Abstract
Heart transplantation has been applied in the clinic as an optimal solution for patients with end stage cardiac failure for a number of years. However, hypothermic preservation of the heart remains limited to 4–6 h and calcium accumulation over time is an important factor resulting in cell death. To provide longer and safer storage for donor hearts, it was demonstrated in our previous study that luteolin, a traditional Chinese medicine used to treat cardiovascular diseases, inhibits cell death and L-type calcium currents during hypothermic preservation. In the current study, the protective role of luteolin in modulating cardiomyocyte calcium cycling was further investigated. Intracellular calcium overload has already been implicated in hypothermia-induced dysfunction of cardiomyocytes. University of Wisconsin (UW) solution supplemented with 7.5, 15 or 30 µmol/l luteolin was used to preserve fresh isolated cardiomyocytes at 4°C. The results demonstrated that all three doses of luteolin supplementation attenuated calcium overload over a 6 h preservation period. Luteolin also suppressed the accumulation of important regulatory proteins and enzymes for cardiomyocyte calcium circulation, mitochondria Ca2+ uniporter and calmodulin, which are normally induced by cold storage in UW solution. Protein Kinase A activity was also suppressed in cardiomyocytes preserved in luteolin supplemented UW solution, while Ca2+-Mg2+-ATPase activity was increased. The results demonstrated that luteolin confers a cardioprotective effect through inhibiting the changes of calcium regulators during cold storage and therefore ameliorates Ca2+ overload in rat cardiomyocytes.
Keywords: cardiomyocyte, luteolin, calcium, hypothermic preservation
Introduction
Heart transplantation (HT) is the most effective treatment for patients with congenital heart disease without suitable alternative treatment options or end-stage heart failure (1,2). Although the demand for HT has increased by 130% over past decades, the number of transplants performed every year increased by 5.2% due to limited organ donation and short-time preservation of the heart (3). Cold ischemic storage of donor hearts is restricted to 4–6 h (4,5), which limits transplantations depending on emergent circumstances and the distance of the donor heart from the potential recipient. Extension of heart preservation may therefore greatly benefit patients waiting for a heart transplantation.
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavone with various pharmacological activities, including scavenging for oxygen free radicals, inhibiting lipid peroxidation, antithrombotic activity and anti-inflammatory responses (6,7). It also has a protective role in ischemia-reperfusion injury by suppressing apoptosis of cardiomyocytes (8,9). As a good candidate for a heart preservation additive, our previous study demonstrated that luteolin enhanced cardiac contractile and diastolic function and coronary flow of rat hearts compared with the control group when used as an additive to University of Wisconsin (UW) solution (10). Luteolin also reduced the L-type calcium current of rat ventricular myocytes in a dose-dependent manner (9). Therefore, it was hypothesized that luteolin may modulate calcium ion homeostasis in order to prolong heart preservation.
Ca2+ homeostasis serves a critical role in maintaining cardiomyocyte function. Intracellular Ca2+ overload may lead to cell death and increased reactive oxygen species (ROS) (11). As a primary transporter for mitochondria Ca2+ influx, mitochondria Ca2+ uniporter (MCU) resides in the inner membrane of mitochondria and allows the cytosolic Ca2+ to be transported into mitochondria (12). Inhibition of MCU has been demonstrated to prevent mitophagy in neurocytes through modulating Ca2+ influx into mitochondria, suggesting low MCU concentration helps maintain mitochondrial morphology and function (13). Protein kinase A (PKA) is a cyclic adenosine monophosphate (cAMP)-dependent protein kinase and regulates L-type voltage-operated calcium channels and intracellular calcium channels by activation of β-adrenergic receptor (β-AR) (14,15). Calmodulin (CaM) is an intracellular Ca2+ sensor, which also serves an indispensible role in intracellular Ca2+ cycling. It transduces intracellular Ca2+ signaling following the binding of Ca2+ and CaM, the product of which is capable of regulating cardiomyocyte excitation-contraction coupling through its binding partner ryanodine receptors (16,17). Ca2+-Mg2+-ATPase, as a calcium pump, regulates ATP-dependent calcium across the sarcoplasmic reticulum (SR) (18). Impairing Ca2+-Mg2+-ATPase activity may lead to increased SR calcium accumulation (19). Understanding the effect of luteolin on these intracellular Ca2+ regulator proteins may shed new light on the process of intracellular Ca2+ overload in cardiomyocytes during hypothermic preservation. In the current study, the effect of different doses of luteolin in UW solution on preserving rat cardiomyocytes was evaluated.
Materials and methods
Reagents
Luteolin (>99% purity) was purchased from Hangzhou FST Pharmaceutical Co., Ltd. (Hangzhou, China). UW solution was purchased from Bristol-Myers Squibb (New York, NY, USA). Dulbecco's modified Eagle's medium (DMEM) solution, pentobarbital sodium and heparin were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The calcium florescent detection kits for mitochondria (cat. no. HL10153.1) and sarcoplasmic reticulum (SR; cat. no. GMS10157.1), and intracellular calcium concentration fluorescent detection kits (cat. no. JM325) were purchased from Shanghai Haling Biological Technology Co., Ltd. (Shanghai, China). Fluo-3 acetoxymethyl (AM) was purchased from Beyotime Institute of Biotechnology (Haimen, China). Collagenase type I was purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Low Ca2+ enzymatic solution (50 µmol/l) was prepared by dissolving 20 mg collagenase type 1, 2 mg protease E (Sigma-Aldrich; Merck KGaA), 10 mg bovine serum albumin (MP Biomedicals, LLC, Santa Ana, CA, USA) and 72 mmol/l calcium chloride into 30 ml calcium free Tyrode's solution, which was composed of 140 mM sodium chloride, 5 mM potassium chloride, 10 mM HEPES, 2 mM BAPTA, 10 mM glucose, and 10 mM Na pyruvate (pH 7.4±0.2).
