Abstract
Vibrio alginolyticus normally has a single polar flagellum whose number and placement are regulated positively by FlhF. FlhF is a GTPase and homolog of a signal recognition particle (SRP) protein called Ffh and SRP receptor FtsY. FlhF is located at the cell pole and directs formation of the flagellum. To study the mechanism of FlhF localization, we introduced random mutations into flhF by means of hydroxylamine and isolated mutants that could not generate the flagellum at the cell pole. The novel mutations were only mapped to the GTPase motif of FlhF. The mutant FlhF proteins showed reduced polar localization as compared to the wild type and still could associate with the membrane. These results support the assumption that the GTPase motif of FlhF plays a critical role in the polar localization of this protein during formation of the flagellum.
Keywords: FlhF, FlhG, polar flagellum, Vibrio
Significance.
In this study, to investigate the mechanism of FlhF localization, we introduced random mutations to flhF by hydroxylamine and isolated mutants that cannot generate flagellum at the cell pole. Mutations were mapped only on the GTPase motif of FlhF. The mutant FlhF proteins showed reduced polar localization compared with wild type and still could associate with membrane. We could isolate the new mutants and suggest that the GTPase motif of FlhF has a critical role for the polar localization.
Many moving bacteria have a flagellum, which is generated on the surface of the cell and functions like a screw for swimming in a liquid. The number and position of flagella vary among bacterial species [1]. Escherichia coli and Salmonella have multiple flagella around the cell (peritrichous flagella), Campylobacter jejuni has a single flagellum at both poles of the cell, whereas Pseudomonas aeruginosa and Vibrio have a single flagellum at one pole.
FlhF and FlhG are known as proteins that control the formation of flagella in bacteria having flagella at cell poles [2–4]. FlhF is involved in determining the position of flagellar formation [5,6]. In Vibrio alginolyticus, which has a single flagellum at the cell pole, overexpression of FlhF or defects in FlhG cause formation of multiple flagella at the cell pole. When FlhF is deficient in the cell or FlhG is overexpressed, then the cell has no flagellum. Thus, FlhF and FlhG control the flagellar number negatively and positively, respectively [3]. Although FlhF is a soluble protein and diffuses into the cytoplasm, some of it is located at the poles of cells with flagella. In addition, because polar localization of FlhF increases when flhG is deleted, polar localization of FlhF is prevented by binding to FlhG [6]. Nevertheless, detailed mechanisms of flagellar number control by FlhF and FlhG have not been elucidated.
MinD, which is a regulator of cell division, constitutes the Min system together with MinC and MinE and controls the Z ring formation at the center of the cell [7]. MinD is an ATPase, and its activity plays an important role in the normal execution of cell division. FlhG is a protein containing an ATPase motif homologous to that of MinD. Previously, we have purified FlhG, measured its ATPase activity, and studied the mutants in which the ATPase motif of FlhG was replaced with alanine [8]. When the ATPase activity of FlhG was lost, the polar localization of FlhF was not prevented and FlhF was strongly localized at the poles. We found that the ATPase motif of FlhG performs an important function: it inhibits the polar localization of FlhF.
FlhF shares homology with FtsY, which serves for targeting nascent membrane proteins to the Sec machinery of E. coli. A protein to be inserted into the membrane has a hydrophobic signal sequence. A signal recognition particle (SRP) called Ffh binds to the signal sequence of the peptide. FtsY functions as the SRP receptor and targets the nascent membrane proteins bound to Ffh to Sec transporter [9,10]. During this process, FtsY and Ffh interact with each other by binding to GTP to form an Ffh-FtsY complex, and the polypeptide on the ribosome can be transferred to the Sec transport device via the Ffh-FtsY complex. FtsY has a GTPase activity and dissociates from Ffh by hydrolyzing GTP and thus switches to the GDP-bound form. The binding of FtsY to GTP and its GTPase activity are important for a series of processes for targeting membrane proteins to membranes [11,12].
FlhF has been reported to contain three domains—B, N, and G—and the C-terminal G domain has a GTPase activity and the N domain determines the polar placement in Vibrio cholerae [13]. However, it was reported in Shewanella that the G domain of FlhF determines the polar placement [14]. It has been shown that the GTPase activity of Shewanella FlhF is essential for motility but not for flagellar positioning [14]. In V. cholerae and P. aeruginosa, the polar localization of FlhF does not depend on its GTPase activity and/or GTP binding [13,15]. On the other hand, in C. jejuni, the flhF mutants lacking GTPase activity cause flagellar misplacement [16]. These observations suggest that the domains of FlhF responsible for its functions may vary among bacterial species [1].
We have shown that the T306A and D439A mutants of V. alginolyticus FlhF completely lack motility and the ability for flagellar formation when expressed in a flhF-deficient strain [17]. In addition, the flagellation of mutants G299A, G304A, K305A, D377A, and K437A is not completely lost, it is only decreased [17]. In contrast, the G380A mutation in flhF does not substantially decrease the flagellar formation, but the motility is reduced when this protein is overproduced in wild-type cells and is more abundantly located at the cell poles. It has been concluded that the polar localization of FlhF is required for formation of flagella, and the GTPase motif seems to be important for polar localization of FlhF in V. alginolyticus [17]. In the present study, we randomly mutated FlhF to gain more new insights into the relation between the FlhF polar localization and formation of the flagellum.
