Abstract
The circadian clock is comprised of a master component situated in the hypothalamic suprachiasmatic nucleus and subordinate clock genes in almost every cell of the body. The circadian clock genes and their encoded proteins govern the organism to follow the natural signals of time, and adapt to external changes in the environment. The majority of physiological processes in mammals exhibit variable circadian rhythms, which are generated and coordinated by an oscillation in the expression of the clock genes. A number of studies have reported that alteration in the expression level of clock genes is correlated with several pathological conditions, including cancer. However, little is known about the role of clock genes in homeostasis of the oral epithelium and their disturbances in oral carcinogenesis. The present review summarizes the current state of knowledge of the implications of clock genes in oral cancer. It has been demonstrated that the development of oral squamous cell carcinoma undergoes circadian oscillation in relation to tumor volume and proliferation rate. The circadian clock gene period (PER)1 has been associated with oral cancer pathogenesis and it is suggested that changes in the expression of PER1 may exhibit an important role in the development, invasion, and metastasis of oral squamous cell carcinoma. However, its role remains elusive and there is a need for further research in order to understand the underlying mechanisms of the clock genes in oral cancer pathogenesis.
Keywords: carcinogenesis, clock genes, circadian rhythm, oral cancer, oral squamous cell carcinoma
1. Introduction
Signals from the overhead pacemaker of the circadian clock, the SCN, mediate the oscillation on a cellular level through clock gene expression and feedback (1). A disruption in these signaling pathways may have a crucial influence on the organism affected. Circadian genes may be involved in regulating cancer-related pathways, including cell proliferation, DNA damage response, and apoptosis (2). Cancer-related genes like c-myc and p53 exhibit a circadian rhythm in vivo (3,4). Oncogenic activity such as excessive cell proliferation, loss of DNA damage control and increased tumor development has been detected in mice with a loss of functioning circadian genes (4). The lifestyle in the twenty-first century has changed due to more industrialization of society, which has altered the endogenous circadian rhythm in ~50% of the world's population. This, among other reasons, has led to increased development of cancer throughout the world (5). There are studies showing the effect of dysfunctional circadian machinery in humans, for example mutations, non-standard expression, and translocation of clock genes, which has led to different cancer types including breast, colorectal, gastric, kidney, lung, prostate, pancreatic, and oral cancer (6). The circadian clock and the cell cycle share some common features in molecular pathways and theoretical stages. It has been hypothesized that clock genes have a crucial role in the cell cycle and with this role they are highly involved in tumorigenesis (4). The underlying molecular mechanisms and the role of clock genes in oral carcinogenesis is elusive. The aim of this review is to summarize the current state of knowledge and to provide insight to guide future research on involvement of clock genes in oral cancer.
2. Circadian clock biology
Physiology of the circadian clock
The circadian clock is an endogenous timekeeping system shared by most organisms. Although there are some differences between species, the underlying molecular mechanisms of the circadian clock are very similar (7). The ability to adapt to a continuously changing environment is an essential key to selective advantage for living creatures to survive and thrive. The circadian clock system is one of these adapting abilities, which organisms have acquired in order to synchronize their daily behavior and internal mechanisms with the most profound environmental signal: The circadian light cycle of 24 h. Body temperature, feeding, hormonal levels, and the sleep-wake cycle all varies synchronized with light-dark cycle (8). Another great ability of this system is adjustment to the 24 h cycle showing a crucial plastic capacity (9). There is a hypothesis called ‘escape from UV’, which is based on the S phase of the cell cycle, which is during night-time. It suggests that ancient lifeforms have adapted to the environment by limiting this UV-sensitive phase of the cycle to nighttime in order to avoid DNA-damage (10).