Animals
A total of 40 6–8 week old healthy male SD rats were purchased from Xi'an Jiaotong University Animal Center (Xi'an, China), weighing 250–300 g. Rats were housed in a pathogen-free environment (temperature, 20–30°C; relative humidity, 45–60%; lighting cycle, 12 h/day) and had ad lbitum access to food and drinking water. All animal experiments were performed according to the Guide of Care and Use of Laboratory Animals (20) and approved by the Animal Care and Use Committee of the Hainan Medical College (Haikou, China).
Heart perfusion and cardiomyocyte isolation
Following sacrifice, rat cardiomyocytes were isolated following a previously described protocol (21). In brief, 30 mg/kg pentobarbital sodium was intraperitoneally injected into the anesthetized rats, followed by 3 mg/kg heparin to prevent blood clot. Heart tissues were quickly isolated following a previously published protocol (10) and were then placed in calcium free Tyrode's solution at 4°C. Isolated rat hearts were first perfused with oxygenated calcium free Tyrode's solution at 37°C until cardiac arrest using the Langendorff technique (22). A low Ca2+ enzymatic solution (50 µmol/l) was then used to further perfuse the rat hearts for 20–40 mins. When the hearts were softened, they were then again perfused with 180–200 µmol/l low Ca2+ enzymatic solution for 5 min. Following the 20 min perfusion process, ventricles were chopped and filtered with Krebs-Bicarbonate (KB) solution composed of 50 mM L-glutamine, 20 mM potassium chloride, 1 mM HEPES, 10 mM glucose, 80 mM potassium hydroxide, 30 mM dipotassium phosphate, 20 mM taurine, 3 mM magnesium sulfate and 0.5 mM EDTA (all Sigma-Aldrich; Merck KGaA) and incubated for 2 h at 37°C. A total of 5×106 harvested cardiomyocytes were then washed with KB solution and preserved in 5 ml UW solution or DMEM. Luteolin was used to supplement UW solution at a low dose of 7.5 µmol/l, middle dose of 15 µmol/l and a high dose of 30 µmol/l. The groups used were as follows: i) A UW group, consisting of cardiomyocytes preserved in UW solution only; ii) a DMEM group, consisting of cardiomyocytes preserved in DMEM only; iii) a 7.5 µmol/l luteolin group, consisting of cardiomyocytes preserved in UW solution supplemented with 7.5 µmol/l luteolin; iv) a 15 µmol/l luteolin group, consisting of cardiomyocytes preserved in UW solution supplemented with 15 µmol/l luteolin; and v) a 30 µmol/l luteolin group, consisting of cardiomyocytes preserved in UW solution supplemented with 30 µmol/l luteolin.
Calcium florescence detection
To detect calcium florescence, 2 µM Fluro-3 AM was incubated with cardiomyocytes for 1 h at 37°C. Samples were washed twice with PBS and observed under a Multiphoton microscope (710 NLO; Carl Zeiss AG, Oberkochen, Germany) with an excitation wavelength of 490 nm and an emission wavelength of 525 nm to detect the level of Ca2+ in the cytosol. DAPI purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China) was used for nuclear staining at room temperature for 5 mins. The same wavelengths were applied for the mitochondria and SR calcium detection kits, which were used according to the manufacturer's protocols. Calcium florescence intensity was analyzed semi-quantitatively by a trained examiner in a blinded manner, with four sections per animal.
ELISA
The levels of CaM and MCU in the cardiomyocytes were examined using a Rat CaM ELISA kit (cat. no. E-EL-M0611) from Elabscience Biotechnology Co., Ltd. (Houston, TX, USA) and Rat MCU ELISA kit (cat. no. ABIN1744117) from Blue Gene Biotech Co., Ltd. (Shanghai, China), respectively. PKA and Ca2+-Mg2+-ATPase activity were quantified by Rat protein kinase A (cat. no. ADI-EKS-390A) and Ca2+-Mg2+-ATPase ELISA kits (cat. no. A070-3) purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA) and Nanjing Jiancheng Bioengineering Institute (Nanjing, China), respectively. Kits were used according to the manufacturers' protocols.
