The existence of a ribosomal stress response in plant cells is revealed by molecular genetic studies that implicate ANAC082 as a crucial mediator of this pathway.
Abstract
Ribosome-related mutants in Arabidopsis thaliana share several notable characteristics regarding growth and development, which implies the existence of a common pathway that responds to disorders in ribosome biogenesis. As a first step to explore this pathway genetically, we screened a mutagenized population of root initiation defective2 (rid2), a temperature-sensitive mutant that is impaired in pre-rRNA processing, and isolated suppressor of root initiation defective two1 (sriw1), a suppressor mutant in which the defects of cell proliferation observed in rid2 at the restrictive temperature was markedly rescued. sriw1 was identified as a missense mutation of the NAC transcription factor gene ANAC082. The sriw1 mutation greatly alleviated the developmental abnormalities of rid2 and four other tested ribosome-related mutants, including rid3. However, the impaired pre-rRNA processing in rid2 and rid3 was not relieved by sriw1. Expression of ANAC082 was localized to regions where phenotypic effects of ribosome-related mutations are readily evident and was elevated in rid2 and rid3 compared with the wild type. These findings suggest that ANAC082 acts downstream of perturbation of biogenesis of the ribosome and may mediate a set of stress responses leading to developmental alterations and cell proliferation defects.
INTRODUCTION
The ribosome is a highly complicated and evolutionarily conserved ribonucleoprotein machinery that executes translation reactions. The eukaryotic ribosome comprises four types of rRNA and 79 to 81 types of ribosomal proteins (Lecompte et al., 2002; McIntosh and Bonham-Smith, 2006; Ben-Shem et al., 2011). One rRNA (18S rRNA) and 33 ribosomal proteins constitute a small subunit (SSU) known as the 40S ribosome, and three rRNAs (the 5S, 5.8S, and 25–28S rRNAs) and 46 to 48 proteins constitute a large subunit (LSU) known as the 60S ribosome. The two subunits join together on mRNAs to form the 80S ribosome, which functions as a precise mechanical device that translates mRNA codons into amino acids to synthesize proteins.
The biogenesis of the ribosome is finely regulated through the orchestration of the synthesis of rRNAs and their assembly with ribosomal proteins into the ribosome subunits, SSU and LSU, in the nucleolus (Henras et al., 2015; de la Cruz et al., 2015). Three of the four rRNAs, the 18S, 5.8S, and 25–28S rRNAs, are transcribed from each unit of rDNA, which is present in tandem repeats, as a single precursor molecule that contains a 5′-external transcribed spacer (5′-ETS) followed by the 18S rRNA sequence, an internal transcribed spacer 1 (ITS1), the 5.8S rRNA sequence, ITS2, the 25–28S rRNA sequence, and a 3′-ETS. The mature forms of these rRNAs are produced by stepwise processing of the precursor. By contrast, the 5S rRNA is transcribed separately from the other rRNAs. Pre-rRNA processing events are tightly linked with the formation of the ribosomal subunits by the action of huge ribonucleoprotein particles termed processomes (Raué, 2004). The SSU processome contains U3 small nucleolar RNA and its associated proteins as core components, as well as many processing/assembly factors (Phipps et al., 2011), whereas the LSU processome contains Nop4/RBM28 as a hub protein and a different set of processing/assembly factors that do not overlap with the processing/assembly factors in the SSU processome (McCann et al., 2015). The SSU processome binds to the nascent rRNA precursor during transcription and carries out early steps of pre-rRNA modification and cleavage (Phipps et al., 2011; Turowski and Tollervey, 2015). After the pre-rRNA is divided into the 18S-rRNA part and the 5.8S and 25–28S part, the former and the latter are further processed and assembled with ribosomal proteins into SSU by the SSU processome and into LSU by the LSU processome, respectively (de la Cruz et al., 2004; Turowski and Tollervey, 2015).
The elaborately regulated biogenesis of ribosomes is disturbed under stress conditions due to various causes, including nutrient starvation, hypoxia, chemical treatments, genetic impairment of ribosome biogenesis factors, and deficiencies in ribosomal proteins (Mayer and Grummt, 2005; Boulon et al., 2010). In animal cells, this perturbation of ribosome biogenesis, regardless of origin, activates a specific signaling pathway leading to cell cycle arrest or apoptosis, which is called the ribosomal stress response or nucleolar stress response. The main pathway of this stress response involves the tumor suppressor p53 and its destabilizer MDM2 as essential players (Deisenroth and Zhang, 2010; Golomb et al., 2014). In brief, this pathway is triggered upon the inhibition of MDM2 function by direct binding of ribosomal proteins, such as RPL5 and RPL11, and the 5S rRNA, which are unusually released from the nucleolus as a result of perturbed ribosome biogenesis; subsequently, stabilized p53 upregulates the transcription of genes that participate in cell cycle arrest and apoptosis. The p53–MDM2 pathway is also responsible for developmental disorders and diseases caused by impaired ribosome biogenesis or ribosomal protein deficiencies (Azuma et al., 2006; Danilova et al., 2008; McGowan et al., 2008; Deisenroth and Zhang, 2010; Sondalle and Baserga, 2014).
In plants, particularly in the model plant Arabidopsis thaliana, many mutants that carry a mutation in a gene encoding a ribosomal protein or a ribosome biogenesis factor have been reported (Byrne, 2009; Horiguchi et al., 2012; Tsukaya et al., 2013; Weis et al., 2015a). Most of these mutants share several notable phenotypes. In mild cases, characteristic effects are often observed in true leaves: a narrow, pointed shape, a pale-green color, conspicuous indentations, obvious venations, and enhanced abaxialization of leaves under the asymmetric leaves1 (as1) or as2 background (Van Lijsebettens et al., 1994; Ito et al., 2000; Petricka and Nelson, 2007; Yao et al., 2008; Fujikura et al., 2009; Horiguchi et al., 2011; Shinohara et al., 2014; Matsumura et al., 2016). In more severe cases, no leaf lamina is formed, root growth is retarded, and the expression of CUP-SHAPED COTYLEDON1/2 (CUC1/2) and SHOOT MERISTEMLESS (STM) is uncontrolled; finally, in the most severe cases, all processes involving cell proliferation are strongly impeded (Szakonyi and Byrne, 2011; Shinohara et al., 2014). The phenotypic similarity among various ribosome-related mutants suggests that a common signaling pathway connects any ribosomal disorder to certain points of plant growth and development, similar to the p53–MDM2-dependent ribosomal stress response pathway in animals. However, if such a pathway is present in plants, it should be quite different from the p53–MDM2 pathway because plants have neither p53 homologs nor MDM family proteins (Huart and Hupp, 2013).
root initiation defective2 (rid2) is a temperature-sensitive mutant of Arabidopsis that was originally isolated by screening for a defect of adventitious root formation from hypocotyl explants in tissue culture (Konishi and Sugiyama, 2003). Under high-temperature conditions, this mutant exhibits impairment of pre-rRNA processing because of a genetic lesion of the RNA methyltransferase-like protein, which has high sequence similarity to the budding yeast BUD23, known to participate in pre-rRNA modification and processing as an SSU processome-associated factor (White et al., 2008; Ohbayashi et al., 2011; Sardana et al., 2013). Cytologically, the rid2 mutant is characterized by cell proliferation defects accompanied by nucleolar enlargement (Ohbayashi et al., 2011). As a first step to address genetically the hypothetical pathway of a plant-unique ribosomal stress response, we isolated a rid2 suppressor mutant named suppressor of rid two1 (sriw1) and identified a mutation in the gene encoding ANAC082, a NAC family transcription factor, as the cause of the sriw1 phenotype. The sriw1 mutation greatly alleviated the growth defects and developmental abnormalities of rid2 without relieving the impaired pre-rRNA processing and was also effective in phenotypically rescuing four other tested ribosome-related mutants. Expression of ANAC082 was found to be elevated under the influence of genetic impairments of pre-rRNA processing. These findings implicate ANAC082 as a mediator in a plant version of the ribosomal stress response pathway.
RESULTS
Isolation of sriw1, a Suppressor Mutant of the rRNA Biosynthesis-Deficient Mutant rid2
Although rid2 exhibits relatively normal growth and development at 22°C or lower temperatures, it displays pleiotropic disorders as the temperature increases. At temperatures as high as 28°C, rid2 shows severe defects in cell proliferation, which are readily obvious in callus induction experiments (Ohbayashi et al., 2011). We took advantage of this temperature-sensitive characteristic to isolate suppressor mutants from rid2. A mutagenized population of rid2 was grown at 22°C, and the self-pollinated progeny were screened for their capacity to form callus from hypocotyl explants at 28°C as an indicator of the suppression of the rid2 phenotype. Repeated screening led to the isolation and establishment of a suppressor mutant line, sr2205. F2 progeny derived from a cross between sr2205 and rid2 segregated into plants that were phenotypically similar to rid2 and sr2205 at a ratio of ∼3 to 1. The suppressor mutation in sr2205 was indicated by this segregation ratio to be recessive and monogenic, and was named sriw1.
