Abstract
An increased neutrophil-to-lymphocyte ratio (NLR) is associated with poorer prognostic outcomes in numerous types of cancer. However, a small number of studies have demonstrated the prognostic role of NLR in patients with laryngeal cancer. The present study evaluated the association between NLR and survival outcomes in patients with laryngeal squamous cancer. All patients were scheduled for follow-up visits. The levels of cytokines from tumor tissues were analyzed by ELISA. A classification and regression tree (CART) was used to determine the optimal cutoff values of NLR. The clinical features and NLR were determined using Kaplan-Meier analysis and Cox regression to analyze the survival outcomes and associated risks. Of the total 654 patients, 70 patients (70/654; 10.7%) failed to receive follow-up. Blood and biochemical parameters, including NLR, platelet-to-lymphocyte ratio and albumin-to-globulin ratio were associated with clinical characteristics of the patients, with the exception of histologic grade. Only one node with NLR at 3.18 divided patients into different categories, according to CART analysis. Survival analysis demonstrated that NLR at cutoff values subdivided patients into different survival outcomes (P<0.001). Subsequent to adjustments for age and other clinical features, NLR was identified to be an independent prognostic factor for overall survival and progression-free survival (P<0.05). Increased levels of cytokines, including IL-6 and IL-8, in tumor tissues were associated with NLR values. In summary, pre-treatment NLR was associated with the prognostic outcomes for patients with laryngeal cancer, and may assist to establish prognostic factors for these patients.
Keywords: laryngeal cancer, survival rate, neutrophils, lymphocytes, interleukin-6, interleukin-8, classification and regression tree
Introduction
According to the current estimates of the American Cancer Society, laryngeal cancer is one of the most common types of cancer of the upper aerodigestive tract (1). It was estimated that ~13,360 incident cases of laryngeal cancer and 3,660 mortalities would occur by 2017 in the United States (2). Changes in diagnostic and therapeutic approaches have increased the rates of larynx preservation and survival (3). However, the 5-year survival rate of patients with advanced-stage laryngeal cancer, particularly stage IV patients, remains low globally, including China (4,5). In addition, patients undergoing aggressive treatments experience a significant reduction in quality of life, including speech, eating, social disruption and aesthetics (4). Therefore, prognostic evaluation and treatment decisions based on clinical pathological features should be implemented according to novel risk stratification, which is based on novel markers for patients with laryngeal cancer, including inflammation-based prognostic scores including the Glasgow Prognostic Score, neutrophil-to-lymphocyte ratio (NLR) and Prognostic Nutritional Index (6).
The inflammatory response serves a vital role in the development and progression of a number of solid tumors. Neutrophils and platelets supply the required bioactive molecules, including angiogenic, epithelial, and stromal growth factors and matrix-remodeling enzymes, for neoplastic progression (7,8). In addition, conditions that induce compromised cell-mediated immunity, such as lymphocytopenia and an impaired T-lymphocytic response, reflect imbalances in the innate and adaptive immune systems, which compromises effective host-tumor immune responses (9,10). Therefore, the combination of neutrophils, platelets and lymphocytes, as markers of host inflammation, has been identified to be an independent prognostic factor in different malignancies (11,12). An increased NLR or platelet-to-lymphocyte ratio (PLR) is associated with poor outcomes in various tumors, including colorectal, primary liver, lung, urinary, cervical, oropharyngeal squamous cell or advanced esophageal cancer (13–15). Furthermore, serum albumin has been identified to be a sensitive and reliable marker of systemic inflammation in patients with cancer (16). However, the application and credibility of albumin level as a marker is limited owing to its interference by numerous factors, including the peritoneal burden of vascular endothelial growth factor (17). The albumin-to-globulin ratio (AGR), which takes into account the level of albumin and globulin, reflects the body nutritional status (18,19). AGR was also identified to be an effective prognostic factor for advanced malignancies, including non-small cell lung cancer (20). Risk stratification based on these factors and clinical pathological features may underlie the optimal treatment decision-making and prognostic evaluation.
However, limited data are available on the prognostic role of these indices in patients with laryngeal squamous cancer (21,22). Rassouli et al (21) demonstrated that systemic inflammatory markers NLR and PLR were independent prognostic factors of head and neck squamous cell carcinoma. However, this was a heterogeneous study that included only a small number of patients with laryngeal cancer. Kum et al (22) indicated that the mean NLR of patients with precancerous laryngeal lesion and laryngeal squamous cell carcinoma was significantly increased compared with patients with benign laryngeal lesion and without prognostic evaluation. At present, there is a lack of data on the evaluation of prognosis with these readily available and inexpensive biomarkers for patients with laryngeal squamous cancer. The present study evaluated the association between these indices and survival outcomes of patients with laryngeal squamous cell cancer.
Materials and methods
Patients and data collection
Patients identified with laryngeal cancer confirmed by pathological diagnosis at the West China Hospital (Chengdu, China) between September 2008 and September 2013 were enrolled in the retrospective study. All the cases were scheduled for regular follow-up visits (1, 2, 3, 6, 12, 24, 36 and 60 months post-operation) at an outpatient department in West China Hospital (Chengdu, China). Patients absent from regular follow-up visits received follow-up by telephone, and the end-point of these patients were collected by the Disease Surveillance Point System in the Sichuan Province Center for Disease Control and Prevention. The current status (succumbed, recurrent or in remission), date of recurrence, date of mortality, and associated cause of recurrence or mortality were recorded.
