Abstract
Systemic inflammatory responses (SIRs) can help predict survival in various cancers. The present study investigated the accuracy of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and prognostic nutritional index (PNI) in predicting survival for patients with recurrent cervical cancer. A retrospective review of prognoses examined the associations among NLR, PLR, and PNI, and clinical characteristics and survival in 79 patients with recurrent cervical cancer after undergoing concurrent chemoradiation therapy (CCRT) or radical hysterectomies with or without CCRT. The Mann-Whitney U-test was used for statistical analyses. In addition, 12-month, 24-month and overall survival were analyzed by the Kaplan-Meier method. Cox's proportional hazard regression was used for univariate and multivariate analyses. Median survival was 15.0 months over follow-up periods of 2–93 months. At the last follow-up point, 54 had succumbed to disease and 25 were alive with disease. In univariate analysis, NLR, PLR and PNI were significantly associated with 12-month, 24 month and overall survival (12 months: P=0.021, P=0.001 and P<0.001; 24 months: P=0.020, P=0.008 and P<0.001; overall; P=0.032, P=0.032 and P<0.001, respectively). In multivariate analyses, PNI was an independent prognostic factor for 12-month, 24-month and overall survival (P=0.001, P=0.001 and P<0.001, respectively). PNI is a useful predictor of survival of recurrent cervical cancer.
Keywords: recurrent cervical cancer, prognostic nutritional index, poor prognosis
Introduction
Recurrence rates for cervical cancer are 11–22% in FIGO stage IB-IIA and 28–64% in FIGO stage IIB-IVA (1). Treatment for patients who suffer recurrent cervical cancer is designed to offer the patient and her family medical, emotional, and spiritual care near the end of life. Predicting these patients' life expectancy is thus important for clinicians and patients. Realistic survival estimates help clinicians decide on appropriate medical interventions, discharge planning and timing of referral to palliative care services.
Systemic inflammatory responses (SIRs), such as relative differences in neutrophil, platelet, lymphocyte counts, and albumin, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and prognostic nutritional index (PNI), have been shown to promote various cancer characteristics (2,3) and to affect survival (4–7). PLR has been shown to predict prognosis in patients with recurrent cervical cancer after undergoing CCRT (8). Although some data on survival outcomes for patients whose cervical cancers recur after undergoing CCRT has been published (8), patients treated only with surgery or surgery plus adjuvant treatment have not been sufficiently investigated. In this study, we investigated the correlation between inflammatory markers (NLR, PLR and PNI) of patients with recurrent cervical cancers.
Patients and methods
Patients
The study population consisted of 79 patients whose cervical cancer recurred after undergoing concurrent chemoradiation therapy (CCRT), or radical hysterectomy with or without CCRT in the Department of Obstetrics and Gynecology of our hospital between April 2004 and December 2015. A total of 79 women underwent positron emission tomography/computed tomography (PET/CT) or CT within the 2 weeks prior to any oncologic treatment. The images were evaluated by 2 diagnostic radiology physicians informed about the clinical data of the patient at the time of the scan.
The study protocol was approved by our hospital's institutional review board [Systemic inflammatory responses were examined prognostic predictor of survival in patients with recurrent cervical cancer (Number: 1605-514)]. Informed consent was obtained from all patients.
Laboratory analysis
Differential white blood cell (WBC) counts and albumin levels were measured within 1 week before their treatments; WBC, neutrophil, lymphocyte, and platelet counts were measured in automated blood cell counters (Bayer HealthCare, Diagnostics Division, Tarrytown, NY, USA). Levels of serum albumin were measured by latex nephelometry (LT Auto Wako, Osaka, Japan). NLR was defined as the absolute neutrophil count (µl) divided by the absolute lymphocyte count (µl); PLR was defined as the absolute platelet count (µl) divided by the lymphocyte count (µl). PNI was calculated as described previously (9); briefly, PNI=[10× albumin (g/dl)]+[0.005× total lymphocyte count (µl)].