A phenotypic comparison between the sriw1 rid2 double mutant and the rid2 single mutant indicated that all visible defects that were characteristic of rid2 were greatly, although not completely, attenuated. In seedlings incubated at 28°C, primary root growth and leaf development, which were very poor in rid2, were considerably improved in sriw1 rid2 (Figure 1A). In tissue cultures performed at 28°C, both adventitious rooting and callus formation were strongly inhibited in hypocotyl explants of rid2, but they occurred well in the sriw1 rid2 explants (Figures 1B and 1C). Stele cells of hypocotyl explants of rid2 cultured on callus-inducing medium (CIM) at 28°C failed to resume cell division and underwent uneven expansion accompanied by extraordinary enlargement of the nucleolus (Figures 1D to 1F; Supplemental Table 1). By contrast, hypocotyl stele cells of sriw1 rid2 actively divided to form callus under the same condition, and the nucleolar enlargement was much smaller in sriw1 rid2 versus rid2 (Figures 1D to 1F). Given that nucleolar dissociation occurs during mitosis in each round of cell cycle, it is possible that the suppression of the nucleolar enlargement by sriw1 is attributable not to a direct effect on the nucleolar structure but, at least partly, to an indirect effect via the recovery of active cell division.
Figure 1.
Phenotypic Comparison between the sriw1 rid2 Double Mutant, the Wild Type, and rid2.
(A) Seedlings of the wild type, rid2, and sriw1 rid2 grown for 14 d at 22°C or 28°C. Bar = 1 cm.
(B) Adventitious root formation induced from hypocotyl explants of the wild type, rid2, and sriw1 rid2 by culture on root-inducing medium (RIM) for 16 d at 22°C or 28°C. Bar = 1 cm.
(C) Callus formation induced from hypocotyl explants of the wild type, rid2, and sriw1 rid2 by culture on CIM for 21 d at 22°C or 28°C. Bar = 1 cm.
(D) Differential interference contrast images of hypocotyl explants of the wild type, rid2, and sriw1 rid2 cultured on CIM for 5 d at 22°C or 28°C. Each yellow bar represents the size (long diameter) of the nucleolus. Bar = 20 μm.
(E) Nucleolar size of stele-derived cells in hypocotyl explants of the wild type, rid2, and sriw1 rid2 cultured on CIM for 5 d at 22°C or 28°C. Mean values obtained from 20 nucleoli are shown, with standard deviations. Values with different letters are significantly different from each other at P < 0.05 (two-tailed Student’s t test; Supplemental Table 1).
(F) Fluorescence micrographs showing nucleoplasms and nucleoli in the wild type, rid2, and sriw1 rid2. Sections were prepared from the hypocotyl explants cultured for 5 d on CIM at 28°C and double-stained with 4’,6-diamidino-2-phenylindole (blue, DNA) and SYTO RNASelect (green, RNA). Nu and No represent the nucleoplasm and nucleolus, respectively. Bar = 20 μm.
Lack of Recovery from the Impaired Pre-rRNA Processing in the sriw1 rid2 Mutant
The rid2 mutation interfered with many steps of pre-rRNA processing in a temperature-dependent manner, which was reflected in the unusual accumulation of many kinds of pre-rRNA processing intermediates in rid2 at high temperatures (Ohbayashi et al., 2011). RNA gel blot analysis detected several rRNA precursors, which were identified according to the pre-rRNA processing pathways known in Arabidopsis (Figures 2A and 2B). The accumulation levels and patterns of the processing intermediates were similar between the sriw1 rid2 double mutant and the rid2 single mutant (Figure 2B). Moreover, compared with the wild type, the ratio of 18S rRNA to 25S rRNA was decreased to a similar degree in sriw1 rid2 and in rid2 (Figure 2C; Supplemental Table 2). These results revealed that the suppression of the visible phenotype of rid2 by the sriw1 mutation is not tied to the recovery of rRNA biosynthesis.
Figure 2.
Lack of Recovery from the Impaired Pre-rRNA Processing in the sriw1 rid2 Mutant.
(A) Schematic representation of the pre-rRNA processing pathways in Arabidopsis according to Weis et al. (2015a, 2015b). Letters above the rDNA unit represent pre-rRNA processing sites. Red bars indicate the positions of hybridization probes used in RNA gel blot analysis.
(B) RNA gel blot analysis of the accumulation of rRNA precursors in the wild type, rid2, and sriw1 rid2. RNA samples were prepared from the wild-type and mutant plants that had been grown for 12 d at 22°C or 28°C and subjected to RNA gel blot analysis with 5′-ETS-, ITS1-, and ITS2-specific probes (a, b, and c, respectively, in [A]) for hybridization. 25S rRNA bands visualized by staining with GelRed (Biotium) are shown as an index of the loaded amounts of total RNA (because 25S rRNA constitutes a large fraction of total RNA, any changes in the 25S rRNA content, which can be caused by RNA biosynthesis-defective mutations such as rid2, are expected to be reflected in changes of the total RNA content). This experiment was repeated three times using independently prepared materials to confirm the reproducibility of the result.
(C) Ratios of 18S rRNA to 25S rRNA in the wild type, rid2, and sriw1 rid2. RNA samples were prepared from the wild-type and mutant plants that had been grown for 12 d at 22°C or 28°C and analyzed by capillary electrophoresis. Relative 18S/25S ratios were calculated with reference to the data of the wild type grown at 22°C. The mean values of the relative ratios obtained from four independently prepared material sets are shown, with standard deviations. Values with different letters are significantly different from each other at P < 0.05 (two-tailed Student’s t test; Supplemental Table 2).
Identification of ANAC082 as the SRIW1 Gene
Chromosomal mapping of sriw1 located this mutation within a 95-kb region at the 25-cM position of chromosome V (Figure 3A). A sequence analysis of this region detected a missense mutation in At5g09330 of the sriw1 genome (Figure 3B). The introduction of a DNA fragment encompassing At5g09330 into the sriw1 rid2 double mutant cancelled the suppression of the temperature sensitivity of rid2 by sriw1, as shown by the low ability to form adventitious roots at 28°C (Figure 3C). These results led us to conclude that At5g09330 corresponds to the SRIW1 gene.
Figure 3.
Positional Identification of ANAC082 as the SRIW1 Gene.
(A) Chromosomal location of the sriw1 mutation and ANAC082. Numbers in parentheses indicate recombinant numbers in F2 plants derived from crossing sriw1 rid2 with the Col plants heterozygous for the T-DNA insertion mutation of RID2 (SALK_014062).
(B) Gene structure of ANAC082 and mutant alleles.
(C) Complementation test for the function of the ANAC082 transgene by adventitious rooting assay. Hypocotyl explants of the sriw1 rid2 double mutant without or with the ANAC082 transgene were cultured on RIM at 28°C for 16 d, and adventitious root formation was compared. Bar = 1 cm.
(D) Suppression of the cell proliferation defect of rid2 by the anac082-6 mutation in tissue culture. Hypocotyl explants of the wild type, anac082-6, rid2, and anac082-6 rid2 were cultured on CIM at 28°C for 21 d, and callus growth was compared. Bar = 1 cm.
At5g09330 encodes ANAC082, a member of the NAC family. The NAC family proteins possess a NAC domain at the N terminus, which comprises ∼160-amino acid residues and contains five subdomains (A to E) (Ooka et al., 2003). The sriw1 mutation causes a substitution of the conserved Gly residue by Asp in subdomain C (Supplemental Figure 1). In Arabidopsis, more than 100 genes encode NAC family proteins. Based on their amino acid sequences, these proteins are classified into two large groups, groups I and II, and each group is further classified into many subgroups (Ooka et al., 2003). ANAC082 belongs to the NAC2 subgroup of group I and is closest to ANAC103. A major difference between ANAC082 and ANAC103 is confined to the C-terminal, nonconserved region (Supplemental Figure 1).
Isolation and Characterization of a Putative Null Allele of ANAC082
Nine additional mutant alleles of ANAC082 were found in the Arabidopsis TILLING collections with the aid of the Seattle TILLING Project (Henikoff et al., 2004); they were named anac082-2 to anac082-10 with sriw1 as the first allele (Supplemental Figure 1). Among these alleles, only anac082-6 was a nonsense mutation that terminated translation at subdomain C of the NAC domain (Figure 3B). As this early termination results in the loss of a large part of the ANAC082 protein, including the NAC subdomains D and E, anac082-6 is expected to be a null allele. When the anac082-6 mutation was introduced into rid2, the resultant double mutant anac082-6 rid2 recovered the ability to form callus at 28°C, as was the case with sriw1 rid2 (Figure 3D). This result confirmed that loss-of-function mutations of the ANAC082 gene can suppress the rid2 phenotype.
