R-loop homeostasis in Arabidopsis plastid determines chloroplast genome stability and development.
Abstract
Maintaining organellar genome integrity is essential for eukaryotic cells, and many factors can threaten genome integrity. R-loops are DNA:RNA duplexes produced during transcription, with the nontemplated DNA forming a single-stranded region. R-loops function in the regulation of transcription, DNA replication, and DNA repair, but can also be susceptible to lesions that form double-stranded breaks and thus induce genome instability. From investigating the function of a plant chloroplast-localized R-loop removing enzyme AtRNH1C, we have found that it is responsible for plastid R-loop homeostasis, chloroplast genome instability, and development. Interactome analysis revealed that AtRNH1C associates with multiple chloroplast-localized DNA and RNA metabolism-related proteins, including the core DNA gyrases complex. The interaction between AtRNH1C and AtGyrases was critical for R-loop homeostasis in chloroplast and important to release the transcription-replication conflicts in the highly transcribed and replication originated cp-rDNA regions and thus to reduce the DNA damage. Our results reveal the plastid R-loop accumulation leads to chloroplast DNA instability and provide insight into the maintenance of genome integrity in chloroplasts, in which the evolutionarily conserved RNase H1 and DNA gyrase proteins are involved.
INTRODUCTION
Chloroplasts are genetically semiautonomous, having their own genome (Jarvis and López-Juez, 2013). The chloroplast DNA (cpDNA) is located around the thylakoid within chloroplasts (Powikrowska et al., 2014) and, given this special location, is sensitive to fluctuations in its surrounding environment, including the presence of DNA damage-inflicting agents that are generated during photosynthesis (Allen and Raven, 1996; Boesch et al., 2011; Gutman and Niyogi, 2009; Oldenburg and Bendich, 2015; Raven, 2015). Indeed, previous pulsed-field gel electrophoresis (PFGE) analysis of chloroplast genomic DNA structure revealed the presence of degraded chloroplast genomes in nature (Deng et al., 1989; Rowan et al., 2010). Recent genome-wide studies have demonstrated that cpDNA rearrangement occurs naturally (Zampini et al., 2015) and that cpDNA maintenance is development and environment (e.g., light) dependent (Kumar et al., 2014; Morley and Nielsen, 2016; Shaver et al., 2008; reviewed in Oldenburg and Bendich, 2015; Raven, 2015).
In spite of numerous potential threats to genome integrity, the architecture of the chloroplast genome is conserved among various green plants, and the chloroplast genome is much more stable than the nuclear genome (Green, 2011; Wolfe et al., 1987). These facts imply that stringent genome maintenance systems exist within plastids. Indeed, several nuclear-encoded and chloroplast-localized proteins including Whirlies, organellar single-stranded DNA (ssDNA) binding proteins, MutS homolog 1, DNA polymerase IB (DNA PolIB), and recombination protein RecA have been identified and shown to play essential roles in maintaining chloroplast genome stability (Maréchal and Brisson, 2010; Oldenburg and Bendich, 2015). The chloroplast-localized, single-stranded DNA binding, Whirly family proteins WHY1 and WHY3 were shown to protect against illegitimate repeat-mediated recombination and thus contribute to plastid genome stability maintenance (Maréchal et al., 2009). The RecA proteins that are targeted to chloroplasts and/or mitochondria are thought to play important roles in DNA damage repair, since the reduction of RecA mRNA leads to a decrease in cpDNA integrity and an increase in cpDNA fragmentation (Rowan et al., 2010). However, there is only limited direct evidence showing that the RecA proteins function specifically in DNA repair rather than in reducing the generation of DNA damage. Although all of these proteins were shown to be necessary for maintaining chloroplast genome integrity, it seems that their functions appear genetically different, as the PFGE results showed different chloroplast genome patterns in knockout mutants of these genes (Rowan et al., 2010; Zampini et al., 2015). Still, the mechanisms in chloroplasts that resist DNA damage and precisely maintain genome integrity remain to be explored.
