Abstract
The present study aimed to investigate the effect of pentraxin 3 (PTX3) on the regulation of proliferation and apoptosis in human glomerular mesangial cells (HMCs). Small interfering (si)RNA was designed and synthesized to inhibit the expression of endogenous PTX3, and the effects on the proliferation and apoptosis of HMCs were detected by flow cytometry and an MTT assay. Western blot analysis was used to detect the activation of mitogen-activated protein kinase (MAPK) proteins in HMCs with PTX3 knockdown. Three siRNAs targeting PTX3 were individually transfected into HMCs for 48 h, and reverse-transcription quantitative PCR demonstrated that the relative mRNA expression of PTX3 was significantly decreased in all groups by up to 79.62% of that in the control group (P<0.05). Following transfection with PTX3-siRNA, the viability of an HMC line was significantly decreased in comparison with that of a control group transfected with scrambled siRNA. However, PTX3-siRNA did not significantly effect early and late apoptotic cell populations in HMCs compared with those in the control. Endogenous PTX3 interference was found to significantly decrease p38 MAPK, extracellular signal-regulated kinase 1/2 and c-Jun N-terminal kinase phosphorylation. In conclusion, silencing of PTX3, inhibited the proliferation of HMCs via MAPK pathways, but exerted no effect on the apoptosis of HMCs.
Keywords: pentraxin 3, glomerular mesangial cells, proliferation, apoptosis
Introduction
Mesangial cells are one of the major components of the nephrocyte population, accounting for 30% of renal cells (1–3). Mesangial cells have important roles in maintaining normal glomerular structure and function in the three glomerular cell types. At present, at least two types of mesangial cell have been identified, namely smooth muscle-like cells, which represent the majority intrinsic glomerular cells, and isa-positive phagocytic cells, belonging to the bone marrow-derived cells (4,5). Mesangial cells serve as a source of a variety of cytokines that they secrete and are simultaneously primary target cells interacting with a variety of cytokines. These cells also possess numerous important physiological functions, such as the secretion of extracellular matrix, cytokine production and regulation of glomerular hemodynamics by supporting the function of contraction and expansion. In addition, the phagocytic function of mesangial cells, which are among the most active intrinsic glomerular cells, have an important role in the clearance of macromolecules within the glomerular mesangial are and regulation of immune function (6,7). Normally, mesangial cells are only associated with functions such as contraction, phagocytosis and maintenance of normal metabolism of substrates. However, under pathological conditions, mesangial cells release various cytokines via autocrine or paracrine signaling, which increases the production of extracellular matrix elevates the levels of certain cytokines. In turn, proliferation of mesangial cells may be promoted by cytokines with a high concentration and thus increases the glomerulus damage. Mesangial cells are a major cell type secreting extracellular matrix, but also have an important role in glomerular sclerosis (8,9).
A previous study by our group found that the expression of pentraxin 3 (PTX3) protein was significantly increased in plasma of hemodialysis patients (10). A previous study also indicated that the expression of PTX3 was significantly different in the glomerular structure, and the phenomenon was epitomized in the membrane structures (11). Inflammatory cytokines and other stimuli significantly promote the secretion of PTX3 protein by mesangial cells (12). The number of mesangial cells was significantly increased following administration of exogenous high concentrations of PTX3 protein, and it was also suggested that dialysis may stimulate mesangial cells to secrete PTX3 protein, which in turn further promotes the proliferation of mesangial cells, inducing the development of atherosclerotic damage through dialysis (13).
To further demonstrate the fact that PTX3 may act on human mesangial cells (HMCs) and is involved in the pathophysiology of atherosclerosis, the present study applied lentiviral technology for silencing PTX3 to further explore the association between proliferation, apoptosis and PTX3. In view of the role of PTX3 in high-cholesterol kidney disease having been indicated by previous studies (14), the present study was implemented to investigate the specific mechanisms of PTX3 involved in early high glucose-induced nephropathy via molecular biology methods, and to provide novel information regarding the role of PTX3 in the blood dialysis field.
Materials and methods
Cell culture
The SGC7901 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were grown at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Beyotime Institute of Biotechnology, Inc., Haimen, China) and 2 mmol/l glutamine (Biofluids, Inc. Rockville, MD, USA).
