Abstract
Non-small-cell lung cancer (NSCLC) is one of the leading causes of cancer mortality worldwide. A growing body of evidence indicates that microRNA (miR) have important and diverse roles in the proliferation, apoptosis and metastasis of human cancer cells. In the present study, the molecular regulation mechanism of miR-30a and its potential target, Myb-related protein B (MYBL2) was investigated in NSCLC. Reverse transcription-quantitative polymerase chain reaction results showed that miR-30a was significantly downregulated in NSCLC tissues compared with adjacent normal tissues (P<0.05). MYBL2 has a putative miR-30a target site in its 3′untranslated region according to previous data, prediction databases and TargetScan software. In the present study, a negative correlation was demonstrated between miR-30a and MYBL2 expression in NSCLC. Direct interaction between miR-30a and MYBL2 was also confirmed via a dual-luciferase reporter assay. miR-30a overexpression inhibited the growth of A549 and H460 cells via MTT and bromodeoxyuridine incorporation assays, whereas miR-30a downregulation promoted cell proliferation. In addition, miR-30a overexpression not only increased cell apoptosis and induced cell cycle arrest in A549 and H460 cell lines, but also attenuated tumor growth, and mRNA and protein expression levels of MYBL2. The present findings suggest that miR-30a may suppress NSCLC by targeting MYBL2.
Keywords: miR-30a, non-small-cell lung cancer, Myb-related protein B, miRNA, cancer
Introduction
Non-small-cell lung cancer (NSCLC) is among the most commonly diagnosed types of cancer, and is the leading cause of cancer mortality worldwide (1). At present, NSCLC is typically diagnosed by screening via cytological examination, and confirmed by histological examination of colposcopy-guided biopsies (2). Subsequent medical imaging is performed to determine whether the cancer has spread. However, this technique is limited to detecting the morphological alterations of tissues and does not elucidate the risk of progression. As such, it is necessary to improve NSCLC diagnostics. Radiotherapy and surgery remain the predominant therapeutic agents for NSCLC treatment, and chemotherapy is performed on patients with metastasis or recurrence (3–5). However, in addition to eliminating cancer cells, these treatments are hazardous to normal cells. At present, it is pertinent to develop novel, efficient therapeutics. A growing body of evidence indicates that microRNA (miRNA or miR) are associated with the molecular pathogenesis of various types of tumor, providing novel insight into the occurrence, development and treatment of NSCLC (6,7).
miRNAs are defined as small non-coding RNA molecules formed of ~22 nucleotides (8–10). By binding to the 3′-untranslated regions (UTRs) of target mRNAs and regulating their expression, miRNAs have important and diverse roles in the cell proliferation, apoptosis and metastasis of human cancer, including lung cancer, breast cancer, glioma, and gastric cancer (2,6–8). Previous studies have detected the presence of aberrant miRNAs in lung tissues using microarrays and miRNA sequencing technologies, suggesting that these miRNAs (or their targets) are important in the development and progression of NSCLC (9–13). However, the targets of these differentially expressed miRNAs have yet to be identified. The aim of the present study was to investigate the functions and targets of miR-30a in NSCLC, which is known to be associated with a number of pathogenic processes including rheumatoid arthritis fibroblasts, Mycobacteria tuberculosis infection and acute myeloid leukemia (14–16). A previous study reported that miR-30 repressed Myb-related protein B (MYBL2) expression during cellular senescence by binding to its 3′-UTR (16). While the molecular regulation mechanism of miR-30a and its potential target, MYBL2, in NSCLC remain undetermined, the present study is the first investigate the functions and targets of miR-30a in human NSCLC.
The aim of the present study was to describe the mechanisms of miR-30a and its potential target, MYBL2, in NSCLC development.
Materials and methods
Clinical samples and cell lines
NSCLC tissues and adjacent non-cancerous tissues were collected between May-October 2013 from 20 patients (11 males and 9 females aged 58.62±10.57 years at The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Tissue samples were frozen and stored at −80°C. The methodology was approved by the Research Ethics Committee of Zhengzhou University. Informed consent was obtained for all samples in accordance with the Declaration of Helsinki. Clinical staging was scored according to the Union for International Cancer Control classification version 6 (17). The 20 patients had not undergone radical prostatectomy and/or any other treatment previously. Patient characteristics are provided in Table I.
Table I.