Materials and Methods
Bacterial strains and growth conditions
The strains of V. alginolyticus and E. coli or plasmids used in this study are listed in Table 1. V. alginolyticus cells were cultured at 30°C in the VC medium [0.5% (w/v) polypeptone, 0.5% (w/v) yeast extract, 0.4% (w/v) K2HPO4, 3% (w/v) NaCl, and 0.2% (w/v) glucose] or VPG medium [1% (w/v) polypeptone, 0.4% (w/v) K2HPO4, 3% (w/v) NaCl, and 0.5% (w/v) glycerol]. E. coli cells were cultured at 37°C in the Luria-Bertani (LB) medium [1% (w/v) bactotryptone, 0.5% (w/v) yeast extract, and 0.5% (w/v) NaCl]. When necessary, the antibiotic chloramphenicol was used at 2.5 μg ml−1 for V. alginolyticus and 25 μg ml−1 for E. coli.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| V. alginolyticus | ||
| VIO5 | VIK4 (Rifr Pof+ Laf−) | [26] |
| LPN1 | VIO5 ΔflhF (Rif r Pof+ Laf−) | [6] |
| LPN2 | VIO5 ΔflhFG (Rifr Pof+ Laf−) | [6] |
| E. coli | ||
| DH5α | Recipient for DNA manipulation | |
| Plasmids | ||
| pBAD33 | Cmr, PBAD | [27] |
| pAK322 | flhF (wt) in pBAD33 | [3] |
| pAK325 | flhF-egfp (wt) in pBAD33 | [6] |
| pSK101 | flhF (T436M) in pBAD33 | This study |
| pSK102 | flhF (E440K) in pBAD33 | This study |
| pSK103 | flhF (Q419ochre) in pBAD33 | This study |
| pSK104 | flhF (Q391amber) in pBAD33 | This study |
| pSK105 | flhF (Q286amber) in pBAD33 | This study |
| pSK106 | flhF (Q160ochre) in pBAD33 | This study |
| pSK107 | flhF (Q110amber) in pBAD33 | This study |
| pSK108 | flhF (K311A) in pBAD33 | This study |
| pSK109 | flhF (G445A) in pBAD33 | This study |
| pSK110 | flhF (D470A) in pBAD33 | This study |
| pSK201 | flhF-egfp (T436M) in pBAD33 | This study |
| pSK202 | flhF-egfp (E440K) in pBAD33 | This study |
| pSK203 | flhF-egfp (Δ419–505) in pBAD33 | This study |
| pSK204 | flhF-egfp (Δ391–505) in pBAD33 | This study |
| pSK208 | flhF-egfp (K311A) in pBAD33 | This study |
| pSK209 | flhF-egfp (G445A) in pBAD33 | This study |
| pSK210 | flhF-egfp (D470A) in pBAD33 | This study |
Cmr, chloramphenicol-resistant; Rifr, Rifampicin-resistant; Pof+, possessing a polar flagellum; Laf−, lack of lateral flagella
Random mutagenesis by hydroxylamine treatment
pAK322 was treated with 2 M hydroxylamine (NH2OH) in 0.5 M potassium phosphate buffer [pH 6.0] with 5 mM EDTA for 2 hours at 50°C. The reaction mixture was placed in a dialysis cassette (Thermo Scientific) and dialyzed against 10 mM Tris-HCl with 1 mM EDTA [pH 7.5] for 24 hours at 4°C. The plasmid DNA was then recovered.
DNA manipulations, mutagenesis, and sequencing
Routine DNA manipulations were carried out according to the standard procedures. Point mutations in flhF were introduced into pAK322 and pAK325 by the “QuikChange” site-directed mutagenesis method as described by Stratagene. Restriction endonucleases and other enzymes for DNA manipulations were purchased from TaKaRa Shuzo, Toyobo, and New England Biolabs. Nucleotide sequences were determined using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) and an ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems).
Transformation of Vibrio cells
V. alginolyticus cells were transformed by electroporation as described previously [18]. The cells were subjected to osmotic shock and washed thoroughly with 20 mM MgSO4. Electroporation was carried out according to the manufacturer’s instructions using a Gene Pulser electroporation apparatus (Bio-Rad Laboratories).
Analysis of motility on a soft agar medium
Two microliters of each V. alginolyticus overnight culture in the VC medium at 30°C was spotted on a VPG 0.25% (w/v) soft agar plate, which was incubated at 30°C for an appropriate period. When we isolated mutants, colonies obtained by transformation were inoculated directly onto VPG soft agar plates. Then, the mutants whose swimming ability was abnormal were isolated.
Immunoblotting
V. alginolyticus cells were cultured overnight in the VC medium. The overnight culture was diluted 1:100 in the VPG medium and then incubated at 30°C for 4 hours. The cells were harvested by centrifugation, and then resuspended in V buffer [50 mM Tris-HCl (pH 7.5), 300 mM NaCl, and 5 mM MgCl2]. The cell suspensions were mixed with one-fifth volume of SDS loading buffer [0.2 M Tris-HCl (pH 6.8), 37.5 % (w/v) glycerol, 6 % (w/v) SDS, and 0.004 % (w/v) bromophenol blue] and one-twentieth volume of 2-mercaptoethanol, and then boiled for 5 min. Proteins in the samples were separated by SDS-PAGE, and immunoblotting was conducted as described previously, using the antibodies: anti-FlhF [6] and anti-PomBC [19].
High-intensity dark-field microscopy
Flagella were examined under a dark-field microscope (Olympus model BHT) equipped with a 100-W mercury lamp (Ushio USH-102). Vibrio cells were cultured overnight in the VC medium at 30°C. The overnight culture was diluted 1:100 in a fresh VPG medium containing 0.02% (w/v) arabinose and 2.5 μg ml−1 chloramphenicol and was further cultivated at 30°C for 4 hours. The cultured cells were harvested and resuspended in V buffer and then examined.