Design and synthesis of small interfering (si)RNA
The complete sequence of PTX3 was obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/), with the gene ID NG_023046.1 (GI: 299473774). The online tool WI siRNA (http://sirna.wi.mit.edu/home.php) was used to design the siRNA sequences and the Basic Local Alignment and Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was applied to confirm the uniqueness of the siRNAs by comparing the selected sequences with relevant sequences in the Genome Database. Finally, three siRNA sequences were selected (Table I).
Table I.
Sequences of small interfering RNAs.
| Start (loci) | GC% | Sequence |
|---|---|---|
| 145 | 47 | Sense, 5′-ATTTGCCCTGGTTATATCA-3′ |
| Anti-sense, 5′-AUAUGUGUGUAUCCGACUGTT-3′ | ||
| 234 | 45 | Sense, 5′-TGGTCTGTGCCCTTCAAGA-3′ |
| Anti-sense, 5′-UUGAAGGUGAGGAUAACGCTT-3′ | ||
| 345 | 45 | Sense, 5′-TGGAAGGAATGAAGGAATG-3′ |
| Anti-sense, 5′-GAGAGUGGGUUUCCAGUAUTT-3′ | ||
| Negative control | 46 | Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Anti-sense, 5′-ACGUGACACGUUCGGAGAATT-3′ |
Plasmid construction and cell transfection
A two-step method of siRNA expression cassette (15) was used to amplify the siRNA under the following conditions: 94°C for 30 sec and 72°C for 90 sec, respectively (16). T4 DNA ligase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was then utilized to combine the PCR products with pRNATU6.1 (GenScript, Piscataway, NJ, USA) for 24 h at 16°C. Escherichia coli DH5α cells (Invitrogen; Thermo Fisher Scientific, Inc.) were transduced with the ligation product. Glycerin bacteria (Invitrogen; Thermo Fisher Scientific, Inc.) were subjected to blue-white selection and endonuclease restriction was performed for gene sequencing (17). The plasmids with the correct sequence of pRNATU6.1-PTX3 were prepared in quantity. DNA in these plasmids was extracted and purified. The siRNA-PTX3 plasmids were transfected into SGC7901 cells with Lipofectamine reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
Cells were harvested and extracted with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total of 2 µg total RNA was reverse-transcribed in a volume of 20 µl for complementary DNA synthesis using TaqMan Reverse Transcription (Applied Biosystems; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. The products were amplified by PCR. Real-time qPCR was performed using Power SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in the Bio-Rad PTC-200 Detection System (Bio-Rad Laboratories, Inc., Hercules, CA). Primers used for PCR were as follows: PTX3 forward, 5′-AAGAACGTGATGGTCCCTGA-3′ and reverse, 5′-AATATACGTGATGGGCTTGG-3′; β-actin forward, 5′-CATTAAGGAGAAGCTGTGCT-3′ and reverse, 5′-GTTGAAGGTAGTTTCGTGGA-3′. The reaction system consisted of 2 µl sense primer, 2 µl anti-sense primer, 12.5 µl SYBR-Green PCR Master mix (2X concentration; Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl template cDNA (25 ng/µl) with double-distilled water to a final volume 25 µl. The PCR protocol was as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec, and a final step at 4°C for 5 min. 2−ΔΔCq method was used to quantify the mRNA levels (18).
Cell proliferation assay
The transfected cells were seeded in a 24-well plate at 4×104 cells per well. After 24, 48 or 72 h of incubation, MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution (5 mg/ml) was added to each well. Following incubation for 4 h at 37°C, the MTT reaction medium was removed and formazan-blue was solubilized with 100 ml of dimethylsulfoxide. The absorbance of the wells was measured using an ELISA plate reader (Bio-Rad Laboratories, Inc.) at 570 nm.
Flow cytometric analysis
Cells were harvested and washed twice with ice-cold PBS and suspended in 500 µl Annexin V binding buffer (Roche Diagnostics, Basel, Switzerland) at a concentration of 5×106 cells/ml. Subsequently, 5 µl Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI; Roche Diagnostics) were added to the cell suspension, followed by incubation at room temperature for 15 min in the dark prior to analysis with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Results were analyzed by using CellQuest software version 2.10 (BD Biosciences).