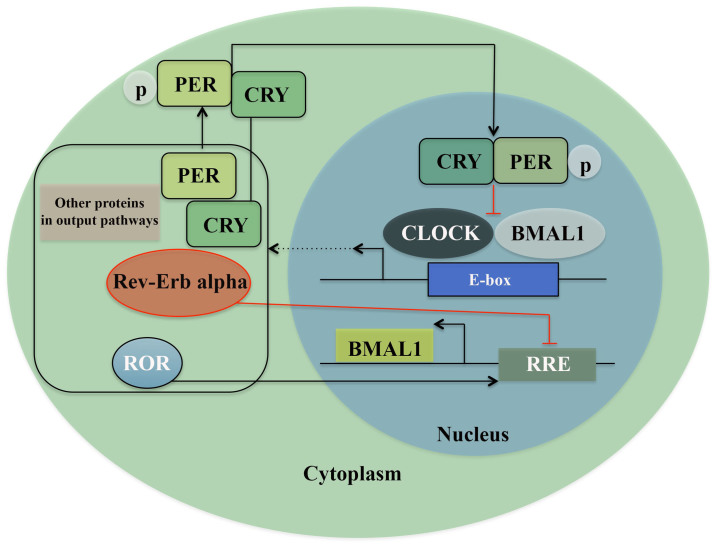
The circadian clock system consists of almost as many individual clocks as there are cells. It is based on different levels and controls the whole rhythmicity of the organism (11). In mammals there is a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus consisting of ~15,000 neurons (12). The input to this central pacemaker comes through different pathways. The primarily input, i.e. light, is registered in the retina by a subset of melanopsin-expressing retinal ganglions and accesses the SCN via the retino-hypothalamic tract (RHT). From the SCN there are output pathways leading to the whole body (11,13,14). These feeding pathways are regulated by interlocked transcription-translation feedback loops (TTFLs) (15), where the clock gene family exerts an important role. In Drosophila and zebrafish, light has a direct influence on the circadian behavior of the peripheral cells (12,16), whereas in mammals the clock genes in peripheral tissues are not light sensitive. Here, they maintain and regulate TTFLs in almost every cell of the body by feeding pathways from the SCN and other molecular processes (11). One of these transcription/translation feedback loops consists of the heterodimeric transcription complex: CLOCK/BMAL1, which in the morning binds to the E-boxes in the promoter region of genes expressing Period proteins (PER1, PER2, and PER3) and Cryptochrome proteins (CRY1 and CRY2). When these proteins accumulate, and reach an acute concentration in cytoplasm, factors like Skp1-Cullin-F-box protein (SCF) E3 ubiquitin ligase complexes, caseinkinase 1ε/δ (CK1ε/δ), and AMP kinase (AMPK) lead to the formation of the PER/CRY complex. These protein complexes translocate into the nucleus and reduce the activity of CLOCK/BMAL1 by direct protein-protein interaction by night, i.e., a negative feedback loop. The robustness of this feedback loop is ensured by a secondary mechanism where two subfamilies of nuclear hormone receptors Rev-erb and Ror, regulate the transcription of Bmal1 and thereby directly regulate the core feedback loop (Fig. 1) (17–19). Further, chromatin remodeling and posttranslational modifications ensure the regulation required for maintenance of the circadian rhythm (8,20,21). A study based on systematic mathematical and computational analysis of the biological rhythms has revealed that oscillations are created by the negative feedback signals, whereas the frequency of these oscillations is adjusted by the positive feedback signals without altering the amplitude of the oscillation (22). Another study has shown that the SCN plays a more significant role in synchronizing the peripheral clocks than regulating their oscillation, which suggests a more cell-independent model of the system (23,24). Accordingly, most circadian genes, except for clock and CKIε, have a rhythmic expression in periods of 24 h. The clock/Bmal1 complex regulates the transcription of many other genes in addition to clock genes. A circadian oscillation is observed in the transcription of >10% of mammalian genes, and naturally the clock gene family exhibits an important role in many physiological functions such as food intake, body temperature, metabolism and synthesis and release of hormones (25–27). Circadian regulators, being directly involved in the circadian machinery, are suggested to control cell cycle. For example, CLOCK/BMAL1 regulates cell cycle gene Wee1 being important in the G2/M phase, 12c-myc in the G0/G1 phase and Cyclin D1, which is important in the G1/S phase (3). Moreover, an interaction is detected between PER1 and checkpoint proteins such as ATM and Chk2 and 17 (28).
Figure 1.
Transcriptional/translational feedback loops (TTFL) model of the molecular clock in mammals. The positive arm of TTFL is constructed by the core clock genes, BMAL1 and CLOCK, which heterodimerize and bind to the E-box element on circadian target genes to activate transcription, including PER 1–3, CRY 1–2, ROR, Rev-Erb α and other genes in output pathways. The complex formed by Phospho-PER and CRY inhibits BMAL1/CLOCK-driven transcription, constructing the core negative feedback loop. ROR increases and Rev-Erb α inhibits the expression of BMAL1 and thus constructs a second feedback loop.
Disruption in the circadian clock and its consequences
It has been suggested that disease caused by circadian rhythm alterations is due to gene dosage changes and failure in controlling gene dosages in TTFLs (29). Alterations and disruption of the circadian clock are a more common problem nowadays due to the industrialization of our society where artificial lighting, working night shifts, and rapid long-distance travelling through several time zones are common features. This is speculated to be directly linked to the increasingly higher risk of acquiring a number of health problems and diseases including cancer (30). Epidemiologic studies of circadian clock alterations have suggested a link between cardiovascular, metabolic, gastrointestinal, and mental disorders as well as numerous cancer forms such as breast, ovarian, lung, pancreatic, prostate, colorectal, and endometrial cancers, non-Hodgkin's lymphoma (NHL), osteosarcoma, acute myeloid leukemia (AML), head and neck squamous cell carcinoma and hepatocellular carcinoma (31–45). The risk of acquiring cancer alters with the frequency and duration of disruption of the endogenous circadian clock. (40,46–49).
The hypothesis of artificial lighting altering the circadian clock and leading to a higher cancer risk is further strengthened by the findings in visually impaired individuals that were not affected by light input and depended on other inputs for regulating their endogenous clock. Studies have shown a lower cancer risk for these individuals than others in the same environment (50–52). In 2007, research and evidence gathered resulted in classifying ‘shiftwork that involves circadian disruption’ as a probable carcinogen by the International Agency for Research in Cancer (44).