Characteristics of patients in the present study.
| Characteristic | Number of patients |
|---|---|
| Male | 11 |
| Female | 9 |
| ≤65 years old | 10 |
| >65 years old | 10 |
| Never smoked | 13 |
| Current smoker | 4 |
| Former smoker | 3 |
| Tumor stage I | 10 |
| Tumor stage II | 3 |
| Tumor stage III | 5 |
| Tumor stage IV | 2 |
Human NSCLC cell lines A549 and H460 were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fischer Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified 5% CO2 incubator.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of miR-30a and MYBL2 expression levels
Total RNAs were isolated from NSCLC tissues or A549 and H460 cell lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol and quantified using an ND1000 spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Expression levels of miR-30a were analyzed by TaqMan® miRNA assays (Applied Biosystems; Thermo Fisher Scientific, Inc.) containing a looped reverse transcriptase primer specific for each miRNA, and measured on an ABI Prism 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). To determine MYBL2 expression, 1 µg total RNA was converted to cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The DNase treatment system contained 1 µl DNase I (Applied Biosystems; Thermo Fisher Scientific, Inc.) and 1 µg total RNA. The reaction was completed at 37°C for 30 min and stopped by heating inactivation, 70°C for 5 min. RT-qPCR was then completed using the SYBR Premix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The qPCR system contained 1 µl cDNA (1:20 dilution), 0.2 µl 10 nM primers (sense and antisense), 1 µl TaqMan MicroRNA assay (20X) and 10 µl TaqMan 2X Universal PCR Master Mix. The qPCR reaction was completed as follows: 95°C for 10 min and 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The fluorophore signals were obtained during 60 sec of 60°C. The experiment was repeated three times and SDS analysis software version 2.3 was used to analyze the results (Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative expression was calculated using the ΔΔCq method [relative expression=2−ΔΔCq; where ΔCT=Cq (Target RNA)-Cq (endogenous control RNA)] (18). Melt curves were performed to confirm whether amplifications were specific. U6 small nuclear RNA and glyceraldehyde 3-phosphate dehydrogenase were used as internal controls for miR-30a and MYBL2, respectively. Primers were designed as follows: MYBL2, forward 5′-ATGTCCAGTGCCTGGAAGAC-3′ and reverse 5′-AGATGAGGGTCCGAGATGTG-3′; and GAPDH, forward 5′-AGGGCTGCTTTTAACTCTGGT-3′ and reverse 5′-CCCCACTTGATTTTGGAGGGA-3′.
Target site prediction and luciferase reporter assay
MYBL2 was determined to be a candidate target of miR-30a; therefore, putative target sites were searched based on the prediction database (19) and TargetScan software version 6.0 (Whitehead Institute for Biomedical Research, Cambridge, MA, USA). A luciferase reporter assay was performed using a dual-luciferase reporter assay system (Promega Corp., Madison, WI, USA) according to the manufacturer's protocol. Wild and mutant 3′-UTRs of MYBL2 containing the target sequence were separately synthesized (Sangon Biotech, Co., Ltd., Shanghai, China) and cloned into the pMIR-Report vector (Applied Biosystems; Thermo Fisher Scientific, Inc.). miR-30a mimics were purchased from Sangon Biotech, Co., Ltd. Prior to transfection, A549 cells were plated in 6-well plates (density, 2×105 cells/well) in RPMI-1640 medium without antibiotics. A549 cell lines were transfected in triplicate with miR-30a mimics at concentrations of 50 and 100 nM, and were subsequently co-transfected with wild or mutant 3′-UTRs of MYBL2 via Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cell lysates were incubated with dual-luciferase reagents (Promega Corp.) at 48 h post-treatment, and luciferase activity was assayed in triplicate by a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
miR-30a overexpression and knockdown for cell proliferation assay
To elucidate the effect of miR-30a on NSCLC cells, cell proliferation following overexpression and knockdown of miR-30a was determined via MTT assay and bromodeoxyuridine (BrdU) incorporation. A549 and H460 cells were divided into control, miR-30a mimics and miR-30a inhibitors groups (n=3/group). Mimics and inhibitors were purchased from Sangon Biotech Co., Ltd. Each group of NSCLC cells were cultured into 6-well plates (density, 1×106 cells/well) and incubated at 37°C for 24 h. Cells were subsequently transfected with 100 nM miR-30a mimics or 100 nM miR-30a inhibitors (concentration, 10 nmol/l) using Lipofectamine® 2000, according to the manufacturer's protocol.
Cells were seeded into 96-well plates (concentration, 5×103 cells/well) at 24 h post-treatment, treated with MTT solution (Sigma-Aldrich; Merck-Millipore, Darmstadt, Germany) according to the manufacturer's protocol, and incubated in the dark at 37°C for 24 h. Absorption at 570 nm was obtained using a Beckman UD800 spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA). BrdU incorporation assay was performed to further analyze cell proliferation. NSCLC cells were incubated with BrdU for 6 h following infection and measured using a Multiskan FC microplate reader (Thermo Fisher Scientific, Inc.).