Treatment
Patients with recurrent cervical cancer had several possible treatments. In general, radiotherapy is the main option for patients with pelvic recurrence, or with solitary localized recurrence outside the radiation field, after previous radiotherapy. Chemotherapy is the main option for patients with recurrence within the radiation field, or metastases to multiple organs, following previous radiotherapy. Conventional TC was also administered as second-line chemotherapy to patients that developed evidence of clinical or radiographic relapse within the 6 months subsequent to completing adjuvant and/or neo-adjuvant chemotherapy. Chemotherapy for the treatment of recurrent disease was continued until complete response (CR) or progressive disease (PD) was identified. Patients with PD received regimens of chemotherapy that were different from the adjuvant and second-line combinations. We used second-line chemotherapy, as weekly TC-paclitaxel; 80 mg/m2 and carboplatin (area under the plasma-concentration curve [AUC]: 2). Third-line chemotherapy consisted of single-agent irinotecan (CPT-11; 70 mg/m2 weekly for 3 weeks followed by 1 week off); fourth-line chemotherapy was single-agent gemcitabine (GEM; 700 mg/m2 weekly for 3 weeks followed by 1 week off). Surgery was considered for solitary distant metastases or local recurrences. Palliative treatment was considered in some cases after comprehensive assessment of the effectiveness of radiotherapy and/or chemotherapy, patients' performance status, and degree of cancer spread. We evaluated 13 patients treated with surgery, 14 with radiation, 48 with chemotherapy, and 4 with palliative care. Patients had follow-up examinations approximately every 1–2 months.
Statistical analysis
Statistical analyses used the Mann-Whitney U-test for comparisons with controls (10). Receiver operating characteristic (ROC) curves were generated for pre-treatment NLR, PLR, and PNI to determine cut-off values that predicted 12-month, 24-months and overall survival that yielded optimal sensitivity and specificity; patients were then grouped by these cut-off values. 12-months, 24-months and overall survival of the groups were analyzed using the Kaplan-Meier method (11). We performed univariate and multivariate analyses using Cox's proportional hazards model to determine which factors predicted 12-months, 24-months and OS after adjusting for effects of known prognostic factors (12,13). Analyses were performed using SPSS Software, version 20.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered significant.
Results
Patients' ages, histology, treatment-free interval, number of metastases, distant metastasis and treatment are listed in Table I. Their median SIR values were NLR: 3.52 (range: 1.28–14.30); PLR: 271.52 (range: 114.30–885.99); and PNI: 45.30 (range: 26.26–56.55; Table II). NLR (P=0.003) and PLR (P<0.001) were significantly associated with treatment type. PNI was significantly associated with number of metastasis (P=0.022), hematogenous metastasis (P=0.027) and treatment type (P<0.001; Mann-Whitney U-test).
Table I.
Patient and tumor characteristics.
| Baseline characteristics Age at diagnosis, years | Numbers | All patients Mean, 52.4; range, 25–78 (%) |
|---|---|---|
| Histology | ||
| SCC | 50 | 63.3 |
| AD | 18 | 22.7 |
| ADSQ | 7 | 8.9 |
| Others | 4 | 5.1 |
| Treatment free intervial | ||
| ≤6 months | 33 | 41.8 |
| 7-12 months | 24 | 30.4 |
| 13-24 months | 11 | 13.9 |
| >25 months | 11 | 13.9 |
| Number of metastasis | ||
| Simple | 44 | 55.7 |
| Multiple | 35 | 44.3 |
| Distant metastasis | ||
| Hematogenous metastasis | 29 | 36.7 |
| Lymphogenous metastasis | 34 | 43 |
| Treatment | ||
| Operation | 13 | 16.4 |
| Radiation | 14 | 17.7 |
| Chemotherapy | 48 | 60.8 |
| Palliative care | 4 | 5.1 |
SCC, squamous cell carcinoma; AD, adnocarcinoma; ADSQ, adenosquamous carcinoma.
Table II.