Statistical analysis
All data are presented as the mean ± standard error of the mean. Two-way analysis of variance followed by Bonferroni's correction post hoc test was used for different preservation solutions and times to determine statistical differences between groups. All statistical analyses were performed using GraphPad Prism 6.0 for Macintosh (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Luteolin attenuates intracellular calcium overload in rat cardiomyocytes during hypothermic preservation
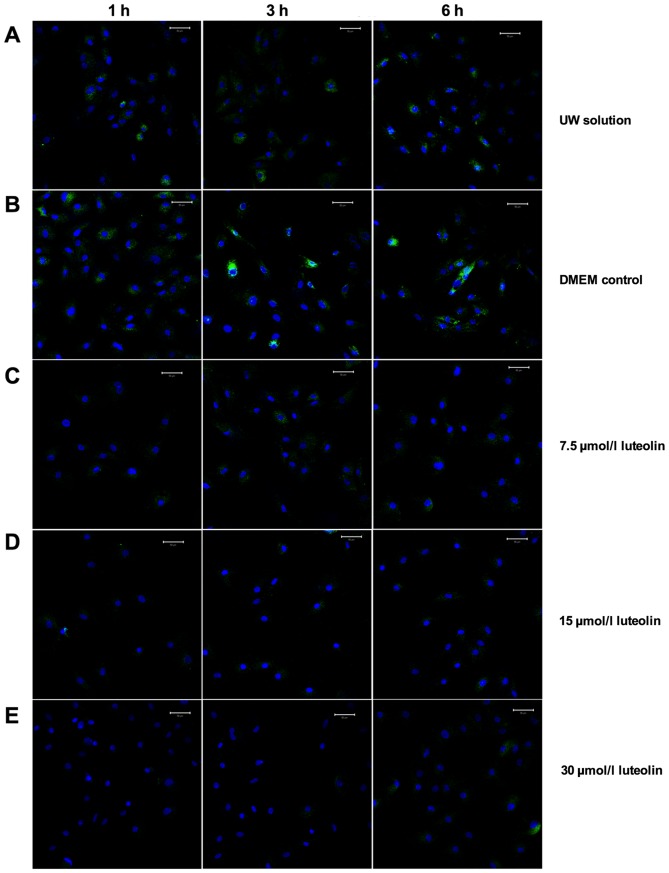
To determine the effect of luteolin on modulating calcium ion cycling, 7.5, 15 and 30 µmol/l luteolin-supplemented UW solution was used to culture cardiomyocytes for 6 h and two control groups consisted of cardiomyocytes preserved in UW solution only or DMEM only. Following the labeling of cytosol free Ca2+ with a Fluro-3 AM fluorescence probe, the confocal microscopy images were captured following 1, 3 and 6 h preservation (Fig. 1). The results demonstrated that universally accepted heart preservation solution, UW solution, had a similar capacity to restrain cytosol free calcium ion load compared with DMEM media (Figs. 1A and B, and 2). There was a significant reduction in cytosol Ca2+ in the three groups using different doses of luteolin to supplement UW solution throughout the 6 h preservation period compared with the UW solution control group (all P<0.01; Figs. 1C-E and 2). The Ca2+ concentration in the cytosol of cardiomyocytes following 1 h UW solution preservation was increased by 31% compared with those preserved with 7.5 µmol/l luteolin for 6 h (Fig. 2). The results therefore demonstrated that luteolin significantly decreases the cytosol Ca2+ concentration in cardiomyocytes during hypothermic preservation (all P<0.01, Fig. 2).
Figure 1.
Luteolin attenuates cytosolic calcium accumulation. Multiphoton microscopy was applied to detect cytosolic Ca2+ in cardiomyocytes preserved in the following solutions: (A) UW solution; (B) DMEM; (C) 7.5 µmol/l luteolin supplemented UW solution; (D) 15 µmol/l luteolin supplemented UW solution and (E) 30 µmol/l luteolin supplemented UW solution. Scale bar, 50 µm. Green fluorescence indicates Fluro-3 labeled Ca2+, while blue fluorescence indicates stained nuclei. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium.
Figure 2.
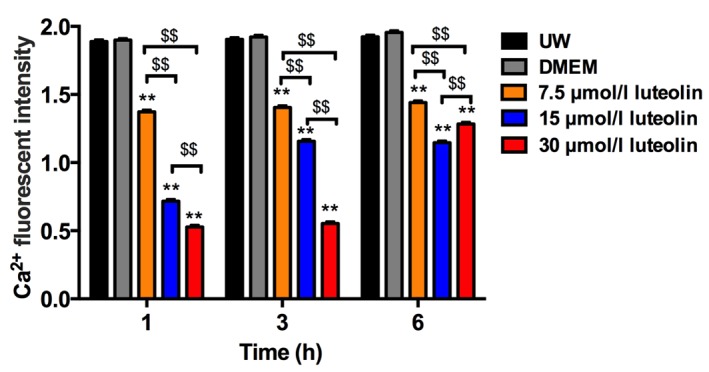
Fluorescence intensity of cytosolic Ca2+ in the different groups. Data are presented as the mean ± standard error of the mean. Experiments were performed in triplicate. **P<0.01 vs. UW solution group; $$P<0.01. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium.
Luteolin inhibits mitochondria calcium overload in rat cardiomyocytes during hypothermic preservation
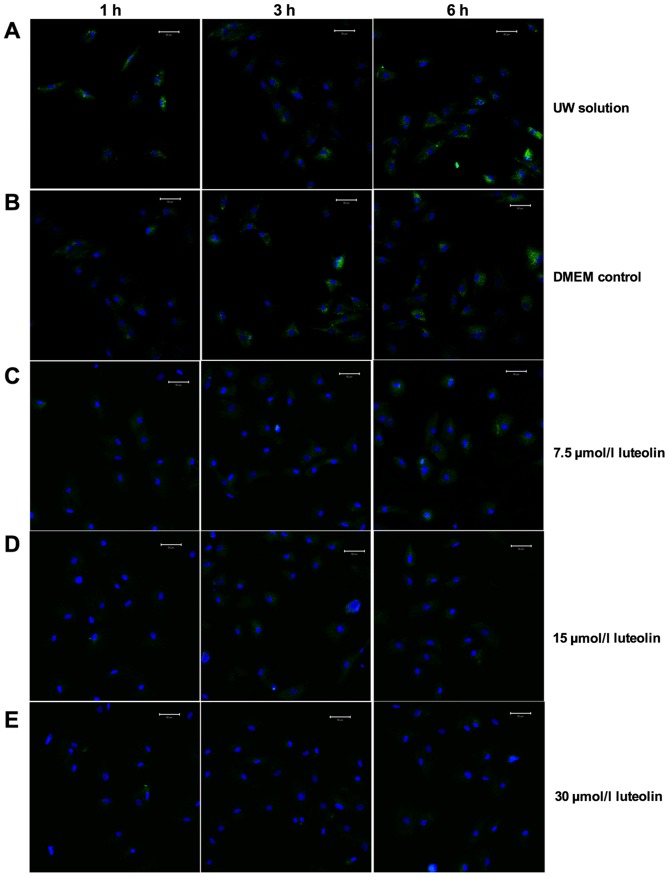
It is known that calcium cycling in cardiomyocytes primarily occurs through calcium channels on the sarcolemma, which allows Ca2+ influx, SR involved intracellular calcium regulation and the transportation pathway of Ca2+ in mitochondria (18). A reduced Ca2+ presence was observed in the cytosol in the treatment groups, therefore, the influence of UW solution supplemented with luteolin on mitochondrial Ca2+ concentration and SR Ca2+ concentration was investigated (Fig. 3). In the DMEM and UW solution control groups, calcium ions accumulated inside the mitochondria during the 6 h cold preservation period (Figs. 3A and B, and 4). The concentration of mitochondrial Ca2+ remained at a significantly lower level following 3 h preservation in cardiomyocytes treated with different luteolin concentrations compared with the UW solution control group (all P<0.01; Figs. 3A and C-E, and 4). When preserved for 6 h in UW solution supplemented with 7.5 µmol/l luteolin, cardiomyocytes exhibited Ca2+ accumulation but remained slightly lower than that in the UW solution group, although this difference was not observed to be significant. The 15 and 30 µmol/l luteolin groups continued to effectively prevent Ca2+ overload in the mitochondria, as the Ca2+ accumulation was significantly decreased compared with that in the UW solution control group (both P<0.01; Figs. 3A, D and E, and 4). When the concentration of luteolin was increased from 7.5 to 15 µmol/l, there was a significant decrease in mitochondria calcium (P<0.01), most markedly following 6 h preservation (Fig. 4). However, the opposite effect was observed when the concentration of luteolin was increased from 15 to 30 µmol/l following 3 and 6 h preservation, suggesting a side effect of using high concentration luteolin for long-term preservation. (Fig. 4).