miR-30a overexpression for cell apoptosis and cell cycle analysis
To analyze the role of miR-30a in the cell cycle and apoptosis of NSCLC cells, A549 and H460 cells pretreated with miR-30a mimics were harvested to calculate the apoptotic rate and determine cell cycle progression. NSCLC cells were fixed in 90% methanol for 2 h and resuspended in phosphate-buffered saline supplemented with 0.1% bovine serum albumin, 0.05% Triton X-100 and 50 µg/ml RNase A (Sigma-Aldrich; Merck-Millipore). Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI; BD Biosciences, San Jose, CA, USA) staining was performed according to the manufacturer's protocol. Cell counting was performed on a FACS Calibur system (BD Biosciences), followed by the calculation of apoptotic rate and analysis of the cell cycle. Cells positive for Annexin V-FITC and negative for PI fluorescence were considered to be apoptotic. The rate of apoptosis was calculated as follows: Apoptotic cells/total cell number. A549 and H460 cells without pretreatment were utilized as the control group. Each experiment was repeated three times.
miR-30a overexpression for in vivo xenograft assay
To observe the effect of miR-30a on tumor growth, a total of eight female nude mice (age, six weeks) were obtained from The Second Affiliated Hospital of Zhengzhou University. The mice were provided with standard food and water with ad libitum access in a maintained environment of 20–25°C, 40% humidity and a 12-h light/dark cycle. Animal experiments were approved by the Research Ethics Committee of Zhengzhou University. A total of 1×107 A549 cells, with or without transfection of miR-30a mimics, were injected into the posterior flank of the mice. The group injected with A549 cells and not infected with miR-30a mimics were regarded as the control group. Tumor volumes were measured [volume=(length × width2)/2]. Mice were sacrificed by cervical dislocation and decapitated after 4 weeks and xenograft tissues were collected for the immunohistochemical staining assay. Tissues were collected and prepared into tissue sections (thickness, 6 µm). Following de-waxing, the tissues were incubated with Anti-MyBL2 primary antibody (1:200; AV100748; Sigma-Aldrich; Merck Millipore) and anti-Rabbit IgG, whole molecule secondary antibody (1:1,000; R5506; Sigma-Adrich; Merck Millipore). Horseradish peroxidase and diaminobenzidine (both Beyotime Institute of Biotechnology, Haimen, China) were employed to visualize the proteins.
mRNA and protein expression levels of MYBL2 in these tissues and their control were analyzed. mRNA expression levels were determined via RT-qPCR analysis of MYBL2, and western blot analysis was used to analyze protein expression levels.
Western blot analysis
Xenograft tissues and controls were lysed using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) and protein quantity was detected via a bicinchoninic acid kit (Beyotime Institute of Biotechnology). For each sample, 15 µg protein was separated by 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerca, MA, USA). Following blocking with 5% non-fat milk, membranes were incubated at 4°C overnight with Anti-MYBL2 antibody produced in a rabbit (SAB1410805; 1:1,000) and anti-GAPDH produced in a rabbit (G9545; 1:1,000) antibodies (Sigma-Aldrich; Merck-Millipore). Membranes were washed with Tris-buffered saline with 0.1% Tween-20 (TBST; Sangon Biotech, Co., Ltd., Shanghai, China) three times for five min. Anti-Rabbit IgG (whole molecule)-Peroxidase antibody produced in a goat was used as a secondary antibody (A6154; 1:2,000) incubated for 1 h at room temperature. This was conjugated to horseradish peroxidase and an enhanced chemiluminescent substrate (ECL plus; GE Healthcare, Chalfont, UK) was used for visualizing the proteins. Images were captured using chemiluminescence Molecular Imager® ChemiDoc™ XRS+ system and analyzed using Bio-Rad Quantity One software version 4.62 (Bio-Rad Laboratories, Inc. Hercules, CA, USA).
Statistical analysis
Each experiment was performed in triplicate. All values were presented as mean ± standard deviation. Differences between or among groups were analyzed by Student's t-test or one-way analysis of variance. All statistical analyses were performed using SPSS version 21 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference. If F ratios exceeded the critical value (P<0.05), the Newman-Keul's post hoc test was preformed to compare the groups.
Results
miR-30a is downregulated in NSCLC tissues
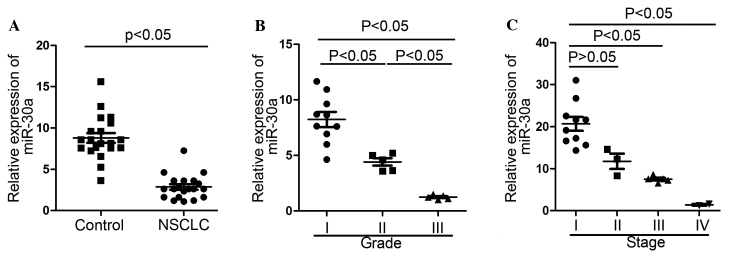
Total RNAs were isolated from 20 NSCLC and adjacent non-cancerous tissues. RT-qPCR indicated that miR-30a expression levels were significantly downregulated (>3-fold decrease) in NSCLC tissues compared with normal tissues (P<0.05; Fig. 1A). A negative correlation was detected as miR-30a expression levels significantly decreased with higher clinical grades and stages of NSCLC (P<0.05). miR-30a exhibited the lowest expression in grade III, followed by grade II, and grade I (P<0.05; Fig. 1B). miR-30a expression in stage I was not significantly different from that in stage II; however, stage I expression levels were significantly increased as compared with stages III and IV (P<0.05; Fig. 1C).