Effects of the sriw1 Mutation in Seedlings of Two Other Temperature-Sensitive, rRNA Biosynthesis-Deficient Mutants
In Arabidopsis, in addition to rid2, rid3 and rh10-1 are available as temperature-sensitive, rRNA biosynthesis-deficient mutations. rid3 and rh10-1 are missense mutations of genes encoding a WD40 repeat protein orthologous to the budding yeast IPI3 and an RNA helicase-like protein orthologous to the budding yeast RRP3, respectively (Shinohara et al., 2014; Matsumura et al., 2016). In budding yeast, in addition to the RID2 homolog, BUD23, both IPI3 and RRP3 are required for normal pre-rRNA processing, but their modes of function are different (O’Day et al., 1996; Krogan et al., 2004; White et al., 2008). BUD23 associates with the SSU processome and RRP3 is a component of the SSU processome, whereas IPI3 is not directly related to the SSU processome, but forms a small protein complex that binds to ITS2 (Krogan et al., 2004; Phipps et al., 2011; Sardana et al., 2013). Based on this information, the rid2, rid3, and rh10-1 mutations are thought to primarily affect different aspects of pre-rRNA processing, which is supported by the fact that they cause different accumulation patterns of pre-rRNA processing intermediates (Ohbayashi et al., 2011; Shinohara et al., 2014; Matsumura et al., 2016).
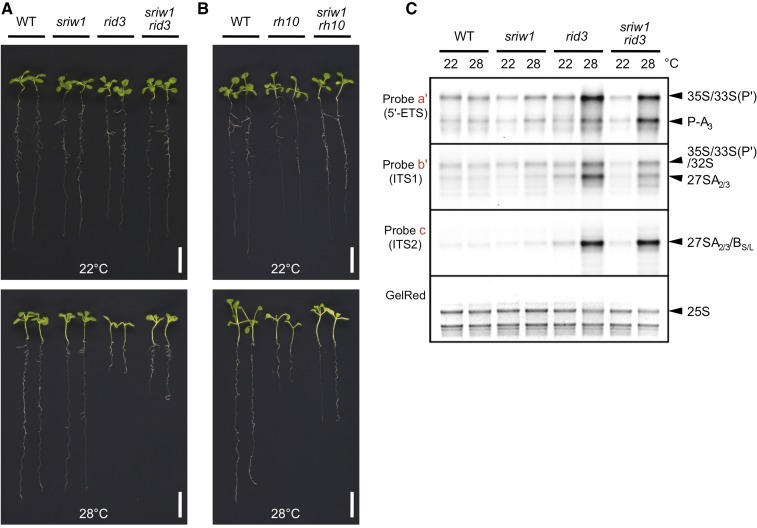
For the reasons stated above, rid3 and rh10-1 were employed as tester mutants to extend the examination of the relationship of the sriw1 mutation to impairment in pre-rRNA processing. Hereafter, the sriw1 single mutant was also included for comparison. Seedlings of the sriw1 single mutant were not distinguishable from the wild-type seedlings, regardless of growth temperature (Figure 4A). However, the sriw1 mutation had a distinctly positive effect on seedling growth and development in the genetic background of rid3 or rh10-1 at 28°C (Figures 4A and 4B). The strong defects of primary root growth and leaf development observed in the rid3 and rh10-1 seedlings were markedly reduced by the introduction of the sriw1 mutation.
Figure 4.
Effects of the sriw1 Mutation on the Defects of Seedling Development of rid3 and rh10 and on Pre-rRNA Processing in rid3.
(A) Seedlings of the Ler wild type, rid3, sriw1, and sriw1 rid3 grown for 14 d at 22°C or 28°C. Bar = 1 cm.
(B) Seedlings of the Col wild type, rh10-1, and sriw1 rh10-1 grown for 14 d at 22°C or 28°C. Bar = 1 cm.
(C) RNA gel blot analysis of the accumulation of rRNA precursors in the wild type, rid2, and sriw1 rid2. RNA samples were prepared from wild-type and mutant plants that had been grown for 14 d at 22°C or 28°C and subjected to RNA gel blot analysis with 5′-ETS-, ITS1-, and ITS2-specific probes (a′, b′, and c, respectively, in Figure 2A) for hybridization. This experiment was repeated twice using independently prepared materials to confirm the reproducibility of the result.
An RNA gel blot analysis of pre-rRNA intermediates was performed for single and double mutants of sriw1 and rid3 (Figure 4C). The levels of the processing intermediates in the sriw1 single mutant were low and equivalent to those of the wild type at both 22°C and 28°C. Aberrantly increased accumulation of the processing intermediates was detected similarly in rid3 and sriw1 rid3 at 28°C. These results thus show that, similar to rid2, the recovery of the seedling morphology of rid3 was achieved by the sriw1 mutation, without recovery of pre-rRNA processing.
Ineffectiveness of the sriw1 Mutation to Suppress Temperature Sensitivity in Ribosome-Unrelated Mutants
root primordium defective1-1 (rpd1-1), rpd1-2, and root growth defective3 (rgd3) are temperature-sensitive mutants that were isolated via the same screening, together with rid2 and rid3 (Konishi and Sugiyama, 2003). RPD1 encodes a plant-unique protein that is implicated in mitochondrial gene expression (Konishi and Sugiyama, 2006; Kroeger et al., 2009). RGD3 encodes a homolog of BTAF1, a specific kind of TATA binding protein-associated factor, and rgd3 is phenotypically distinct in several aspects from rid2 and rid3 and normal regarding pre-rRNA accumulation levels (Konishi and Sugiyama, 2006; Tamaki et al., 2009; Shinohara et al., 2014). It is therefore most likely that neither RPD1 nor RGD3 is related to ribosome biogenesis or ribosomal function.
To assess whether the sriw1 mutation can affect ribosome-unrelated mutants, sriw1 was crossed with rpd1-1, rpd1-2, and rgd3, and the resultant double mutants were assessed for their temperature sensitivity by inducing adventitious root formation at 28°C (Figure 5). The sriw1 mutation did not afford a recognizable improvement of adventitious rooting in any of these ribosome-unrelated mutants. Accordingly, we conclude that the suppressive effect of the sriw1 mutation does not work widely on temperature sensitivity per se, but is selectively connected with impairment of rRNA biosynthesis.
Figure 5.
Effects of the sriw1 Mutation on Adventitious Root Formation in Hypocotyl Explants of Ribosome-Unrelated, Temperature-Sensitive Mutants.
(A) Hypocotyl explants of the wild type, sriw1, rid2, sriw1 rid2, rpd1-1, sriw1 rpd1-1, rpd1-2, and sriw1 rpd1-2 were cultured on RIM for 16 d at 28°C. Bar = 1 cm.
(B) Hypocotyl explants of the wild type, sriw1, rid2, sriw1 rid2, rgd3, and sriw1 rgd3 were cultured on RIM for 16 d at 28°C. Bar = 1 cm.
Effects of the sriw1 Mutation on Shoot Regeneration in the rid2 and rid3 Mutants
When callused explants of rid2 and rid3 are cultured on shoot-inducing medium (SIM), they regenerate shoots at low temperatures (such as 19°C or 22°C), but not at high temperatures (such as 25°C or 28°C) (Tamaki et al., 2009; Shinohara et al., 2014). The failure of shoot regeneration at high temperatures in these mutants is characteristically accompanied by uncontrolled expression of the CUC1 and STM genes and occasional formation of irregularly large cell mounds, probably arising from aberrant development of pre-meristematic cell mounds (Tamaki et al., 2009; Shinohara et al., 2014). With a focus on this peculiar disorder, the effect of the sriw1 mutation was further investigated.
In these experiments, hypocotyl explants of the wild-type, rid2, and rid3 seedlings with or without the sriw1 mutation were cultured on CIM at 19°C and then cultured on SIM at 19°C, 25°C, or 28°C for the induction of shoot regeneration. Morphological observation showed a clear restoration of the ability of regenerating shoots at high temperatures, both in rid2 and rid3 (Figure 6A). It is notable that even in rid2, which displays much severer defects than does rid3, the sriw1 mutation recovered shoot regeneration to a level that was comparable to that of the wild type.
Figure 6.
Effects of the sriw1 Mutation on Shoot Regeneration in Hypocotyl Explants of rid2 and rid3.
(A) Hypocotyl explants of the wild type, rid2, rid3, sriw1 rid2, and sriw1 rid3 cultured on SIM at 19°C, 25°C, or 28°C for 14 d after 6 d of CIM culture at 19°C. Bar = 1 cm.
(B) Relative quantities of CUC1 and STM mRNAs in hypocotyl explants of the wild type, rid2, rid3, sriw1 rid2, and sriw1 rid3. Explants were cultured on CIM at 19°C for 6 d and then cultured on SIM at 19°C, 25°C, or 28°C. Total RNA samples were isolated from the explants collected immediately after CIM culture (0 d) and after 5 d of SIM culture (5 d) and subjected to RT-qPCR analysis. mRNA relative quantities were calculated with reference to the data of the wild type at 0 d after normalization. The mean values of the relative quantities obtained from four independent cultures are shown, with standard deviations. Statistical analyses of these data are shown in Supplemental Tables 3 and 4.