Recent evidence from different organisms has shown that transcription is an important source of triggering genome instability (Aguilera and García-Muse, 2013; Gaillard and Aguilera, 2016; García-Muse and Aguilera, 2016; Hamperl and Cimprich, 2016). A three-stranded nucleic acid structure is universally formed during transcription, as the nascent RNA molecule may hybridize with the template DNA strand forming a DNA:RNA hybrid and leaving the nontemplated DNA single-stranded (Costantino and Koshland, 2015; Santos-Pereira and Aguilera, 2015; Skourti-Stathaki and Proudfoot, 2014). Such structures are termed R-loops and have been found in various organisms (El Hage et al., 2014; Sanz et al., 2016; Sun et al., 2013; Wahba et al., 2016; Yu et al., 2003). R-loops can have beneficial and detrimental effects in cells (reviewed in Costantino and Koshland, 2015; Santos-Pereira and Aguilera, 2015; Skourti-Stathaki and Proudfoot, 2014). On the one hand, R-loops play important roles in regulating gene expression by influencing transcription termination and DNA and chromatin modifications and are also involved in the processes of DNA replication, DNA repair, and Ig class-switch recombination. On the other hand, R-loops can lead to DNA damage as the ssDNA formed from RNA/DNA hybridization is susceptible to mutagenesis and lesions, leading to double-strand breaks and recombination that further induces genome instability. Accumulation of R-loops is associated with many human diseases such as neurodegenerative diseases, nucleotide expansion diseases, and cancer (Groh and Gromak, 2014). Formation and resolution of R-loops are regulated by a number of different factors. DNA structures such as negative DNA supercoiling and GC skew as well as transcription are known to positively affect formation of R-loops (Chédin, 2016; Costantino and Koshland, 2015; Santos-Pereira and Aguilera, 2015; Skourti-Stathaki and Proudfoot, 2014; Sollier and Cimprich, 2015). R-loops can be resolved by DNA:RNA hybrid helicases such as Rho in bacteria, Sen1 in yeast, and senataxin in humans (Costantino and Koshland, 2015; Santos-Pereira and Aguilera, 2015; Skourti-Stathaki and Proudfoot, 2014; Sollier and Cimprich, 2015). R-loops can also be removed by the ribonucleases such as RNase H. RNase H enzymes, including RNase H1 and RNaseH2, are evolutionally conserved and specifically degrade the RNA moiety of the DNA:RNA hybrids; therefore, they are likely to be primarily responsible for the efficient dissolution of R-loops (Cerritelli and Crouch, 2009). A recent study in yeast revealed unexpectedly that RNase H enzymes are essential during homologous recombination-mediated DNA repair, which pointed to a surprising role of DNA:RNA hybrids in maintaining genomic stability (Ohle et al., 2016). Mammals have one RNase H1 protein with dual localization, in both the nucleus and mitochondria (Cerritelli et al., 2003). Mitochondrion-localized RNase H1 is essential for mitochondrial DNA replication, RNA processing, and development (Akman et al., 2016; Cerritelli et al., 2003; Holmes et al., 2015). Although studies have shown the important roles of RNase H1 in R-loop resolution and genomic stability maintenance in the nucleus of eukaryotes, the detailed molecular functions of organelle-localized RNase H1 remain unclear.
In plants, R-loops were first reported to repress the expression of the antisense long noncoding RNA COOLAIR to promote flowering in Arabidopsis thaliana (Sun et al., 2013). Recently, we revealed that the maintenance of nuclear inherent R-loops in rice (Oryza sativa) auxin-related genes by OsTOP1 is essential for root development (Shafiq et al., 2017) and uncovered the distinct R-loop profile in the Arabidopsis nuclear genome (Xu et al., 2017). However, we still have a limited understanding of the mechanisms of R-loop formation and resolution or their biological functions in plants, especially in DNA-containing organelles such as chloroplasts and mitochondria.