Figure 1.
miR-30a is downregulated in NSCLC tissues (n=20). (A) Relative expression of miR-30a in the NSCLC tissues and the control. (B) Relative expression of miR-30a in different histological grades. (C) Relative expression of miR-30a in different tumor stages. miR, microRNA; NSCLC, non-small cell lung cancer.
MYBL2 is a direct target of miR-30a
Putative target sites of MYBL2 were searched using the TargetScan database. MYBL2 has a putative target region in its 3′-UTR for human miR-30a (Fig. 2A). In addition, miR-30a and MYBL2 expression levels were investigated by RT-qPCR in 20 clinical samples, and MYBL2 expression was negatively correlated with miR-30a in NSCLC tissues (P=0.02; R=−0.69; Fig. 2B). Luciferase activity was measured in A549 cells following transfection with miR-30a mimics and wild or mutant 3′-UTR of MYBL2. Transfection of miR-30a mimics resulted in a significant repression (2.5-fold) of luciferase activity of wild 3′-UTR of MYBL2, but had no effect on the mutant (P<0.05; Fig. 2C).
Figure 2.
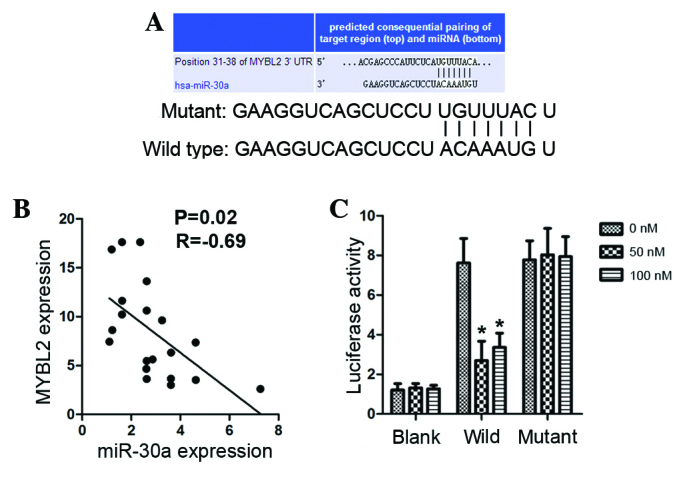
MYBL2 is a direct target of miR-30a in NSCLC tissues (n=20). (A) Target region in the 3′-UTR of MYBL2 and miR-30a, predicted by TargetScan software, version 6.0. (B) Correlation between miR-30a expression and MYBL2 expression in NSCLC tissues. (C) Luciferase activity in A549 cells after co-transfection with miR-30a mimics and wild 3′-UTR of MYBL2. MYBL2, Myb-related protein B; miR, microRNA; NSCLC, non-small cell lung cancer; UTR, untranslated region. *P<0.05 vs. blank.
Overexpression of miR-30a inhibits cell proliferation, and knockdown of miR-30a promotes cell proliferation in NSCLC cells
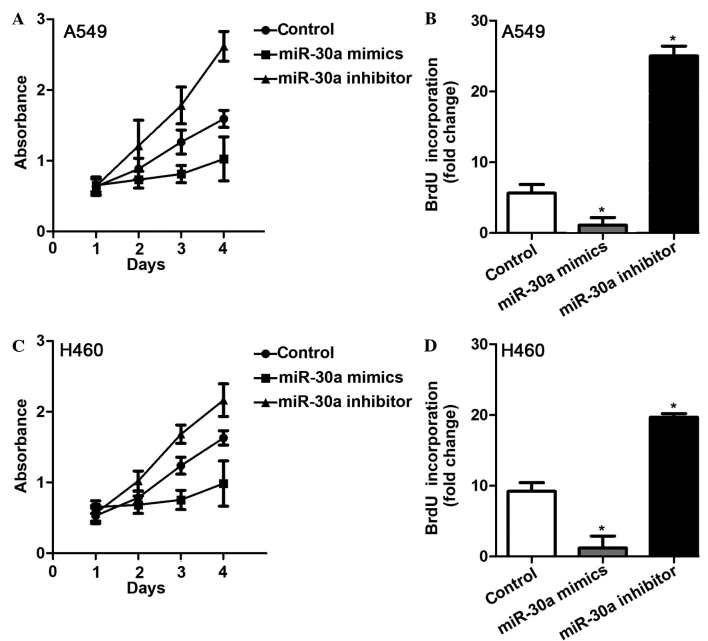
Considering the significant decrease of miR-30a in NSCLC tissues, the role of miR-30a in tumor development was investigated. The effect of overexpression and knockdown of miR-30a on cell proliferation was studied via the transfection of miR-30a mimics and miR-30a inhibitors. MTT assay revealed that transfection with miR-30a mimics significantly inhibited the proliferation of A549 and H460 cells (P<0.05; Fig. 3). NSCLC cells transfected with miR-30a inhibitor exhibited significantly accelerated cell proliferation compared with the control (P<0.05; Fig. 3A and C). BrdU incorporation also indicated that overexpression of miR-30a inhibited cell growth, and that knockdown of miR-30a promoted cell proliferation (Fig. 3B and D). These results indicate that miR-30a may function as a suppressive factor in NSCLC.