The inclusion criteria were as follows: i) Pathological diagnosis of squamous cell cancer; and ii) routine complete blood counts (CBCs) tests with differential counting and serum biochemical analysis. The exclusion criteria were: i) Presence of infection, connective tissue diseases or any other disease affecting blood cells; ii) patients who discontinued treatment or were treated outside of West China Hospital; iii) absence of CBC and serum biochemical analysis prior to treatment; iv) patients who succumbed prior to discharge from hospital following initial treatment; v) presence of symptoms and signs of hepatic function damage that may affect AGR; and vi) and presence of other tumors. The present study was approved by the Institutional Ethics Committee of the West China Hospital, Sichuan University (Chengdu, China), and written informed consent were obtained.
A total of 654 patients were enrolled for the present study, and 85 patients were enrolled for cytokine testing in tumor tissues. Patient characteristics such as age, sex, American Joint Committee on Cancer histologic grade (23), pathological diagnosis [including Tumor Node Metastasis (TNM) Classification of Malignant Tumors staging] (23), tumor location, date of diagnosis and treatment were recorded from the electronic hospital information system (HIS). In addition, hospital examination included CBC with differential counting, and several biochemical indices such as globulin and albumin were also recorded from HIS at the West China Hospital.
Freshly obtained laryngeal cancer tissue specimens, obtained during the operation, were processed and assayed for interleukin (IL)-6 and IL-8 levels. Briefly, the samples were homogenized and centrifuged at 3,000 × g for 10 min at 4°C, and the supernatants were stored at −80°C until analysis. The samples were assayed for IL-6 (cat. no. D6050; R&D Systems China Co., Ltd., Shanghai, China) and IL-8 (cat. no. D8000C; R&D Systems China Co., Ltd.) using commercially available ELISA kits (R&D Systems China Co., Ltd.), according to the manufacturer's protocol.
NLR and PLR ratio
NLR was calculated by dividing the number of neutrophils by the number of lymphocytes obtained from the CBCs. PLR was calculated by dividing the number of platelets by the number of lymphocytes, and AGR was calculated by dividing the level of albumin by the level of globulin obtained from biochemical analysis of the blood. All three ratios were obtained from the CBCs prior to the initiation of treatment. Progression-free survival (PFS) and overall survival (OS) are presented in months.
A review of the literature revealed heterogeneity in the NLR, PLR and AGR cutoff points used in various malignances (14,24), including head and neck squamous cancer (21,22). Patients were grouped according to the cut-off points for NLR, PLR, AGR and age. The classification and regression tree (CART) algorithm was used to produce predictive rules and improve the accuracy of survival prediction (25–27).
Statistical analysis
All parameters including CBCs with differential counting, biochemical indices, and NLR, PLR and AGR were compared among different clinical characteristics by Kruskal-Wallis test. CART analysis was performed in SPSS (version no. 17.0; SPSS, Inc., Chicago, IL, USA) to generate predictive rules and optimal cutoff points. Survival outcomes were compared using Kaplan-Meier analyses with log-rank tests. All the clinical pathological features and NLR were included in the univariate analysis, and then factors with significance values of P<0.10 in a univariate analysis were included in the multivariable analyses using Cox's proportional hazards model to determine the hazard ratio of survival. The differences in the level of cytokines between groups with different NLR values were compared using the Mann-Whitney test, and the correlation between cytokines with NLR was analyzed using Pearson's correlation test. For statistical analysis, Statistical Package for Social Sciences (version 18.0; SPSS, Inc.) was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
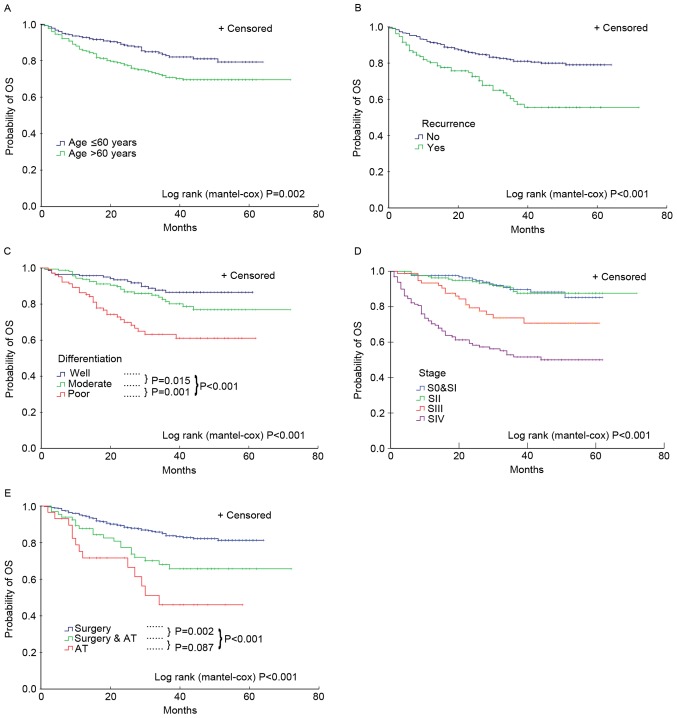
Of the total 654 patients enrolled in the present study, 70 patients (70/654; 10.7%) were not followed up for overall survival (median follow-up, 36 months; interquartile range (IQR), 28–46 months). A total of 49 patients (7.5%) failed to receive follow-up for PFS at a median follow-up of 33 months (IQR, 18–41 months). Overall, 200 patients (200/654; 30.6%) exhibited disease progression, and 128 patients (128/654; 19.6%) succumbed to cancer. The 5-year survival rates were 85, 81, 70 and 51% for patients with laryngeal cancer at TNM stages I, II, III and IV, respectively. Additionally, the 3-year survival rate was 87%, and the survival rate of glottic carcinoma was increased significantly compared with other types of cancer in the larynx, which were 87 and 53% (P<0.05), respectively. Finally, factors, including age >60 years, non-glottic carcinoma, high histologic grade, high staging, extensive treatment and recurrence were associated with increased probability of overall mortality (Fig. 1) and disease progression (data not shown).