To extend our understanding of R-loops in plant organelles, we started to analyze the biological functions of the organelle-localized RNase H1 enzymes, which can remove R loops. We provide evidence that restriction/resolution of R-loop levels by AtRNH1C is essential for the maintenance of chloroplast genome stability, and this process requires the collaboration of AtRNH1C and AtGyrases. As RNase H1 and DNA gyrases are evolutionarily conserved, our findings suggest a general regulatory mechanism for resolving R-loop-mediated DNA stress, especially in prokaryotes and eukaryotic DNA-containing organelles.
RESULTS
Identification of RNase H1-Like Proteins in Arabidopsis
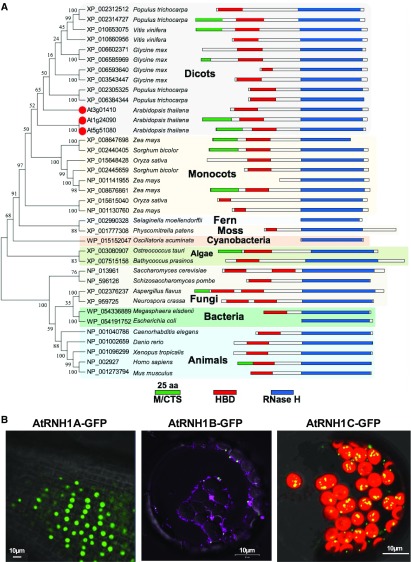
Our interests in plant R-loop homeostasis promoted us to focus on the function of RNase H1 proteins because (1) in contrast to RNase H2 family proteins, which are composed of three subunits, RNase H1 has only RNase H activity; (2) RNase H1 is essential for removing aberrant R-loops without sequence specificity (Cerritelli and Crouch, 2009; Nowotny et al., 2005); and (3), the function and protein architecture of RNase H1 are highly conserved from prokaryotes to eukaryotes (Cerritelli and Crouch, 2009), but little is known about the roles in plants. In order to compare plant RNase H1 proteins with those in other organisms, we constructed a phylogenetic tree from the alignment of full-length protein sequences (Figure 1A). Similar to RNase H1 proteins in other organisms, all the plant RNase H1 proteins harbor a DNA:RNA hybrid binding domain and an RNase H1 activity domain, suggesting conserved functions of this protein in different organisms. However, the topology of the tree clearly separates plants from other organisms and splits dicot and monocot species within plant kingdoms, which reflects the speciation history during evolution.
Figure 1.
RNase H1-Like Proteins in Arabidopsis.
(A) Phylogenetic analysis and protein structures of RNase H1 proteins. RNase H1 proteins from Arabidopsis are marked with red dot. Protein structures are drawn to scale. The RNase H domains are highlighted in blue, DNA:RNA hybrid binding domains (HBD) are highlighted in red, and mitochondrion and/or chloroplast targeting sequences are predicted using TargetP and highlighted in green.
(B) Subcellular localization of RNase H1 proteins AtRNH1A, AtRNH1B, and AtRNH1C in Arabidopsis are shown. Subcellular localization of AtRNH1A and AtRNH1B is analyzed from the 2-week-old stably transformed seedling roots and protoplast, respectively; transiently expressed GFP-tagged AtRNH1C were analyzed from protoplasts. Mitochondrion was stained by MitoTracker Red CMXRos (magenta). Chloroplasts can be distinguished by the chlorophyll autofluorescence (red). Bars = 10 µm.
While other organisms have only one RNase H1 gene in their genome, plant species have multiple genes, particularly in the higher plants. Nearly all paralogs of RNase H1 proteins grouped together with high bootstrap values, indicating that the gene duplication was a recent evolutionary event that occurred in higher plants. Three RNase H1-like proteins, named AtRNH1A (AT3G01410), -B (AT5G51080), and -C (AT1G24090) (Figure 1A), that showed similar domain architectures to mammalian RNase H1 were identified from Arabidopsis genome. Two of these (AtRNH1B and -C) had predicted mitochondrion/chloroplast-targeting signals (MTS/CTS) (Figure 1A). We tested the subcellular localization of the three nuclear-encoded Arabidopsis RNase H1 proteins by fusion with eGFP (Figure 1B) and found that, while AtRNH1A localized in the nucleus, AtRNH1B colocalized within mitochondria, and AtRNH1C was predominately found in chloroplasts. These results indicated that, unlike other organisms such as human (Cerritelli and Crouch, 2009), plants have evolved more than one RNase H1 proteins and had already diverged with preferential subcellular localizations. The increased numbers of RNase H1 proteins and their specific subcellular localizations in Arabidopsis suggest that they may play important roles in nucleic acid-containing cellular compartments.