Fluorescence microscopy
Vibrio cells were cultured overnight in VC medium at 30°C. The overnight culture was diluted 1:100 in a fresh VPG medium containing 0.02% (w/v) arabinose and 2.5 μg ml−1 chloramphenicol and was cultivated further at 30°C for 4 hours. Fluorescence microscopic observations were carried out as described previously [6]. In brief, cultured cells were harvested and resuspended in V buffer. These cells were fixed on slides via poly-L-lysine, washed with V buffer and examined under a BX-50 microscope (Olympus). Fluorescent images were captured and processed using a digital camera (Hamamatsu photonics ORCA-Flash4.0) and imaging software HSR (Hamamatsu photonics). Polar localization of FlhF-GFP was evaluated by the percentage of cells that have fluorescent foci at the cell pole in the population being examined. We conducted at least three independent experiments and examined at least 200 cells for each strain in each experiment.
Cell fractionation
Vibrio cells were cultured overnight in the VC medium at 30°C. The overnight culture was diluted 1:100 in 40 ml of a fresh VPG medium containing 0.006% (w/v) arabinose and 2.5 μg ml−1 chloramphenicol and was cultivated further at 30°C for 4 hours. The cultured cells were harvested and resuspended in 1 ml of a buffer consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and complete protease inhibitor (Roche) and were sonicated for lysis. Undisrupted cells were removed by centrifugation (6,100×g for 5 min). The supernatant was ultracentrifuged (154,000×g for 30 min) to separate it into a cytoplasmic fraction (supernatant) and a membrane fraction (precipitate). The precipitate was resuspended in the same amount of the buffer as the supernatant was, and the concentration of the membrane protein was measured by the BCA method. Triton X-100 was added to the final concentration of 1.5% (w/v), and the mixture was rotated at 4°C for 90 min after dilution with the buffer so that the membrane protein concentration became 1.0 mg ml−1. After that, ultracentrifugation (154,000×g for 30 min) was carried out again to separate the supernatant and the precipitate. The precipitate was resuspended in the same amount of the buffer as the supernatant was. A 1/5 amount of 5×SDS loading buffer was added to each fraction and incubated at 95°C for 5 minutes to prepare a sample for SDS-PAGE. Proteins were separated by SDS-PAGE, and FlhF was detected by immunoblotting.
Results
Random mutagenesis of the flhF gene
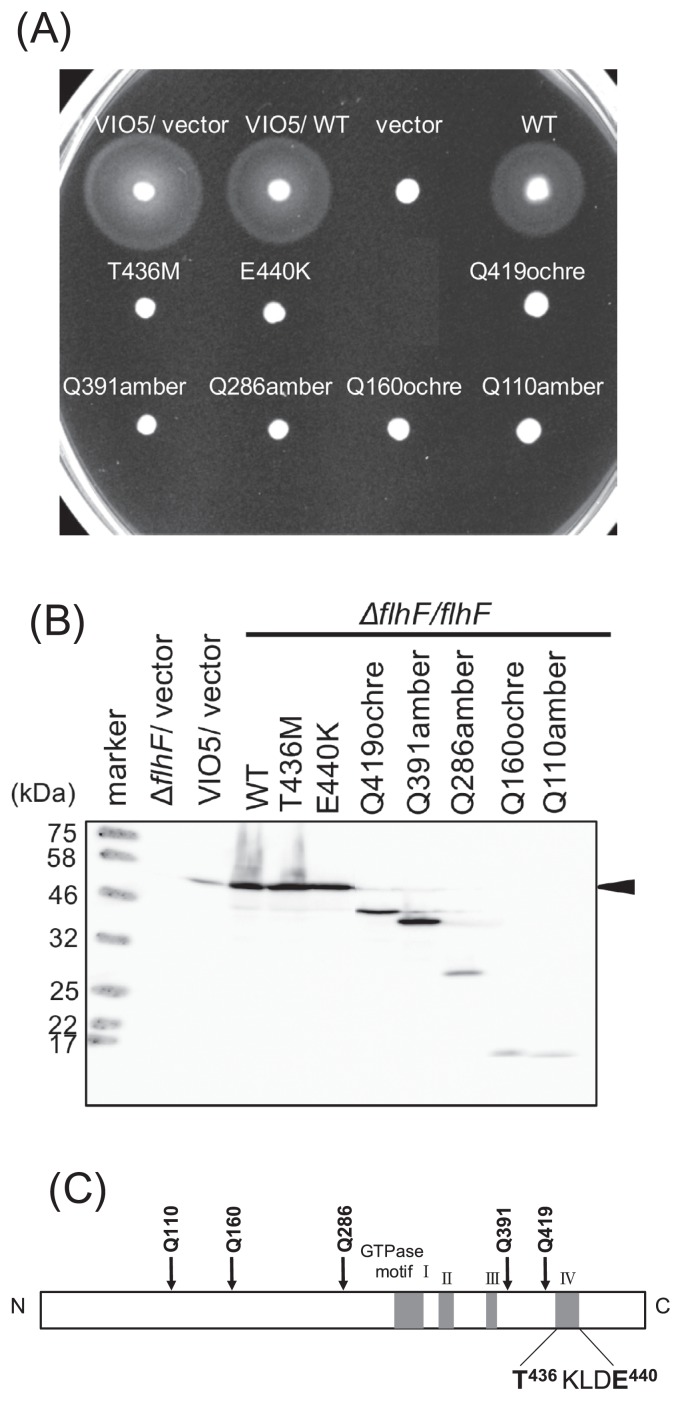
Hydroxylamine (NH2OH) is a mutagen that converts cytosine to thymine (and guanine to adenine in the complementary strand) by hydroxylating cytosine to facilitate pairing with adenine. The pBAD-derived plasmid pAK322, into which the flhF gene was ligated downstream of the araBAD promoter, was treated with hydroxylamine, and the resulting plasmid was introduced into a Vibrio flhF deletion strain (LPN1). We obtained 8534 colonies of transformants and transferred some of them to a soft agar plate containing 0.02% of arabinose using a toothpick and incubated the plates at 30°C for 3 hours. As a result, seven colonies were isolated as the mutants lacking the motility because of the plasmid mutations (Figs. 1A and 2). We sequenced the plasmid and found novel two point mutant strains, T436M and E440K, and five nonsense mutant strains: Q110amber, Q160ochre, Q286amber, Q391amber, and Q419ochre. Immunoblotting of whole-cell lysates revealed that point mutations did not affect protein amounts in the cell but mutations that cause a larger C-terminal truncation (Q110amber, Q160ochre, and Q286amber) greatly reduced protein expression (Fig. 1B). T436 is located in GTPase motif IV, and E440 is in the vicinity of this motif (Fig. 1C). Previously, mutants in which residues of the GTPase motif of FlhF were substituted with alanine have been generated and characterized [17], including mutations K437A and D439A in motif IV.