Figure 3.
Effects of overexpression and knockdown of miR-30a on the proliferation of A549 and H460 cell lines. (A) MTT assay of A549 transfected with miR-30a mimics and inhibitors. (B) BrdU incorporation assay of A549 transfected with miR-30a mimics and inhibitors. (C) MTT assay of H460 transfected with miR-30a mimics and inhibitors. (D) BrdU incorporation assay of H460 transfected with miR-30a mimics and inhibitors. *P<0.05 vs control. miR, microRNA; BrdU, bromodeoxyuridine.
Overexpression of miR-30a increases apoptosis in NSCLC cells and arrests the cell cycle
Based on the MTT and BrdU incorporation assays, overexpression of miR-30a was used to study the apoptotic rate and cell cycle of NSCLC cells, and elucidate its suppressive effect in NSCLC. A549 and H460 cells were transfected with miR-30a mimics and analyzed by flow cytometry. Overexpression of miR-30a markedly increased the apoptotic rates in the two cell lines (P<0.05; Fig. 4A). Cell cycle analysis indicated that transfection with miR-30a mimics significantly increased the number of cells in G1 phase and decreased those in S phase in A549 and H460 cells (P<0.05; Fig. 4B and C), as compared with the mean values of control and mimics.
Figure 4.
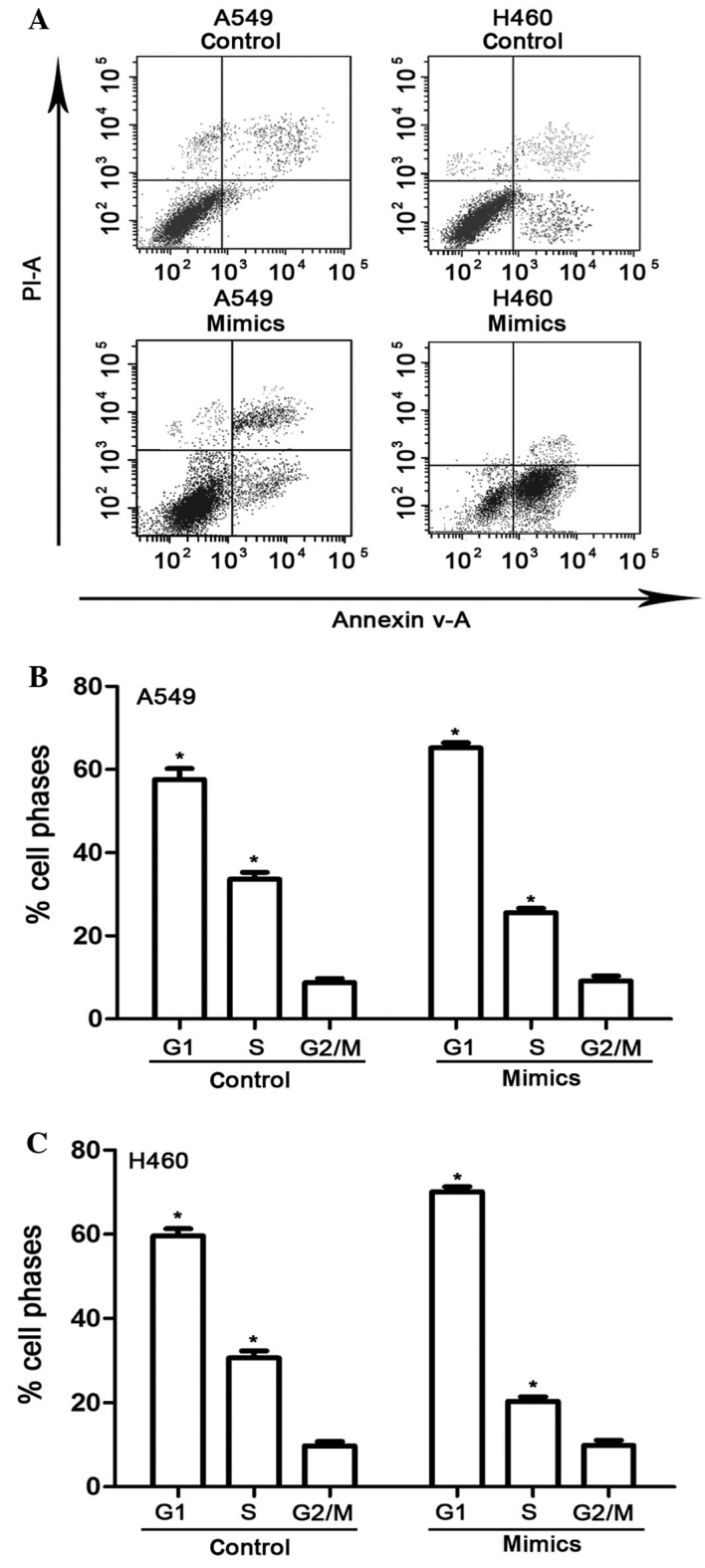
