Abstract
Osimertinib, a third-generation epithelial growth factor receptor (EGFR) tyrosine kinase inhibitor, has been demonstrated to be effective for treating patients with T790M-positive advanced non-small cell lung cancer (NSCLC) with a relatively good performance status (grade 0–1). Reports of therapeutic response to osimertinib in advanced NSCLC patients with poor performance status are infrequent. The present case report discusses a patient with advanced lung adenocarcinoma harboring EGFR exon 19 deletion and T790M mutation with central nervous system involvement and poor performance status. The patient had a past history of partial lung resection due to lung adenocarcinoma, positive genetic test for EGFR exon 19 deletion in post-surgical tumor specimens, and therapy with erlotinib and onartuzumab for the appearance of a lung metastatic tumor during the post-surgical follow-up. The combined therapy was continued until the discovery of metastatic tumors in bones and the central nervous system. The Cobas test performed using tissue from bone metastatic tumor was positive for exon 19 deletion and for T790M mutation. The patient was treated with osimertinib and adverse effects or hematological toxicity were not observed. Performance status of the patient improved from grade 4 to 2. Subsequent studies revealed remission of bone metastasis and reduced central nervous system lesions. This report provides evidence on the safety and efficacy of osimertinib for treating NSCLC patients with progressive disease, central nervous system lesion and poor performance status.
Keywords: lung adenocarcinoma, epithelial growth factor receptor mutant, tyrosine kinase inhibitor resistance, performance status, mutation
Introduction
Therapy with first-generation of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) shows significant clinical response and provides progression-free survival benefits vs. chemotherapy in patients with EGFR mutant-positive non-small cell lung cancer (NSCLC) (1). However, most patients have progressive disease after 9 to 14 months of treatment because of acquired resistance to TKI (2). About 60% of acquired resistance is attributable to EGFR T790M mutation (2). Ascending doses of osimertinib (AZD9291) have shown significant efficacy in patients with T790M-positive advanced NSCLC with progressive disease (3,4). However, the therapeutic efficacy and safety of osimertinib in T790M-positive NSCLC patients with central nervous system (CNS) metastasis and poor performance status is not completely clear.
In the present report we presented a case of EGFR-T790M mutant positive NSCLC patient with CNS lesion and poor performance status based on the criteria of the Eastern Cooperative Oncology Group (ECOG-PS4) that effectively responded to osimertinib therapy.
Case report
Seven years before admission, a 72-year old non-smoker female, with no relevant family history, received the diagnosis of lung adenocarcinoma, stage pT2N2M0 and underwent partial resection of the upper and middle lobes of the right lung. The post-surgical clinical stage was IIIA and genetic testing disclosed EGFR exon 19 deletion. Thereafter, the patient was treated with standard chemotherapy including cisplatin/carboplatin in combination with gemcitabine but, during the follow-up evaluation 3 years before admission, a metastatic tumor in the right lung was detected by positron emission tomography (PET) study. The patient was then treated with a combination of erlotinib and onartuzumab and then followed up on a maintenance therapy with oral erlotinib (100 mg). Nine months before admission PET examination disclosed the presence of bone metastasis and the patient was then treated with four cycles of chemotherapy including carboplatin and pemetrexed followed by a maintenance therapy with pemetrexed. One month previous to admission, the patient complained of dizziness and a serum test showed increased values of carcinoembryonic antigen (CEA). A magnetic resonance image of the brain revealed multiple brain metastasis with neurological alterations. Combined therapy with docetaxel and ramucirumab was started but a subsequent magnetic resonance imaging showed enlargement of ventricles (Fig. 1). Based on these observations the malignant disease was judged as being in progressive stage, and the performance status of the patient was grade 4 based on the criteria of the Eastern Cooperative Oncology Group (ECOG). At this clinical stage, the patient was referred to our hospital.
Figure 1.