Figure 1.
Characterization of flhF mutations. (A) Cells of strain VIO5 (wild type) or LPN1 (ΔflhF mutant) harboring a plasmid— “vector” (pBAD33), +”WT” (pAK322), or a mutant plasmid (T436M, E440K, Q419ochre, Q391amber, Q286amber, Q160ochre, Q110amber) —were inoculated into a soft agar plate containing 0.02% arabinose and incubated for 4 hours at 30°C. (B) Detection of the FlhF protein. Proteins were induced by 0.02% arabinose in the cells as in panel (A) and immunoblotting was performed using an anti-FlhF antibody. (C) A schematic diagram of FlhF. The mutations obtained in this study are indicated. T436 and E440 are located in the putative GTPase motif of FlhF.
Figure 2.
Alignment of FlhF sequences of various bacteria. Multiple sequence alignment was carried out in the Clustal W2 software application on the European Bioinformatics Institute website (http://www.ebi.ac.uk/Tools/clustalw2/). The mutations obtained in this study are indicated by arrowheads with the residue number, and the GTP-binding motifs are indicated by gray boxes.
Cells were examined under a high-intensity dark-field microscope to evaluate the flagella, and we confirmed that the LPN1 cells expressing the flhF mutant protein had a nonflagellate phenotype (data not shown). The LPN1 cells expressing the K437A mutant protein showed slight but significant motility on a soft agar plate and had a single flagellum at the polar region of the cell [17]. To study the dominance of the mutation, a plasmid carrying mutant flhF was introduced into wild-type strain VIO5. All the flhF mutants showed the same motility as did the wild-type FlhF strain. These mutant proteins do not have dominant effects as is the case for the K437A and D439A mutants, which do not exert dominant effects [17].
Localization of FlhF
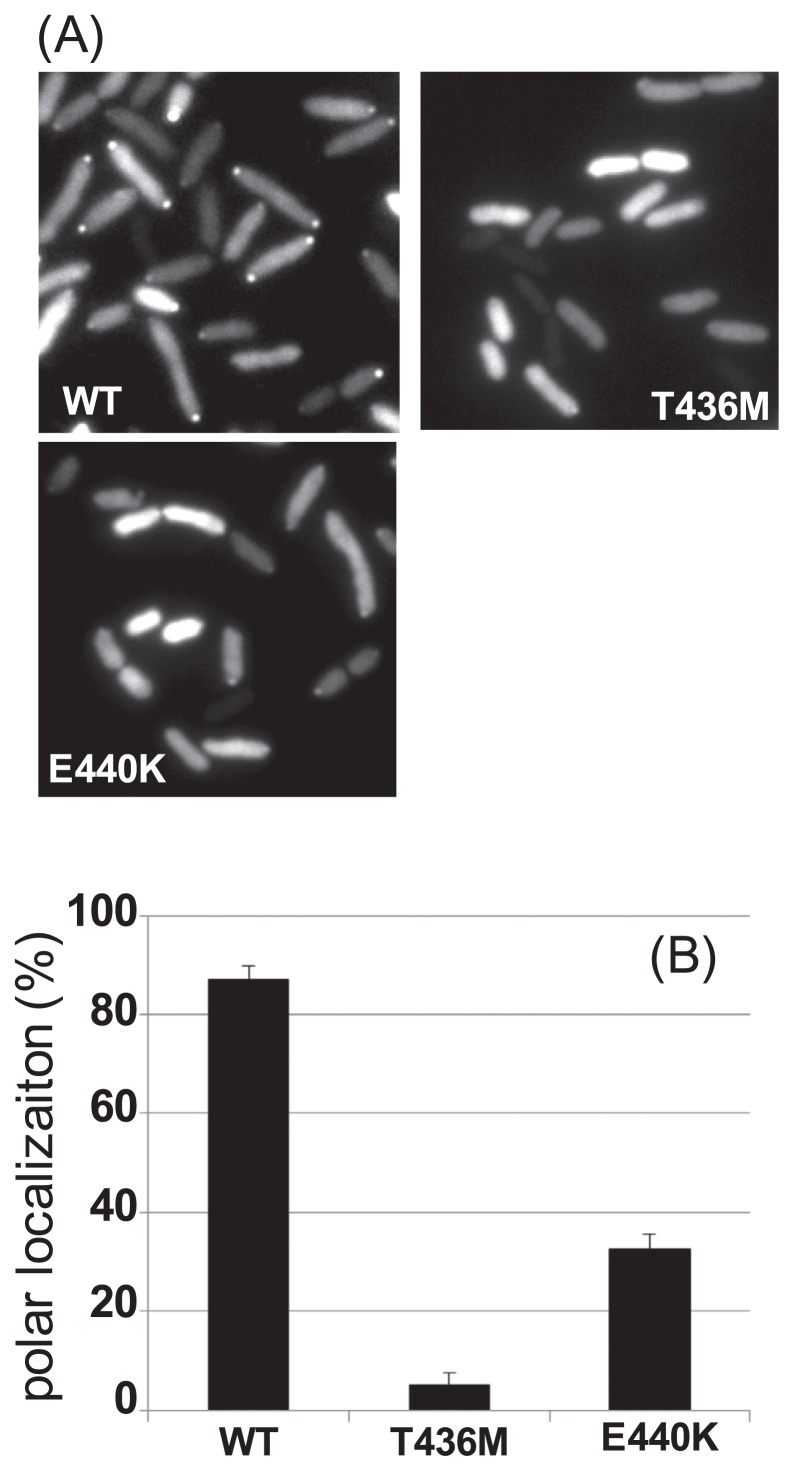
In V. alginolyticus, it has been shown that wild-type FlhFGFP, which is functional, diffuses into the cytoplasm and is located at the flagellate pole [6]. We assessed the subcellular localization of the FlhF mutants created in the present study. FlhF mutants T436M, E440K, Δ391–505(Q391amber), and Δ419–505(Q419ochre) fused to GFP at the C terminus were expressed in the ΔflhF mutant (LPN1) cells and were examined under a fluorescence microscope (Figs. 2 and 3A). The wild-type FlhF-GFP protein diffused into the cytoplasm, and the fluorescent dots at the cell pole were observed in many cells (ca. 85%; Fig. 3B). In the case of the T436M mutant, the dots were rarely observed, and only ca. 5% of the cells had the dots at the cell poles though the fluorescent signals from the mutant protein were detected in the cytosol. In the E440K mutant, the polar dots were observed in ca. 33% of