Figure 1.
Association between clinical features of patients and increased probability of overall mortality. The patients were divided distinct overall survival outcomes by (A) age (cutoff value, age ≤60 and >60), (B) whether recurrence occurred, (C) pathology grades, (D) tumor-node-metastasis stages and (E) treatment methods. AT, adjuvant therapy; OS, overall survival; S, stage.
Association of blood and biochemical parameters with clinical characteristics of patients with laryngeal cancer
There were no differences observed among the histologic grades for the median of whole white blood cell (WBC) and various differential counts from CBC analysis, including hemoglobin, globulin and albumin levels, and NLR, PLR and AGR. All increased parameters (including WBC count and various differential counts from CBC analysis, including hemoglobin, globulin and albumin levels, and NLR, PLR and AGR) were associated with the higher T classification and TNM stage (P<0.01). All increased parameters, with the exception of WBC, lymphocyte and monocyte counts, were associated with higher N classifications (P<0.01; Table I).
Table I.
Blood and biochemical parameters of patients with laryngeal squamous cell cancer.
| Parameters (IQR) | WBC (×109/l) (IQR) | Neutrophil (×109/l) (IQR) | Lymphocyte (×109/l) (IQR) | Monocyte (×109/l) (IQR) | Platelet (×109/l) (IQR) | Hb (g/l) (IQR) | Globulin level (g/l) (IQR) | Albumin level (g/l) (IQR) | NLR (IQR) | PLR (IQR) | GAR (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=654) | 6.31 (5.39–7.46) | 3.89 (3.13–4.86) | 1.75 (1.39–2.19) | 0.37 (0.29–0.47) | 162 (127–207) | 145 (135–154) | 26.6 (24.1–29.5) | 43.6 (41–45.6) | 2.18 (1.58–3.10) | 92.4 (67.9–122.8) | 0.48 (0.38–0.63) |
| Histologic grade | |||||||||||
| Well (n=149) | 6.35 (5.38–7.26) | 3.81 (3.08–4.61) | 1.77 (1.45–2.22) | 1.77 (1.45–2.22) | 164 (130–208) | 147 (136–155) | 27.1 (24.1–30) | 43.9 (41.2–46.2) | 2 (1.55–2.81) | 94.1 (72.3–113.6) | 0.61 (0.56–0.68) |
| Moderately (n=224) | 6.34 (5.43–7.44) | 3.83 (3.05–4.92) | 1.74 (1.39–2.19) | 1.74 (1.39–2.19) | 176 (135–220) | 145 (137–153) | 26.1 (23.5–28.9) | 43.7 (41–45.4) | 2.18 (1.57–3.18) | 101 (70.8–127.1) | 0.61 (0.53–0.68) |
| Poorly (n=143) | 6.22 (5.23–7.67) | 3.89 (3.15–4.88) | 3.89 (3.15–4.88) | 1.72 (1.35–2.05) | 162 (124–212) | 145 (135–154) | 27 (24.8–29.7) | 42.9 (40.5–45.1) | 2.34 (1.71–3.18) | 93.5 (67.8–129.7) | 0.62 (0.57–0.7) |
| P-value | 0.916 | 0.754 | 0.346 | 0.519 | 0.246 | 0.37 | 0.061 | 0.121 | 0.128 | 0.154 | 0.143 |
| T stage | |||||||||||
| T1 (n=186) | 6.21 (5.16–7.05) | 3.65 (2.93–4.42) | 1.81 (1.46–2.31) | 0.36 (0.29–0.44) | 149 (119–183) | 147 (136–155) | 26.4 (23.7–28.7) | 44.3 (41.7–46) | 1.96 (1.43–2.64) | 80.5 (60.7–109.9) | 0.6 (0.53–0.67) |
| T2 (n=213) | 6.11 (5.33–7.04) | 3.69 (2.99–4.33) | 1.82 (1.49–2.18) | 0.35 (0.28–0.46) | 164 (128–204) | 149 (139–156) | 26.1 (23.8–28.4) | 44 (41.7–46) | 1.94 (1.51–2.61) | 88.4 (66.1–115.8) | 0.6 (0.54–0.67) |
| T3 (n=103) | 6.34 (5.43–7.73) | 3.92 (3.24–4.88) | 1.8 (1.38–2.15) | 0.38 (0.29–0.47) | 167 (126–211) | 146 (138–153) | 27.2 (24.6–29.7) | 44.4 (42.2–45.7) | 2.18 (1.75–2.88) | 94.7 (69.3–125.2) | 0.61 (0.55–0.7) |
| T4 (n=136) | 6.97 (5.5–8.62) | 4.53 (3.41–6.22) | 1.57 (1.24–1.99) | 0.42 (0.32–0.55) | 176 (135–226) | 140 (131–150) | 27.9 (24.5–30.5) | 41.9 (39.3–44.3) | 3.01 (1.98–4.29) | 108.5 (80.6–159.1) | 0.66 (0.58–0.75) |
| P-value | 0.001 | <0.001 | <0.001 | 0.002 | 0.002 | <0.000 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 |
| N stage | |||||||||||