Effects of miR-30a overexpression on the apoptosis and cell cycle of A549 and H460 cell lines. (A) Analysis of apoptosis by flow cytometry and proportion of (B) A549 and (C) H460 cells in various cell cycle phases.*P<0.05 compared with the mean values of control and mimic-treated cells. miR, microRNA; mimics, cells transfected with miR-30a mimic.
Overexpression of miR-30a suppresses tumor growth and mRNA and protein expression levels of MYBL2 in vivo
In vivo xenograft assays were performed to observe the effect of miR-30a on tumor growth. Three weeks after injection, the tumor volume in groups injected with A549 cells increased significantly in the control compared with the miR-30a mimics group (P<0.05; Fig. 5A). In accordance with the analysis in NSCLC tissues and A549 and H460 cell lines, overexpression of miR-30a decreased the mRNA expression and protein expression levels of MYBL2 in xenograft tissues (Fig. 5B and C). Overexpression of miR-30a not only retarded tumor growth, but also downregulated mRNA and protein expression of MYBL2 in xenograft tumors, suggesting that miR-30a may suppress NSCLC by targeting MYBL2.
Figure 5.
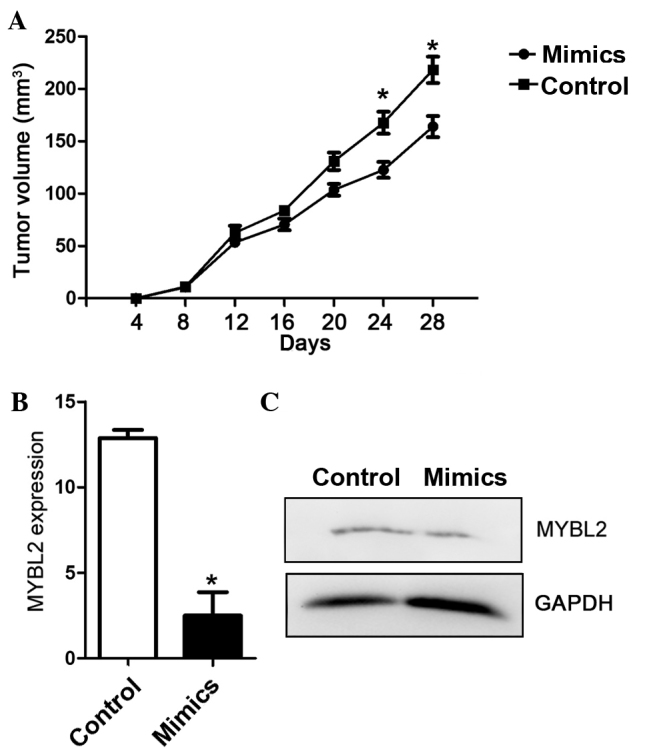
Effects of overexpression of miR-30a on tumor growth. (A) Tumor volume in vivo injected with A549 cells. Relative (B) mRNA and (C) protein expression of MYBL2 in xenograft tumor and control tissues. *P<0.05 compared with the mean values of control and mimic-treated cells. miR, microRNA; MYBL2, Myb-related protein B.
