viennois E, Xu C, et al. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4(2):e1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6:19514–19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Pérez-Bermúdez P, Blesa J, Soriano JM, et al. Extracellular vesicles in food: experimental evidence of their secretion in grape fruits. Eur J Pharm Sci. 2017;98:40–50. [DOI] [PubMed] [Google Scholar]
- [43]. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Sunkara V, Woo HK, Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst. 2016;141:371–381. [DOI] [PubMed] [Google Scholar]
- [45]. Liga A, Vliegenthart ADB, Oosthuyzen W, et al. Exosome isolation: A microfluidic road-map. Lab Chip. 2015;15:2388–2394. [DOI] [PubMed] [Google Scholar]
- [46]. Andreu Z, Rivas E, Sanguino-Pascual A, et al. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracell Vesicles. 2016;5:31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Lane RE, Korbie D, Anderson W, et al. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep. 2015;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Tauro BJ, Greening DW, Mathias RA, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. [DOI] [PubMed] [Google Scholar]
- [49]. Greening DW, Xu R, Ji H, et al. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods In: Posch A, editor. Proteomic profiling: methods and protocols. New York: Springer; 2015. p. 179–209. [DOI] [PubMed] [Google Scholar]
- [50]. Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013;6:659–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Yamada T, Inoshima Y, Matsuda T, et al. Comparison of methods for isolating exosomes from bovine milk. J Vet Med Sci. 2012;74:1523–1525. [DOI] [PubMed] [Google Scholar]
- [52]. Böing AN, van der Pol E, Grootemaat AE, et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Davies RT, Kim J, Jang SC, et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. [DOI] [PubMed] [Google Scholar]
- [54]. He M, Crow J, Roth M, et al. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14:3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Welton JL, Webber JP, Botos LA, et al. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;4:27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. [DOI] [PubMed] [Google Scholar]
- [57]. Lee J, Lee H, Goh U, et al. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mat Interfaces. 2016;8:6790–6795. [DOI] [PubMed] [Google Scholar]
- [58]. Fuhrmann G, Serio A, Mazo M, et al. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. [DOI] [PubMed] [Google Scholar]
- [59]. Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles. 2016;5:31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Yim N, Ryu SW, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun. 2016;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Stickney Z, Losacco J, McDevitt S, et al. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. 2016;472(1):53–59. [DOI] [PubMed] [Google Scholar]
- [63]. Delcayre A, Estelles A, Sperinde J, et al. Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells, Mol Dis. 2005;35:158–168. [DOI] [PubMed] [Google Scholar]
- [64]. Delcayre A inventor, Le PJB inventor; Methods and compounds for the targeting of protein to exosomes. European patent WO2003016522 A2. 2003.
- [65]. Zeelenberg IS, Ostrowski M, Krumeich S, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–1235. [DOI] [PubMed] [Google Scholar]
- [66]. Hartman ZC, Wei J, Glass OK, et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. [DOI] [PubMed] [Google Scholar]
- [68]. Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. [DOI] [PubMed] [Google Scholar]
- [69]. Ohno SI, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Bolukbasi MF, Mizrak A, Ozdener GB, et al. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Lee J, Kim J, Jeong M, et al. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15:2938–2944. [DOI] [PubMed] [Google Scholar]
- [73]. Chang PV, Prescher JA, Sletten EM, et al. Copper-free click chemistry in living animals. Proc Natl Acad Sci USA. 2010;107:1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Aqil F, Kausar H, Agrawal AK, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol. 2016;101:12–21. [DOI] [PubMed] [Google Scholar]
- [77]. Weaver JC. Electroporation: A general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51:426–435. [DOI] [PubMed] [Google Scholar]
- [78]. Kooijmans SAA, Stremersch S, Braeckmans K, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172:229–238. [DOI] [PubMed] [Google Scholar]
- [79]. Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Smyth T, Petrova K, Payton NM, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25:1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Hermanson GT. Zero-length crosslinkers In: Hermanson GT, editor. Bioconjugate Techniques. 3rd ed. Boston: Academic Press; 2013. p. 259–273. [Google Scholar]