Western blot analysis
Cells were harvested and protein was extracted using a Total Protein Extraction kit (Sigma-Aldrich; Merck KGaA). After centrifugation at 9,000 × g at 4°C for 10 min, the protein content of the supernatant was quantified using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. A total of 25 µg protein per lane was separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Inc.) using an electro-blotting apparatus (Bio-Rad Laboratories, Inc.). The membranes were then blocked with 5% (w/v) skimmed milk in Tris-buffered saline containing Tween-20 (TBST) for 2 h at 37°C, followed by incubation overnight at 4°C with the following primary antibodies: Anti-p38 (cat. no. 9212), anti-phospho-p38 (cat. no. 9211), anti-c-Jun N-terminal kinase (JNK; cat. no. 9252), anti-phospho-JNK (cat. no. 9251), anti-extracellular signal-regulated kinase (ERK; cat. no. 9102), anti-phospho-ERK (cat. no. 9101; all 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA). Subsequently, the membranes were washed with TBST and incubated with goat anti-rabbit IgG (cat. no. 8885; 1:1,000; Cell Signaling Technology, Inc.) at room temperature for 2 h. The protein bands were visualized by enhanced chemiluminescence system (ECLTM PRN 2106; GE Healthcare Life Sciences, Little Chalfont, UK) according to the manufacturer's instructions. The images were captured using a Luminescence/Fluorescence Imaging System (GE Healthcare Life Sciences). Quantitative analysis of western blots was performed using ImageJ 1.41 software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experiments were performed three times. Data were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) and values are expressed as the mean ± standard deviation. Differences among groups were analyzed using one-way analysis of variance followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Silencing of PTX3 in HMCs
To investigate the role of PTX3 in mesangial cells, changes in the cellular activity of various sub-clone cell lines stably transfected with vectors expressing siRNA-1, −2 or −3 was assessed. Stable transfection efficiency was confirmed via assessing the mRNA levels of PTX3 in HMCs transfected with siPTX3-1, siPTX3-2 or siPTX3-3 by RT-qPCR. The mRNA levels in the cells in the three PTX3 groups were found to be significantly reduced in comparison with those in the control group (P<0.05; Fig. 1). Of these, siRNA1 had the highest knockdown efficiency and inhibited the expression of PTX3 mRNA by 79.62%.
Figure 1.
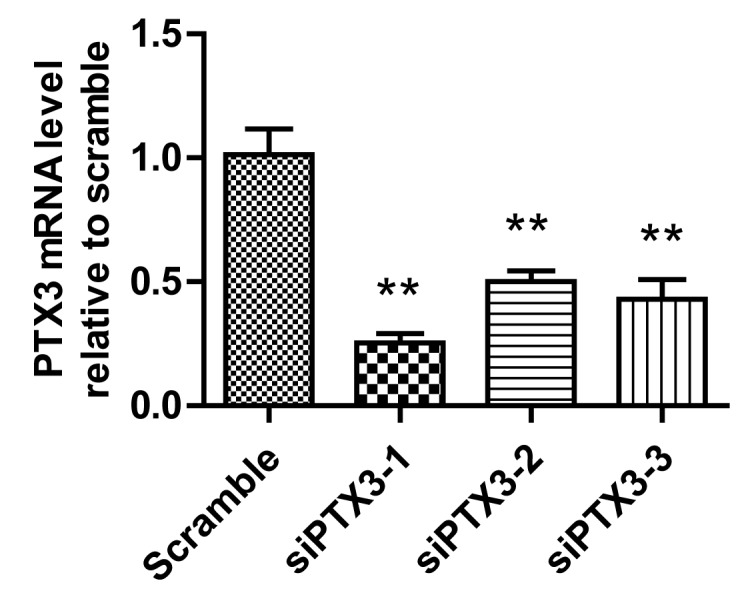
siPTX3 significantly reduced the expression of PTX3 mRNA in human glomerular mesangial cells. **P<0.01 vs. Scramble. PTX3, pentraxin 3; siPTX3, small interfering RNA targeting PTX3.