With respect to the effects of sriw1 on the expression of CUC1 and STM, there was a considerable difference between rid2 and rid3 (Figure 6B). The sriw1 mutation tended to reduce the overexpression of CUC1 and STM caused by the rid3 mutation. By contrast, in the rid2 background, the sriw1 mutation did not affect CUC1 expression much; rather, it enhanced STM expression. As the expression data varied between experiments, however, these effects of the sriw1 mutation were only partially supported by statistical analysis (Supplemental Tables 3 and 4). From this complicated and variable result, currently we can say only that the altered gene expression and the developmental disorder in rRNA biosynthesis-deficient mutants are not always modified in parallel by the sriw1 mutation.
Suppression of Leaf Morphological Alterations by the sriw1 Mutation in Various Ribosome-Related Mutants
Leaf development is highly susceptible to various ribosome-related mutations, including ribosomal protein gene mutations, and characteristic effects of these mutations can be easily detected in leaf morphology (Byrne, 2009; Horiguchi et al., 2012). In an attempt to further investigate the effectiveness of sriw1, oligocellula5-1 (oli5-1) and rpl4d-3, which are mutated in the genes that encode the ribosomal proteins RPL5A and RPL4D, respectively (Fujikura et al., 2009; Horiguchi et al., 2011), were added to tester mutants, and leaf morphology was examined for single tester mutants and double mutants carrying tester and sriw1 mutations (Figure 7). When grown at 22°C, seedlings of the rid2, rid3, rh10-1, oli5-1, and rpl4d-3 single mutants formed pointed and slightly to moderately narrow true leaves with a little simpler and more obvious venation compared with the wild type. At 28°C, leaves of the temperature-sensitive mutants rid2, rid3, and rh10-1 were much narrower in shape and venations were much simpler. These phenotypes were clearly suppressed in every mutant by the introduction of the sriw1 mutation, indicating that sriw1 is effective as a general suppressor of developmental disorders in a broad range of ribosome-related mutants.
Figure 7.
Effects of the sriw1 Mutation on Leaf Morphology in rid2, rid3, rh10, oli5, and rpl4d Seedlings.
The first or second true leaves of seedlings of the wild type (Col and Ler), sriw1, rid2, rid3, rh10-1, oli5-1, rpl4d-3, sriw1 rid2, sriw1 rid3, sriw1 rh10-1, sriw1 oli5-1, and sriw1 rpl4d-3 grown at 22°C or 28°C on vermiculite for 16 d. After chlorophyll was removed, leaf samples were mounted with water and photographed. L, C, and M in parentheses indicate genetic backgrounds of Ler, Col, and mixture of Ler and Col, respectively. For each strain, more than 10 leaves were observed, and a representative one is shown. Bars = 2 mm.
Hyposensitivity of Callus Formation of the sriw1 Mutant to Agents That Interfere with Ribosome Biogenesis or Ribosomal Function
A variety of agents, such as antibiotics and synthetic substrate analogs, interfere with ribosome biogenesis or ribosomal function. The sriw1 single mutant was compared with the wild type regarding sensitivity of callus formation to these agents. The first group of agents used in this experiment included actinomycin D, 5-fluorouracil, and camptothecin, which are known to trigger a ribosomal stress response in animal cells (Boulon et al., 2010). These drugs have different action mechanisms and can affect many biochemical processes such as RNA transcription and DNA replication, among which their common and sensitive target is rRNA biosynthesis (Hsiang et al., 1985; Sobell, 1985; Longley et al., 2003). Actinomycin D, 5-fluorouracil, and camptothecin were reported to prevent rRNA biosynthesis at nanomolar, submicromolar, and micromolar concentrations, respectively, in human fibrosarcoma cell cultures (Burger et al., 2010). For actinomycin D and 5-fluorouracil, preferential inhibition of rRNA biosynthesis was previously reported in plants as well (Key, 1966; Cheng and Hagen, 1971; Fraser, 1975). According to these reports, the effective concentration of actinomycin D was two to three orders of magnitude higher in plants than in human carcinoma cells. As a second group of agents, we employed cycloheximide and puromycin, which inhibit translation via different mechanisms. Although these chemicals were shown to affect rRNA biosynthesis in animal cells at relatively high concentrations such as micromolar to submillimolar (Holland, 1963; Burger et al., 2010), neither of them has been listed as a ribosomal stress inducer.
Compared with the wild type, sriw1 appeared somewhat less sensitive to all of the above agents at marginal concentrations, regardless of group, with respect to callus formation, which was supported by statistical analysis of fresh weight data of callus, except for the case of cycloheximide (Figure 8; Supplemental Table 5). DTT and 2,6-dichlorobenzonitrile were also tested for their effects on callus formation in the wild-type and sriw1 explants. These chemicals have no known action on ribosome biogenesis or ribosomal function, but they induce ribosome-unrelated stresses: DTT induces endoplasmic reticulum stress by unfolding proteins and 2,6-dichlorobenzonitrile induces cell wall stress by inhibiting cellulose synthesis (Seifert and Blaukopf, 2010; Deng et al., 2013). The sensitivity of callus formation of sriw1 to these ribosome-unrelated agents was not different from or even higher than that of the wild type (Figure 8). These results indicated that the sriw1 mutation selectively relieved the weak repression of cell proliferation caused by the pharmaceutical perturbation of ribosomes.
Figure 8.
Comparison of Sensitivities of Callus Formation to Agents That Interfere with rRNA Biosynthesis or Ribosomal Function between the Wild Type and sriw1.
(A) Hypocotyl explants of the wild type and sriw1 were cultured at 22°C for 28 d on CIM containing actinomycin D (Act D), 5-fluorouracil (5-FU), camptothecin (CPT), cycloheximide (CHX), puromycin (Puro), DTT, or 2,6-dichlorobenzonitrile (DCB) at various concentrations. For each agent, cultures were performed at least three times and a representative one is shown. Bars = 1 cm.
(B) Fresh weights of hypocotyl explants cultured at 22°C for 28 d on CIM containing the indicated agent. For a solvent control, explants were cultured with 0.1% DMSO. Cultures were set up in six-well plates or small dishes. In one well or dish, eight explants in total, consisting of four wild type and four sriw1, were cultured. The four explants of each strain cultured in the same well or dish were gathered together and weighed. Mean values obtained from three sets of cultures are shown, with standard deviations. Asterisks and double asterisks represent significant differences at P < 0.05 and P < 0.01, respectively (two-tailed Student’s t test; Supplemental Table 5).
Expression Patterns of ANAC082 as Influenced by the rid2 and rid3 Mutations
The spatial patterns of expression driven by the ANAC082 promoter were analyzed using ANAC082pro:GUS as a reporter gene. In young wild-type seedlings, a relatively strong GUS activity was detected in the shoot apical region, particularly around the vascular or provascular strands of developing leaves and leaf primordia, and modest activity was observed in the root apical meristem (Figure 9A). In both rid2 and sriw1 rid2, GUS activity in these regions was a little higher than that of the wild type at 22°C and markedly elevated at 28°C (Figure 9A), a result that indicated that the expression of ANAC082 is increased by the rid2 mutation.
Figure 9.
Expression of ANAC082 as Influenced by the rid2 and rid3 Mutations.
(A) Expression patterns of ANAC082pro:GUS in seedlings of the wild type, rid2, and sriw1 rid2 grown for 12 d at 22°C and then for an additional 4 d at 22°C or 28°C. Bar = 100 μm.
(B) Effects of the rid2 and rid3 mutations on the level of ANAC082 expression. Total RNAs were isolated from wild-type, rid2, and rid3 seedlings that were grown for 12 d at 22°C and then for an additional 4 d at 22°C or 28°C, and subjected to RT-qPCR analysis. The relative quantities of the ANAC082 mRNA were calculated with reference to the data of the wild type at 22°C after normalization. The mean values of the relative quantities obtained from three independently prepared material sets are shown, with standard deviations. Values with different letters are significantly different from each other at P < 0.05 (two-tailed Student’s t test; Supplemental Table 6).
(C) GUS activity of ANAC082pro:GUS in hypocotyl explants cultured on CIM at 22°C for the indicated times. Bar = 200 μm.
The expression of ANAC082 was also examined using RT-qPCR analysis of rid2 and rid3 (Figure 9B; Supplemental Table 6). The data obtained showed a temperature-dependent enhancement of ANAC082 expression in both rid2 and rid3. Additionally, in the public microarray data GEO-GSE23940, we found that, compared with the wild type, the expression of ANAC082 was approximately two times higher in the T-DNA insertion mutant of APUM23, which encodes a nucleolar Puf domain protein involved in pre-rRNA processing (Abbasi et al., 2010). Consequently, ANAC082 expression proved to be stimulated in response to the impairment of rRNA biosynthesis.