| N0 (n=535) | 6.22 (5.38–7.32) | 3.81 (3.05–4.63) | 1.78 (1.43–2.21) | 0.37 (0.3–0.47) | 158 (126–200) | 147 (137–155) | 26.5 (23.8–28.9) | 44 (41.5–45.9) | 2.09 (1.55–2.88) | 88.4 (65.4–117.6) | 0.61 (0.54–0.68) |
| N1 (n=57) | 6.49 (5.07–7.46) | 3.95 (3.07–5.19) | 1.63 (1.3–2) | 0.34 (0.29–0.47) | 189 (141–226) | 140 (130–152) | 27.8 (25.8–30.1) | 41.8 (38.9–44.5) | 2.19 (1.66–3.15) | 104.7 (83.6–139) | 0.66 (0.59–0.73) |
| N2 and N3 (n=55) | 7.13 (5.47–8.25) | 4.3 (3.52–5.91) | 1.79 (1.32–2.13) | 0.42 (0.33–0.54) | 184 (128–252) | 140 (134–150) | 28.1 (25.3–30.9) | 42.2 (39.5–44.8) | 2.58 (1.82–3.83) | 101.5 (68.3–169) | 0.5 (0.42–0.65) |
| P-value | 0.056 | 0.011 | 0.092 | 0.061 | 0.003 | 0.004 | 0.001 | <0.001 | 0.006 | <0.001 | <0.001 |
| TNM stage | |||||||||||
| 0 and I (n=182) | 6.21 (5.13–7.05) | 3.66 (2.90–4.42) | 1.80 (1.46–2.31) | 0.36 (0.29–0.44) | 149 (119–183) | 147 (137–155) | 26.4 (23.7–28.7) | 44.3 (41.7–46.0) | 1.96 (1.43–2.64) | 81.3 (60.7–109.9) | 0.60 (0.53–0.67) |
| S II (n=199) | 6.11 (5.34–7.06) | 3.66 (2.99–4.35) | 1.82 (1.49–2.19) | 0.36 (0.30–0.46) | 160 (127–203) | 149 (139–156) | 26.0 (23.8–28.5) | 44.0 (41.9–46.0) | 1.95 (1.51–2.63) | 87.2 (64.2–115.0) | 0.60 (0.54–0.67) |
| S III (n=104) | 6.22 (5.33–7.31) | 3.83 (3.12–4.64) | 1.80 (1.47–2.14) | 0.37 (0.28–0.47) | 168 (132–217) | 146 (137–152) | 27.0 (24.7–29.4) | 44.1 (41.0–45.5) | 2.16 (1.73–2.70) | 95.2 (70.0–120.5) | 0.62 (0.55–0.70) |
| S IV (n=164) | 7.11 (5.69–8.62) | 4.65 (3.52–6.25) | 1.58 (1.23–2.06) | 0.42 (0.32–0.54) | 180 (135–227) | 140 (130–150) | 28.0 (14.7–30.5) | 42.0 (39.3–44.5) | 2.94 (1.92–4.37) | 106.5 (79.4–159.4) | 0.66 (0.58–0.76) |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
For each index, some data could not be collected, thus the total number differs. WBC, white blood cells; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; GAR, globulin-to-albumin ratio; TNM, tumor node metastasis; IQR, interquartile range.
NLR value categorizes patients into groups with different survival and clinical features
Using the recursive partitioning statistical approach, with OS status as a dependent variable, while age, NLR, PLR and AGR were independent variables, only one node with a NLR value of 3.18 divided patients into different groups. Patients with a NLR >3.18 exhibited significantly decreased OS and PFS compared with patients with ≤3.18, as demonstrated by the Kaplan-Meier survival curve (Fig. 2). Patients with NLR ≤3.18 exhibited lower probability of overall mortality compared with patients with NLR >3.18 (3-year OS for NLR ≤3.18 vs. >3.18, 84.36 vs. 58.58%; log-rank, P<0.001; Fig. 2A) and disease progression (3-year PFS for NLR ≤3.18 vs. >3.18, 71.87 vs. 48.41%; log-rank, P<0.001; Fig. 2B).
Figure 2.
Survival curves for OS and PFS between patients with NLR ≤3.18 and >3.18. The cutoff values of NLR differentiated patients into two survival outcomes with log-rank P<0.001. NLR at cutoff value subdivided the patients into distinct (A) OS outcomes and (B) PFS outcomes. HR, hazard ratio; OS, overall survival; PFS, progression-free survival; NLR, neutrophil-to-lymphocyte ratio.
There was no significant difference in median age and histologic grade between patients with NLR below and above the cutoff value (Table II). The NLR cutoff value subdivided patients into different proportion of T and N classification and TNM stage (P<0.001; Table II). Patients with a NLR >3.18 experienced significantly more invasive procedures including surgery (P<0.001) and neck dissection (P=0.012) (Table II).
Table II.