Mutation of Chloroplast-Localized AtRNH1C Affects Chloroplast Development
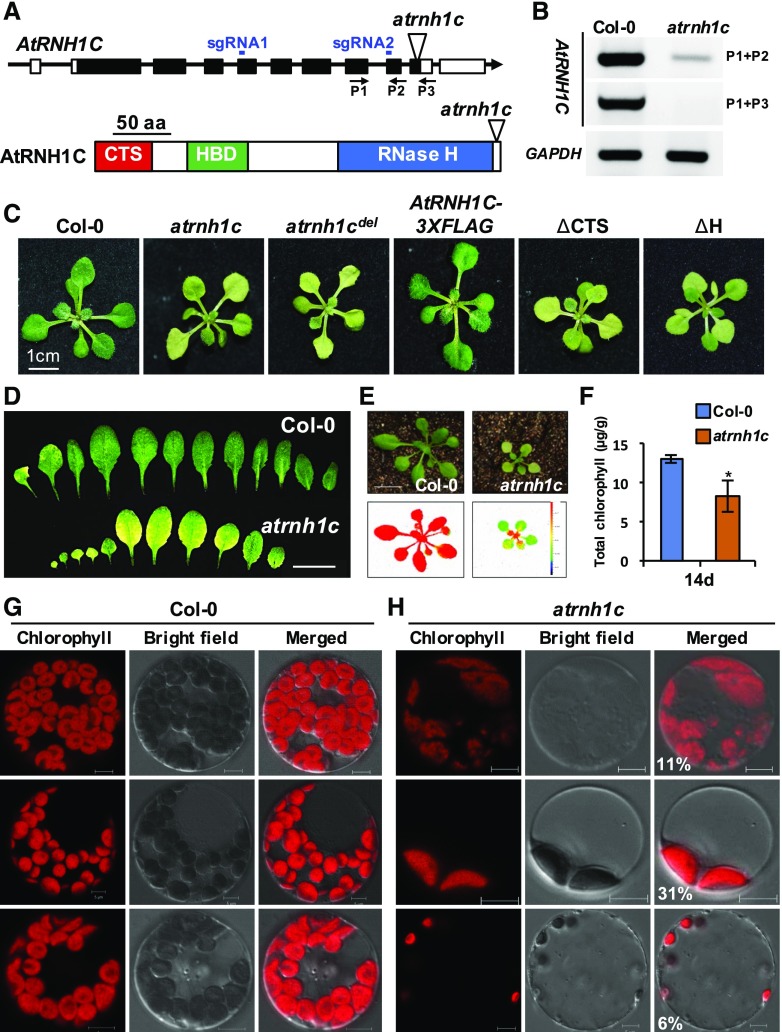
To further explore the functions of RNase H1 proteins in Arabidopsis, we obtained T-DNA knockout mutants and analyzed their phenotypes. Interestingly, knockout mutation of AtRNH1C (SAIL_97_E11, here named atrnh1c ; Figure 2A) showed obvious dwarf and yellowish (pale-green) leaf phenotypes compared with the wild-type Col-0 (Figures 2C to 2E; Supplemental Figures 1A to 1D). The atrnh1c mutant has dramatically decreased AtRNH1C expression (Figure 2B). Photosynthetic efficiency (PSII signal) and chlorophyll content were dramatically decreased in atrnh1c (Figures 2E and 2F). Further detailed observations showed the presence of a large portion of protoplasts containing abnormal chloroplasts in atrnh1c (calculated in Figure 2H and Supplemental Figure 2), including a reduction in chloroplast number and defects in chloroplast division. The intensity of chlorophyll autofluorescence was also lower compared with Col-0 (Figures 2G and 2H). Complementation analysis confirmed that the atrnh1c pale-green leaf phenotype was caused by the AtRNH1C mutation (Figure 2C; Supplemental Figure 1D). To further confirm the atrnh1c phenotype, we generated CRISPR/Cas9-based (Wang et al., 2015) AtRNH1C genomic deletion mutants (Figures 2A and 2C; Supplemental Figure 1E; here named atrnh1cdel). Consistent with the atrnh1c mutant phenotype, atrnh1cdel mutants showed a similar pale-green leaf phenotype (Figure 2C; Supplemental Figure 1E). Versions of AtRNH1C with various domain truncations, including deletion of the chloroplast targeting signals (ΔCTS) or catalytic RNase H domain (ΔH), failed to complement the atrnh1c defect (Figure 2C), further confirming that the chloroplast localization