Effect of siRNA targeting PTX3 on the proliferation of HMCs
To study the effect of PTX3 silencing on the proliferation of HMCs, growth curves of cells in the siPTX3 and control groups were determined by using an MTT assay. After the expression of PTX3 protein was inhibited, the cell proliferation was significantly reduced within 3 or 4 days compared with that in the scrambled siRNA group (Fig. 2).
Figure 2.
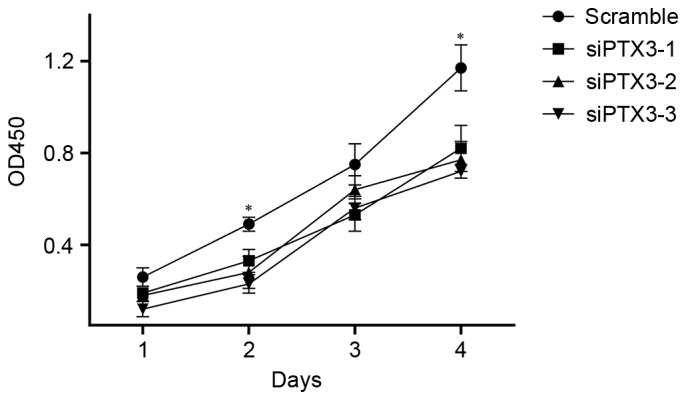
Human glomerular mesangial cell proliferation in Scramble, siPTX3-1, siPTX3-2 and siPTX3-3 groups. OD450, optical density at 450 nm; siPTX3, small interfering RNA targeting pentraxin 3. *P<0.05 vs. siPTX3-1, −2 and −3.
Effect of siRNA targeting PTX3 on the apoptosis of HMCs
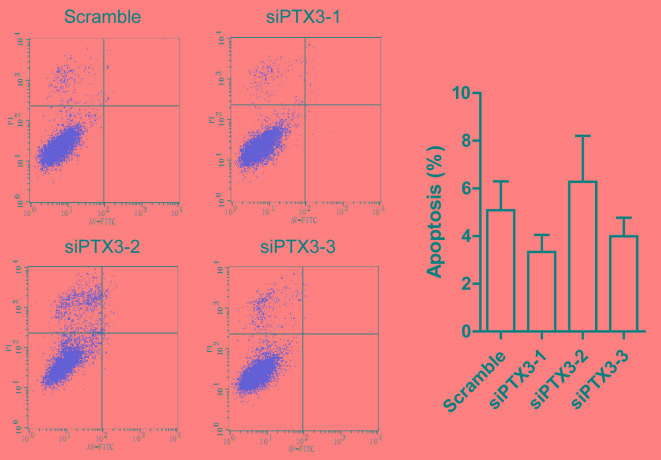
Apoptosis is a type of cell death associated with cell shrinkage, chromatin and nuclear condensation as well as membrane blebbing. In order to examine whether knockdown of PTX3 is associated with apoptosis of HMCs, the apoptotic rate was assessed by flow cytometry following Annexin V-FITC/PI double staining. As presented in Fig. 3, no statistically significant differences in cell apoptosis between the siPTX3 groups and the scrambled group were observed, and no significant differences in Annexin V-FITC−/PI− (upper left quadrant) cell percentage was observed.
Figure 3.
siPTX3 had no significant effect on the apoptosis of human glomerular mesangial cells. siPTX3, small interfering RNA targeting pentraxin 3; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Effects of siPTX3 on the activation of the mitogen-activated protein kinase (MAPK) pathway
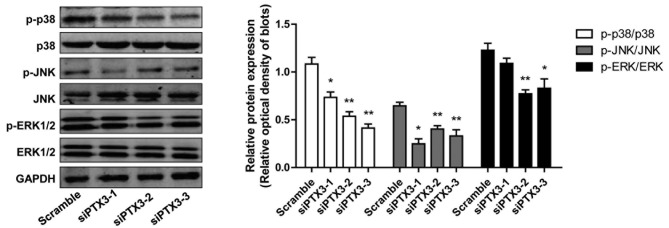
To investigate the possible signaling pathways associated with PTX3 knockdown, the phosphorylation of JNK, p38 MAPK and ERK1/2 in HMCs was assessed by western blot analysis. As displayed in Fig. 4, the expression levels of total protein of p38 MAPK, JNK and ERK1/2 were not changed by siPTX3, while the phosphorylation of p38 MAPK, JNK and ERK was significantly decreased (P<0.05). These results suggested that downregulation of p38 MAPK, ERK and JNK phosphorylation was associated with the knockdown of PTX3.