Figure 3.
Luteolin inhibits calcium accumulation in mitochondria. A mitochondrial calcium detection kit was used to measure Ca2+ changes in the mitochondria of cardiomyocyte preserved in the following solutions: (A) UW solution; (B) DMEM; (C) 7.5 µmol/l luteolin supplemented UW solution; (D) 15 µmol/l luteolin supplemented UW solution and (E) 30 µmol/l luteolin supplemented UW solution. Representative multiphoton microscopy images from indicated time points were captured over the hypothermic preservation period. Scale bar, 50 µm. Green fluorescence indicates fluo-3 labeled Ca2+ while blue fluorescence indicates stained nuclei. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium.
Figure 4.
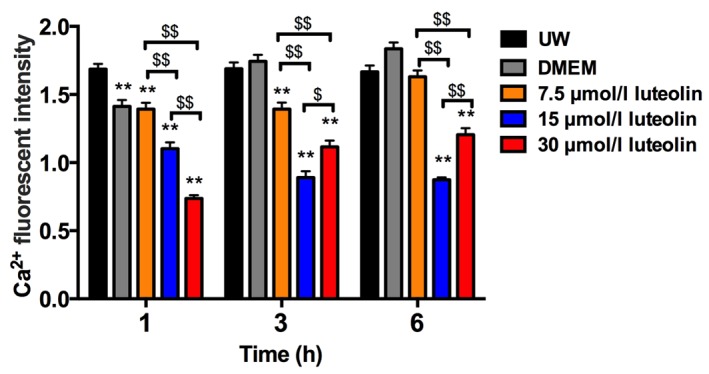
Fluorescence intensity of mitochondrial Ca2+ in the different groups. Data are presented as the mean ± standard error of the mean. Experiments were performed in triplicate. **P<0.01 vs. UW solution group. $$P<0.01, $P<0.05. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium.
Luteolin suppresses SR calcium overload in rat cardiomyocytes during hypothermic preservation
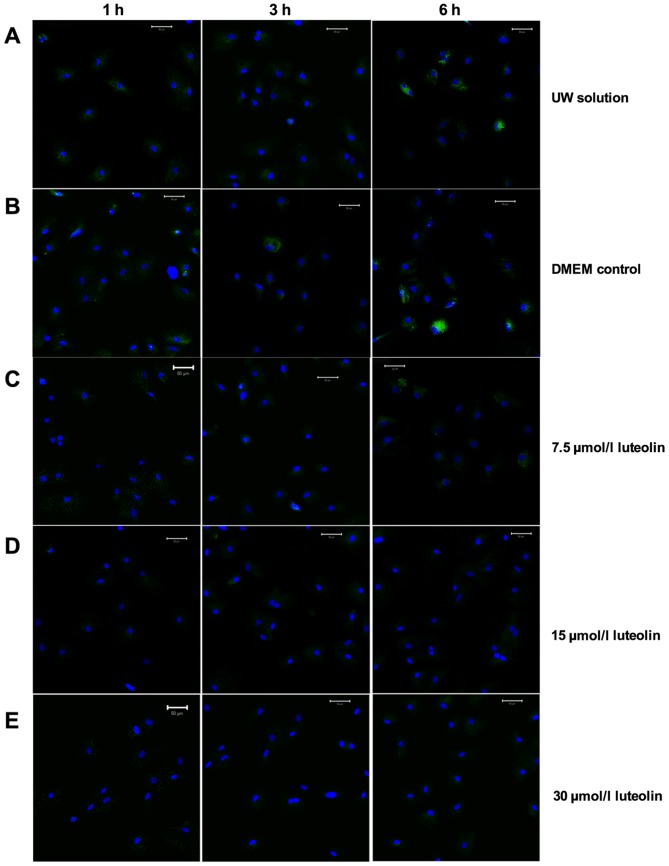
The Ca2+ concentration changes in the SR of cardiomyocytes during the 6 h cold preservation using the same solutions was then investigated (Fig. 5). Similar to the Ca2+ accumulation in the mitochondria, the Ca2+ concentration inside the SR accumulated over time when cardiomyocytes were preserved in UW, and this accumulation was significantly exacerbated in the DMEM group at all three time points (P<0.01; Figs. 5A and B, and 6). The Ca2+ accumulation inside the SR remained at a significantly decreased level in cardiomyocytes preserved in UW solution with 15 or 30 µmol/l luteolin throughout the 6 h preservation period compared with the UW solution control group (P<0.01; Figs. 5A and D, and 6). Even though cardiomyocytes preserved in UW solution with 7.5 µmol/l luteolin started to build up intracellular calcium slightly in the SR after 6 h, there was no significant difference between all three time points (Fig. 6). In addition, compared with the UW solution control group, Ca2+ concentration was significantly decreased in the 7.5 µmol/l luteolin group at all three time points (all P<0.01; Figs. 5A and C, and 6). Together, these results indicate that adding luteolin to UW solution to preserve heart myocytes may inhibit intracellular calcium overload in the SR.