the all cells (Fig. 3B). Mutant proteins Δ391–505 and Δ419–505 were hardly located at the pole, as expected, with the localization ratios ca. 3.5% and ca. 2.5%, respectively (Supplementary Fig. S1A). For mutant proteins K437A and D439A, which are alanine substitution mutants of the GTPase motif, the localization rates were lower relative to the wild type, as observed before, at ca. 50% and ca. 10% of cells, respectively [17]. The expression levels of mutant FlhF proteins were confirmed by immunoblotting of whole-cell lysates, and the amount of FlhF was almost the same for all the mutations (Supplementary Fig. S1B). From these and previous results, we concluded that the GTPase motif is important for the polar localization of FlhF.
Figure 3.
Location of FlhF-GFP in the cell. (A) Cells of strain LPN1 (ΔflhF mutant) harboring a plasmid—pAK325 (WT) or the mutant derivatives (T436M and E440K)—induced by 0.02% arabinose were examined under a fluorescence microscope. (B) The polar localization of FlhF-GFP in cells was evaluated as described in Materials and Methods. Independent experiments were conducted three times to count the cells in which fluorescent dots were observed at the poles. Bars in the columns show the standard deviation.
Fractionation of FlhF mutants
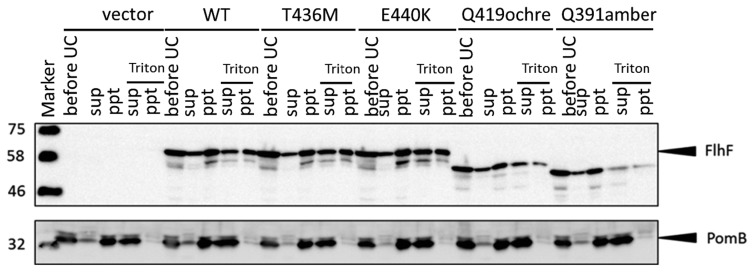
Mutant proteins T436M, E440K, Q391amber, and Q419ochre showed defects of polar localization compared to wild-type FlhF. Thus, we hypothesized that the mutant FlhF proteins may have different properties, such as affinity for the membrane. We fractionated cells into a cytoplasmic fraction and membrane fraction (Fig. 4). Almost the same amounts of wild-type FlhF were detected in the cytoplasmic fraction and membrane fraction. This finding seems to be consistent with the observation that wild-type FlhF diffuses into the cytoplasm and some of it is located at cell poles by attaching to membrane. The fraction profiles of mutant proteins T436M, E440K, Q391amber, and Q419ochre are similar to the profile of wild-type FlhF.
Figure 4.
Fractionation of the FlhF mutant proteins. Cells of strain LPN1 (ΔflhF mutant) harboring a plasmid—”vector” (pBAD33), “WT” (pAK322), or a mutant plasmid (T436M, E440K, Q419ochre, Q391amber)—induced by 0.02% arabinose were separated into a membrane fraction (ppt) and soluble fraction (sup) by ultracentrifugation. before UC is the sample before ultracentrifugation. To detect the interaction of FlhF and the membrane, the membrane fraction was solubilized with Triton X-100 and then fractionated into the supernatant and the pellet by ultracentrifugation. Bottom columns show the immunoblot of membrane protein PomB, to confirm complete solubilization of membrane.