The disruption of the circadian clock has not only been shown to increase the risk of disease, but also to affect the prognosis and treatment outcome of patients. Studies have demonstrated that variation in circadian cortisol value in blood and sleeping patterns of the patients with metastatic breast, colorectal, or lung cancer are linked to overall survival of these patients (53–59). Although these findings suggest that the circadian clock undergoes significant changes in human tumorigenesis, the direct links between aberrant circadian clock gene expression and human malignancies, including oral and head and neck carcinomas, remain largely elusive. The present review focuses on the role of clock genes in oral squamous cell cancer.
3. Clock genes in cancer
Effects of clock gene expression level in cancer tissue
The molecular process in how clock genes expression levels prevent or enhance tumorigenesis is not yet fully understood; however, several studies have registered a correlation between different expression levels of each clock gene and different cancer types. NPAS2 has shown a significant association with a lack of metastasis and survival prognosis in breast cancer patients (60–62). Similar results were presented in colorectal cancer, and decreased expression of NPAS2 was strongly correlated to tumor size, TNM stage and metastasis rate (63). In ERα-positive breast cancer tissue the high expression of the clock gene was reduced through a knockout technique and a reduction in proliferation was observed. In contrast, administration of estrogen resulted in increased expression of clock and the proliferation of breast cancer cells (64). Also, in colorectal cancer a higher expression of clock is registered in diseased tissue (65). These findings present a diagnostic value for both genes and the adverse effects of two different clock genes where a high level of NPAS2 expression is correlated to better prognosis whereas a high level of clock expression correlates to increased proliferation of cancerous tissue.
A lower expression level of PER1, PER2, and PER3 has been registered in diseased tissue compared to normal adjacent tissue in breast cancer, prostate cancer, colorectal cancer, pancreatic ductal adenocarcinoma, gastric cancer, kidney cancer and non-small-cell lung cancer (61,66–71). PER1 and PER2 have shown a tumor-suppressing effect in a number of studies. Higher expression of PER2 in breast cancer tissue correlated with a lack of metastasis (61). In a study where the PER2 expression was downregulated, a substantial increase in tumor growth rate and higher proliferation in diseased cells were observed both in vivo and in vitro (72). In gastric cancer tissue a suppressing role of PER1 and PER2 on tumor progression and metastasis was registered and a low expression of PER1 and PER2 was correlated with poorer prognosis (64). Overexpression of PER1 showed a great inhibition of growth and stimulated apoptosis in prostate cancer cell lines (71). A correlation between decreased levels of PER1 and a lower survival rate and liver metastasis in gastric cancer patients has also been detected (65,66).
A link has been discovered between CRY2 and breast cancer progression and prognosis where lower expression levels were registered in diseased tissue (73). Moreover, the CRY1 gene was associated with fatal prostate cancer (74). Results from an animal study with Bmal1 knockout mice showed that circadian behavior in total darkness came to a complete stop (75). Research has registered lower expression of BMAL1 in diseased tissue compared to normal adjacent tissue in patients with colorectal cancer, pancreatic cancer, and pancreatic ductal adenocarcinoma (67,76,77). A knockdown of Bmal1 in pancreatic cancer cell lines led to increased cell proliferation and decreased apoptosis (77). In vivo and in vitro studies of colorectal cancer patients show that a higher level of BMAL1 expression correlates to less tumor cell proliferation and higher survival (76). Collectively, these data open avenues for novel diagnostic and therapeutic models for different cancer forms and prove the need for, and importance of, further research on clock genes.
4. Clock genes in oral cancer
Oral cancer
Worldwide, almost 300,000 people are annually diagnosed with oral cancer, which makes it the 10th most common type of cancer (78). Oral cancer incidence and mortality rates vary widely across the world, and the highest rates are generally registered in a few developing countries, i.e., Sri Lanka, India, Pakistan, and Bangladesh (79). The etiology of oral cancer is multifactorial. The main risk factors are tobacco use and alcohol consumption with combined multiplicative effects possibly leading to DNA damage or mutations. Human papilloma virus infection and genetic polymorphism can also be mentioned as risk factors (80–82). Men are overrepresented in this patient group and high age and lower socioeconomic status may have an impact (83).
Oral squamous cell carcinoma (OSCC), one type of oral cancer, is the eighth most common cancer worldwide (84,85). Over 90% of oral malignancies are squamous cell carcinomas and its variants (45,86). This cancer type usually emerges from the tongue, floor of the mouth, buccal mucosa, gingiva and hard palate. Cancer located in the tongue is associated with poorer prognosis (87,88).
Clock genes in healthy oral mucosa
Clock genes have been detected in healthy oral mucosa and their diurnal oscillations are mapped (89,90). Rhythmical oscillation of the genes and their different peaks has been shown to occur simultaneously with different phases of the cell-cycle. PER1 peaked simultaneously as p53, which is a G1-marker and an important gene in oncogenesis. BMAL1 peaked simultaneously with the M-phase marker cyclinβ1 (90). Studies have confirmed that cyclinβ1 and p53 are targets of human clock genes where loss of BMAL1 reduces the expression of p53 along with PER1, PER2, and PER3. It has also been hypothesized that p53 is involved in regulating PER2 expression by blocking the CLOCK/BMAL1 complex from binding to a promotor region (77,91–93). This further supports the theory that there is a connection between clock gene activity and the cell cycle (2).