GUS activity from the ANAC082pro:GUS reporter was absent in the hypocotyl tissues of seedlings but became detectable in the stele when hypocotyls were excised from seedlings and cultured on CIM to induce callus formation (Figure 9C). In hypocotyl explants cultured for 4 d, strong GUS activity was observed in the stele-derived callus cells. This result suggested a link between the expression of ANAC082 and the reactivation of cell division. It is also of note that the regions in which GUS activity was evident in the ANAC082pro:GUS plants largely agree with the regions where effects of the ribosome-related mutations on growth and development are evident.
Potential of ANAC082 as a Transcriptional Activator
Most of the NAC family proteins studied to date have been demonstrated to function as transcription factors. In typical NAC transcription factors, the NAC domain and C-terminal regions serve as DNA binding and regulatory domains, respectively (Olsen et al., 2005). In light of this knowledge, we examined the C-terminal region of ANAC082 for its potential for transcriptional activation in the GAL4 transactivation system of budding yeast, which is designed in such a manner that if a protein sequence of interest has the ability to activate transcription, its expression as a fusion with the GAL4 DNA binding domain enables yeast cells to proliferate in the absence of histidine (James et al., 1996). When the full-length protein of ANAC082 and the N-terminal half of ANAC082 protein, which retains the NAC domain but lacks the C-terminal region, were tested using this system, the full-length protein showed a clearly positive result, whereas the N-terminal half did not (Figure 10). This pattern indicated that ANAC082 is able to function as a transcriptional activator and that its regulatory domain lies within the C-terminal region.
Figure 10.
GAL4 Transactivation Assay of ANAC082 in Budding Yeast.
Constructs for expression of the GAL4 DNA binding domain alone (#1) and a chimeric protein consisting of the GAL4 DNA binding domain fused with the NAC domain of ANAC082 (#2) or with full-length ANAC082 (#3) were transformed into the PJ69-4A cells of budding yeast. The transformed cells were incubated for 3 d at 28°C with or without histidine. Growth in the absence of histidine indicated transactivation of the HIS3 reporter by the chimeric protein.
DISCUSSION
Possible Involvement of ANAC082 in a Plant Version of the Ribosomal Stress Response Pathway
In this study, through the analysis of the rid2 suppressor mutant sriw1, we discovered that loss-of-function mutations of the NAC transcription factor gene ANAC082, such as sriw1 (anac082-1) and anac082-6, greatly alleviate the developmental alterations and cell proliferation defects caused by pre-rRNA processing-related mutations, including rid2, rid3, and rh10-1, without restoring the ability of pre-rRNA processing. This finding shows that ANAC082 is involved downstream of the impairment of pre-rRNA processing in the process of cellular and developmental reactions to such impairment. The sriw1 mutation also suppressed the pointed and narrow leaf phenotype of the ribosomal protein mutants oli5-1 and rpl4d-3 and rendered callus cell proliferation slightly tolerant to agents that interfere with ribosome biogenesis or ribosomal function. Taken together, these findings indicate that ANAC082 plays a critical role in the common pathway that connects a wide range of ribosome-related disorders to their effects on cell proliferation and development.
Genetic impairment of pre-rRNA processing is expected to reduce the number of functional ribosomes, which should lower the capacity for translation and globally affect cellular activities. However, this view is in disagreement with the result that the sriw1 mutation rescued growth and development of the pre-rRNA processing-deficient mutants, although it did not compensate for the defects of rRNA biosynthesis. The simplest interpretation of our findings is that these mutants, in which rRNA biosynthesis is not completely blocked but partially impaired, maintain a sufficient level, even though not a full level, of functional ribosomes and that a partial impairment of pre-rRNA processing and/or a small reduction of the amount of functional ribosomes are sensed as a kind of stress to stimulate the ANAC082-dependent pathway, with little direct influence on global translation required for normal cellular activities (Figure 11A). This hypothesis is applicable to various other ribosomal disorders and can account for all the effects of the sriw1.
Figure 11.
Hypothetical Model for the Role of ANAC082 and Ribosomal Stress Response.
(A) Activation of the ANAC082-dependent pathway and direct influence of the deficiency of functional ribosomes on global translation at different degrees of ribosomal disorders. This model assumes that the ANAC082-dependent pathway responds sensitively to mild disorders of ribosome biogenesis even when the number of functional ribosomes is still sufficiently large and direct influence on global translation is negligible.
(B) Comparison of the framework of ribosomal stress signaling between animals and plants. The ribosomal stress signaling pathway of animals involves the transcription factor p53 as a key mediator. In plants, which have no p53 homologs, ANAC082, a member of the plant-specific transcription factor family, plays a role comparable to p53 in the ribosome stress signaling pathway.
In animals, a set of cellular reactions, such as cell cycle arrest and apoptosis, that follow deregulation of ribosome biogenesis because of either genetic or pharmaceutical effects are recognized as a specific type of stress response called the ribosomal stress response or nucleolar stress response (Mayer and Grummt, 2005; Boulon et al., 2010). Release of the transcription factor p53 from the destabilizing effect of MDM2 lies at the core of this stress response pathway (Deisenroth and Zhang, 2010; Golomb et al., 2014). Our findings suggest that in plants, the perturbation of ribosome biogenesis or ribosomal function triggers a broad but particular spectrum of responses by way of a signaling pathway that involves ANAC082. This ANAC082-dependent pathway is comparable to the p53–MDM2 pathway of the ribosomal stress response of animals, as their triggers mostly overlap. However, there may be some difference in the range of triggers between these two pathways, considering the reduced sensitivity of sriw1 to puromycin, which is generally not viewed as a ribosomal stressor in animal cells.
p53 has a pivotal role not only in the ribosomal stress response but also in the DNA damage response (Horn and Vousden, 2007). In Arabidopsis, the DNA damage response is governed by SUPPRESSOR OF GAMMA RESPONSE1 (SOG1), a NAC transcription factor belonging to a subfamily different from the one containing ANAC082 (Yoshiyama et al., 2009). Based on its analogous function as a key transcriptional regulator in the DNA damage response, SOG1 is often discussed as a possible counterpart of p53, although the two proteins share no sequence similarity (Mannuss et al., 2012). From the same point of view, ANAC082 might be considered as a functional counterpart of p53 in ribosomal stress response (Figure 11B).
Characteristics of ANAC082 and Their Possible Implications in the Ribosomal Stress Response
Few studies have investigated ANAC082. Yamaguchi et al. (2010) identified ANAC082 via yeast two-hybrid screening as a protein that potentially interacts with VASCULAR-RELATED NAC-DOMAIN7 (VND7), a NAC transcription factor that acts as a master regulator of xylem vessel differentiation, and designated it VND-INTERACTING1 (VNI1). Unlike what was observed for VND7, however, the expression of ANAC082/VNI1 was not closely related to xylem differentiation (Yamaguchi et al., 2010), and the interaction between ANAC082/VNI1 and VND7 has not been proven in planta. Therefore, it remains uncertain whether ANAC082/VNI1 has a function in xylem differentiation.
Subsequently, several additional experiments were performed for the molecular characterization of ANAC082/VNI1, together with its closest homolog, ANAC103 (Yamaguchi et al., 2015). An in vitro assay with recombinant proteins indicated that ANAC082/VNI1 and ANAC103 are able to bind efficiently to VND7 and many other NAC domain proteins, such as VND1, VND2, VND3, NAC1, and CUC2, while they bind only weakly to themselves and each other. Moreover, a transient transfection assay in Arabidopsis leaves was used to demonstrate that both ANAC082/VNI1 and ANAC103 have the ability to activate transcription of the cotransfected reporter gene in the GAL4-derived system, which is consistent with our results for ANAC082 obtained using the GAL4 transactivation assay in yeast cells. These pieces of information imply that ANAC082 forms a heterodimer with another member of the NAC family and functions as a transcriptional activator, rather than as a transcriptional repressor.
Our analyses of ANAC082 expression using the ANAC082pro:GUS reporter and RT-qPCR showed that ANAC082 expression was enhanced when rRNA biosynthesis was impaired. This enhancement is of great interest, as it may provide a clue regarding the molecular framework of the ANAC082-dependent pathway of ribosomal stress response. Our current hypothesis is that perturbations in ribosome biogenesis or ribosomal function stimulate ANAC082 expression to upregulate the ANAC082 protein, which, possibly together with an unidentified NAC partner, activates the transcription of genes that encode regulators of cell proliferation and development.
To understand the molecular mechanisms of the plant ribosomal stress response, the next key question is how ribosomal perturbations stimulate ANAC082 expression. The elevated expression of the ANAC082pro:GUS reporter in rid2 appeared to reflect transcriptional upregulation, but it may also be attributed to posttranscriptional regulation. In this respect, the 5′-untranslated region of ANAC082, which was contained in the ANAC082pro:GUS reporter gene, is particularly notable, as it possesses a conserved upstream open reading frame (uORF) that encodes a peptide of 37 amino acids and has a peptide-sequence-dependent, negative impact on the expression of the main ORF (Takahashi et al., 2012; Ebina et al., 2015). Most of the previously characterized regulatory uORFs cause ribosome stalling at their termination codon, which restricts the access of ribosomes to the main ORF and induces nonsense-mediated mRNA decay particularly in the case of them being longer than 35 amino acids (Cao and Geballe, 1996; Law et al., 2001; Gaba et al., 2005; Nyikó et al., 2009; Uchiyama-Kadokura et al., 2014). If shortage of ribosomes or imbalance of ribosome subunits renders this uORF-based control leaky, it might account for the enhancement of ANAC082 expression under the influence of ribosomal perturbations. Analysis using a uORF-deletion mutant of ANAC082 would be helpful for testing whether the uORF-based control is responsible for the upregulation of ANAC082 in ribosomal stress signaling.