The putative GTPase activity of mutant FlhF proteins
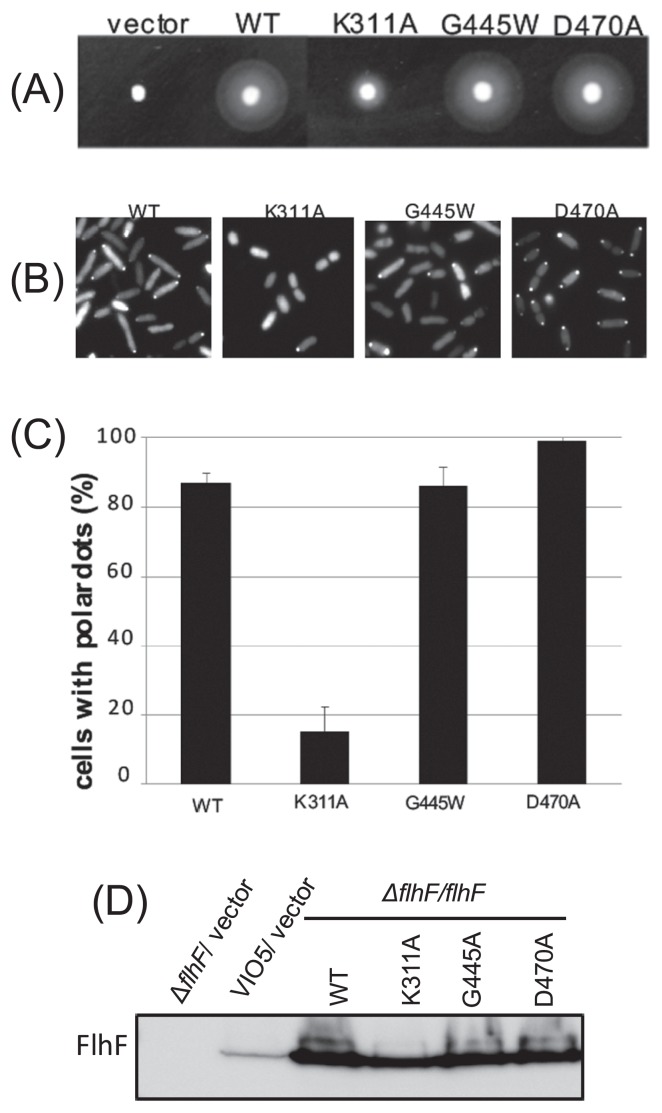
We created mutations K311A, G445W, and D470A, which are not located in the GTPase motif, but the corresponding mutations in E. coli FtsY decrease the GTPase activity (Fig. 5) [20]. The swimming ring of K311A was found to be much smaller than that of the wild type. When the cells were examined by high-intensity dark-field microscopy, few cells had flagella, i.e., most cells were nonflagellate. On the other hand, the swimming ring of mutants G445W and D470A was similar to that of the wild type, as was the case for flagellar formation (Fig. 5A).
Figure 5.
(A) The FlhF mutants with a putative defect of the GTPase activity. Cells of strain LPN1 (ΔflhF mutant) harboring a plasmid—” vector” (pBAD33), “WT” (pAK322), or the mutant plasmid (K311A, G445A, D470A)—were inoculated into a soft agar plate containing 0.02% arabinose and incubated for 6 hours at 30°C. (B) Subcellular localization of FlhF-GFP. Proteins were induced by means of 0.02% arabinose in the cells as in Figure 2A. (C) The rate of polar dot formation was determined by calculating the ratio of the number of cells that have a fluorescent dot at the cell pole, to the total number of cells seen in the image, as in (B). Bars in the columns show the standard deviation. (D) Immunoblotting was performed using the anti-FlhF antibody.
Next, we examined intracellular location of the GFP fusion protein as described above. The K311A mutation reduced the polar dots, and the dots were observed only in 15% of the cells. In contrast, polar localization of mutant proteins G445W and D470A was observed in ca. 86% and ca. 99% of the cells, respectively. The polar dots seemed to be more intense in the D470A mutant cells than in the wild type (Fig. 5B and 5C). Protein amounts of these mutant FlhF versions in the cells were comparable to that of the wild type (Fig. 5D).
Finally, cells expressing these mutants were fractionated into the cytoplasmic fraction and membrane fraction. Mutant proteins K311A, G445W, and D470A had a distribution similar to that of the wild type (Supplementary Fig. S2). This result suggests that these mutations do not affect either the protein’s intracellular distribution or structure.
Discussion
In this study, a random mutation was introduced into full-length flhF using hydroxylamine, and we screened the mutants to find those defective in motility, to identify the domain related to polar localization of FlhF. We expected that mislocalization of FlhF would cause abnormal flagellar positioning and/or formation so that motility of the mutants in a soft agar plate would be reduced as compared to the wild-type strain. Among the 8534 colonies obtained by introducing the plasmid treated with the mutagen, only 7 kinds of mutants had abnormal motility. Hydroxylamine causes only the nucleotide change from cytosine to thymine (guanine to adenine in the complementary strand), and 5 out of 7 variants obtained in this study show mutation of a glutamine residue into a stop codon. It seems that the same kind of mutations tend to occur during hydroxylamine treatment. Therefore, if random mutagenesis is performed using other mutagens such as EMS (ethylmethane sulfonate), distinct mutations other than the C to T (G to A) change may be obtained.
Mutant proteins Q110amber, Q160ochre, Q286amber, Q391amber, and Q419ochre lost the C-terminal region via a nonsense mutation. The Q419ochre mutant protein with the shortest truncation still caused a loss of flagellation. Because FlhF consists of 505 amino acid residues, at least we can say that 90 residues of the C terminus are necessary for formation of the flagellum. In V. alginolyticus, the GTP-binding motif of FlhF is thought to be important for the formation of the flagellum [17], and all the missense mutations, T436M and E440K, obtained in this study were mapped to motif IV. These mutant proteins cannot reside at the cell poles. We extensively mutated flhF, but FlhF mutations that caused the loss of polar localization were still mapped only to the GTPase motif. Thus, our current results support the idea that polar localization of FlhF is necessary for flagella formation and that the GTP-binding motif is important for polar localization of FlhF [17].