Associations of NLR, PLR, and PNI with clinical factors in recurrent cervical cancer.
| Variable | Numbers | NLR | P-value | PLR | P-value | PNI | P-value |
|---|---|---|---|---|---|---|---|
| Histology | 0.564 | 0.408 | 0.076 | ||||
| SCC | 50 | 3.86±2.76 | 283.05±124.66 | 44.85±6.29 | |||
| Non-SCC | 29 | 3.45±3.46 | 247.45±209.18 | 47.27±4.7.53 | |||
| Treatment free interval | 0.541 | 0.422 | 0.124 | ||||
| ≤6 months | 34 | 3.88±3.29 | 293.28±154.56 | 44.98±6.95 | |||
| >7 months | 45 | 3.46±2.79 | 263.97±164.01 | 47.35±6.53 | |||
| Number of metastasis | 0.472 | 0.706 | 0.022a | ||||
| Simple | 44 | 3.46±1.98 | 267.39±130.4 | 47.13±6.34 | |||
| Multiple | 35 | 3.97±3.77 | 281.45±186.92 | 43.61±7.11 | |||
| Hematogenous metastasis | 0.564 | 0.915 | 0.027a | ||||
| Absent | 50 | 3.49±2.97 | 273.37±147.79 | 46.98±6.70 | |||
| Present | 29 | 3.90±3.14 | 277.37±181.31 | 43.43±6.93 | |||
| Lymphogenous metastasis | 0.282 | 0.634 | 0.34 | ||||
| Absent | 45 | 3.90±2.87 | 287.451±169.91 | 46.76±6.86 | |||
| Present | 34 | 3.15±3.28 | 270.01±147.18 | 45.28±6.69 | |||
| Treatment | 0.003a | <0.001a | <0.001a | ||||
| Operation or radiation | 27 | 2.53±1.90 | 196.34±114.55 | 48.61±4.98 | |||
| Chemotherapy or palliative cares | 52 | 4.28±3.20 | 319.25±167.62 | 42.45±6.98 |
P<0.05. NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PNI, prognostic nutritional index.
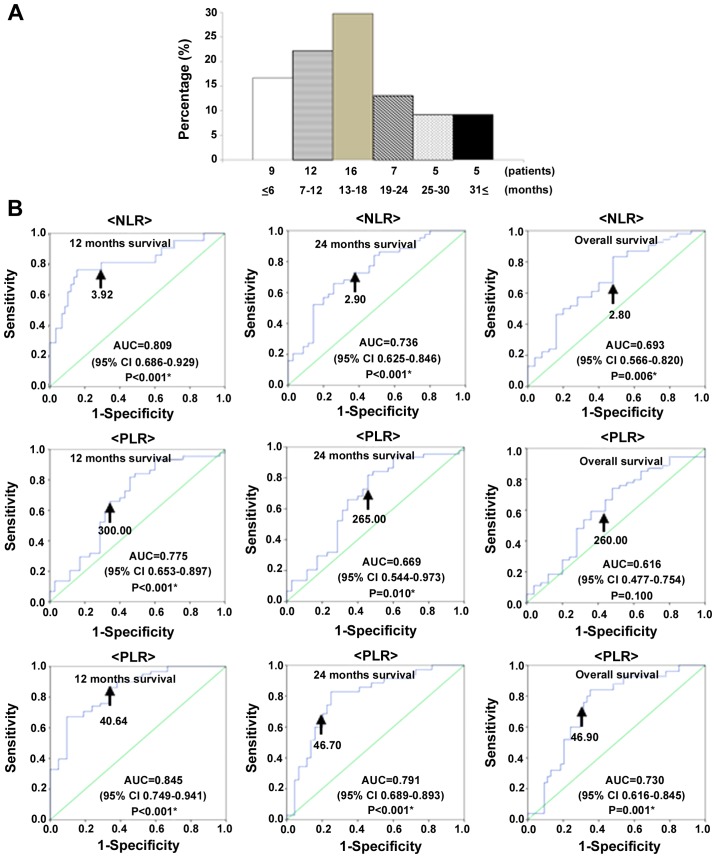
Patients with recurrent cervical cancer had median OS of 15.0 months over follow-up periods of 2–93 months. At the last follow-up point, 54 had died of their disease, and 25 were alive with disease. The time between diagnosis and mortality was ≤6 months in 9 patients (16.7%), 7–12 months in 12 patients (22.2%), 13–18 months in 16 patients (29.7%), 19–24 months in 7 patients (13.0%), 19–24 months in 5 patients (9.2%), and ≥31 months in 5 patients (9.2%; Fig. 1A).