and RNase H activity of AtRNH1C are essential for its physiological function in plant development. As there is little known about regulation of plastid R-loop homeostasis and its biological functions, we therefore started to investigate the function of chloroplast localized RNase H1 protein AtRNH1C in linking R-loop homeostasis and chloroplast development.
Figure 2.
Characterization of atrnh1c Mutant.
(A) The AtRNH1C gene and its encoded protein domains are shown. The T-DNA insertion site of SAIL_97_E11 and the primers used for RT-PCR analysis and sgRNAs of atrnh1cdel (see Supplemental Figure 1E for details) are indicated. CTS, chloroplast target sequence; HBD, DNA:RNA hybrid binding domain.
(B) RT-PCR analysis of AtRNH1C expression level in atrnh1c. GAPDH was included as an equal loading control.
(C)The phenotype of 20-d-old plants of Col-0, atrnh1c, CRISPR-mediated AtRNH1C deletion (atrnh1cdel), and transgenic lines with full-length or truncations of AtRNH1C in atrnh1c mutant.
(D) Rosette leaves of 2-week-old Col-0 (upper panel) and atrnh1c mutant (lower panel). Bar = 1 cm.
(E) Photographs and chlorophyll fluorescence images of Col-0 (left) and atrnh1c (right) plants. The color scale representing Fv/Fm is given at the right of lower panel. Bar = 1 cm.
(F) The chlorophyll contents in the leaves of 14-d-old Col-0 and atrnh1c plants. Asterisk represents significant difference to Col-0, P < 0.05. Thirty independent seedlings were analyzed from three biological experiment.
(G) and (H) Comparison of protoplasts from the leaves of 14-d-old Col-0 and atrnh1c plants. Chloroplasts can be distinguished by the chlorophyll autofluorescence (red). Percentage of various types of atrnh1c chloroplast were statistically calculated and indicated on the merged photos. Bar = 10 µm.