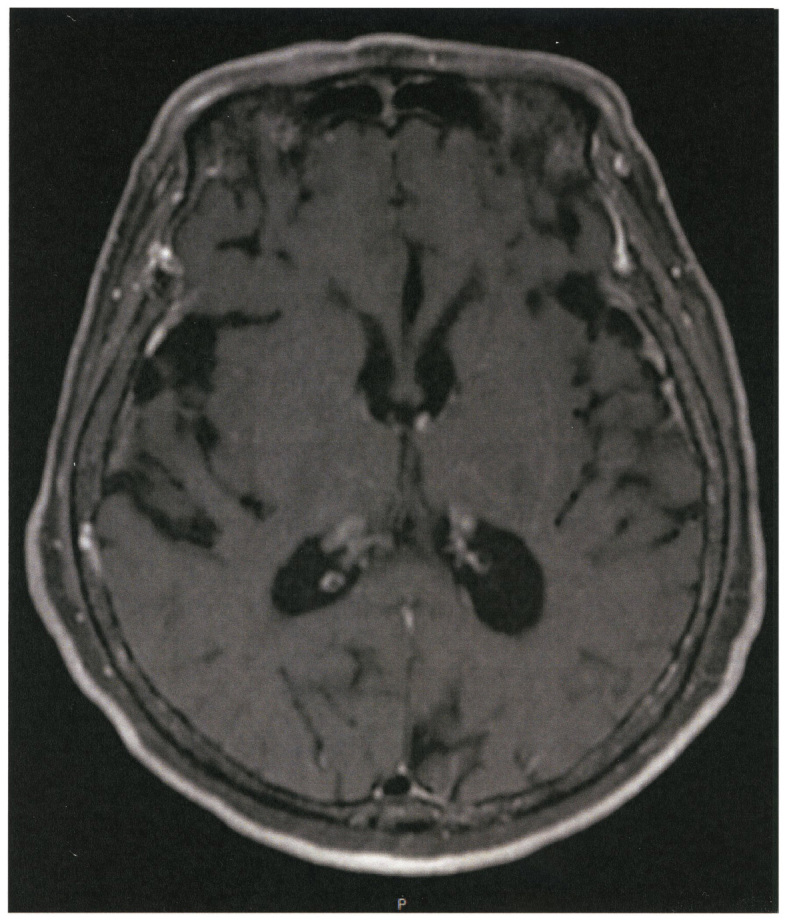
Magnetic resonance imaging of the brain. There was a slight enlargement of the brain ventricles on admission.
On admission, the patient was lucid, heart rate 71/min, blood pressure 164/85 mmHg, oxygen saturation 97% at room air, respiratory and heart sounds were normal, neck stiffness or lymph node enlargement was absent. The chest X-ray and whole body computed tomography (CT) revealed multiple bone metastasis in vertebra and right iliac but no metastasis in the lungs, lymph nodes or abdominal cavity. The blood values of serum albumin were 3.2 mg/dl, lactate dehydrogenase 430 IU/l, sodium 130 mEq/l and CEA 20.0 ng/ml.