Figure 1.
(A) Time between diagnosis and mortality in patients with recurrent cervical cancer. (B) Receiver operating characteristic curve analyses to determine optimal cut-off values for NLR, PLR, and PNI to predict 12-month and overall survival with recurrent cervical cancers in 79 patients. Optimal cutoff values to predict 12-month survival were NLR: 3.92 (AUC=0.809; 95% CI: 0.636–0.929; P<0.001); PLR: 300.00 (AUC:=0.775; 95% CI: 0.653–0.891; P<0.001); and PNI: 40.64 (AUC=0.845; 95% CI: 0.749–0.941; P<0.001). Optimal cutoff values to predict 24-month survival were NLR: 2.90 (AUC=0.736; 95% CI: 0.625–0.846; P<0.001); PLR: 265.00 (AUC=0.669; 95% CI: 0.554–0.793; P=0.010); and PNI: 46.70 (AUC=0.791; 95% CI: 0.689–0.893; P<0.001). Optimal cutoff values to predict overall survival were NLR: 2.80 (AUC=0.693; 95% CI: 0.566–0.820; P=0.006); PLR: 260.00 (AUC=0.616; 95% CI: 0.477–0.754; P=0.100); and PNI: 46.90 (AUC=0.730; 95% CI: 0.616–0.845; P=0.001).
We used receiver operating characteristic (ROC) curve analyses to determine optimal cut-off values of NLR, PLR, and PNI to predict 12-month, 24-month and overall survival. The analyses identified NLR ≥3.92 (AUC: 0.809, 81.0% sensitive, 56.1% specific), PLR ≥300.00 (AUC: 0.775, 76.2% sensitive, 70.7% specific), and PNI ≤40.64 (AUC: 0.845, 84.5% sensitive, 66.7% specific), as the most accurate cut-off values for predicting 12-month survival; NLR ≥2.90 (AUC: 0.736, 72.7% sensitive, 57.1% specific), PLR ≥265.00 (AUC: 0.669, 65.9% sensitive, 60.0% specific), and PNI ≤46.70 (AUC: 0.791, 80.0% sensitive, 75.0% specific), as the most accurate cutoff values for predicting 24-month survival; and NLR ≥2.80 (AUC: 0.693, 70.4% sensitive, 52.0% specific), PLR ≥260.00 (AUC: 0.616, 61.1% sensitive, 56.0% specific), and PNI ≤46.90 (AUC: 0.730, 72.0% sensitive, 68.5% specific), as the most accurate cutoff values for predicting overall survival (Fig. 1B).