Clock genes and oral squamous cell carcinoma
Clock genes have a clear role in cancer development, prognosis, and therapy. From the perspective of biological rhythms, focusing on clock genes may provide novel ideas and methods for a better understanding of the occurrence and development of tumors, and for individualized treatment of cancer. So far, the results suggest that the PER1 gene may be used as a marker to determine clinical staging and the metastatic risk, and as a novel target for the prevention and treatment of oral cancer. (94–96) However, future studies are warranted in order to concentrate on the translational and post-translational levels and to illustrate the molecular function and the regulatory effects in the clock gene network and the tumor-suppression mechanisms of PER1, providing new and effective molecular targets for the treatment of oral cancer.
A few studies have investigated the role of clock genes in OSCCs (Table I). It has been demonstrated that OSCC in vivo development undergoes circadian oscillation in relation to tumor volume and proliferation rate (97). Hsu et al observed similar results in head and neck squamous cell carcinoma (HNSCC). Cancerous and non-cancerous adjacent tissues from 40 patients diagnosed with HNSCC were obtained, and they detected the expression of nine core clock genes, PER1, PER2, PER3, CRY1, CRY2, CK1ε, TIM, CLOCK, and BMAL1. The results also showed a significantly decreased expression of PER1, PER2, PER3, BMAL1, and especially CRY2 in cancerous tissue compared to healthy adjacent tissue. In more advanced stages, they observed lower expression levels of PER3, CRY2, and BMAL1. Downregulation of PER1 and PER3 correlated with poor survival in these patients (45).
Table I.
Clock genes and oral cancer.
| Authors, year | Cell type/origin | Methods | Results/conclusions | (Refs.) |
|---|---|---|---|---|
| Zhao et al, 2016 | In vitro: SCC15 cell line; In vivo: Nude mice | PER1 knockdown in SCC15 cells; In vivo tumerigenisity of SCC cells evaluated in mice | Enhanced proliferation, reduced apoptosis and enhanced the tumorigenic capacity of SCC15 cells in vivo and in vitro; mRNA expression of PER3, TIM, RORα and REV-ERBα was significantly up-regulated; mRNA expression of PER2, CRY1, CRY2 and NPAS2 was significantly down-regulated | (96) |
| Fu et al, 2016 | In vitro: SCC15 cell line; In vivo: Nude mice | Quantitative real-time PCR; In vivo tumerigenisity of SCC cells evaluated in mice | Increased expression of Cyclin D1, Cyclin E, Cyclin b1, CDK1 and WEE1; Decreased expression of P53, Cyclin A2, P16, P21, and CDC25; Fewer cells in S phase and more cells in G2/M phase; Enhanced proliferation and reduced apoptosis; Enhanced tumorigenicity of PER1 downregulated SCC15 cells in vivo | (100) |
| Li et al, 2016 | In vitro: SCC15 cell line; In vivo: Nude mice | PER1 knockdown in SCC15 cells; In vivo tumerigenisity of SCC cells evaluated in mice | Profeleration, migration and invasion increased whereas apoptosis decreased; Up-regulated expression of tumorrelated genes; Enhanced in vivo tumerogenesis | (95) |
| Wang et al, 2016 | OSCC cell line Tca8113 | Per2 downregulation; Quantitative real-time PCR | Increased Cyclin A2, B1 and D1, CDK4, CDK6 and E2F1; Decreased p53, p16 and p21; Increased profileration; Decreased apoptosis | (101) |
| Zhao et al, 2013 | 32 mice injected with human OSCC cell line BcaCD885 | Measured tumor progression after 3 weeks | Circadian rhythm in tumor volume and proliferative index; Not in apoptotic index | (97) |
| Chen et al, 2012 | Human cancerous and healthy adjacent tissue from 41 OSCC patients | PER1 protein and mRNA expression and clinicopathological features | Significantly decreased expression of PER1 in diseased tissue;Gradually decreased expression during cancer development | (94) |
| Hsu et al, 2012 | Human cancerous and noncancerous tissue from 40 HNSCC patients | Quantitative real-time PCR | PER1, PER2, PER3, CRY2, BMAL were significantly downregulated; The expression levels were most changed for CRY2 | (45) |
| Sato et al, 2011 | Cell line from human gingival cancer CA9-22; Tumor and non tumor tissue from 13 patients | Knockdown and overexpression of PER1 and PER3; Quantitative real-time PCR | PER1 knockdown enhanced apoptosis while PER3 knockdown inhibited apoptosis in CA9-22; PER1 overrepresented in cancerous tissue; PER3 overrepresented in non cancerous tissue | (104) |
SCC, squamous cell carcinoma; OSCC, oral squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma.