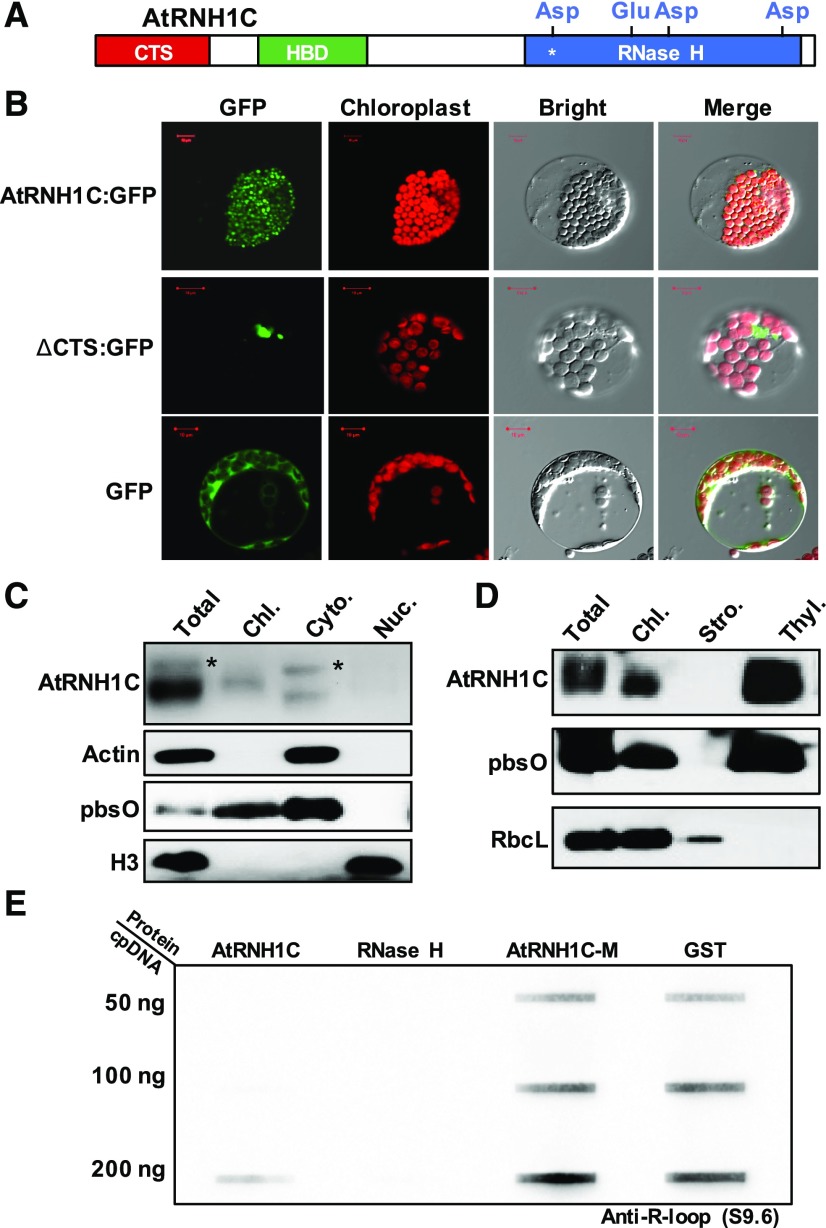
AtRNH1C Is Associated with Thylakoids and Has RNase H Activity in Vitro
To fully understand the function of AtRNH1C in chloroplast development, we investigated the detailed localization of AtRNH1C within chloroplasts. First, we found that the chloroplast localization of AtRNH1C was dependent on the CTS domain, as the GFP-tagged ΔCTS fusion protein aggregated in the cytoplasm rather than being targeted to chloroplasts (Figures 3A and 3B; Supplemental Figures 3A to 3C). This result was consistent with results from subcellular fractionation followed by immunoblot analysis (Figure 3C). A smaller band (CTS cleaved) could be detected only in the chloroplast fraction but additional bands, likely representing proteins that still harbored the CTS, could be detected in total protein and cytoplasmic fractions (Figure 3C). To explore the localization of AtRNH1C protein with the chloroplast, we separated different components of the chloroplast from the FLAG-tagged AtRNH1C complementation plants (Figure 3D) and used different protein markers to determine AtRNH1C localization. As shown in Figure 3D, AtRNH1C predominately colocalized with the thylakoid, where the chloroplast nucleoid is located (Powikrowska et al., 2014), in line with the potential role of AtRNH1C in R-loop regulation.
Figure 3.
AtRNH1C Is a Novel Chloroplast-Localized RNase H1 Protein.
(A) AtRNH1C protein domain architecture. Chloroplast target sequence, DNA:RNA hybrid binding domain, and RNase H enzyme activity domains are represented by green, red, and blue bars, respectively. The protein was purified together with GST tag without CTS. Four conserved amino acids within RNase H enzyme activity domain are shown. The asterisk indicates the amino acid mutagenized for purifying a GST-AtRNH1C-M protein lacking RNase H activity.