Figure 5.
Luteolin suppresses calcium overload in the SR. An SR calcium kit was used to detect Ca2+ influx in the SR in myocytes preserved in the following solutions: (A) UW solution; (B) DMEM; (C) 7.5 µmol/l luteolin supplemented UW solution; (D) 15 µmol/l luteolin supplemented UW solution; and (E) 30 µmol/l luteolin supplemented UW solution. Representative multiphoton microscopy images from indicated time points were captured over the hypothermic preservation period. Scale bar, 50 µm. Green fluorescence indicates fluo-3 labeled Ca2+, while blue fluorescence indicates stained nuclei. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium; SR, sarcoplasmic reticulum.
Figure 6.
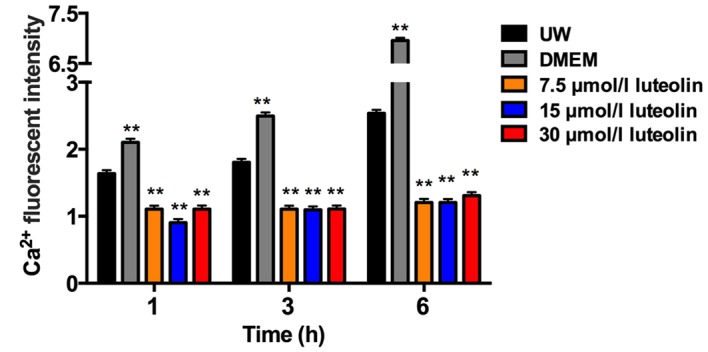
Fluorescence intensity of Ca2+ in the SR in different groups. Data are presented as the mean ± standard error of the mean. Experiments were performed in triplicate. **P<0.01 vs. UW solution group. UW solution, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium; SR, sarcoplasmic reticulum.
Luteolin represses CaM and MCU accumulation in rat cardiomyocytes during hypothermic preservation
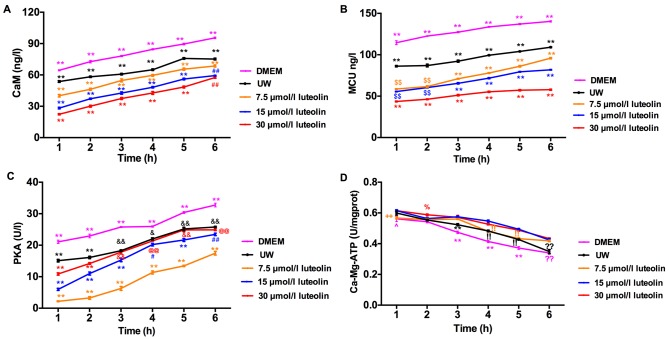
The effect of luteolin supplementation on calcium signaling-related proteins during preservation was investigated. CaM concentration in rat cardiomyocytes during 6 h hypothermic preservation was determined first (Fig. 7). Myocytes preserved with DMEM in the control group expressed a significantly higher CaM compared with UW solution (P<0.01). All three groups of cardiomyocytes preserved in UW solution supplemented with different doses of luteolin had a significantly lower CaM concentration compared with the UW solution control group (all P<0.01; Fig. 7A). The higher the dose of luteolin used to treat cardiomyocytes, the lower the CaM concentration throughout the 6 h preservation period. The differences between luteolin treatment groups at each time point were significant (P<0.01) except for cardiomyocytes preserved with 15 and 30 µmol/l luteolin during the 6 h preservation period (Fig. 7A). The differences between the CaM concentration of cardiomyocytes preserved in the UW solution control group at 1 h and the cardiomyocytes preserved in UW solution supplemented with 30 µmol/l luteolin for 6 h, or cardiomyocytes preserved in UW solution supplemented with 15 µmol/l luteolin for 5 h were not significant. This suggests that the presence of luteolin during hypothermic preservation may repress CaM accumulation in cardiomyocytes and thus prolong preservation time.
Figure 7.
Luteolin exerts a cardioprotective effect on inhibiting the changes of intracellular calcium regulatory proteins, including MCU, CaM, PKA and Ca2+-Mg2+-ATPase during hypothermic preservation. ELISA was used to determine the concentration of (A) CaM and (B) MCU, and the activity of (C) PKA and (D) Ca2+-Mg2+-ATPase in myocytes during the 6 h preservation with DMEM (magenta line), UW solution (black line), 7.5 µmol/l luteolin (orange line), 15 µmol/l luteolin (blue line) and 30 µmol/l luteolin (red line), respectively. Data are presented as the mean ± standard error of the mean. Experiments were performed in triplicate. **P<0.01 vs. all other groups; ##P<0.01 vs. DMEM, UW and 7.5 µmol/l luteolin; $$P<0.01 vs. DMEM, UW and 30 µmol/l luteolin; &&P<0.01 vs. DMEM, and 7.5 and 15 µmol/l luteolin; %P<0.05 vs. UW and DMEM; !!P<0.01 vs. DMEM, and 15 and 30 µmol/l luteolin; @@P<0.01 vs. DMEM and 7.5 µmol/l luteolin; ^P<0.05 vs. UW, and 15 and 30 µmol/l luteolin; ++P<0.01 vs. 15 and 30 µmol/l luteolin. ??P<0.01 vs. UW, 7.5 µmol/l, 15 and 30 µmol/l luteolin. UW, University of Wisconsin solution; DMEM, Dulbecco's modified Eagle's medium; ns, not significant; MCU, mitochondria Ca2+ uniporter; CaM, calmodulin; PKA, protein kinase A.