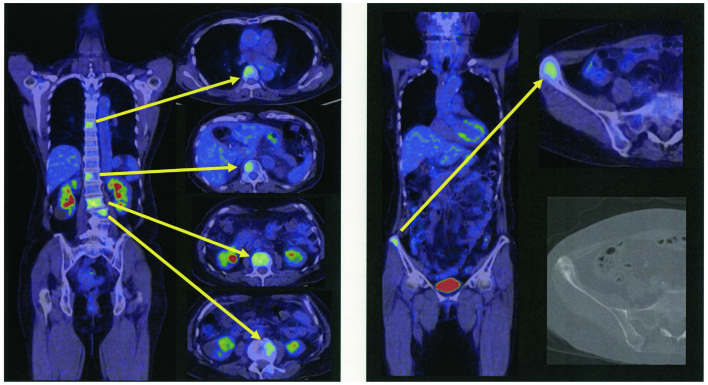
Intravenous hyperalimentation was initiated because the patient was unable to take meals due to poor performance status and nausea. The diagnosis of NSCLC with EGFR exon 19 deletion, resistance to erlotinib, the presence of CNS lesions and the poor performance status of the patient predicted a poor response to therapy with first-generation EGFR-TKIs. The patient was then treated only with best supportive care. In addition, positron emission tomography CT (PET/CT) images from a previous hospital (Fig. 2) revealed several metastatic hot spots in vertebra (Th7, 12, L2, 3, 5) and right ilium. Because the patient and her relatives preferred tumor-reductive therapy rather than palliative treatment, we performed liquid biopsy using plasma and cerebrospinal fluid (CSF) and tissue biopsy from the right ilium on day 6 after admission to rule out EGFR-T790M-related TKI resistance. The biopsy specimen analysis for EGFR mutation performed by peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp was negative for all mutations in plasma samples, positive for exon 19 deletion and for exon 21 mutation but negative for T790M mutation in CSF samples. Cobas test (Cobas EGFR Mutation Test, version 2) performed using CSF samples was positive for exon 19 deletion, but negative for exon 21 and T790M mutations. However, Cobas test performed using tissue from the right ilium was positive for exon 19 deletion, negative for exon 21 mutation but positive for T790M mutation.
Figure 2.
PET and CT study. Bone metastases were evident in both PET and CT studies.
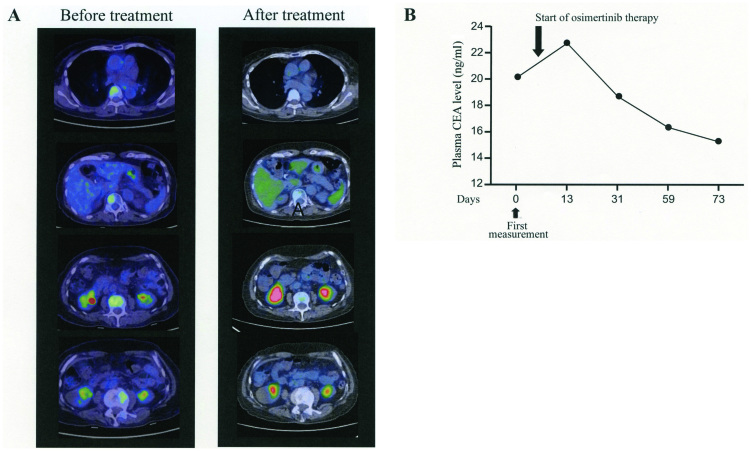
Based on these findings, therapy with osimertinib was initiated on day 9 after admission. Adverse effects including diarrhea, rash, dry skin or tiredness were absent and appetite was significantly increased. There was no hematological toxicity and a magnetic resonance image examination revealed no progression of central nervous system lesions, chest CT showed no drug-induced pneumonia and her performance status gradually improved to grade 2. She was discharged from the hospital. On day 20 of osimertinib therapy, the PET/CT examination revealed remission of bone metastasis with undetectable lesions (Fig. 3). Oral administration of osimertinib was continued and the patient clinical course was followed up in the outpatient department. After 2 months of therapy, MRI and CT images showed no disease lesions and the plasma CEA was gradually decreasing.
Figure 3.
PET study and plasma CEA before and after therapy. (A) PET images prior and after treatment with osimertinib. (B) Plasma CEA values prior and after treatment with osimertinib. CEA, carcinoembryonic antigen.
Discussion
EGFR-TKIs are currently the standard first-line therapy for NSCLC patients with EGFR mutations, but most patients eventually acquire resistance within one year of starting treatment (2). Recurrence of NSCLC tumors after EGFR-TKI therapy has been previously treated with cytotoxic drugs or with different EGFR-TKIs (5). However, cytotoxic drugs are generally ineffective and the clinical efficacy of EGFR-TKIs in patients with poor performance status is very limited. Gefitinib, a first generation EGFR TKI, has shown clinical benefits in terms of response rate and progression-free survival in NSCLC patients with poor performance status harboring EGFR mutations (deletion, L858R, L861Q) other than the T790M mutation (6).