PER1 expression was detected in 41 OSCC patients where diseased tissue was compared with healthy adjacent mucosa. In addition, the correlation with clinicopathological features was investigated in these patients. The results showed a significantly decreased level of expression in cancerous tissue compared to adjacent healthy tissue; also, the expression level decreased with the tumor progression. Patients with no lymph-node metastasis expressed a higher level of PER1 than those with metastasis (94). It is suggested that the clock gene PER1 possesses a tumor suppressing quality, which may have diagnostic and therapeutic use. PER1 gene knockdown in OSCC cell line SCC15 led to immediate abnormal behavior in terms of cell growth, proliferation, apoptosis resistance, migration and invasion in vitro. Mice injected with these modified cells subcutaneously experienced enhanced tumor development (95,96). Another interesting finding again is the connection between PER1 and p53, where knockdown of PER1 was followed by decreased expression of p53. Moreover, the daily oscillation of PER1 and tumor-related genes such as p53, but also VEGF and c-myc, is correlated with cancer development (98). Suppression of PER1 leads to disturbance in the cell cycle and inhibits DNA damage control, which again makes clock genes and PER1 a key research subject in the field of carcinogenesis (99). The molecular mechanism behind the tumor-suppressing quality of PER1 is the regulation of the Cyclin-CDK-cyclin-dependent kinase inhibitor regulatory network (100). Tumorigenesis is highly due to disorders in the normal cell cycle, and maintaining a functional cell cycle is dependent on the Cyclin-CDK-cyclin-dependent kinase inhibitor regulatory network (2). Studies on the OSCC cell line SCC15 have shown decreased expression of PER1, leading to downstream regulation by increasing the level of CyclinD1, CyclinE, CyclinB1, CDK1, and WEE1 while decreasing the levels of P53, CyclinA2, P16, P21, and CDC25 (100). Knockdown of PER1 led to a downregulation of the PER2, DEC1, DEC2, CRY1, CRY2, and NPAS2 mRNA level, while PER3, TIM, RORα, and REV-ERBα mRNA were upregulated (96). This suggests that PER1 not only regulates the downstream genes, but it also plays a role in the synergy of the rest of the clock genes in the circadian machinery in SCC15 cell lines.
Investigation of the role of PER2 in OSCC cell line Tca8113 cells, showed a lower level of expression than in healthy tissue. The expression of PER2 was down regulated, and cell cycle, cell proliferation and apoptosis was analyzed using flow cytometry and RT-qPCR. The down-regulation of PER2 expression had a great effect on the CDK/CKI cell cycle network and altered the expression levels of many factors including decreasing p53. A significantly higher cell proliferation and lower apoptosis were observed (101). Tan et al investigated the circadian pattern of PER2 and various cell cycle genes in golden hamsters. PER2 and P53 had a decreased level while Cyclin D1, CDK1, and Cyclin B1 levels increased during cancer development (102).
Reports show that PER1 has a pro-apoptotic role in many cancer types, for example in human colon cancer and prostate cancer (71,99). But it has also been reported that PER1 has an anti-apoptotic role in pancreatic and hepatocellular cancer cells (103). A pro-apoptotic role of PER1 is suggested in OSCCs, but in contrast, in the gingival cancer cell line CA9-22, PER1 had an increased level of expression in cancer cells compared to healthy gingival cells, while PER3 had a decreased level of expression in diseased cells compared to normal cells. An anti-apoptotic role was observed for PER1 and pro-apoptotic role for PER3 (104). These results emphasize the importance of the variation in cancer cell properties and indicate that the same gene may play a substantially different role in different parts of the body and that thorough research is crucial for obtaining useful results concerning cancer diagnosis and therapeutic methods.
Acknowledgements
We are grateful to the Department of Oral Biology, University of Oslo and the Department of Medical Biochemistry, Oslo University Hospital for the funding.
References
- 1.Moore RY. The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biol Transl Sci. 2013;119:1–28. doi: 10.1016/B978-0-12-396971-2.00001-4. [DOI] [PubMed] [Google Scholar]
- 2.Soták M, Sumová A, Pácha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 2014;46:221–232. doi: 10.3109/07853890.2014.892296. [DOI] [PubMed] [Google Scholar]
- 3.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/S0092-8674(02)01223-0. [DOI] [PubMed] [Google Scholar]
- 4.Hunt T, Sassone-Corsi P. Riding tandem: Circadian clocks and the cell cycle. Cell. 2007;129:461–464. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Hrushesky WJ, Grutsch J, Wood P, Yang X, Oh EY, Ansell C, Kidder S, Ferrans C, Quiton DF, Reynolds J, et al. Circadian clock manipulation for cancer prevention and control and the relief of cancer symptoms. Integr Cancer Ther. 2009;8:387–397. doi: 10.1177/1534735409352086. [DOI] [PubMed] [Google Scholar]
- 6.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9:1097–1103. doi: 10.4161/cc.9.6.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roenneberg T, Merrow M. Circadian clocks-the fall and rise of physiology. Nat Rev Mol Cell Biol. 2005;6:965–971. doi: 10.1038/nrm1766. [DOI] [PubMed] [Google Scholar]