Discussion
There has been a marked increase in the numbers of NSCLC cases every year in China (20). Despite the availability of novel diagnostic and genetic technologies and advancements in surgical techniques, improved survival rates have yet to be achieved in NSCLC, and novel biological treatments are yet to be developed. Various genetic and epigenetic alternations occur during NSCLC tumorigenesis; therefore, to pursue more efficient therapeutic strategies, contemporary studies are focusing on the molecular mechanisms underlying the genetic and epigenetic processes involved in NSCLC (21–23). miR-30 family has provided insight into the prevention, diagnosis and treatment of human diseases (24–26). In a previous study, it was demonstrated that miR-155 downregulation was able to induce cell apoptosis and cell cycle arrest by targeting various anti-apoptotic factors (27). Moreover, transfection with a miR-155 inhibitor increased the percentage of cells in the G1 phase, suggesting that low expression of miR-155 induced G1-phase cell cycle arrest (27). The targets of miR-199a, V-Ki-Ras2 Kirsten rat sarcoma viral oncogene homolog and mitogen-activated protein kinase kinase 4 have been identified (28–31), and the inhibitory effects of miR-199a and/or miR-214 on the expression of these target genes have been demonstrated (31). miR-199a and miR-214 may have important roles in cell growth and proliferation via the mitogen-activated protein kinase pathway (31,32).
miR-30a expression has been explored in various types of human cancer. It has been demonstrated that miR-30a-5p expression is upregulated in glioma cell lines and glioma specimens (33). Knockdown of miR-30a-5p with antisense oligonucleotide in LN229 and SNB19 glioblastoma (GBM) cells inhibited cell growth and invasion, and induced apoptosis (34). In contrast, when miR-30a-5p mimics were transfected into LN229 and SNB19 GBM cells, cell growth and invasion were promoted and the expression of relevant proteins increased (34). Furthermore, MYBL2 was found that participated in lung developmental pathways in lung cancer, and found to be amplified and overexpressed in various tumor types (35). A previous study demonstrated that miR-30a repressed the expression of MYBL2 during cellular senescence by binding to its 3′-UTR (16). However, the roles of miR-30a and its potential target in disease progression of NSCLC remain poorly understood. The present study aimed to elucidate the roles of miR-30a and its potential target, MYBL2, in NSCLC.
The present results demonstrated that miR-30a was significantly downregulated in NSCLC tissues compared with the adjacent normal tissues. MYBL2 was a direct target of miR-30a and has a putative target site in its 3′-UTR. A negative correlation was detected between miR-30a and MYBL2 expression levels. miR-30a overexpression inhibited the growth of A549 and H460 cells, whereas miR-30a downregulation promoted cell proliferation. Furthermore, in addition to increasing cell apoptosis and inducing cell cycle arrest in A549 and H460 cell lines, miR-30a overexpression also attenuated tumor growth and the mRNA and protein expression levels of MYBL2. It was demonstrated that miR-30a overexpression inhibited cells and arrested the cell cycle during G1 phase, providing a novel molecular mechanism for the regulation of tumor cells by upregulating miR-30a. To the best of our knowledge, there is no evidence to support the hypothesis that miR-30a mediates NSCLC progression. Therefore, the present study is the first to indicate that miR-30a is able to induce the apoptosis of NSCLC in vivo and in vitro.
In conclusion, the present findings suggest that miR-30a may be able to suppress NSCLC by targeting MYBL2. These findings may be beneficial to the development of miR-30a as a potential therapeutic agent in the treatment of NSCLC.
Acknowledgements
The present study was partially supported by grants from the Xiamen City Major Joint Research Projects (no. 3502Z20159013), the Fujian Department of Science and Technology (nos. 2014D020, 2015J01546 and 2016J01636).
References
- 1.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, Alattar M, Deepak J, Stass SA, Jiang F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paz-Ares L. Beyond first-line NSCLC therapy: Chemotherapy or erlotinib? Lancet Oncol. 2012;13:225–227. doi: 10.1016/S1470-2045(12)70001-3. [DOI] [PubMed] [Google Scholar]
- 4.Pilotti V, Mastrorilli M, Pizza G, De Vinci C, Busutti L, Palareti A, Gozzetti G, Cavallari A. Transfer factor as an adjuvant to non-small cell lung cancer (NSCLC) therapy. Biotherapy. 1996;9:117–121. doi: 10.1007/BF02628668. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZX. Therapy of NSCLC. Med Recapitulate. 2007;24:690–692. [Google Scholar]