In B. subtilis, P. aeruginosa, and C. jejuni, the GTPase activity of FlhF has been detected [15,16,21]. Although the GTPase activity of V. alginolyticus FlhF has not been shown, all the GTPase motifs, which include motifs I, II, III, and IV and play a key part in the hydrolysis of GTP [22], are conserved. When mutations are introduced into FtsY residues corresponding to V. alginolyticus FlhF T306, G299, and K305, these mutants cannot hydrolyze GTP and cannot form a complex with Ffh [20,23]. In B. subtilis FlhF, FtsY, and Ffh, the residue corresponding to D377 was shown to bind to Mg2+ via a water molecule and to be necessary to maintain the GTPase activity and for formation of the complex with Ffh [20]. Regarding G380, the corresponding residue of a GTPase called p21ras (which is involved mainly in cell proliferation, differentiation, apoptosis signaling) forms a hydrogen bond with the γ-phosphate group of GTP [22]. V. alginolyticus FlhF mutations K437 and D439 are located in motif IV, and this motif determines the specificity for nucleotides. In general, the aspartic acid residue corresponding to D439 is predicted to determine the nucleotide specificity of GTPase by forming two hydrogen bonds with the guanine base of a nucleotide [11]. When a mutation was introduced into a residue corresponding to K437 or D439 in FtsY, the ability to bind to GTP and to hydrolyze GTP was impaired, and FtsY could not form a complex with Ffh [20]. We previously reported that the D439A mutant protein, which had been predicted to form a hydrogen bond with the guanine base of a nucleotide, was not located at the pole [17]. This mutant probably cannot bind GTP and cannot form a homodimer. K318 and E321 of B. subtilis FlhF, which correspond to V. alginolyticus FlhF K437 and E440, are inferred to interact with T184 of the partner FlhF for formation of a homodimer. Based on the evidence, we hypothesize that the dimer is stabilized by the two interactions and either mutation K437A or E440K disrupts such an interaction, thus the K437A and E440K mutant proteins show reduced polar localization.
Our current extensive mutagenesis analysis revealed point mutations only at T436 and E440 in motif IV, and as expected, C-terminal truncation mutants that lack motif IV (Q391amber and Q419ochre) yield a phenotype similar to that of T436 or E440. Moreover, because these mutations in motif IV yielded the protein fractionation profile similar to that of wild-type FlhF (Fig. 4 and Supplementary Fig. S2), we assumed that GTP binding and/or recognition is involved in polar localization of FlhF, and thereby in its function. The mutants of G445W and D470A, whose corresponding mutants in E. coli FtsY decrease the GTPase activity [20], have similar function of the wild type FlhF. This suggests that the GTPase activity may not be essential for the function. In Vibrio, we recently proposed a model for flagellar assembly that is initiated at the position where a putative initiation factor (denoted as X) is located, and this initiation factor X is recruited to the cell pole by FlhF/FlhG and HubP [24]. HubP, which has been identified as a polar landmark membrane protein and has an N-terminal periplasmic peptidoglycan-binding motif with a large cytoplasmic domain, seems to interact with FlhG to regulate the flagellar number at the cell pole [25]. We speculate that FlhF activates factor X to generate flagella. We are now trying to identify the factor X which should interact with FlhF.
Conclusion
By the random mutagenesis of the flhF gene on a plasmid using hydroxylamine, the motility defective cells were isolated. The mutations of stop codon were introduced in a various positions and only two mutations were isolated in the C-terminal GTPase motif IV. Taking into accounting the previous mutational analysis of GTPase motifs and the results of newly introduced mutations in the GTPase motifs, it was strongly supported that the GTPase motifs of FlhF plays a critical role in the polar localization of this protein during formation of the flagellum.
Supplementary Information
Acknowledgments
This research was supported by Grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan (JP26115705 and JP16H04774 to SK).
Abbreviations
- EMS
ethylmethane sulfonate
- SRP
signal recognition particle
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
Sh. K., and Se. K. designed experiments. Sh. K performed experiments. Sh. K., Se. K., and M. H. analyzed the data. Sh. K., and M. H. wrote the manuscript. Se. K. supervised the study.