- [83]. Sessa G, Freer JH, Colacicco G, et al. Interaction of a lytic polypeptide, melittin, with lipid membrane systems. J Biol Chem. 1969;244:3575–3582. [PubMed] [Google Scholar]
- [84]. Pan H, Myerson JW, Ivashyna O, et al. Lipid membrane editing with peptide cargo linkers in cells and synthetic nanostructures. Faseb J. 2010;24:2928–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Morris GJ, McGrath JJ. The response of multilamellar liposomes to freezing and thawing. Cryobiology. 1981;18:390–398. [DOI] [PubMed] [Google Scholar]
- [87]. Jo W, Kim J, Yoon J, et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056–12064. [DOI] [PubMed] [Google Scholar]
- [88]. Jo W, Jeong D, Kim J, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14:1261–1269. [DOI] [PubMed] [Google Scholar]
- [89]. Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2015;10:711–719. [PubMed] [Google Scholar]
- [91]. Szoka JF, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9(1):467–508. [DOI] [PubMed] [Google Scholar]
- [92]. Abdus S, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4:297–305. [DOI] [PubMed] [Google Scholar]
- [93]. Antonietti M, Förster S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv Mat. 2003;15:1323–1333. [Google Scholar]
- [94]. Lasic DD. The mechanism of vesicle formation. Biochem J. 1988;256:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. García-Manrique P, Matos M, Gutiérrez G, et al. Using factorial experimental design to prepare size-tuned nanovesicles. Ind Eng Chem Res. 2016;55:9164–9175. [Google Scholar]
- [96]. Gregoriadis G, editor. Entrapment of drugs and other materials into liposomes.Volume II, Liposome Technology. Boca Ratonn: CRC Press; 2007. [Google Scholar]
- [97]. Avanti Polar Lipids products http://www.avantilipids.com Available from:
- [98]. Sullivan SM, Connor J, Huang L. Immunoliposomes: preparation, properties, and applications. Med Res Rev. 1986;6:171–195. [DOI] [PubMed] [Google Scholar]
- [99]. Hermanson GT. The reactions of bioconjugation In: Hermanson GT, editor. Bioconjugate techniques. 3rd ed. Boston: Academic Press; 2013. p. 229–258. [Google Scholar]
- [100]. Nobs L, Buchegger F, Gurny R, et al. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93:1980–1992. [DOI] [PubMed] [Google Scholar]
- [101]. Schuber F. Chemistry of ligand-coupling to liposomes In: Schuber F, Philippot JR, editors. Liposomes as tools in basic research and industry. Boca Raton: CRC Press; 1995. p. 21–39. [Google Scholar]
- [102]. Martinez-Lostao L, García-Alvarez FC, Basáñez G, et al. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010;62:2272–2282. [DOI] [PubMed] [Google Scholar]
- [103]. Li K, Chang S, Wang Z, et al. A novel micro-emulsion and micelle assembling method to prepare DEC205 monoclonal antibody coupled cationic nanoliposomes for simulating exosomes to target dendritic cells. Int J Pharm. 2015;491:105–112. [DOI] [PubMed] [Google Scholar]
- [104]. Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Throm Haemost. 2011;9:1216–1224. [DOI] [PubMed] [Google Scholar]
- [105]. Gardiner C, Shaw M, Hole P, et al. Measurement of refractive index by nanoparticle tracking analysis reveals heterogeneity in extracellular vesicles. J Extracell Vesicles. 2014;3:25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. De Miguel D, Basáñez G, Sánchez D, et al. Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol Pharm. 2013;10:893–904. [DOI] [PubMed] [Google Scholar]
- [107]. de Miguel D, Gallego-Lleyda A, Galan-Malo P, et al. Immunotherapy with liposome-bound TRAIL overcomes partial protection to soluble TRAIL-induced apoptosis offered by down-regulation of Bim in leukemic cells. Clin Trans Oncol. 2015;17:657–667. [DOI] [PubMed] [Google Scholar]
- [108]. Pagnan G, Stuart DD, Pastorino F, et al. Delivery of c-myb antisense oligodeoxynucleotides to human neuroblastoma cells via disialoganglioside GD2-Targeted immunoliposomes: antitumor effects. J Natl Cancer Inst. 2000;92:253–261. [DOI] [PubMed] [Google Scholar]
- [109]. Pastorino F, Brignole C, Marimpietri D, et al. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63:86–92. [PubMed] [Google Scholar]
- [110]. Marianecci C, Di Marzio L, Rinaldi F, et al. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci. 2014;205:187–206. [DOI] [PubMed] [Google Scholar]
- [111]. Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J.Control Release. 2014;185:22–36. [DOI] [PubMed] [Google Scholar]
- [112]. Capretto L, Carugo D, Mazzitelli S, et al. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Adv Drug Deliv Rev. 2013;65:1496–1532. [DOI] [PubMed] [Google Scholar]
- [113]. Birnbaumer G, Kupcu S, Jungreuthmayer C, et al. Rapid liposome quality assessment using a lab-on-a-chip. Lab Chip. 2011;11:2753–2762. [DOI] [PubMed] [Google Scholar]
- [114]. Bhise NS, Ribas J, Manoharan V, et al. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release. 2014;190:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Hu CMJ, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