Patient characteristics.
| A, Patient characteristics | ||||
|---|---|---|---|---|
| NLR | ||||
| Total, (n=654) | ≤3.18 | >3.18 | P-value | |
| Age | ||||
| Median | 61 (54–67) | 60 (54–67) | 62 (54–68) | 0.184 |
| ≤60, n (%) | 324 (49.5) | 207 (52.1) | 117 (45.5) | 0.098 |
| >60, n (%) | 330 (50.5) | 210 (47.9) | 141 (54.7) | |
| Sex | ||||
| Female, n (%) | 17 (2.6) | 8 (1.8) | 9 (3.5) | 0.159 |
| Male, n (%) | 637 (97.4) | 436 (98.2) | 248 (96.5) | |
| B, Disease characteristics | ||||
| NLR | ||||
| Total, (n=654) | ≤3.18 | >3.18 | P-value | |
| Region, n (%) | ||||
| Glottic laryngeal cancer | 478 (73.1) | 332 (81.9) | 144 (57.8) | <0.001 |
| Histologic grade | ||||
| Well-differentiated | 149 (28.9) | 105 (32.1) | 44 (23.7) | 0.100 |
| Moderately differentiated | 224 (43.4) | 138 (42.2) | 83 (44.6) | |
| Poorly differentiated | 143 (27.7) | 84 (25.7) | 59 (31.7) | |
| T stage, n (%) | ||||
| pT0, T1 | 186 (29.2) | 136 (32.9) | 49 (22.4) | <0.001 |
| pT2 | 213 (33.4) | 159 (38.5) | 54 (24.7) | |
| pT3 | 103 (16.1) | 68 (16.5) | 35 (16) | |
| pT4 | 136 (21.3) | 50 (12.1) | 81 (37) | |
| N stage, n (%) | ||||
| pN0 | 535 (82.7) | 357 (85.5) | 176 (78.2) | 0.011 |
| pN1 | 57 (8.8) | 33 (7.9) | 22 (9.8) | |
| pN2 | 49 (7.6) | 24 (5.8) | 24 (10.7) | |
| pN3 | 6 (0.9) | 2 (0.5) | 3 (1.3) | |
| Stage, n (%) | ||||
| Early 0 | 31 (4.8) | 20 (4.8) | 11 (4.7) | <0.001 |
| I | 151 (23.3) | 114 (27.3) | 37 (15.9) | |
| II | 199 (30.7) | 146 (35) | 53 (22.8) | |
| Late III | 104 (16) | 71 (10.9) | 33 (14.2) | |
| IV | 164 (25.3) | 66 (15.8) | 98 (42.2) | |
| Recurrence, n (%) | 157 (24) | 81 (20.4) | 76 (29.5) | 0.008 |
| C, Treatment characteristics | ||||
| NLR | ||||
| Total, (n=654) | ≤3.18 | >3.18 | P-value | |
| Surgery, n (%) | ||||
| Larynscopy | 227 (32.3) | 170 (41) | 66 (28.1) | <0.001 |
| Partial laryngectomy | 253 (36) | 180 (43.4) | 73 (31.1) | |
| Total laryngectomy | 164 (23.3) | 65 (15.7) | 96 (40.9) | |
| Neck dissection, n (%) | ||||
| None | 515 (78.7) | 310 (74.9) | 159 (66.2) | 0.012 |
| Unilateral | 117 (16.6) | 71 (17.1) | 45 (18.8) | |
| Bilateral | 71 (10.1) | 33 (8.0) | 36 (15.0) | |
| Chemotherapy, n (%) | ||||
| Yes | 96 (14.7) | 62 (14.4) | 34 (18.0) | 0.253 |
| Radiotherapy, n (%) | ||||
| Yes | 59 (9.0) | 32 (7.9) | 27 (10.8) | 0.212 |
For each index, some data could not be collected, thus the total number differs. All data are presented as the median (interquartile range) or number (percent). NLR, neutrophil-to-lymphocyte ratio; pT, pathological T stage; pN, pathological N stage.
Univariate and multivariable analysis indicates that NLR is a risk factor for OS and PFS
All the factors analyzed in univariate analysis with Cox's proportional hazards model were identified to be associated with an increased risk of mortality and disease progression, including histologic grade, pathological diagnosis, TNM staging, tumor location, recurrence, treatment and NLR (P<0.05; Tables III and IV). When adjusted in multivariable Cox's models, non-glottic cancer, poor cancer cell differentiation, late stage, recurrence and NLR >3.18 were identified to be associated with an increased risk of mortality [NLR HR, 1.901; 95% confidence interval (CI), 1.153–3.135; P=0.012; Table III]. Furthermore, NLR >3.18 was also identified to be associated with increased risk of disease progression (NLR HR, 1.621; 95% CI, 1.094–2.404; P=0.016; Table IV).
Table III.