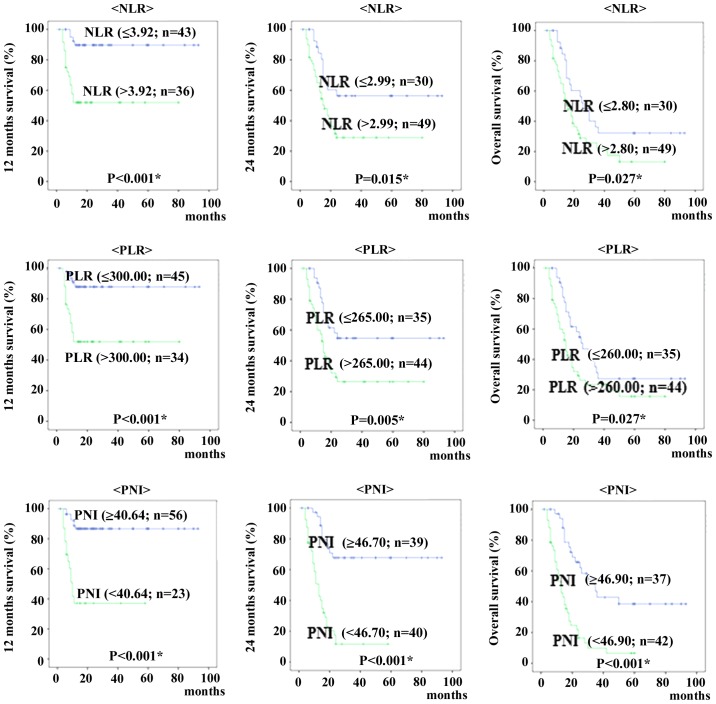
When patients were classified into those above and below each cut-off value for 12 months, 24 months and overall survival, Kaplan-Meyer curves of survival show patients with high NLR and PLR were significantly shorter than rates of patients with low NLR and PLR (12 months survival; P<0.001 and P<0.001, 24 months survival; P=0.015 and P=0.005, overall survival; P=0.027 and P=0.027, respectively). The Kaplan-Meier curves showed that patients with low PNI were shorter than for patients with high PNI (12 months survival; P<0.001, 24 months survival; P<0.001, overall survival; P<0.001, respectively) (Fig. 2).
Figure 2.
Kaplan-Meier plots for 12 months, 24 months and overall survival in 79 patients with recurrence cervical cancer, according to neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and prognostic nutritional index (PNI).
Correlations between clinical factors and 12-month, 24-month and overall survival were assessed in univariate and multivariate analyses (Table III). Treatment-free interval (P=0.036), treatment type (P=0.015), NLR (P=0.001), PLR (P=0.001) and PNI (P<0.001) were significantly associated with 12-month survival in univariate analyses; treatment-free interval (P=0.012) and PNI (P=0.001) were independent predictors of 12-month survival in multivariate analyses.
Table III.
Prognostic factors for 12, 24 months survival and overall survival with recurrent cervical cancer selected by Cox's univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| 12 months survival | ||||||
| Histology | 0.808 | 0.326–2.002 | 0.645 | |||
| Treatment free interval | 2.568 | 1.064–6.201 | 0.036a | 3.322 | 1.300–8.489 | 0.012a |
| Multiple metastasis | 1.852 | 0.780–4.397 | 0.162 | |||
| Hematogenous metastasis | 1.295 | 0.545–3.074 | 0.558 | |||
| Lymphogenous metastasis | 1.355 | 0.575–3.191 | 0.487 | |||
| Treatment (Cx or PC) | 12.115 | 1.625–90.298 | 0.015a | 5.588 | 0.680–45.943 | 0.109 |
| NLR (>3.92) | 6.422 | 2.156–19.125 | 0.001a | 2.332 | 0.457–11.894 | 0.308 |
| PLR (>300.00) | 5.19 | 1.897–14.199 | 0.001a | 1.475 | 0.348–6.252 | 0.598 |
| PNI (<40.64) | 7.205 | 2.887–17.987 | <0.001a | 4.983 | 1.889–13.146 | 0.001a |
| 24 months survival | ||||||
| Histology | 0.595 | 0.310–1.140 | 0.118 | |||
| Treatment free interval | 2.748 | 1.500–5.035 | 0.001a | 2.44 | 1.316–4.526 | 0.005a |
| Multiple metastasis | 2.3 | 1.258–4.206 | 0.007a | 1.099 | 0.565–2.139 | 0.781 |
| Hematogenous metastasis | 1.302 | 0.717–2.366 | 0.385 | |||
| Lymphogenous metastasis | 1.301 | 0.718–2.357 | 0.386 | |||
| Treatment (Cx or PC) | 6.772 | 2.649–17.314 | <0.001a | 4.17 | 1.463–11.885 | 0.008a |
| NLR (>2.90) | 2.252 | 1.136–4.464 | 0.020a | 1.002 | 0.342–2.931 | 0.997 |
| PLR (>265.00) | 2.378 | 1.258–4.495 | 0.008a | 1.093 | 0.408–2.927 | 0.859 |
| PNI (<46.70) | 5.495 | 2.749–10.984 | <0.001a | 3.767 | 1.750–8.109 | 0.001a |
| Overall survival | ||||||
| Histology | 0.713 | 0.406–1.252 | 0.239 | |||
| Treatment free interval | 2.446 | 1.421–4.209 | 0.001a | 2.244 | 1.285–3.918 | 0.004a |
| Multiple metastasis | 2.564 | 1.478–4.447 | 0.001a | 1.07 | 0.578–1.980 | 0.829 |
| Hematogenous metastasis | 1.245 | 0.726–2.136 | 0.426 | |||
| Lymphogenous metastasis | 1.302 | 0.760–2.231 | 0.336 | |||