- 6.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jay C, Nemunaitis J, Chen P, Fulgham P, Tong AW. miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol. 2007;26:293–300. doi: 10.1089/dna.2006.0554. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didiano D, Hobert O. Molecular architecture of a miRNA-regulated 3′ UTR. RNA. 2008;14:1297–1317. doi: 10.1261/rna.1082708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Solomides CC, Evans BJ, Navenot JM, Vadigepalli R, Peiper SC, Wang Z. MicroRNA Profiling in Lung Cancer Reveals New Molecular Markers for Diagnosis. Acta Cytol. 2012;56:645–654. doi: 10.1159/000343473. [DOI] [PubMed] [Google Scholar]
- 12.Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol. 2014;5:604–620. doi: 10.5306/wjco.v5.i4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsaleh G, Francois A, Philippe L, Gong YZ, Bahram S, Cetin S, Pfeffer S, Gottenberg JE, Wachsmann D, Georgel P, Sibilia J. MiR-30a-3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PLoS One. 2014;9:e111266. doi: 10.1371/journal.pone.0111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Wang T, Liu Z, Zhang G, Wang J, Feng S, Liang J. Inhibition of autophagy by miR-30A induced by Mycobacteria tuberculosis as a possible mechanism of immune escape in human macrophages. Jpn J Infect Dis. 2015;68:420–424. doi: 10.7883/yoken.JJID.2014.466. [DOI] [PubMed] [Google Scholar]
- 16.Fuster O, Llop M, Dolz S, García P, Such E, Ibáñez M, Luna I, Gómez I, López M, Cervera J, et al. Adverse prognostic value of MYBL2 overexpression and association with microRNA-30 family in acute myeloid leukemia patients. Leuk Res. 2013;37:1690–1696. doi: 10.1016/j.leukres.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Gospodarowicz MK, Miller D, Groome PA, Greene FL, Logan PA, Sobin LH. The process for continuous improvement of the TNM classification. Cancer. 2004;100:1–5. doi: 10.1002/cncr.11898. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang Y, Zhao H, Wu J, Zhang Y, Zhao L, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Baer C, Claus R, Plass C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013;73:473–477. doi: 10.1158/0008-5472.CAN-12-3731. [DOI] [PubMed] [Google Scholar]
- 23.Ji M, Guan H, Gao C, Shi B, Hou P. Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC) BMC Cancer. 2011;11:147. doi: 10.1186/1471-2407-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guess MG, Barthel KK, Harrison BC, Leinwand LA. miR-30 family microRNAs regulate myogenic differentiation and provide negative feedback on the microRNA pathway. PLoS One. 2015;10:e0118229. doi: 10.1371/journal.pone.0118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Z, Wang K, Wang G, Zhang A, Pu P. miR-30a-5p Antisense Oligonucleotide Suppresses Glioma Cell Growth by Targeting SEPT7. PLoS One. 2013;8:e55008. doi: 10.1371/annotation/9a8447a4-bffe-445f-b323-7e200896aea9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Le Guen CL, Friedman JR, Hand NJ. Novel targets of miR-30, a microRNA required for biliary development. F1000 Res. 2013;2:197. doi: 10.12688/f1000research.2-197.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lao G, Liu P, Wu Q, Zhang W, Liu Y, Yang L, Ma C. mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol. 2014;35:11933–11938. doi: 10.1007/s13277-014-2479-7. [DOI] [PubMed] [Google Scholar]
- 28.Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22:1054–1062. doi: 10.1016/j.cellsig.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurai K, Furukawa C, Haraguchi T, Inada K, Shiogama K, Tagawa T, Fujita S, Ueno Y, Ogata A, Ito M, et al. MicroRNAs miR-199a-5p and-3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 2011;71:1680–1689. doi: 10.1158/0008-5472.CAN-10-2345. [DOI] [PubMed] [Google Scholar]
- 30.Yi H, Liang B, Jia J, Liang N, Xu H, Ju G, Ma S, Liu X. Differential roles of miR-199a-5p in radiation-induced autophagy in breast cancer cells. FEBS Lett. 2013;587:436–443. doi: 10.1016/j.febslet.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 31.el Azzouzi H, Leptidis S, Dirkx E, Hoeks J, van Bree B, Brand K, McClellan EA, Poels E, Sluimer JC, Van Den Hoogenhof MM, et al. The Hypoxia-Inducible MicroRNA Cluster miR-199a-214 Targets Myocardial PPARδ and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18:341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Salim H, Akbar NS, Zong D, Vaculova AH, Lewensohn R, Moshfegh A, Viktorsson K, Zhivotovsky B. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br J Cancer. 2012;107:1361–1373. doi: 10.1038/bjc.2012.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Jia Z, Zou J, Zhang A, Wang G, Hao J, Wang Y, Yang S, Pu P. Analysis of hsa-miR-30a-5p Expression in Human Gliomas. Pathol Oncol Res. 2013;19:405–411. doi: 10.1007/s12253-012-9593-x. [DOI] [PubMed] [Google Scholar]
- 34.Jia Z, Wang K, Wang G, Zhang A, Pu P. miR-30a-5p Antisense Oligonucleotide Suppresses Glioma Cell Growth by Targeting SEPT7. PLoS One. 2013;8:e55008. doi: 10.1371/journal.pone.0055008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Borczuk A, Powell C. Expression profiling and lung cancer development; Proc Am Thorac Soc; 2007; pp. 127–132. [DOI] [PubMed] [Google Scholar]