Figure 4.
Protein levels of p38 MAPK, JNK and ERK1/2 as well as their phosphorylated forms in human glomerular mesangial cells. Phosphorylation of p38 MAPK, JNK and ERK1/2 were significantly reduced after knockdown of PTX3. *P<0.05, **P<0.01 vs. Scramble. MAPK, mitogen-activated protein kinase; p-ERK, phosphorylated extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; PTX3, pentraxin 3; siPTX3, small interfering RNA targeting PTX3.
Discussion
Various molecular biological studies have demonstrated that the effect of extracellular and intracellular factors on cell proliferation and growth occurs at the cell cycle level (12–15). Abnormalities of the cell cycle of kidney cells was reported to not only induce the kidney disease in hemodialysis patients but to also regulate disease progression (6).
Renal cell hypertrophy as well as abnormal proliferation and apoptosis are important processes in the development of hemodialysis-associated nephropathy (17–19). Studies have indicated that hemodialysis-associated nephropathy occurred due to early high-glucose dialysis and a variety of factors affecting the cell cycle. Under high glucose, several factors stimulating kidney cell proliferation and hypertrophy lead to early pathological changes, including irreversible changes in the organizational structure, such as glomerular sclerosis and interstitial fibrosis, finally causing hemodialysis-associated nephropathy (20). Under normal conditions, the proliferation rate of renal cells is rather low at no more than 1%. The remaining cells are quiescent and rest in G0 phase, the inactive stage of the cell cycle. Upon stimulation by growth factors or cytokines, these cells are activated and resume to G1 phase; during this period, the cell volume increases, protein is augmented and mRNA synthesis is performed to prepare for DNA replication. After a short pause, if the conditions are suitable for cells, they enter the S phase by passing the G1/S-phase checkpoint to undergo cell proliferation. However, if the expression of checkpoint inhibitors increases during the G1 phase of the cell cycle, the cells fail to pass through the checkpoint and proliferation is paused, while cells remain to have an increased volume and are hypertrophic. A series of complex signaling processes induces the expression of genes, which regulate division and determine cell fate (21).
The present study demonstrated that PTX3 has no significant effect on apoptosis in HMCs; however, it did induce proliferation. Further investigation using western blotting revealed that the expression of p-p38, p-JNK and p-ERK was decreased to different extents.
MAPKs are serine/threonine protein kinases that are ubiquitous in mammalian cells and are involved in cell growth, development, division, death and functional synchronization as well as physiological reactions. Furthermore, MAPKs mediate cell proliferation and differentiation, and represent an intersection of common cell signaling pathways (22). Since the discovery of ERK in mammalian cells in 1991, studies on MAPK signaling pathways have led to rapid developments (23). To date, at least four MAPK family members have been identified: ERK, JNK/stress-activated protein kinase (SAPK), p38 MAPK and ERK5/big mitogen-activated protein kinase 1 (BMK1) (24,25).