This study has demonstrated the presence of a kind of ribosomal stress response in plant cells and implicated ANAC082 as a crucial mediator of its pathway. This is a first, but important, step in the research of the frontier field of plant ribosome biology. Future studies focusing on ANAC082 could be expected to reveal the molecular mechanisms and physiological significance of the ribosome stress response in plants.
METHODS
Plant Materials and Growth Conditions
rid2, rid3, rpd1-1, rpd1-2, rgd3, and sriw1 (anac082-1) were derived from the Landsberg erecta (Ler) strain of Arabidopsis thaliana (Konishi and Sugiyama, 2003; this study), and Ler carrying no additional mutations was used as the wild type for comparison with these mutants. rh10-1, oli5-1, and rpl4d-3 originated from the Columbia (Col) strain (Matsumura et al., 2016; Fujikura et al., 2009; Horiguchi et al., 2011). anac082-2 to anac082-10 were identified from the TILLING collection in the Col er-105 background (Seattle TILLING Project).
The original line of sriw1 isolated from rid2 was backcrossed three times with rid2 and then self-pollinated. From the resultant progeny, a double homozygous line was established and used as sriw1 rid2 for further characterization. For the establishment of the sriw1 single mutant line, the original sriw1 line was backcrossed twice with rid2, crossed with Ler, and self-pollinated to genetically purify the sriw1 mutation.
Unless otherwise indicated, seedlings were grown aseptically at 22°C or 28°C on Murashige and Skoog-based germination medium solidified with 1.5% (w/v) agar (GMA; Konishi and Sugiyama, 2003) under continuous light from white fluorescence lamps. The photon fluence rate was set to 30 to 50 μmol m–2 s–1 for the seedlings used for phenotypic or pre-rRNA processing analysis, and to 10 to 20 μmol m–2 s–1 for the donor seedlings used in tissue culture. For the inspection of leaf morphology, seedlings were grown under continuous light (20–40 μmol m–2 s–1) on vermiculite watered with 1/1000-diluted Hyponex 6-10-5 fertilizer (Hyponex Japan).
Reporter Gene and Plant Transformation
The genome fragment of the 2.0-kb sequence upstream of the translation start site of ANAC082 (At5g09330) was amplified with the primers ANAC082_2.0kb-pro_Fw and ANAC082_2.0kb-pro_Rv (Supplemental Table 7) and cloned into the entry vector pENTR/D-TOPO (Invitrogen). The cloned fragment was then transferred to the binary vector pGWB3 (Nakagawa et al., 2007) by the Gateway LR reaction (Invitrogen) to construct ANAC082pro:GUS. The construct was introduced into the Col strain using a simplified version of the floral dip method (Clough and Bent, 1998).
Tissue Culture
Tissue culture was performed as described by Konishi and Sugiyama (2003) and Shinohara et al. (2014). Hypocotyl segments were excised from 12-d-old seedlings and cultured at 22°C or 28°C on CIM for the induction of callus and on RIM for the induction of roots. For the induction of shoot regeneration, hypocotyl segments of 12-d-old seedlings were cultured on CIM for 6 d at 19°C and then cultured on SIM at 19°C, 25°C, or 28°C. Tissue culture was performed normally under continuous light (15–25 μmol m–2 s–1), while it was performed in the dark only in the inhibitor experiments. The culture media were based on Gamborg’s B5 medium, supplemented with 2.0% (w/v) glucose and phytohormones (0.5 mg L–1 2,4-D and 0.1 mg L–1 kinetin in CIM; 0.5 mg L–1 indole-3-butyric acid in RIM; 0.04 mg L–1 indole-3-acetic acid and 1.5 mg L–1 N6-Δ2-isopentenyladenine in SIM), buffered to pH 5.7 with 0.05% (w/v) MES, and solidified with 0.25% (w/v) gellan gum. In the experiment in which fresh weights of cultured explants were measured, explants were placed on nylon mesh sheets laid on the culture medium to avoid adhesion of the medium gel to the explants.
Histological and Histochemical Analyses
Explants were fixed overnight at 4°C in a 9:1 (v/v) mixture of ethanol and acetic acid, and then rehydrated through a graded series of aqueous ethanol solutions. Samples were mounted with a few drops of an 8:1:2 (w/v/v) mixture of 2,2,2-trichloroethane-1,1-diol (chloral hydrate), glycerol, and water. Differential interference contrast images were taken under a microscope equipped with Nomarski optics (BX50-DIC; Olympus).
Microscopic examination of the nucleolar structure was performed as previously described (Ohbayashi et al., 2011). Hypocotyl explants were fixed with 3:1 (v/v) mixture of methanol and acetic acid, dehydrated through a graded series of aqueous ethanol solutions, and embedded in Technovit 7100 resin (Heraeus Kulzer). The resin blocks were cut into 7-µm sections and stained with 1 µg mL–1 4’,6-diamidino-2-phenylindole and 500 nM SYTO RNASelect (Invitrogen) following the manufacturer’s protocol. Fluorescence images were captured and deconvolved to reduce haze with the Biozero microscope system (Keyence).
For observation of leaf morphology, shoots were fixed at 4°C in a 9:1 (v/v) mixture of ethanol and acetic acid, and then rehydrated through a graded series of aqueous ethanol solutions. Leaves were excised from the rehydrated samples, mounted with water, and subjected to microscopic inspection.
Histochemical detection of the GUS activity signal was performed as previously described (Ohtani and Sugiyama, 2005). After the GUS reaction, samples were cleared with an 8:1:2 (w/v/v) mixture of 2,2,2-trichloroethane-1,1-diol, glycerol, and water and examined under a microscope equipped with Nomarski optics (BX50-DIC; Olympus).
Sequence Analysis
DNA sequences were amplified by PCR from genomic DNA. The PCR product was subjected to a cyclic sequencing reaction using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) after treatment with ExoSAP-IT (USB Corporation). The sequence was determined using an ABI PRISM 377 Sequencer or 3130 Genetic Analyzer (Applied Biosystems) and analyzed using the GENETYX-MAC software (Software Development).
Chromosomal Mapping
The sriw1 rid2 double mutant of the Ler strain was crossed with Col plants that were heterozygous for the T-DNA insertion allele rid2-2 of the SALK_014062 strain (Ohbayashi et al., 2011). F1 plants carrying the rid2-2 mutation were selected and self-pollinated. The resultant F2 progeny were used for chromosome mapping of sriw1. Each individual F2 plant was checked for its ability to form adventitious roots at 28°C and for DNA polymorphisms. The chromosome location of the sriw1 mutation was determined on the basis of linkage events between the mutations and the Ler alleles of polymorphic marker loci.
Complementation Test
A DNA fragment encompassing At5g09330 and its upstream sequence was amplified with the primers ANAC082_2.0kb-pro_Fw and ANAC082_Rv (Supplemental Table 7) from the wild-type genome by PCR and inserted into the pGWB1 vector (Nakagawa et al., 2007) via pENTR/D-TOPO (Invitrogen). The genomic construct was introduced into the Col plants as described above. The resultant transformants were crossed with the sriw1 rid2 double mutant. Each individual of the F2 plants was examined for adventitious rooting ability at 28°C and for the presence of the sriw1-linked Ler polymorphic allele, the rid2 mutant allele, and the At5g09330 transgene.
RNA Gel Blot Analysis
Total RNA samples were prepared using the TRIzol reagent (Invitrogen) and separated electrophoretically in 1.0% agarose/MOPS/formaldehyde gels according to the conventional method. After RNAs were blotted onto a nylon membrane, the membrane was hybridized with a digoxigenin-labeled probe specific for 5′-ETS, ITS1, or ITS2 of pre-rRNA, as described previously (Ohbayashi et al., 2011). The primers used for probe synthesis are listed in Supplemental Table 8. Hybridization signals were detected using the DIG system (Roche Diagnostics).
Measurement of the 18S/25S rRNA Ratio
Total RNAs were extracted with the TRIzol reagent (Invitrogen) and analyzed by capillary electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies). The 18S/25S rRNA ratios were obtained from the peak areas of the 18S and 25S rRNAs in the electrophoretograms.