| Treatment (Cx or PC) | 7.196 | 3.293–15.727 | <0.001a | 5.709 | 2.297–14.190 | <0.001a |
| NLR (>2.80) | 1.89 | 1.056–3.380 | 0.032a | 1.051 | 0.435–2.539 | 0.912 |
| PLR (>260.00) | 1.821 | 1.051–3.155 | 0.032a | 0.817 | 0.353–1.891 | 0.637 |
| PNI (<46.90) | 3.621 | 2.026–6.473 | <0.001a | 3.229 | 1.689–6.173 | <0.001a |
P<0.05. HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PNI, prognostic nutritional index; Cx, chemotherapy; PC, palliative cares.
Treatment-free interval (P=0.001), multiple metastases (P=0.007), treatment type (P<0.001), NLR (P=0.020), PLR (P=0.008) and PNI (P<0.001) were significantly associated with 24-month survival in univariate analyses; treatment-free interval (P=0.005), treatment type (P=0.008) and PNI (P=0.001) were independent predictors of 24-month survival in multivariate analyses.
Treatment-free interval (P=0.001), multiple metastases (P=0.001), treatment type (P<0.001), NLR (P=0.032), PLR (P=0.032) and PNI (P<0.001) were significantly associated with overall survival in univariate analyses; treatment-free interval (P=0.004), treatment type (P<0.001) and PNI (P<0.001) were independent predictors of overall survival in multivariate analyses.
Discussion
Among patients with recurrent cancer, prognosis is based on different criteria for those with advanced disease than for those with earlier-stage disease, in whom prognosis depends mainly on the primary site and histology. However, the prognostic values of SIRs are still unknown for recurrence of cervical cancer. This is the first study to evaluate whether NLR, PLR and PNI are predictors of survival for patients whose cervical cancer has recurred after undergoing treatment.
SIRs have been examined as possible predictors of prognosis in various types of cancer. Neutrophils release inflammatory cytokines, leukocyte chemotactic factors and other phagocytic mediators that can damage cellular DNA, inhibit apoptosis and promote angiogenesis (14–17). Platelets can release potent mitogens or adhesive glycoprotein, such as platelet-derived growth factor, transforming growth factor-β, and vascular endothelial growth factor (18–20). Albumin levels decrease with increased levels of pro-inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor, which modulate albumin production (21). Lymphocytes can affect growth and metastasis such as CD3+ T cells and NK cells (22). Recent evidence has shown that relative differences in neutrophil, platelet, albumin and lymphocyte counts, NLR, PLR, and PNI are systemic indicators of prognosis. The PNI is based on albumin and absolute lymphocyte count, which are measured routinely in clinical practice. It was originally derived to assess immunologic and nutritional condition of patients undergoing surgical treatment for disease of the digestive tract. Mizunuma et al reported that NLR was a significant prognostic factor for PFS and OS in patients with cervical cancer who had been treated with CCRT or RT (23). Reportedly, PLR is an important predictor of prognosis in patients whose cervical cancers recur after undergoing CCRT (8). The current investigation of correlations among clinicopathological parameters and NLR, PLR and PNI found NLR and PLR were significant associated with treatment type; and PNI was significantly associated with multiple metastases, hematogenous metastasis and treatment type.