The mechanism of activation is similar for the different MAPKs family members, as they are all activated by phosphorylation via three enzymatic linking reactions. ERK is a major member of the MAPK family and comprises five subtypes: ERKl/2, −3/4 and −5. ERK1 and −2 constitute a highly homologous subclass and may be activated by extracellular stimuli, including factors such as epithelial growth factor. These factors utilize G protein receptors, growth factor receptors, tyrosine kinase receptors and the Raf/MAPK kinase/ERK signaling cascade to active ERKl/2 and regulate transcription factors as well as the activity of a number of kinases. The ERK pathway regulates cell growth, development, proliferation and differentiation, and may further be involved in malignant transformation of cells and other physiological and pathological processes. ERK has the ability of promoting protein synthesis and cell proliferation (26). JNK mainly includes three subclasses (JNKl-3). The JNK/SAPK pathway may be activated by numerous sources of stress, pro-inflammatory cytokines and components of the inflammatory response, including macrophage colony stimulating factor, platelet-derived growth factor and transforming growth factor β, high blood sugar, lipopolysaccharide, osmotic stress and heat shock. Dual-specificity kinases MAPK kinase 7 and 4 act on the tyrosine and threonine sites of JNK to specifically activate the JNK pathway, the downstream c-Jun, activating transcription factor 2 (ATF-2) and c-fos, are able to cause the cell growth change, produce prostate substances and cell dysfunction (27). p38 MAPK includes a total of six subclasses (p38α, p38β, p38λ, p38δ), which may be activated by various stimuli, including hormones, inflammatory cytokines, endotoxin, G protein-coupled receptor ligands, cell tension (cell stretch) and ultraviolet light. After activation of p38 MAPK kinase and translocation into cell nuclei, phosphorylated ATF-2 participates in the phosphorylation and activation of heat shock protein via its targets MAPK activated protein kinase (MAPKAPK)-2, MAPKAPK3 and MAPKAPK25 (28). ERK5/BMKl is an important regulator of the cellular response for growth factors and may be activated by environmental stress, but not by any vasoactive substances or inflammatory factors (29,30).
Serum inflammatory factors act as pathogenic inducers in kidney dialysis patients and may be activated by a variety of factors, such as oxidative stress and polyol release. In addition, accumulation of advanced glycation end products in glomerular mesangial cells of hemodialysis patients results in the activation of MAPKs, which then phosphorylate transcription factors regulating gene expression and, to a certain extent, cause and accelerate the occurrence of hemodialysis-associated kidney necrosis (31). PTX3 protein is involved in energy metabolism and proliferation of intestinal and lung cells via the MAPK pathway (32). In line with this, the present study also found that silencing of PTX3 in HMCs decreased the activity of phosphorylated p38 MAPK, ERK and JNK. However, in line with the results of previous clinical studies, PTX3 knockdown had no significant effect on the apoptosis of mesangial cells (3,4).
In conclusion, the results of the present study suggest that PTX3 may affect MAPK pathways to regulate renal mesangial cell proliferation and participate in the pathophysiological development of early hemodialysis-associated nephropathy.
Acknowledgements
The present study was supported by the fund from Wu Jieping's Medical Foundation, Beijing, China (grant no. 320.6750.14201).
References
- 1.Suzuki D, Toyoda M, Kimura M, Miyauchi M, Yamamoto N, Sato H, Tanaka E, Kuriyama Y, Miyatake H, Abe M, et al. Effects of liraglutide, a human glucagon-like peptide-1 analogue, on body weight, body fat area and body fat-related markers in patients with type 2 diabetes mellitus. Intern Med. 2013;52:1029–1034. doi: 10.2169/internalmedicine.52.8961. [DOI] [PubMed] [Google Scholar]