- 8.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: The interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41:81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Aschoff J, Fatranská M, Giedke H, Doerr P, Stamm D, Wisser H. Human circadian rhythms in continuous darkness: Entrainment by social cues. Science. 1971;171:213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 10.Rosato E, Kyriacou CP. Origins of circadian rhythmicity. J Biol Rhythms. 2002;17:506–511. doi: 10.1177/0748730402238232. [DOI] [PubMed] [Google Scholar]
- 11.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/S0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 12.Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- 13.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 14.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 15.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 16.Giebultowicz JM. Peripheral clocks and their role in circadian timing: Insights from insects. Philos Trans R Soc Lond B Biol Sci. 2001;356:1791–1799. doi: 10.1098/rstb.2001.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 18.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337:599–602. doi: 10.1126/science.1221592. [DOI] [PubMed] [Google Scholar]
- 21.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–1439. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novák B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9:981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishide SY, Honma S, Nakajima Y, Ikeda M, Baba K, Ohmiya Y, Honma K. New reporter system for Per1 and Bmal1 expressions revealed self-sustained circadian rhythms in peripheral tissues. Genes Cells. 2006;11:1173–1182. doi: 10.1111/j.1365-2443.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues; Proc Natl Acad Sci USA; 2004; pp. 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/S0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 26.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/S0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 27.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unsal-Kaçmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol. 2005;25:3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1; Proc Natl Acad Sci USA; 2011; pp. 16451–16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa G, Haus E, Stevens R. Shift work and cancer-considerations on rationale, mechanisms and epidemiology. Scand J Work Environ Health. 2010;36:163–179. doi: 10.5271/sjweh.2899. [DOI] [PubMed] [Google Scholar]
- 31.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 32.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 33.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 34.Lahti TA, Partonen T, Kyyrönen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 35.Stevens RG. Artificial lighting in the industrialized world: Circadian disruption and breast cancer. Cancer Causes Control. 2006;17:501–507. doi: 10.1007/s10552-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 36.Touitou Y, Bogdan A, Lévi F, Benavides M, Auzéby A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br J Cancer. 1996;74:1248–1252. doi: 10.1038/bjc.1996.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panzer A. Melatonin in osteosarcoma: An effective drug? Med Hypotheses. 1997;48:523–525. doi: 10.1016/S0306-9877(97)90123-7. [DOI] [PubMed] [Google Scholar]
- 38.Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, Skibola DR, Hegedus C, Smith MT. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:779–786. [PubMed] [Google Scholar]
- 39.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26:108–125. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- 40.Bhatti P, Mirick DK, Davis S. Invited commentary: Shift work and cancer. Am J Epidemiol. 2012;176:760–765. doi: 10.1093/aje/kws311. [DOI] [PubMed] [Google Scholar]
- 41.Buzzelli G, Dattolo P, Pinzani M, Brocchi A, Romano S, Gentilini P. Circulating growth hormone and insulin-like growth factor-I in nonalcoholic liver cirrhosis with or without superimposed hepatocarcinoma: Evidence of an altered circadian rhythm. Am J Gastroenterol. 1993;88:1744–1748. [PubMed] [Google Scholar]
- 42.Rafnsson V, Tulinius H, Jónasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: A population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/A:1008983416836. [DOI] [PubMed] [Google Scholar]
- 43.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment; Proc Natl Acad Sci USA; 2009; pp. 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straif K, Baan R, Grosse Y, Secretan B, Ghissassi FE, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, WHO. International Agency for Research on Cancer Monograph Working Group Carcinogenicity of shift-work, painting and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 45.Hsu CM, Lin SF, Lu CT, Lin PM, Yang MY. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33:149–155. doi: 10.1007/s13277-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 46.Haus EL, Smolensky MH. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night and sleep deprivation. Sleep Med Rev. 2013;17:273–284. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 48.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L, et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154–162. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- 49.Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafnkelsson J, Kyyrönen P, Linnersjö A, et al. Incidence of cancer among Nordic airline pilots over five decades: Occupational cohort study. BMJ. 2002;325:567. doi: 10.1136/bmj.325.7364.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feychting M, Osterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiology. 