(B) Subcellular localization of GFP fused to the full-length AtRNH1C protein (upper panel) or to the AtRNH1C protein lacking the CTS (middle panel) and GFP alone (bottom). Bar = 10 µm.
(C) Immunoblots of total protein (Total) and protein fractions isolated from chloroplasts (Chl.), cytoplasm (Cyto.) and nuclei (Nuc.) of AtRNH1C-3XFLAG-complemented plants (shown in Figure 2C and Supplemental 1D) analyzed with antibodies against FLAG, Actin, pbsO, and H3, respectively. The asterisks indicate the full-length AtRNH1C protein with uncleaved CTS.
(D) Immunoblots of total protein (Total) and protein fractions isolated from chloroplasts (Chl.), stroma (Stro.), and thylakoids (Thyl.) of AtRNH1C-3XFLAG-complemented plants analyzed with antibodies against FLAG, pbsO (thylakoid marker), and RbcL (stroma marker).
(E) Analysis of AtRNH1C in vitro RNase H activity by slot-blot hybridization with chloroplast DNA as the substrate. Chloroplast DNA (50, 100, and 200 ng) was incubated with GST-AtRNH1C (Supplemental Figure 3A), commercial RNase H from NEB, GST-AtRNH1C-M, and GST alone, slotted onto nylon membrane and detected using anti-R-loop antibody S9.6. Loading control is shown in Supplemental Figure 3B.
RNase H1 family proteins specifically remove DNA:RNA hybrids in different organisms (Cerritelli and Crouch, 2009), and four conserved amino acids in the RNase H domain are essential for this activity (Figure 3A; Nowotny et al., 2005). To test whether the chloroplast-localized AtRNH1C protein has indeed an RNase H activity, we expressed and purified the AtRNH1C protein without its CTS (Figure 3E; Supplemental Figure 4A). In parallel, we mutated one of the four conserved amino acids essential for its catalytic activity (AtRNH1C-M, D222N; Figure 3E). The RNase H activities of purified proteins were analyzed by slot-blot assays using the chloroplast genomic DNA purified from Col-0 plants as the substrates (Supplemental Figure 4B). Commercial RNase H was used as the positive control, while the GST protein was used as the negative control (Figure 3E). Our data showed that, AtRNH1C, but not AtRNH1C-M, had a comparable in vitro RNase H activity to the commercial RNase H (Figure 3E), as the DNA:RNA hybrids were almost undetectable in the presence of either AtRNH1C protein or commercial RNase H. These data demonstrated that AtRNH1C has R-loop cleavage activity. Taken together, these results indicated that AtRNH1C is potentially involved in chloroplast R-loop resolution.