References
- 1.Kazmierczak BI, Hendrixson DR. Spatial and numerical regulation of flagellar biosynthesis in polarly flagellated bacteria. Mol Microbiol. 2013;88:655–663. doi: 10.1111/mmi.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa NE, Peng F, Klose KE. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol. 2005;187:6324–6332. doi: 10.1128/JB.187.18.6324-6332.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusumoto A, Kamisaka K, Yakushi T, Terashima H, Shinohara A, Homma M. Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. J Biochem. 2006;139:113–121. doi: 10.1093/jb/mvj010. [DOI] [PubMed] [Google Scholar]
- 4.Balaban M, Hendrixson DR. Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni. PLoS Pathog. 2011;7:e1002420. doi: 10.1371/journal.ppat.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandza S, Baetens M, Park CH, Au T, Keyhan M, Matin A. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol Microbiol. 2000;36:414–423. doi: 10.1046/j.1365-2958.2000.01859.x. [DOI] [PubMed] [Google Scholar]
- 6.Kusumoto A, Shinohara A, Terashima H, Kojima S, Yakushi T, Homma M. Collaboration of FlhF and FlhG to regulate polar-flagella number and localization in Vibrio alginolyticus. Microbiology. 2008;154:1390–1399. doi: 10.1099/mic.0.2007/012641-0. [DOI] [PubMed] [Google Scholar]
- 7.de Boer PA, Crossley RE, Hand AR, Rothfield LI. The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. EMBO J. 1991;10:4371–4380. doi: 10.1002/j.1460-2075.1991.tb05015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono H, Takashima A, Hirata H, Homma M, Kojima S. The MinD homolog FlhG regulates the synthesis of the single polar flagellum of Vibrio alginolyticus. Mol Microbiol. 2015;98:130–141. doi: 10.1111/mmi.13109. [DOI] [PubMed] [Google Scholar]
- 9.Herskovits AA, Bochkareva ES, Bibi E. New prospects in studying the bacterial signal recognition particle pathway. Mol Microbiol. 2000;38:927–939. doi: 10.1046/j.1365-2958.2000.02198.x. [DOI] [PubMed] [Google Scholar]
- 10.Pohlschroder M, Hartmann E, Hand NJ, Dilks K, Haddad A. Diversity and evolution of protein translocation. Annu Rev Microbiol. 2005;59:91–111. doi: 10.1146/annurev.micro.59.030804.121353. [DOI] [PubMed] [Google Scholar]
- 11.Bange G, Petzold G, Wild K, Parlitz RO, Sinning I. The crystal structure of the third signal-recognition particle GTPase FlhF reveals a homodimer with bound GTP. Proc Natl Acad Sci USA. 2007;104:13621–13625. doi: 10.1073/pnas.0702570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross BC, Sinning I, Luirink J, High S. Delivering proteins for export from the cytosol. Nat Rev Mol Cell Biol. 2009;10:255–264. doi: 10.1038/nrm2657. [DOI] [PubMed] [Google Scholar]
- 13.Green JC, Kahramanoglou C, Rahman A, Pender AM, Charbonnel N, Fraser GM. Recruitment of the earliest component of the bacterial flagellum to the old cell division pole by a membrane-associated signal recognition particle family GTP-binding protein. J Mol Biol. 2009;391:679–690. doi: 10.1016/j.jmb.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Shi M, Ju L, Gao H. Investigation into FlhFG reveals distinct features of FlhF in regulating flagellum polarity in Shewanella oneidensis. Mol Microbiol. 2015;98:571–585. doi: 10.1111/mmi.13141. [DOI] [PubMed] [Google Scholar]
- 15.Schniederberend M, Abdurachim K, Murray TS, Kazmierczak BI. The GTPase activity of FlhF is dispensable for flagellar localization, but not motility, in Pseudomonas aeruginosa. J Bacteriol. 2013;195:1051–1060. doi: 10.1128/JB.02013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balaban M, Joslin SN, Hendrixson DR. FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J Bacteriol. 2009;191:6602–6611. doi: 10.1128/JB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusumoto A, Nishioka N, Kojima S, Homma M. Mutational analysis of the GTP-binding motif of FlhF which regulates the number and placement of the polar flagellum in Vibrio alginolyticus. J Biochem. 2009;146:643–650. doi: 10.1093/jb/mvp109. [DOI] [PubMed] [Google Scholar]
- 18.Kawagishi I, Okunishi I, Homma M, Imae Y. Removal of the periplasmic DNase before electroporation enhances efficiency of transformation in a marine bacterium Vibrio alginolyticus. Microbiology. 1994;140:2355–2361. [Google Scholar]
- 19.Terauchi T, Terashima H, Kojima S, Homma M. A conserved residue, PomB-F22, in the transmembrane segment of the flagellar stator complex, has a critical role in conducting ions and generating torque. Microbiology. 2011;157:2422–2432. doi: 10.1099/mic.0.048488-0. [DOI] [PubMed] [Google Scholar]
- 20.Shan SO, Stroud RM, Walter P. Mechanism of association and reciprocal activation of two GTPases. PLoS Biol. 2004;2:e320. doi: 10.1371/journal.pbio.0020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bange G, Kummerer N, Grudnik P, Lindner R, Petzold G, Kressler D, et al. Structural basis for the molecular evolution of SRP-GTPase activation by protein. Nat Struct Mol Biol. 2011;18:1376–1380. doi: 10.1038/nsmb.2141. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldgaard M, Nyborg J, Clark BF. The GTP binding motif: variations on a theme. FASEB J. 1996;10:1347–1368. [PubMed] [Google Scholar]
- 23.Dong HJ, Tao SM, Li YQ, Chan SH, Shen XL, Wang CX, et al. Analysis of the GTPase activity and active sites of the NG domains of FtsY and Ffh from Streptomyces coelicolor. Acta Biochim Biophys Sin. 2006;38:467–476. doi: 10.1111/j.1745-7270.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 24.Inaba S, Nishigaki T, Takekawa N, Kojima S, Homma M. Localization and domain characterization of the SflA regulator of flagellar formation in Vibrio alginolyticus. Genes Cells. 2017;22:619–627. doi: 10.1111/gtc.12501. [DOI] [PubMed] [Google Scholar]
- 25.Takekawa N, Kwon S, Nishioka N, Kojima S, Homma M. HubP, a polar landmark protein, regulates flagellar number by assisting in the proper polar localization of FlhG in Vibrio alginolyticus. J Bacteriol. 2016;198:3091–3098. doi: 10.1128/JB.00462-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okunishi I, Kawagishi I, Homma M. Cloning and characterization of motY, a gene coding for a component of the sodium-driven flagellar motor in Vibrio alginolyticus. J Bacteriol. 1996;178:2409–2415. doi: 10.1128/jb.178.8.2409-2415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.