Luteolin had an effect on limiting the accumulation of Ca2+ in the mitochondria, therefore, it was hypothesized that MCU, which is a vital transporter for mitochondria Ca2+ entry, may be decreased during luteolin preservation to suppress Ca2+ influx into the mitochondria. MCU concentration was determined by ELISA and the results indicated that luteolin supplementation at all doses reduced MCU levels compared with the UW solution control group (all P<0.01; Fig. 7B). Compared with DMEM, utilizing UW solution reduced MCU expression over the 6 h preservation period, whereas luteolin supplemented UW solution further significantly reduced the MCU content in a dose dependent manner, from 3–6 h, for luteolin treated cardiomyocytes (all P<0.01; Fig. 7B). The concentration of MCU was significantly lower following the 6 h preservation period with 30 µmol/l luteolin compared with 1 h preservation in the UW solution control group (P<0.01). However, no significant difference was observed between myocytes preserved with 15 µmol/l luteolin for 5 h and the UW solution group following 1 h preservation. The results imply that cold preservation of cardiomyocytes in UW solution supplemented with luteolin results in decreased MCU concentration, which serves a role in restricting mitochondrial Ca2+ influx.
Luteolin suppresses PKA activity and enhances Ca2+-Mg2+-ATPase activity during cold preservation
The role of PKA in luteolin prolonged heart preservation was investigated by measuring PKA enzymatic activity, as PKA is known to phosphorylate calcium regulatory proteins, including phospholamban (PLB) and ryanodine receptor (RyR) 2 (23,24). Cardiomyocytes preserved in UW solution supplemented with different doses of luteolin exhibited a reduction in PKA activity compared with the UW solution control group (Fig. 7C); however, unlike MCU and CaM, cardiomyocytes treated with 7.5 µmol/l luteolin exhibited the biggest reduction compared with cardiomyocytes treated with 15 and 30 µmol/l luteolin (Fig. 7C). Nevertheless, even in cardiomyocytes treated with 30 µmol/l luteolin, which may have a toxic effect on PKA activity, the PKA activity was significantly decreased compared with the UW solution control group at 1–2 h (P<0.01; Fig. 7C). Compared with the UW solution control group, PKA was significantly decreased in the 7.5 and 15 µmol/l luteolin treatment groups at all time points (P<0.01). When cardiomyocytes were preserved in DMEM, PKA activity was significantly increased compared with the UW solution control group (P<0.01), while adding luteolin to UW solution further suppressed PKA activity.
Ca2+-Mg2+-ATPase activity was measured using ELISA. The results indicated that luteolin supplementation increased Ca2+-Mg2+-ATPase activity, with the most marked differences compared with the UW control group observed with 15 and 30 µmol/l luteolin treatment, and significant differences at 4–6 h (P<0.01; Fig. 7D). There was a significant increase in Ca2+-Mg2+-ATPase activity when comparing the response in cardiomyocytes treated with 7.5 and 15 µmol/l at 1, 4 and 5 h (P<0.01), however, there was no difference in Ca2+-Mg2+-ATPase activity when comparing cardiomyocytes preserved with 15 to 30 µmol/l luteolin at the different time points during the 6 h preservation period.
Discussion
The current accepted method of preservation for donor hearts is to cold preserve them in UW solution for 4–6 h prior to transplantation (4). However, short-term preservation limits the feasibility of transplantation surgery when cytotoxic events are initiated in cardiomyocytes, including the accumulation of ROS and Ca2+, which eventually results in cell death (25–27). The results of the present study demonstrated that hypothermia may induce Ca2+ accumulation in the cytosol, mitochondria and SR. Using a guinea pig heart as a model, a previous study indicated that cardioplegia may decrease cytosolic calcium accumulation and lead to better cardiac function following reperfusion (25). This suggests that suppression of Ca2+ overload during preservation may provide better protection to the heart and prolong storage time. The current study demonstrates the protective role of the flavonoid compound luteolin as an additive to UW solution, in modulating calcium cycling during hypothermal preservation of rat cardiomyocytes. Luteolin exists widely in plants, including peanut and chrysanthemum (28). The cardioprotective role of luteolin has been established in rabbit and rat hearts associated with ischemia (8,29). However, to the best of our knowledge, there are no reports on the role served by luteolin in heart preservation and calcium signaling of cardiomyocytes. Our previous work demonstated that supplementation of luteolin into UW solution effectively inhibits the L-type calcium currents in hypoxia treated rat cardiomyocytes and provides better protection for rat hearts following 12 or 18 h hypothermic preservation in terms of contractile and diastolic function, enhancing coronary flow and preventing creatine kinase leakage (10). Consistent with this, the results of the current study demonstrated the role of luteolin in directly ameliorating intracellular Ca2+ overload through modulating regulatory proteins, including CaM, PKA, MCU and Ca2+-Mg2+-ATPase during a 6 h preservation period.