Osimertinib is a third-generation EGFR-TKI that is able to inactivate EGFR harboring T790M mutation. The AURA1/2 clinical study has demonstrated effectiveness of osimertinib in EGFR-T790M mutation-positive NSCLC patients with 66% response rate and a median PFS of 11 months (7). In addition, osimertinib was significantly superior to platinum-pemetrexed treatment in the AURA3 study (8). Osimertinib may also suppress brain metastases from NSCLC tumors compared to gefitinib, afatinib and rociletinib (9). It is worthy to note that most patients that were included in previous clinical trials had performance status of grade 0 to 1. The clinical efficacy of osimertinib in patients with EGFR harboring T790M mutation and with poor performance status has been previously reported in only two cases (10,11). Both previous cases had also advanced lung adenocarcinoma with brain metastasis, received therapy with combined chemotherapy or with first-line TKI (gefitinib, erlotinib or afatinib), were diagnosed as having tumor positive for T790M mutation during disease progression, and showed dramatic response to osimertinib therapy despite poor performance status (10,11). Osimertinib-related adverse effect (leukopenia) was reported only in one of the two previously reported cases (11). Our current patient showed no adverse effect during osimertinib administration.
T790M mutation was detected in samples taken from a metastatic pleural tumor or primary lung tumor but results from liquid biopsy was unavailable in the two previous case reports (10,11). We detected T790M mutation in metastatic bone tumor but the result of liquid biopsy was negative. Although liquid biopsy of the spinal fluid was negative for T790M mutation, the detection rate of T790M in patients with brain metastases has been shown to be as low as 17%. In addition, the AURA study has demonstrated that osimertinib is effective in cases with CNS metastasis and in T790M-positive NSCL tumors with good performance status (9,12). The present report further demonstrates the clinical efficacy of osimertinib in NSCLC patients with brain lesion harboring EGFR T790M mutation and with poor performance status.
This report provides evidence on the safety and efficacy of osimertinib for treating NSCLC patients with CNS lesion and poor performance status.
Written informed consent was obtained from the patient and the report was approved by the Ethical Committee of Matsusaka Municipal Hospital.
References
- 1.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 2.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 4.Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, Yang JC, Barrett JC, Jänne PA. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in Advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Ramalingam SS, Jänne PA, Cantarini M, Mitsudomi T. LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: Updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol. 2016;11(4 Suppl):S152–S153. doi: 10.1016/S1556-0864(16)30325-2. [DOI] [Google Scholar]
- 8.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-Mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 10.Sakai H, Hayashi H, Iwasa T, Hasegawa Y, Takeda M, Nakagawa K. Successful osimertinib treatment for leptomeningeal carcinomatosis from lung adenocarcinoma with the T790M mutation of EGFR. ESMO Open. 2017;2(Suppl 1):e000104. doi: 10.1136/esmoopen-2016-000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uemura T, Oguri T, Okayama M, Furuta H, Kanemitsu Y, Takakuwa O, Ohkubo H, Takemura M, Maeno K, Ito Y, Niimi A. Dramatic intracranial response to osimertinib in a poor performance status patient with lung adenocarcinoma harboring the epidermal growth factor receptor T790M mutation: A case report. Mol Clin Oncol. 2017;6:525–528. doi: 10.3892/mco.2017.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata A, Katakami N, Yoshioka H, Kaji R, Masago K, Fujita S, Imai Y, Nishiyama A, Ishida T, Nishimura Y, Yatabe Y. Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J Thorac Oncol. 2015;10:1553–1559. doi: 10.1097/JTO.0000000000000647. [DOI] [PubMed] [Google Scholar]