This study mainly evaluated correlations between the SIR parameters, such as NLR, PLR and PNI, and survival (12 months, 24 months and overall survival) of patients whose cervical cancer recurred after undergoing CCRT or radical hysterectomy (with or without CCRT). We found that 12-month, 24-month and overall survival for patients with higher NLR and PLR were significantly shorter than for patients with lower NLR and PLR. We also found that 12-month, 24-month and overall survival for patients with lower PNI were significantly shorter than for patients with higher PNI. We not only found PNI to be an independent prognostic factor for 12-month, 24-month and overall survival in multivariate analysis, but PNI was also superior to NLR or PLR as a predictor of survival in all patient cohorts with recurrent cervical cancer.
Each patient underwent varied treatment combinations, some of which are subjected to alter the bone marrow hematopoietic function, further to affect the results of blood tests, like dosage of radiotherapy, coverage of irradiated field, numbers of chemotherapies, regimens of chemotherapies and different combination. In this study, pretreatment of SIR was also superior to during treatment as a predictor of survival with recurrent cervical cancer. Therefore, the PNI may be useful in reflecting the frailty and nutritional decline in patients with recurrent cervical cancer. Our results suggest that the PNI will provide additional value to the routine assessment of survival of patients with recurrent cervical cancer.
We acknowledge that our study has some limitations. The number of patients was relatively few, and the duration of follow-up was relatively short. Further prospective studies with more patients and longer follow-up periods would provide more definitive data to clarify the significance of our findings.
In conclusion, our results show that PNI can serve as a useful indicator of survival in patients with recurrent cervical cancer.
References
- 1.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43–S103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 2.Acmaz G, Aksoy H, Unal D, Ozyurt S, Cingillioglu B, Aksoy U, Muderris I. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac J Cancer Prev. 2014;15:1689–1692. doi: 10.7314/APJCP.2014.15.4.1689. [DOI] [PubMed] [Google Scholar]
- 3.Babu SN, Chetal G, Kumar S. Macrophage migration inhibitory factor: A potential marker for cancer diagnosis and therapy. Asian Pac J Cancer Prev. 2012;13:1737–1744. doi: 10.7314/APJCP.2012.13.5.1737. [DOI] [PubMed] [Google Scholar]
- 4.Absenger G, Szkandera J, Stotz M, Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H, Samonigg H, Gerger A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013;33:4591–4594. [PubMed] [Google Scholar]
- 5.Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 6.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Nishida T, Haruma T, Haraga J, Omichi C, Ogawa C, Kusumoto T, Seki N, Masuyama H, Hiramatsu Y. Pretreatment platelet-lymphocyte ratio is an independent predictor of cervical cancer recurrence following concurrent chemoradiation therapy. Mol Clin Oncol. 2015;3:1001–1006. doi: 10.3892/mco.2015.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 10.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assn. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 12.Breslow NE. Analysis of survival data under the proportional hazards model. Int Stat Rev. 1975;43:45–57. doi: 10.2307/1402659. [DOI] [Google Scholar]
- 13.Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 14.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: Release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102:1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol. 1997;17:2293–2305. doi: 10.1161/01.ATV.17.10.2293. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: Studies on release and subcellular localization. Blood. 1979;53:604–618. [PubMed] [Google Scholar]
- 21.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X, Li W, Lai J, Okazaki M, Sugimoto S, Yamamoto S, Wang X, Gelman AE, Kreisel D, Krupnick AS. Five-year update on the mouse model of orthotopic lung transplantation: Scientific uses, tricks of the trade, and tips for success. J Thorac Dis. 2012;4:247–258. doi: 10.3978/j.issn.2072-1439.2012.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizunuma M, Yokoyama Y, Futagami M, Aoki M, Takai Y, Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol. 2015;20:989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]