Calcium serves a critical role in regulating cardiomyocyte function including contraction, transcription regulation and cell death (14,23). Hypothermia induces intracellular Ca2+ accumulation, which may promote the activation of Ca2+-dependent proteases, leading to the apoptosis and necrosis of cardiomyocytes (26). Calcium ion influx into cardiomyocytes through a Ca2+ current is dependent on L-type voltage-operated calcium channels, which are regulated by the activation of β-AR, downstream PKA and Na+/Ca2+ exchange (NCX) (23,30). The major pathway used by calcium to exit cells is through plasma membrane NCX and the Ca2+-Mg2+-ATPase pump. The SR and mitochondria are also critical for the removal of cytosolic calcium. Cytosolic Ca2+ is transported through the MCU into the mitochondria, while SR Ca-ATPase (SERCA) mediates the majority of calcium ion uptake (14,23). Using a fluorescent probe to detect Ca2+ in the current study, confocal microscopy data indicated that Ca2+ was decreased in the cytosol, mitochondria and SR during the 6 h cold preservation period with luteolin-supplemented UW solution. Furthermore, the Ca2+ load in the cytosol, mitochondria and SR of cardiomyocytes following 6 h preservation with luteolin was decreased compared with that in those preserved with UW solution alone for 1 h. There was no significant accumulation of Ca2+ in SR over time observed in the cardiomyocytes preserved with UW solution supplemented with different doses of luteolin during 6 h preservation. By contrast, Ca2+ accumulated in the SR over time in the UW solution control group. Even though the dose response was not significant between the luteolin-treated groups in preventing intracellular Ca2+ overload, supplementing luteolin to cardiomyocytes was able to effectively suppress Ca2+ accumulation in the SR which stayed at a basal level of Ca2+ concentration at the end of the 6 h preservation period.
Hypothermia-induced mitochondria Ca2+ accumulation is harmful to mitochondrial functions, including the generation of ATP (25). Suppression of mitochondrial Ca2+ overload may ameliorate abnormal function in the mitochondria following reperfusion (31). A recent study also demonstrated the inhibition of MCU, which is responsible for mitochondrial Ca2+ uptake and prevents mitophagy in neurocytes following ischemia/reperfusion injury (13). In the current study, during hypothermic preservation with luteolin-supplemented UW solution, upregulation of MCU over time was significantly delayed in cardiomyocytes. Bonferroni post-hoc comparison indicated that myocytes preserved for 5 h in 7.5 µmol/l luteolin supplemented UW solution expressed similar MCU to those preserved for 1 h with UW solution only. Delayed MCU increase was extended to 6 h in the two groups preserved with higher concentrations of luteolin. This further indicated the protective role of luteolin in slowing hypothermia-induced dysfunction in cardiomyocytes.
Besides mitochondria, the importance of the SR in modulating calcium homeostasis in cardiomyocytes has been established previously. The SR absorbs Ca2+ through SERCA, which requires energy consumption and is regulated through the phosphorylation of PLB and the release of Ca2+ through the RyR (14). PKA, Ca2+-Mg2+-ATPase and CaM are involved in this SR mediated calcium exchanging. The activation of PKA, which is mediated through an increasing level of cAMP, modulates L-type calcium channel opening (32,33). In the current study, it was demonstrated that luteolin inhibits PKA activity in cardiomyocytes preserved with luteolin. This inhibition may restrict L-type calcium channel opening and thereby L-type calcium currents. Furthermore, PLB is polymerized through phosphorylation mediated by PKA. Polymerized PLB is unable to interact with SERCA and results in Ca2+ accumulation in the SR (34,35). Thus, suppression of PKA activity by luteolin may lead to the depolymerization of PLB, which decreases pump activity and subsequently inhibits Ca2+ influx into the SR (23). CaM is a calcium binding protein that regulates Ca2+ efflux via RyR and Ca-dependent inactivation of the L-type calcium current (14). The results of the ELISAs performed in the current study indicated that luteolin decreased CaM concentration and delayed its accumulation in cardiomyocytes during cold storage in a dose-dependent manner. Free calcium ions from the cytosol bind to form a Ca-CaM complex, therefore the decreased CaM concentration in myocytes preserved in luteolin supplemented UW solution may be due to the enhanced interaction between Ca-CaM, which inactivates the L-type calcium channels and decelerates Ca2+ influx (36). This increased Ca-CaM formation may also consume the cytosolic Ca2+ and subsequently decrease Ca2+ accumulation. As a critical enzyme in the SR that participates in Ca2+ influx, Ca2+-Mg2+-ATPase positively regulates the transportation of Ca2+ across the cell membrane via the hydrolysis of ATP (37). The current study indicated that there was enhanced Ca2+-Mg2+-ATPase activity in cardiomyocytes preserved in UW solution supplemented with luteolin, although the dose effect was only observed between cardiomyocytes preserved with 7.5 and 15 µmol/l luteolin-supplemented UW solution. The deactivation of Ca2+-Mg2+-ATPase is exacerbated during hypothermic preservation, leading to intracellular calcium accumulation through modulating SR Ca2+ uptake (19). However, the results of the current study suggest that the addition of luteolin to UW solution to preserve cardiomyocytes may repress this process and ameliorate Ca2+ overload. The activity of Ca2+-Mg2+-ATPase following 3 h preservation with the three different doses of luteolin was similar to that of cardiomyocytes preserved with UW solution for 1 h, which indicates that luteolin serves a protective role in the extension of rat heart preservation. This novel effect of luteolin on cardiomyocyte calcium cycling may shed new light on extending heart hypothermic preservation.
In conclusion, there were decreased concentrations of MCU and CaM, decreased PKA enzyme activity and enhanced Ca2+-Mg2+-ATPase activity in the cardiomyocytes preserved with luteolin and UW solution for 6 h. These results indicate that luteolin may reduce intracellular Ca2+ through restrained Ca2+ influx and enhanced Ca2+ extruding. The results indicated that intracellular Ca2+ overload in rat cardiomyocytes was suppressed by the addition of luteolin. Together, the alteration of Ca2+ homeostasis-regulating proteins by luteolin may be the reason why it inhibits intracellular Ca2+ accumulation and subsequently prolongs heart preservation. This novel effect of luteolin on cardiomyocyte calcium cycling may shed new light on extending heart hypothermic preservation. Adding luteolin to UW solution to preserve the heart may extend the preservation time which may greatly facilitate clinical heart transplantation.
Acknowledgements
This study was supported by a grant from the Natural Science Foundation of China (grant no. 81360029) and Natural Science Foundation of Hainan Province (grant no. 809042). The authors would like to thank BersinBio (GuangZhou, China) for their technical assistance.