Univariate and multivariable analyses of hazard ratio for overall survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
| NLR | ||||
| >3.18 vs. ≤3.18 | 3.254 (2.171–4.877) | <0.0001 | 1.901 (1.153–3.135) | 0.012 |
| Age, years | ||||
| >60 vs. ≤60 | 1.720 (1.153–2.567) | 0.008 | 0.077 | |
| Tumor location | ||||
| Glottic vs. non-glottic | 4.833 (3.260–7.163) | <0.001 | 1.858 (1.071–3.223) | 0.028 |
| Histologic grade | 1.528 (1.036–2.254) | 0.032 | ||
| Moderately vs. well differentiated | 2.822 (1.281–6.220) | 0.010 | ||
| Poorly vs. well differentiated | 5.662 (2.552–12.565) | <0.001 | ||
| TNM stage | 1.582 (1.208–2.072) | 0.001 | ||
| II vs. I | 1.163 (0.538–2.515) | 0.701 | ||
| III vs. I | 3.464 (1.654–7.255) | 0.001 | ||
| IV vs. I | 6.654 (3.524–12.566) | <0.001 | ||
| Recurrence | ||||
| Yes vs. no | 2.610 (1.745–3.904) | <0.001 | 1.884 (1.122–3.163) | 0.017 |
| Surgery | 0.842 | |||
| Total LE vs. partial LE | 3.669 (2.397–5.616) | <0.001 | ||
| Non-surgery vs. Partial LE | 6.968 (3.975–12.217) | <0.001 | ||
| Neck dissection | ||||
| Yes vs. no | 3.391 (2.224–5.168) | <0.001 | 0.075 | |
| Chemotherapy | ||||
| Yes vs. no | 2.396 (1.422–4.037) | 0.001 | 0.402 | |
| Radiotherapy | ||||
| Yes vs. no | 2.474 (1.608–3.808) | <0.001 | 0.239 | |
Data analyzed using Cox's proportional hazard regression model. HR for multivariate analyses for categories with a P>0.05 (age, surgery, neck dissection, chemotherapy and radiotherapy) were not calculated. CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; LE, laryngectomy; TNM, tumor-node-metastasis; HR, hazard ratio.
Table IV.
Univariate and multivariable analyses of hazard ratio for progression-free survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| NLR | ||||
| >3.18 vs. ≤3.18 | 2.191 (1.582–3.035) | <0.001 | 1.621 (1.094–2.404) | 0.016 |
| Age | ||||
| >60 vs. ≤60 | 0.081 | 0.609 | ||
| Tumor location | ||||
| Non-glottic vs. glottic | 2.518 (1.867–3.398) | <0.001 | 1.604 (1.062–2.422) | 0.025 |
| Histologic grade | 1.485 (1.139–1.938) | 0.004 | ||
| Moderately vs. well differentiated | 1.576 (0.978–2.539) | 0.061 | ||
| Poorly vs. well differentiated | 2.821 (1.731–4.598) | <0.001 | ||
| TNM stage | 0.692 | |||
| II vs. I | 0.839 (0.530–1.329) | 0.454 | ||
| III vs. I | 1.609 (0.985–2.629) | 0.058 | ||
| IV vs. I | 2.548 (1.702–3.814) | <0.001 | ||
| Surgery | 1.445 (0.967–2.159) | 0.073 | ||
| Total LE vs. partial LE | 2.158 (1.560–2.986) | <0.001 | ||
| Non-surgery vs. partial LE | 3.323 (2.009–5.494) | <0.001 | ||
| Neck dissection | ||||
| Yes vs. no | 1.665 (1.207–2.298) | 0.002 | 0.596 | |
| Chemotherapy | ||||
| Yes vs. no | 1.999 (1.298–3.079) | 0.002 | 0.063 | |
| Radiotherapy | ||||
| Yes vs. no | 1.755 (1.224–2.516) | 0.002 | 0.221 | |
Data analyzed using Cox's proportional hazards regression model. HR for multivariate analyses for categories with a P>0.05 (age, surgery, neck dissection, chemotherapy and radiotherapy) were not calculated. CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; LE, laryngectomy; TNM, tumor-node-metastasis; HR, hazard ratio.
NLR is associated with the levels of IL-6 and IL-8
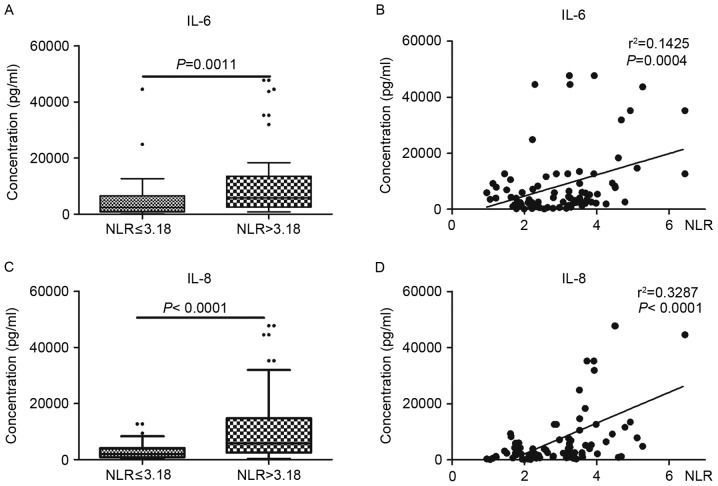
To confirm the association between NLR and the level of cytokines in tumor tissues, the levels of inflammatory mediators in laryngeal cancer tissues were examined. As indicated in Fig. 3, the levels of IL-6 and IL-8 were significantly increased in tumor tissues with higher NLR values (NLR >3.18) compared with lower NLR values (NLR ≤3.18) (P<0.01). Additionally, there was a significant association between the levels of cytokines and NLR value (P<0.001).
Figure 3.
NLR is associated with the level of IL-6 and IL-8. (A) The level of IL-6 was significantly increased in tumor tissues with higher NLR values (NLR >3.18) compared with lower NLR values (NLR ≤3.18). (B) There was a significant association between the levels of IL-6 and NLR values. (C) The level of IL-8 was significantly increased in tumor tissues with higher NLR values compared with lower NLR values. (D) There was a significant association between the levels of IL-8 and NLR values. NLR, neutrophil-to-lymphocyte ratio; IL, interleukin.