1998;9:490–494. doi: 10.1097/00001648-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Kliukiene J, Tynes T, Andersen A. Risk of breast cancer among Norwegian women with visual impairment. Br J Cancer. 2001;84:397–399. doi: 10.1054/bjoc.2000.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verkasalo PK, Pukkala E, Stevens RG, Ojamo M, Rudanko SL. Inverse association between breast cancer incidence and degree of visual impairment in Finland. Br J Cancer. 1999;80:1459–1460. doi: 10.1038/sj.bjc.6690544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Lévi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 54.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 55.Kim KS, Kim YC, Oh IJ, Kim SS, Choi JY, Ahn RS. Association of worse prognosis with an aberrant diurnal cortisol rhythm in patients with advanced lung cancer. Chronobiol Int. 2012;29:1109–1120. doi: 10.3109/07420528.2012.706767. [DOI] [PubMed] [Google Scholar]
- 56.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando X, Mormont MC, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer. 2012;131:2684–2692. doi: 10.1002/ijc.27574. [DOI] [PubMed] [Google Scholar]
- 58.Wu HS, Davis JE, Natavio T. Fatigue and disrupted sleep-wake patterns in patients with cancer: A shared mechanism. Clin J Oncol Nurs. 2012;16:E56–E68. doi: 10.1188/12.CJON.E56-E68. [DOI] [PubMed] [Google Scholar]
- 59.Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S, Genet D, et al. Circadian rhythm in rest and activity: A biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009;69:4700–4707. doi: 10.1158/0008-5472.CAN-08-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi C, Mu L, de la Longrais IA, Sochirca O, Arisio R, Yu H, Hoffman AE, Zhu Y, Katsaro D. The circadian gene NPAS2 is a novel prognostic biomarker for breast cancer. Breast Cancer Res Treat. 2010;120:663–669. doi: 10.1007/s10549-009-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, Marchan R, Schmidt M, Rahnenführer J, Oster H, Hengstler JG. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13:3282–3291. doi: 10.4161/15384101.2014.954454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, Brown HN, Zheng T. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat. 2008;107:421–425. doi: 10.1007/s10549-007-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue X, Liu F, Han Y, Li P, Yuan B, Wang X, Chen Y, Kuang Y, Zhi Q, Zhao H. Silencing NPAS2 promotes cell growth and invasion in DLD-1 cells and correlated with poor prognosis of colorectal cancer. Biochem Biophys Res Commun. 2014;450:1058–1062. doi: 10.1016/j.bbrc.2014.06.104. [DOI] [PubMed] [Google Scholar]
- 64.Xiao L, Chang AK, Zang MX, Bi H, Li S, Wang M, Xing X, Wu H. Induction of the CLOCK gene by E2-ERα signaling promotes the proliferation of breast cancer cells. PLoS One. 2014;9:e95878. doi: 10.1371/journal.pone.0095878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oshima T, Takenoshita S, Akaike M, Kunisaki C, Fujii S, Nozaki A, Numata K, Shiozawa M, Rino Y, Tanaka K, et al. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25:1439–1446. doi: 10.3892/or.2011.1207. [DOI] [PubMed] [Google Scholar]
- 66.Mazzoccoli G, Panza A, Valvano MR, Palumbo O, Carella M, Pazienza V, Biscaglia G, Tavano F, Di Sebastiano P, Andriulli A, Piepoli A. Clock gene expression levels and relationship with clinical and pathological features in colorectal cancer patients. Chronobiol Int. 2011;28:841–851. doi: 10.3109/07420528.2011.615182. [DOI] [PubMed] [Google Scholar]
- 67.Relles D, Sendecki J, Chipitsyna G, Hyslop T, Yeo CJ, Arafat HA. Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg. 2013;17:443–450. doi: 10.1007/s11605-012-2112-2. [DOI] [PubMed] [Google Scholar]
- 68.Hu ML, Yeh KT, Lin PM, Hsu CM, Hsiao HH, Liu YC, Lin HY, Lin SF, Yang MY. Deregulated expression of circadian clock genes in gastric cancer. BMC Gastroenterol. 2014;14:67. doi: 10.1186/1471-230X-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mazzoccoli G, Piepoli A, Carella M, Panza A, Pazienza V, Benegiamo G, Palumbo O, Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother. 2012;66:175–179. doi: 10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Liu B, Xu K, Jiang Y, Li X. Aberrant expression of Per1, Per2 and Per3 and their prognostic relevance in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:7863–7871. [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang X, Wood PA, Oh EY, Du-Quiton J, Ansell CM, Hrushesky WJ. Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res Treat. 2009;117:423–431. doi: 10.1007/s10549-008-0133-z. [DOI] [PubMed] [Google Scholar]
- 73.Mao Y, Fu A, Hoffman AE, Jacobs DI, Jin M, Chen K, Zhu Y. The circadian gene CRY2 is associated with breast cancer aggressiveness possibly via epigenomic modifications. Tumour Biol. 2015;36:3533–3539. doi: 10.1007/s13277-014-2989-3. [DOI] [PubMed] [Google Scholar]
- 74.Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, Tamimi RM, Batista JL, Haneuse S, Flynn-Evans E, Lockley SW, et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25–33. doi: 10.1007/s10552-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng ZL, Luo HY, Yang J, Wu WJ, Chen DL, Huang P, Xu RH. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res. 2014;20:1042–1052. doi: 10.1158/1078-0432.CCR-13-0171. [DOI] [PubMed] [Google Scholar]
- 77.Jiang W, Zhao S, Jiang X, Zhang E, Hu G, Hu B, Zheng P, Xiao J, Lu Z, Lu Y, et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016;371:314–325. doi: 10.1016/j.canlet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 79.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Sanderson RJ, de Boer MF, Damhuis RA, Meeuwis CA, Knegt PP. The influence of alcohol and smoking on the incidence of oral and oropharyngeal cancer in women. Clin Otolaryngol Allied Sci. 1997;22:444–448. doi: 10.1046/j.1365-2273.1997.00049.x. [DOI] [PubMed] [Google Scholar]