References
- 1.Alsoufi B, Deshpande S, McCracken C, Kogon B, Vincent R, Mahle W, Kanter K. Outcomes and risk factors for heart transplantation in children with congenital heart disease. J Thorac Cardiovasc Surg. 2015;150:1455–1462.e3. doi: 10.1016/j.jtcvs.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Vitali E, Colombo T, Fratto P, Russo C, Bruschi G, Frigerio M. Surgical therapy in advanced heart failure. Am J Cardiol. 2003;91:88F–94F. doi: 10.1016/S0002-9149(02)03343-X. [DOI] [PubMed] [Google Scholar]
- 3.Colvin M, Smith JM, Skeans MA, Edwards LB, Uccellini K, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 annual data report: Heart. Am J Transplant. 2017;17(Suppl 1):S286–S356. doi: 10.1111/ajt.14128. [DOI] [PubMed] [Google Scholar]
- 4.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Jahania MS, Sanchez JA, Narayan P, Lasley RD, Mentzer RM., Jr Heart preservation for transplantation: Principles and strategies. Ann Thorac Surg. 1999;68:1983–1987. doi: 10.1016/S0003-4975(99)01028-0. [DOI] [PubMed] [Google Scholar]
- 6.Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Kim YS, Shin CH, Lee HJ, Kim S. Antithrombotic activities of luteolin in vitro and in vivo. J Biochem Mol Toxicol. 2015;29:552–558. doi: 10.1002/jbt.21726. [DOI] [PubMed] [Google Scholar]
- 8.Qi L, Pan H, Li D, Fang F, Chen D, Sun H. Luteolin improves contractile function and attenuates apoptosis following ischemia-reperfusion in adult rat cardiomyocytes. Eur J Pharmacol. 2011;668:201–207. doi: 10.1016/j.ejphar.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan QF, Yan GF, Yang DK. Myocardial protective effects of luteolin on isolated rat heart in hypothermic preservation. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28:154–158. (In Chinese) [PubMed] [Google Scholar]
- 11.Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: At the heart of mitochondrial calcium signalling. J Mol Cell Cardiol. 2009;46:781–788. doi: 10.1016/j.yjmcc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn GW, II, Maack C. SR and mitochondria: Calcium cross-talk between kissing cousins. J Mol Cell Cardiol. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Yu S, Zheng S, Leng J, Wang S, Zhao T, Liu J. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci Lett. 2016;628:24–29. doi: 10.1016/j.neulet.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol. 2011;3:a004242. doi: 10.1101/cshperspect.a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:1–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Membr Biol. 2002;185:1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 17.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura J. pH and temperature resolve the kinetics of two pools of calcium bound to the sarcoplasmic reticulum Ca2+ -ATPase. J Biol Chem. 1989;264:17029–17031. [PubMed] [Google Scholar]
- 19.Morris GL, Cheng HC, Colyer J, Wang JH. Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca(2+)-Mg2+)-ATPase. Mechanism of regulation and site of monoclonal antibody interaction. J Biol Chem. 1991;266:11270–11275. [PubMed] [Google Scholar]
- 20.National Research Council, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academy Press; Washington, DC: 2011. [Google Scholar]
- 21.Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 24.Müller BA, Dhalla NS. Mechanisms of the beneficial actions of ischemic preconditioning on subcellular remodeling in ischemic-reperfused heart. Curr Cardiol Rev. 2010;6:255–264. doi: 10.2174/157340310793566118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stowe DF, Varadarajan SG, An J, Smart SC. Reduced cytosolic Ca(2+) loading and improved cardiac function after cardioplegic cold storage of guinea pig isolated hearts. Circulation. 2000;102:1172–1177. doi: 10.1161/01.CIR.102.10.1172. [DOI] [PubMed] [Google Scholar]
- 26.Wakayama K, Fukai M, Yamashita K, Kimura T, Hirokata G, Shibasaki S, Fukumori D, Haga S, Sugawara M, Suzuki T, et al. Successful transplantation of rat hearts subjected to extended cold preservation with a novel preservation solution. Transpl Int. 2012;25:696–706. doi: 10.1111/j.1432-2277.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- 27.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17 degrees C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 29.Rump AF, Schüssler M, Acar D, Cordes A, Ratke R, Theisohn M, Rösen R, Klaus W, Fricke U. Effects of different inotropes with antioxidant properties on acute regional myocardial ischemia in isolated rabbit hearts. Gen Pharmacol. 1995;26:603–611. doi: 10.1016/0306-3623(94)00209-6. [DOI] [PubMed] [Google Scholar]
- 30.Weber CR, Piacentino V, III, Houser SR, Bers DM. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation. 2003;108:2224–2229. doi: 10.1161/01.CIR.0000095274.72486.94. [DOI] [PubMed] [Google Scholar]
- 31.Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am J Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 32.Fusi F, Manetti F, Durante M, Sgaragli G, Saponara S. The vasodilator papaverine stimulates L-type Ca(2+) current in rat tail artery myocytes via a PKA-dependent mechanism. Vascul Pharmacol. 2016;76:53–61. doi: 10.1016/j.vph.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 33.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 34.Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- 35.Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- 36.Tang W, Halling DB, Black DJ, Pate P, Zhang JZ, Pedersen S, Altschuld RA, Hamilton SL. Apocalmodulin and Ca2+ calmodulin-binding sites on the CaV1.2 channel. Biophys J. 2003;85:1538–1547. doi: 10.1016/S0006-3495(03)74586-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson JE, Lokesh BR, Kinsella JE. Ca2+-Mg2+ ATPase of mouse cardiac sarcoplasmic reticulum is affected by membrane n-6 and n-3 polyunsaturated fatty acid content. J Nutr. 1989;119:364–372. doi: 10.1093/jn/119.3.364. [DOI] [PubMed] [Google Scholar]