- 2.Zaza G, Granata S, Rascio F, Pontrelli P, Dell'Oglio MP, Cox SN, Pertosa G, Grandaliano G, Lupo A. A specific immune transcriptomic profile discriminates chronic kidney disease patients in predialysis from hemodialyzed patients. BMC Med Genomics. 2013;6:17. doi: 10.1186/1755-8794-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witasp A, Rydén M, Carrero JJ, Qureshi AR, Nordfors L, Näslund E, Hammarqvist F, Arefin S, Kublickiene K, Stenvinkel P. Elevated circulating levels and tissue expression of pentraxin 3 in uremia: A reflection of endothelial dysfunction. PLoS One. 2013;8:e63493. doi: 10.1371/journal.pone.0063493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Ni Z, Zhang J, Zhang W, Wu Q, Shen G, Wang Y, Qian J. Plasma pentraxin 3 may be a better marker of peripheral artery disease in hemodialysis patients than C-reactive protein. Vasc Med. 2013;18:85–91. doi: 10.1177/1358863X13483864. [DOI] [PubMed] [Google Scholar]
- 5.Lech M, Römmele C, Gröbmayr R, Eka Susanti H, Kulkarni OP, Wang S, Gröne HJ, Uhl B, Reichel C, Krombach F, et al. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013;83:647–661. doi: 10.1038/ki.2012.463. [DOI] [PubMed] [Google Scholar]
- 6.Lech M, Rommele C, Anders HJ. Pentraxins in nephrology: C-reactive protein, serum amyloid P and pentraxin-3. Nephrol Dial Transplant. 2013;28:803–811. doi: 10.1093/ndt/gfs448. [DOI] [PubMed] [Google Scholar]
- 7.Sjöberg B, Qureshi AR, Anderstam B, Alvestrand A, Bárány P. Pentraxin 3, a sensitive early marker of hemodialysis-induced inflammation. Blood Purif. 2012;34:290–297. doi: 10.1159/000342630. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Matzuk MM, Zhou XJ, Lu CY. Endothelial pentraxin 3 contributes to murine ischemic acute kidney injury. Kidney Int. 2012;82:1195–1207. doi: 10.1038/ki.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp A, Strobel S, Tortajada A, Rodríguez de Córdoba S, Sánchez-Corral P, Prohászka Z, López-Trascasa M, Józsi M. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor h and factor h-related protein 1 to pentraxin 3. J Immunol. 2012;189:1858–1867. doi: 10.4049/jimmunol.1200357. [DOI] [PubMed] [Google Scholar]
- 10.Suliman ME, Qureshi AR, Carrero JJ, Bárány P, Yilmaz MI, Snaedal-Jonsdottir S, Alvestrand A, Heimbürger O, Lindholm B, Stenvinkel P. The long pentraxin PTX-3 in prevalent hemodialysis patients: Associations with comorbidities and mortality. QJM. 2008;101:397–405. doi: 10.1093/qjmed/hcn019. [DOI] [PubMed] [Google Scholar]
- 11.Vitale A, Tosoni A, Zawada L, Genderini F, Caruso S, Vago G, Barbiano di Belgiojoso G, Nebuloni M. Role and distribution of PENTRAXIN 3 (PTX3) in glomerular lesions of HIV positive patients. Nazionale SIAPEC-IAP. 2011 [Google Scholar]
- 12.Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol. 2003;170:1466–1472. doi: 10.4049/jimmunol.170.3.1466. [DOI] [PubMed] [Google Scholar]
- 13.Gilardini L, Zulian A, Girola A, Redaelli G, Conti A, Invitti C. Predictors of the early impairment of renal disease in human obesity. Int J Obes (Lond) 2010;34:287–294. doi: 10.1038/ijo.2009.227. [DOI] [PubMed] [Google Scholar]
- 14.da Silva PM. Are all statins the same? Focus on the efficacy and tolerability of pitavastatin. Am J Cardiovasc Drugs. 2011;11:93–107. doi: 10.2165/11591190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Castanotto D, Li H, Rossi JJ. Functional siRNA expression from transfected PCR products. RNA. 2002;8:1454–1460. doi: 10.1017/S1355838202021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu SQ, Lv YE, Lin BH, Luo LM, Lv SL, Bi AH, Jia YS. Silencing of periostin inhibits nicotine-mediated tumor cell growth and epithelial-mesenchymal transition in lung cancer cells. Mol Med Rep. 2013;7:875–880. doi: 10.3892/mmr.2013.1267. [DOI] [PubMed] [Google Scholar]