- 81.Scully C, Field J. Genetic aberrations in squamous cell carcinoma of the head and neck (SCCHN), with reference to oral carcinoma (review) Int J Oncol. 1997;10:5–21. doi: 10.3892/ijo.10.1.5. [DOI] [PubMed] [Google Scholar]
- 82.Yuan H, Li H, Ma H, Niu Y, Wu Y, Zhang S, Hu Z, Shen H, Chen N. Genetic polymorphisms in key DNA repair genes and risk of head and neck cancer in a Chinese population. Exp Ther Med. 2012;3:719–724. doi: 10.3892/etm.2012.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Gao F, Yang AK, Chen WK, Chen SW, Li H, Zhang X, Yang ZY, Chen XL, Song M. Epidemiologic characteristics of oral cancer: Single-center analysis of 4097 patients from the Sun Yat-sen University Cancer Center. Chin J Cancer. 2016;35:24. doi: 10.1186/s40880-016-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amit M, Yen TC, Liao CT, Chaturvedi P, Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Kreppel M, Zöller J, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: An international collaborative study. Cancer. 2013;119:4242–4248. doi: 10.1002/cncr.28357. [DOI] [PubMed] [Google Scholar]
- 85.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 86.Sand L, Wallström M, Hirsch JM. Smokeless tobacco, viruses and oral cancer. Oral Health Dent Manag. 2014;13:372–378. [PubMed] [Google Scholar]
- 87.Listl S, Jansen L, Stenzinger A, Freier K, Emrich K, Holleczek B, Katalinic A, Gondos A, Brenner H, GEKID Cancer Survival Working Group Survival of patients with oral cavity cancer in Germany. PLoS One. 2013;8:e53415. doi: 10.1371/journal.pone.0053415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, Køtlum JE, Olafsdóttir E, Pukkala E, Storm HH. NORDCAN-a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 89.Zieker D, Jenne I, Koenigsrainer I, Zdichavsky M, Nieselt K, Buck K, Zieker J, Beckert S, Glatzle J, Spanagel R, et al. Circadian expression of clock- and tumor suppressor genes in human oral mucosa. Cell Physiol Biochem. 2010;26:155–166. doi: 10.1159/000320547. [DOI] [PubMed] [Google Scholar]
- 90.Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: Association with specific cell-cycle phases. Am J Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One. 2009;4:e4798. doi: 10.1371/journal.pone.0004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miki T, Matsumoto T, Zhao Z, Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun. 2013;4:2444. doi: 10.1038/ncomms3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng ZL, Wu MW, Sun J, Sun YL, Cai YC, Huang YJ, Xian LJ. Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. J Biochem. 2010;148:319–326. doi: 10.1093/jb/mvq069. [DOI] [PubMed] [Google Scholar]
- 94.Chen R, Yang K, Zhao NB, Zhao D, Chen D, Zhao CR, Tang H. Abnormal expression of PER1 circadian-clock gene in oral squamous cell carcinoma. Onco Targets Ther. 2012;5:403–407. doi: 10.2147/OTT.S38508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li HX, Fu XJ, Yang K, Chen D, Tang H, Zhao Q. The clock gene PER1 suppresses expression of tumor-related genes in human oral squamous cell carcinoma. Oncotarget. 2016;7:20574–20583. doi: 10.18632/oncotarget.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Q, Zheng G, Yang K, Ao YR, Su XL, Li Y, Lv XQ. The clock gene PER1 plays an important role in regulating the clock gene network in human oral squamous cell carcinoma cells. Oncotarget. 2016;7:70290–70302. doi: 10.18632/oncotarget.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao N, Tang H, Yang K, Chen D. Circadian rhythm characteristics of oral squamous cell carcinoma growth in an orthotopic xenograft model. Onco Targets Ther. 2013;6:41–46. doi: 10.2147/OTT.S39955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye H, Yang K, Tan XM, Fu XJ, Li HX. Daily rhythm variations of the clock gene PER1 and cancer-related genes during various stages of carcinogenesis in a golden hamster model of buccal mucosa carcinoma. Onco Targets Ther. 2015;8:1419–1426. doi: 10.2147/OTT.S83710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 100.Fu XJ, Li HX, Yang K, Chen D, Tang H. The important tumor suppressor role of PER1 in regulating the cyclin-CDK-CKI network in SCC15 human oral squamous cell carcinoma cells. Onco Targets Ther. 2016;9:2237–2245. doi: 10.2147/OTT.S100952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Wang Q, Ao Y, Yang K, Tang H, Chen D. Circadian clock gene Per2 plays an important role in cell proliferation, apoptosis and cell cycle progression in human oral squamous cell carcinoma. Oncol Rep. 2016;35:3387–3394. doi: 10.3892/or.2016.4724. [DOI] [PubMed] [Google Scholar]
- 102.Tan XM, Ye H, Yang K, Chen D, Wang QQ, Tang H, Zhao NB. Circadian variations of clock gene Per2 and cell cycle genes in different stages of carcinogenesis in golden hamster buccal mucosa. Sci Rep. 2015;5:9997. doi: 10.1038/srep09997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato F, Nagata C, Liu Y, Suzuki T, Kondo J, Morohashi S, Imaizumi T, Kato Y, Kijima H. PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem. 2009;146:833–838. doi: 10.1093/jb/mvp126. [DOI] [PubMed] [Google Scholar]
- 104.Sato F, Wu Y, Bhawal UK, Liu Y, Imaizumi T, Morohashi S, Kato Y, Kijima H. PERIOD1 (PER1) has anti-apoptotic effects, and PER3 has pro-apoptotic effects during cisplatin (CDDP) treatment in human gingival cancer CA9-22 cells. Eur J Cancer. 2011;47:1747–1758. doi: 10.1016/j.ejca.2011.02.025. [DOI] [PubMed] [Google Scholar]