RT-qPCR Analysis
Gene expression levels were measured by RT-qPCR as described previously (Shinohara et al., 2014). Briefly, total RNA was isolated from plant samples using the TRIzol reagent (Invitrogen) and reverse transcribed using the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa). Real-time PCR was performed using the resultant cDNA as a template and gene-specific primers (Supplemental Table 9) with SYBR Premix ExTaq II (TaKaRa) on a StepOne Real Time PCR System (Life Technologies). The expression level of a target gene was normalized to that of TUBULIN ALPHA-4 CHAIN (TUA4).
Transactivation Assay in Budding Yeast
The sequences encoding full-length ANAC082 and the NAC domain of ANAC082 were amplified with the primer pairs ANAC082_BamHI_Fw plus ANAC082_PstI_Rv and ANAC082_BamHI_Fw plus ANAC082_NACdom_PstI_Rv, respectively (Supplemental Table 7). They were cloned into the BamHI/PstI site of pGBD-C1 (James et al., 1996) to create constructs for expression of GAL4 DNA binding domain-ANAC082 fusion proteins. These plasmids were transformed into the yeast strain PJ69-4A, which was mutated in HIS3 and harbored the HIS3 reporter gene GAL1-HIS3 (James et al., 1996), according to the method of Gietz et al. (1995). The transformed yeast strains were incubated on SD medium (Sherman, 1991) supplemented with or without histidine for 3 d at 28°C.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: ANAC082, At5g09330; CUC1, At3g15170; STM, At1g62360; and TUA4, At1g04820.
Supplemental Data
Supplemental Figure 1. Alignment of NAC Transcription Factors and Mutant Alleles of ANAC082.
Supplemental Table 1. P Values of Two-Tailed Student’s t test for Paired Comparisons of the Nucleolar Diameter Data of Figure 1E.
Supplemental Table 2. P Values of Two-Tailed Student’s t test for Paired Comparisons of the 18S/25S rRNA Ratio Data of Figure 2C.
Supplemental Table 3. P Values of Two-Tailed Student’s t test for Paired Comparisons of the CUC1 Expression Data of Figure 6B.
Supplemental Table 4. P Values of Two-Tailed Student’s t test for Paired Comparisons of the STM Expression Data of Figure 6B.
Supplemental Table 5. P Values of Two-Tailed Student’s t test for Paired Comparisons of the Callus Fresh Weight Data of Figure 8B.
Supplemental Table 6. P Values of Two-Tailed Student’s t test for Paired Comparisons of the ANAC082 Expression Data of Figure 9B.
Supplemental Table 7. List of Primers Used for Plasmid Construction.
Supplemental Table 8. List of Primers Used for Probe Synthesis.
Supplemental Table 9. List of Primers Used in Real-Time PCR.
Acknowledgments
We thank Yoshiko Kikuchi (University of Tokyo) for providing the budding yeast strain PJ69-4A and the plasmid pGBD-C1 and for her helpful advice, Tsuyoshi Nakagawa (Shimane University) for providing pGWB vector plasmids, Yukiko Sugisawa (University of Tokyo) for capillary electrophoresis of rRNAs, Hitoshi Onouchi (Hokkaido University) and Mary Byrne (University of Sydney) for valuable discussion, and the Seattle TILLING Project for identifying mutations in ANAC082. This work was funded by the Ministry of Education, Culture, Sports, Science, and Technology, Japan, Grant-in-Aid for Scientific Research on Priority Areas (no.19060001 to M.S. and no.19060003 to Y. Machida) and Grant-in-Aid for Scientific Research on Innovative Areas (no.25113002 to G.H. and H.T.), and by the Japan Society for the Promotion of Science, Japan, Grant-in-Aid for Scientific Research (no.25291057 to M.S.). I.O. and N.S. were supported by grant no.1906001 and Y. Matsumura was supported by grant no.1906003.
AUTHOR CONTRIBUTIONS
I.O. and M.S. conceived and designed the project. I.O. performed most of the experiments. C.-Y.L., N.S., and M.S. performed the RT-PCR analysis of gene expression, the characterization of the sriw1 rid3 double mutant, and inhibitor experiments, respectively. Y. Matsumura and Y. Machida contributed to the research design and data interpretation, especially in the part dealing with rh10. G.H. and H.T. contributed to the research design and data interpretation, especially in the part dealing with oli5 and rpl4d. I.O. and M.S. wrote the article. All authors read, revised, and approved the article.
References
- Abbasi N., Kim H.B., Park N.-I., Kim H.-S., Kim Y.-K., Park Y.-I., Choi S.-B. (2010). APUM23, a nucleolar Puf domain protein, is involved in pre-ribosomal RNA processing and normal growth patterning in Arabidopsis. Plant J. 64: 960–976. [DOI] [PubMed] [Google Scholar]
- Azuma M., Toyama R., Laver E., Dawid I.B. (2006). Perturbation of rRNA synthesis in the bap28 mutation leads to apoptosis mediated by p53 in the zebrafish central nervous system. J. Biol. Chem. 281: 13309–13316. [DOI] [PubMed] [Google Scholar]
- Ben-Shem A., Garreau de Loubresse N., Melnikov S., Jenner L., Yusupova G., Yusupov M. (2011). The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334: 1524–1529. [DOI] [PubMed] [Google Scholar]
- Boulon S., Westman B.J., Hutten S., Boisvert F.-M., Lamond A.I. (2010). The nucleolus under stress. Mol. Cell 40: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger K., Mühl B., Harasim T., Rohrmoser M., Malamoussi A., Orban M., Kellner M., Gruber-Eber A., Kremmer E., Hölzel M., Eick D. (2010). Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J. Biol. Chem. 285: 12416–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M.E. (2009). A role for the ribosome in development. Trends Plant Sci. 14: 512–519. [DOI] [PubMed] [Google Scholar]
- Cao J., Geballe A.P. (1996). Coding sequence-dependent ribosomal arrest at termination of translation. Mol. Cell. Biol. 16: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.-Y., Hagen G.L. (1971). Ribosomal RNA precursor synthesis in tobacco tissue culture. Biochim. Biophys. Acta 228: 503–508. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Danilova N., Sakamoto K.M., Lin S. (2008). Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood 112: 5228–5237. [DOI] [PubMed] [Google Scholar]
- Deisenroth C., Zhang Y. (2010). Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene 29: 4253–4260. [DOI] [PubMed] [Google Scholar]
- de la Cruz J., Kressler D., Linder P. (2004). Ribosomal subunit assembly. In The Nucleolus, Olson M.O.J., ed (New York: Kluwer Academic/Plenum Publishers; ), pp. 258–285. [Google Scholar]
- de la Cruz J., Karbstein K., Woolford J.L. Jr (2015). Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu. Rev. Biochem. 84: 93–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Srivastava R., Howell S.H. (2013). Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int. J. Mol. Sci. 14: 8188–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina I., et al. (2015). Identification of novel Arabidopsis thaliana upstream open reading frames that control expression of the main coding sequences in a peptide sequence-dependent manner. Nucleic Acids Res. 43: 1562–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R.S.S. (1975). Studies on messenger and ribosomal RNA synthesis in plant tissue cultures induced to undergo synchronus cell division. Eur. J. Biochem. 50: 529–537. [DOI] [PubMed] [Google Scholar]
- Fujikura U., Horiguchi G., Ponce M.R., Micol J.L., Tsukaya H. (2009). Coordination of cell proliferation and cell expansion mediated by ribosome-related processes in the leaves of Arabidopsis thaliana. Plant J. 59: 499–508. [DOI] [PubMed] [Google Scholar]
- Gaba A., Jacobson A., Sachs M.S. (2005). Ribosome occupancy of the yeast CPA1 upstream open reading frame termination codon modulates nonsense-mediated mRNA decay. Mol. Cell 20: 449–460. [DOI] [PubMed] [Google Scholar]
- Gietz R.D., Schiestl R.H., Willems A.R., Woods R.A. (1995). Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11: 355–360. [DOI] [PubMed] [Google Scholar]
- Golomb L., Volarevic S., Oren M. (2014). p53 and ribosome biogenesis stress: the essentials. FEBS Lett. 588: 2571–2579. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Till B.J., Comai L. (2004). TILLING. Traditional mutagenesis meets functional genomics. Plant Physiol. 135: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henras A.K., Plisson-Chastang C., O’Donohue M.-F., Chakraborty A., Gleizes P.-E. (2015). An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip. Rev. RNA 6: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J.J. (1963). Effects of puromycin on RNA synthesis in mammalian cells. Proc. Natl. Acad. Sci. USA 50: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G., Van Lijsebettens M., Candela H., Micol J.L., Tsukaya H. (2012). Ribosomes and translation in plant developmental control. Plant Sci. 191-192: 24–34. [DOI] [PubMed] [Google Scholar]
- Horiguchi G., Mollá-Morales A., Pérez-Pérez J.M., Kojima K., Robles P., Ponce M.R., Micol J.L., Tsukaya H. (2011). Differential contributions of ribosomal protein genes to Arabidopsis thaliana leaf development. Plant J. 65: 724–736. [DOI] [PubMed] [Google Scholar]
- Horn H.F., Vousden K.H. (2007). Coping with stress: multiple ways to activate p53. Oncogene 26: 1306–1316. [DOI] [PubMed] [Google Scholar]
- Hsiang Y.-H., Hertzberg R., Hecht S., Liu L.F. (1985). Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 260: 14873–14878. [PubMed] [Google Scholar]