- 17.Müller A, Bialek R, Kämper A, Fätkenheuer G, Salzberger B, Franzen C. Detection of microsporidia in travelers with diarrhea. J Clin Microbiol. 2001;39:1630–1632. doi: 10.1128/JCM.39.4.1630-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Imai N, Nishi S, Yoshita K, Ito Y, Osawa Y, Takahashi K, Nakagawa Y, Saito K, Takahashi K, Narita I. Pentraxin-3 expression in acute renal allograft rejection. Clin Transplant. 2012;26(Suppl 24):S25–S31. doi: 10.1111/j.1399-0012.2012.01641.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Nascimento MM, Hayashi SY, Qureshi AR, Waniewski J, Brodin LÅ, Anderstam B, Lind B, Riella MC, Seeberger A, Lindholm B. Changes in circulating biomarkers during a single hemodialysis session. Hemodial Int. 2013;17:59–66. doi: 10.1111/j.1542-4758.2012.00720.x. [DOI] [PubMed] [Google Scholar]
- 21.Argani H, Ghorbanihaghjo A, Panahi G, Rashtchizadeh N, Safa J, Meimand SM. Serum Fetuin-A and Pentraxin3 in hemodialysis and renal transplant patients. Clin Biochem. 2012;45:775–779. doi: 10.1016/j.clinbiochem.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, Node K. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto T, Rashid Qureshi A, Heimbürger O, Bárány P, Carrero K, Sjöberg B, Lindholm B, Stenvinkel P, Carrero JJ. Inverse relationship between the inflammatory marker pentraxin-3, fat body mass, and abdominal obesity in end-stage renal disease. Clin J Am Soc Nephrol. 2011;6:2785–2791. doi: 10.2215/CJN.02320311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavin-Gómez BA, Palomar-Fontanet R, Gago-Fraile M, Quintanar-Lartundo JA, Gómez-Palomo E, González-Lamuño D, García-Unzueta MT, Arias-Rodríguez MA, Gómez-Gerique JA. Inflammation markers, chronic kidney disease, and renal replacement therapy. Adv Perit Dial. 2011;27:33–37. [PubMed] [Google Scholar]
- 25.Xu Y, Ding X, Zou J, Liu Z, Jiang S, Xu S, Shen B, Chen Y, Shan Y, Cao X. Plasma pentraxin 3 is associated with cardiovascular disease in hemodialysis patients. Ren Fail. 2011;33:998–1004. doi: 10.3109/0886022X.2011.618969. [DOI] [PubMed] [Google Scholar]
- 26.Outinen TK, Mäkelä S, Huhtala H, Hurme M, Meri S, Pörsti I, Sane J, Vaheri A, Syrjänen J, Mustonen J. High pentraxin-3 plasma levels associate with thrombocytopenia in acute Puumala hantavirus-induced nephropathia epidemica. Eur J Clin Microbiol Infect Dis. 2012;31:957–963. doi: 10.1007/s10096-011-1392-x. [DOI] [PubMed] [Google Scholar]
- 27.Oldani S, Finazzi S, Bottazzi B, Garlanda C, Baldassarre E, Valaperta S, Cuccovillo I, Albini M, Child M, Montanelli A, et al. Plasma pentraxin-3 as a marker of bioincompatibility in hemodialysis patients. J Nephrol. 2012;25:120–126. doi: 10.5301/JN.2011.8432. [DOI] [PubMed] [Google Scholar]
- 28.Pradeep AR, Kathariya R, Arjun Raju P, Sushma Rani R, Sharma A, Raghavendra NM. Risk factors for chronic kidney diseases may include periodontal diseases, as estimated by the correlations of plasma pentraxin-3 levels: A case-control study. Int Urol Nephrol. 2012;44:829–839. doi: 10.1007/s11255-011-9997-7. [DOI] [PubMed] [Google Scholar]
- 29.Lech M, Römmele C, Kulkarni OP, Susanti HE, Migliorini A, Garlanda C, Mantovani A, Anders HJ. Lack of the long pentraxin PTX3 promotes autoimmune lung disease but not glomerulonephritis in murine systemic lupus erythematosus. PLoS One. 2011;6:e20118. doi: 10.1371/journal.pone.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meuwese CL, Carrero JJ, Stenvinkel P. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol. 2011;171:120–126. doi: 10.1159/000327228. [DOI] [PubMed] [Google Scholar]
- 31.Nishi K, Imamura T, Kitamura K, Ogawa T, Fujimoto S, Kakitsubata Y, Ishikawa T, Asada Y, Kodama T. Associations of plasma pentraxin 3 and monocyte chemoattractant protein-1 concentrations with cardiovascular disease in patients with chronic kidney disease. Ren Fail. 2011;33:398–404. doi: 10.3109/0886022X.2011.568136. [DOI] [PubMed] [Google Scholar]
- 32.Zanetti M, Barazzoni R, Gortan Cappellari G, Burekovic I, Bosutti A, Stocca A, Bianco F, Ianche M, Panzetta G, Guarnieri G. Hemodialysis induces p66(shc) gene expression in nondiabetic humans: Correlations with oxidative stress and systemic inflammation. J Ren Nutr. 2011;21:401–409. doi: 10.1053/j.jrn.2010.12.006. [DOI] [PubMed] [Google Scholar]