Discussion
Association of systemic inflammation with adverse outcomes in malignancies
Systemic hematological markers that represent the inflammatory response of the body, including neutrophils, lymphocytes and platelet counts, either alone or expressed as ratios, have been used as prognostic factors associated with malignancies (28). The prognostic role of these markers is attributed to the infiltration of the immune cells such as neutrophils and lymphocytes in solid tumors and inflammation at the majority of cancer stages (28). Previously, studies on different malignancies have demonstrated that higher NLR and PLR values were associated with poorer prognoses in terms of mortality and recurrence (24,29). Serum albumin and globulin belong to a separate class of biochemical markers included in clinical routine blood examinations. These are also used as prognostic factors in various types of cancer. Serum albumin generally reflects the severity of disease and the nutritional status of the body (30). In addition, it is also used to assess the progression and prognosis of certain malignancies such as operable colorectal cancer, advanced non-small cell lung cancer and ovarian cancer (16,27,28). Concurrently, globulin was identified to be associated with certain types of hormone-associated cancer with poor survival outcomes (19,31,32), and AGR (serum chemistry indexes for globulin and albumin levels together) has been identified to function as an effective prognostic factor for patients with cancer (20). In the present study, the levels of cytokines (IL-6 and IL-8) were examined in tumor tissues, and it was identified that the level of NLR was associated with the levels of IL-6 and IL-8 in tumor tissues. As previously reported (33), cytokines may be secreted into blood as chemokines of neutrophils to elevate neutrophil levels.
Association of NLR, PLR and AGR with the severity of laryngeal cancer
The results of the present study indicated that the medians of NLR, PLR and AGR were significantly increased with increased T and N classifications as well as TNM stage, respectively. Additionally, patients with NLR above the cutoff values (NLR >3.18) demonstrated higher proportions of higher T, N and clinical stage tumors. However, NLR was not associated with histologic grades. The findings of the present study were different from the results by Rassouli et al (21), where NLR and PLR values were analysed in head and neck squamous cell carcinoma. Rassouli et al (21) demonstrated that there was no significant increase in NLR values with high T and N classifications, and TNM stage. It was also demonstrated that a higher NLR was associated with an increased proportion of higher T classification in patients with other types of cancer (21), which was consistent with the data of the present study.
Identification of optimal cutoff with minimum bias with CART analysis
CART analysis has been used to estimate the survival probability of individual patients with tumor (breast, head and neck tumor) and to select immune markers for tumor diagnosis (25–27). CART analysis also has been used in the study of unknown primary carcinoma to estimate the survival probability of individual patients and additionally in the analysis of recurrence in breast cancer following radiation and chemotherapy (24,25). A number of previous studies have revealed the heterogeneity of NLR (cut-off, 1.9–7.2) (29) and PLR (cut-off, 100–300) (24) when predicting prognostic outcomes (23,28). This may be attributed to the use of different approaches in determining cutoff values in different populations. In a study on head and neck squamous cell carcinoma by Rassouli et al (21), a recursive partitioning statistical approach was used to determine the cut-off points of NLR (cut-off value, 3) and PLR (cut-off value, 170).
However, in other studies investigating cancer, mean (22), median (34) and ROC curve (20) were used to determine the cut-off points, which may have led to heterogeneity in data. These cut-off values may differ from the optimal original values for adverse outcomes. The present study used a CART algorithm to produce the predictive rules. Age, NLR, PLR and AGR were entered into the analysis as independent variables, while overall survival status was considered as a dependent variable, and only one node with NLR at 3.18 divided patients into different groups. PLR, AGR and age were excluded. Therefore, in the present study, the optimal cutoff for NLR was identified as 3.18.
NLR predicts prognosis in patients with laryngeal cancer
To the best of our knowledge, the present study investigated the largest sample size for the prognostic ability of NLR as an independent factor in patients with laryngeal squamous cell cancer. NLR, at the determined cutoff points (NLR ≤3.18 and >3.18), was able to differentiate the patients into two groups with significantly different prognoses for OS and PFS. The groups above the cutoff value (NLR >3.18) exhibited poorer prognoses in the survival analysis. This result is consistent with previous studies in a number of other malignancies (24,29). Furthermore, NLR above the cutoff points was also associated with an increased risk of mortality and disease progression in the univariate analysis with Cox's regression model. Subsequent to adjustment for age, pathological grade, TNM stage, treatment and recurrence, a higher HR for OS was demonstrated for NLR values above the cutoff point (NLR >3.18).
Previous studies with large patient populations have also indicated the prognostic role of NLR in various malignancies (29). Therefore, the authors of the present study hypothesized that with a large sample size, collection of more detailed information and reduction in the loss of follow-up, consistent or more accurate results may be obtained. The results of the present study indicate that NLR, as an index of the systemic inflammatory response, may predict prognosis in patients with laryngeal cancer.
In conclusion, the results of the present study have demonstrated that pretreatment NLR, PLR and serum AGR may be associated with the severity of laryngeal cancer, and NLR may serve as a useful prognostic predictor for patients with laryngeal cancer. With these readily available and inexpensive biomarkers, prognostic factors may be established for clinical decisions, including stringent follow-up and additional adjunctive therapy, to improve the stratification of patients with laryngeal cancer.
Acknowledgements
The authors would like to thank Professor Yajia Lan (School of Public Health, West China Medical Center, Sichuan University, Chengdu, China) for his assistance in statistical analysis, and Xiaofang Chen (Sichuan Province Center for Disease Control and Prevention, Chengdu, China) for her assistance in investigating the survival status of patients using the Disease Surveillance Point System. The authors would also like to thank Professor Hengyi Xiao (Laboratory for Aging Research, Center for Medical Stem Cell Biology, State Key Laboratory of Biotherapy, West China Hospital, China) for her assistance in reviewing the manuscript.