- Huart A.-S., Hupp T.R. (2013). Evolution of conformational disorder & diversity of the p53 interactome. Biodiscovery 8: 5. [Google Scholar]
- Ito T., Kim G.T., Shinozaki K. (2000). Disruption of an Arabidopsis cytoplasmic ribosomal protein S13-homologous gene by transposon-mediated mutagenesis causes aberrant growth and development. Plant J. 22: 257–264. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., Craig E.A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144: 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J.L. (1966). Effect of purine and pyrimidine analogues on growth and RNA metabolism in the soybean hypocotyl-the selective action of 5-fluorouracil. Plant Physiol. 41: 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Sugiyama M. (2003). Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development 130: 5637–5647. [DOI] [PubMed] [Google Scholar]
- Konishi M., Sugiyama M. (2006). A novel plant-specific family gene, ROOT PRIMORDIUM DEFECTIVE 1, is required for the maintenance of active cell proliferation. Plant Physiol. 140: 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger T.S., Watkins K.P., Friso G., van Wijk K.J., Barkan A. (2009). A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing. Proc. Natl. Acad. Sci. USA 106: 4537–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N.J., et al. (2004). High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell 13: 225–239. [DOI] [PubMed] [Google Scholar]
- Law G.L., Raney A., Heusner C., Morris D.R. (2001). Polyamine regulation of ribosome pausing at the upstream open reading frame of S-adenosylmethionine decarboxylase. J. Biol. Chem. 276: 38036–38043. [DOI] [PubMed] [Google Scholar]
- Lecompte O., Ripp R., Thierry J.-C., Moras D., Poch O. (2002). Comparative analysis of ribosomal proteins in complete genomes: an example of reductive evolution at the domain scale. Nucleic Acids Res. 30: 5382–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley D.B., Harkin D.P., Johnston P.G. (2003). 5-Fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3: 330–338. [DOI] [PubMed] [Google Scholar]
- Mannuss A., Trapp O., Puchta H. (2012). Gene regulation in response to DNA damage. Biochim. Biophys. Acta 1819: 154–165. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., et al. (2016). A genetic link between epigenetic repressor AS1-AS2 and a putative small subunit processome in leaf polarity establishment of Arabidopsis. Biol. Open 5: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C., Grummt I. (2005). Cellular stress and nucleolar function. Cell Cycle 4: 1036–1038. [DOI] [PubMed] [Google Scholar]
- McCann K.L., Charette J.M., Vincent N.G., Baserga S.J. (2015). A protein interaction map of the LSU processome. Genes Dev. 29: 862–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K.A., Li J.Z., Park C.Y., Beaudry V., Tabor H.K., Sabnis A.J., Zhang W., Fuchs H., de Angelis M.H., Myers R.M., Attardi L.D., Barsh G.S. (2008). Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat. Genet. 40: 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K.B., Bonham-Smith P.C. (2006). Ribosomal protein gene regulation: what about plants? Can. J. Bot. 84: 342–362. [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Nyikó T., Sonkoly B., Mérai Z., Benkovics A.H., Silhavy D. (2009). Plant upstream ORFs can trigger nonsense-mediated mRNA decay in a size-dependent manner. Plant Mol. Biol. 71: 367–378. [DOI] [PubMed] [Google Scholar]
- O’Day C.L., Chavanikamannil F., Abelson J. (1996). 18S rRNA processing requires the RNA helicase-like protein Rrp3. Nucleic Acids Res. 24: 3201–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi I., Konishi M., Ebine K., Sugiyama M. (2011). Genetic identification of Arabidopsis RID2 as an essential factor involved in pre-rRNA processing. Plant J. 67: 49–60. [DOI] [PubMed] [Google Scholar]
- Ohtani M., Sugiyama M. (2005). Involvement of SRD2-mediated activation of snRNA transcription in the control of cell proliferation competence in Arabidopsis. Plant J. 43: 479–490. [DOI] [PubMed] [Google Scholar]
- Olsen A.N., Ernst H.A., Leggio L.L., Skriver K. (2005). NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 10: 79–87. [DOI] [PubMed] [Google Scholar]
- Ooka H., et al. (2003). Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 10: 239–247. [DOI] [PubMed] [Google Scholar]
- Petricka J.J., Nelson T.M. (2007). Arabidopsis nucleolin affects plant development and patterning. Plant Physiol. 144: 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps K.R., Charette J., Baserga S.J. (2011). The small subunit processome in ribosome biogenesis—progress and prospects. Wiley Interdiscip. Rev. RNA 2: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué H.A. (2004). Pre-rRNA processing and assembly in Saccharomyces cerevisiae. The machine that makes the machine. In The Nucleolus, Olson M.O.J., ed (New York: Kluwer Academic/Plenum Publishers; ), pp. 199–222. [Google Scholar]
- Sardana R., White J.P., Johnson A.W. (2013). The rRNA methyltransferase Bud23 shows functional interaction with components of the SSU processome and RNase MRP. RNA 19: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G.J., Blaukopf C. (2010). Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 153: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. (1991). Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Ohbayashi I., Sugiyama M. (2014). Involvement of rRNA biosynthesis in the regulation of CUC1 gene expression and pre-meristematic cell mound formation during shoot regeneration. Front. Plant Sci. 5: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H.M. (1985). Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 82: 5328–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondalle S.B., Baserga S.J. (2014). Human diseases of the SSU processome. Biochim. Biophys. Acta 1842: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakonyi D., Byrne M.E. (2011). Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J. 65: 269–281. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Takahashi A., Naito S., Onouchi H. (2012). BAIUCAS: a novel BLAST-based algorithm for the identification of upstream open reading frames with conserved amino acid sequences and its application to the Arabidopsis thaliana genome. Bioinformatics 28: 2231–2241. [DOI] [PubMed] [Google Scholar]
- Tamaki H., Konishi M., Daimon Y., Aida M., Tasaka M., Sugiyama M. (2009). Identification of novel meristem factors involved in shoot regeneration through the analysis of temperature-sensitive mutants of Arabidopsis. Plant J. 57: 1027–1039. [DOI] [PubMed] [Google Scholar]
- Tsukaya H., Byrne M.E., Horiguchi G., Sugiyama M., Van Lijsebettens M., Lenhard M. (2013). How do ‘housekeeping’ genes control organogenesis?--Unexpected new findings on the role of housekeeping genes in cell and organ differentiation. J. Plant Res. 126: 3–15. [DOI] [PubMed] [Google Scholar]
- Turowski T.W., Tollervey D. (2015). Cotranscriptional events in eukaryotic ribosome synthesis. Wiley Interdiscip. Rev. RNA 6: 129–139. [DOI] [PubMed] [Google Scholar]
- Uchiyama-Kadokura N., Murakami K., Takemoto M., Koyanagi N., Murota K., Naito S., Onouchi H. (2014). Polyamine-responsive ribosomal arrest at the stop codon of an upstream open reading frame of the AdoMetDC1 gene triggers nonsense-mediated mRNA decay in Arabidopsis thaliana. Plant Cell Physiol. 55: 1556–1567. [DOI] [PubMed] [Google Scholar]
- Van Lijsebettens M., Vanderhaeghen R., De Block M., Bauw G., Villarroel R., Van Montagu M. (1994). An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. EMBO J. 13: 3378–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis B.L., Kovacevic J., Missbach S., Schleiff E. (2015a). Plant-specific features of ribosome biogenesis. Trends Plant Sci. 20: 729–740. [DOI] [PubMed] [Google Scholar]
- Weis B.L., Palm D., Missbach S., Bohnsack M.T., Schleiff E. (2015b). atBRX1-1 and atBRX1-2 are involved in an alternative rRNA processing pathway in Arabidopsis thaliana. RNA 21: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Li Z., Sardana R., Bujnicki J.M., Marcotte E.M., Johnson A.W. (2008). Bud23 methylates G1575 of 18S rRNA and is required for efficient nuclear export of pre-40S subunits. Mol. Cell. Biol. 28: 3151–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Nagahage I.S.P., Ohtani M., Ishikawa T., Uchimiya H., Kawai-Yamada M., Demura T. (2015). Arabidopsis NAC domain proteins VND-INTERACTING1 and ANAC103 interact with multiple NAC domain proteins. Plant Biotechnol. 32: 119–123. [Google Scholar]
- Yamaguchi M., Ohtani M., Mitsuda N., Kubo M., Ohme-Takagi M., Fukuda H., Demura T. (2010). VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 22: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Ling Q., Wang H., Huang H. (2008). Ribosomal proteins promote leaf adaxial identity. Development 135: 1325–1334. [DOI] [PubMed] [Google Scholar]
- Yoshiyama K., Conklin P.A., Huefner N.D., Britt A.B. (2009). Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 106: 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]