Glossary
Abbreviations
- NLR
neutrophil-to-lymphocyte ratio
- IL-6
interleukin-6
- IL-8
interleukin-8
- OS
overall survival
- PFS
progression-free survival
- CART
classification and regression tree
References
- 1.Cahlon O, Lee N, Le QT, Kaplan MJ, Colevas AD. https://clinicalgate.com/cancer-of-the-larynx/ [Apr 9;2015 ];Cancer of the larynx. [Google Scholar]
- 2.American Cancer Society, corp-author. What are the key statistics about laryngeal and hypopharyngeal cancers. https://www.cancer.org/cancer/laryngeal-and-hypopharyngeal-cancer/about/key-statistics.html. [Jan 5;2017 ]. https://www.cancer.org/cancer/laryngeal-and-hypopharyngeal-cancer/about/key-statistics.html
- 3.Barzan L, Talamini R, Franchin G, Vaccher E, Politi D, Minatel E, Gobitti C. Changes in presentation and survival of head and neck carcinomas in Northeastern Italy, 1975–1998. Cancer. 2002;95:540–552. doi: 10.1002/cncr.10682. [DOI] [PubMed] [Google Scholar]
- 4.Woodard TD, Oplatek A, Petruzzelli GJ. Life after total laryngectomy: A measure of long-term survival, function, and quality of life. Arch Otolaryngol Head Neck Surg. 2007;133:526–532. doi: 10.1001/archotol.133.6.526. [DOI] [PubMed] [Google Scholar]
- 5.Ji W, Guan C, Pan Z. Analysis of curative effects on laryngeal carcinoma patients in the northeast region of China. Acta Otolaryngol. 2008;128:574–577. doi: 10.1080/00016480701596104. [DOI] [PubMed] [Google Scholar]
- 6.Hermanns T, Bhindi B, Wei Y, Yu J, Noon AP, Richard PO, Bhatt JR, Almatar A, Jewett MA, Fleshner NE, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788–793. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- 11.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, Garvey C, Hughes M, Raraty M, Campbell F, Neoptolemos JP. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658–666. doi: 10.1016/j.surg.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 14.Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: A meta-analysis. PLoS One. 2014;9:e96072. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: A meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 17.Bizzo SM, Meira DD, Lima JM, Mororó Jda S, Moreira FC, Casali-da-Rocha JC, Ornellas MH. Serum albumin and vascular endothelial growth factor in epithelial ovarian cancer: Looking at adnexal tumor drainage. Arch Gynecol Obstet. 2011;283:855–859. doi: 10.1007/s00404-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 18.Fujii T, Sutoh T, Morita H, Katoh T, Yajima R, Tsutsumi S, Asao T, Kuwano H. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012;64:1169–1173. doi: 10.1080/01635581.2012.718034. [DOI] [PubMed] [Google Scholar]
- 19.Adly L, Hill D, Sherman ME, Sturgeon SR, Fears T, Mies C, Ziegler RG, Hoover RN, Schairer C. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer. 2006;119:2402–2407. doi: 10.1002/ijc.22203. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Zhao M, Yuan D, Gu X, Liu H, Song Y. Elevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patients. J Thorac Dis. 2014;6:1261–1270. doi: 10.3978/j.issn.2072-1439.2014.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015;37:103–110. doi: 10.1002/hed.23567. [DOI] [PubMed] [Google Scholar]
- 22.Kum RO, Ozcan M, Baklaci D, Kum NY, Yilmaz YF, Gungor V, Unal A. Elevated neutrophil-to-lymphocyte ratio in squamous cell carcinoma of larynx compared to benign and precancerous laryngeal lesions. Asian Pac J Cancer Prev. 2014;15:7351–7355. doi: 10.7314/APJCP.2014.15.17.7351. [DOI] [PubMed] [Google Scholar]
- 23.American Joint Committee on Cancer, corp-author. AJCC Cancer Staging Manual. 7th. Springer; New York, NY: 2010. Larynx; pp. 57–62. [Google Scholar]
- 24.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman GM, Hanlon AL, Fowble BL, Anderson PR, Nicolaou N. Recursive partitioning identifies patients at high and low risk for ipsilateral tumor recurrence after breast-conserving surgery and radiation. J Clin Oncol. 2002;20:4015–4021. doi: 10.1200/JCO.2002.03.155. [DOI] [PubMed] [Google Scholar]
- 26.Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5:3403–3410. [PubMed] [Google Scholar]
- 27.Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015;37:103–110. doi: 10.1002/hed.23567. [DOI] [PubMed] [Google Scholar]
- 28.Grivennikov SI, Karin M. Inflammation and oncogenesis: A vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 30.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, Serna-Thomé MG, Flores-Estrada D, Diaz-Romero C, Rodríguez CM, Martínez L, Sánchez-Lara K. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC cancer. 2010;10:50. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29:2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 32.Kristal AR, Schenk JM, Song Y, Arnold KB, Neuhouser ML, Goodman PJ, Lin DW, Stanczyk FZ, Thompson IM. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am J Epidemiol. 2008;168:1416–1424. doi: 10.1093/aje/kwn272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Wang L, Liu Y, Wang S, Shang P, Gao Y, Chen X. Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Int J Gynecol Cancer. 2014;24:1319–1325. doi: 10.1097/IGC.0000000000000219. [DOI] [PubMed] [Google Scholar]