AtRNH1C Is Important for R-Loop Homeostasis and Genome Integrity in Chloroplast
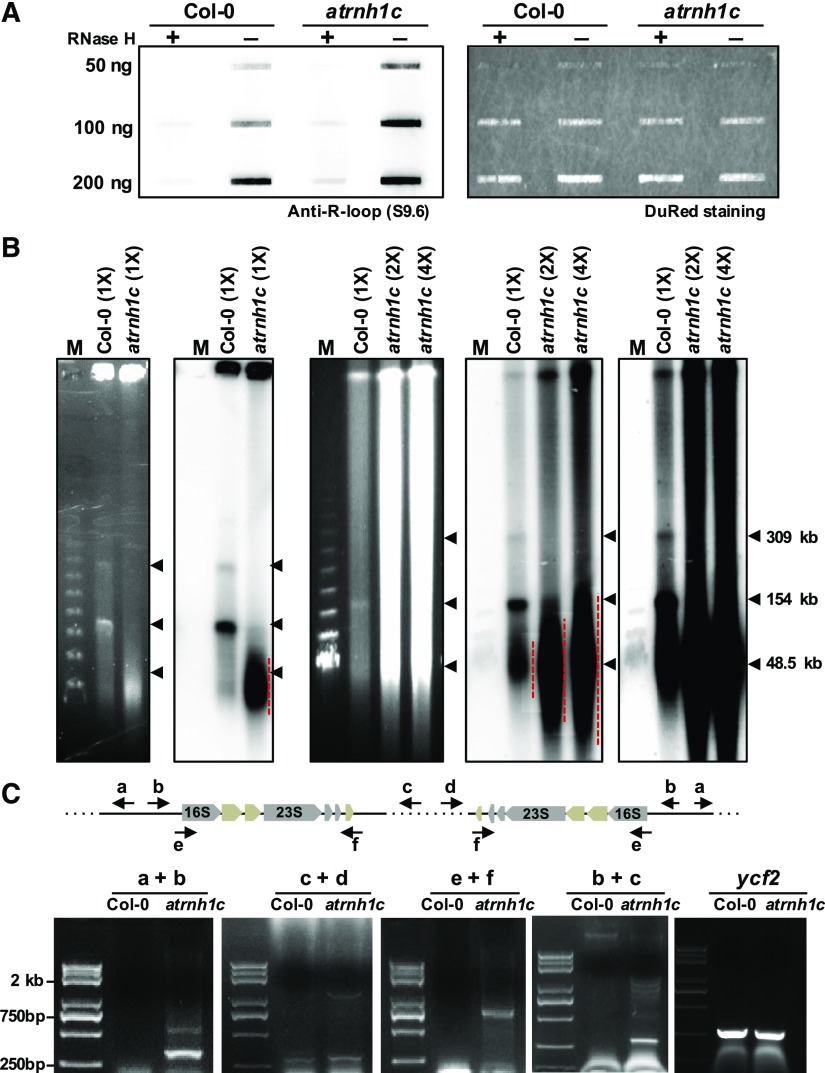
The results showed in Figure 3E also suggested the inherent R-loop levels presented in chloroplast genome. Accordingly, we then tested whether maintenance of the overall R-loop levels requires AtRNH1C activity. The overall R-loop levels in atrnh1c were much higher than those in Col-0, based on slot-blot assays (Figure 4A). These results indicated that AtRNH1C is involved in R-loop homeostasis in Arabidopsis chloroplasts.
Figure 4.
AtRNH1C Is Important for R-Loop Homeostasis and Genome Integrity in Chloroplast.
(A) Slot-blot assay analyzing overall R-loop levels in the chloroplast genome (left panel). Serial dilution of chloroplast DNAs (50, 100, and 200 ng) extracted from Col-0 and atrnh1c plants with or without RNase H treatment were slotted onto a nylon membrane and detected using the DNA:RNA hybrid antibody S9.6. DNA was stained using DuRed to show equal loading (right panel).
(B) PFGE of cpDNA obtained from the same (panel 1 from left) and different numbers (panel 3 from left) of chloroplasts (“1×” = 1.5 × 107) from 2-week-old Col-0 and atrnh1c plants after staining with ethidium bromide. Panel 2 and panels 4 and 5 are blot hybridization of the probe (a 505-bp rbcL gene fragment) that corresponds to panels 1 and 3, respectively (panels 4 and 5 correspond to panel 3 with a different exposure time). Red dotted lines indicate the destabilized cpDNA, and arrows indicate DNA molecular size. A Lambda Ladder (New England Biolabs; N0341) was used to indicate the molecular weight.
(C) PCR-based detection of chloroplast DNA rearrangement events in 2-week-old Col-0 and atrnh1c plants. Information on primers (a to f) used for the PCR reactions is referred to in the upper panel. ycf2 was used as the DNA quality control, and detailed primer sequences can be found in Supplemental Table 2.
As increased R-loop levels can trigger genome instability in other organisms (Aguilera and García-Muse, 2013; Gaillard and Aguilera, 2016; García-Muse and Aguilera, 2016; Hamperl and Cimprich, 2016), we were then interested to know whether the accumulation of R-loops in atrnh1c mutant could cause chloroplast genome instability. Strikingly, PFGE results showed that a large proportion of degraded DNA molecules accumulated in atrnh1c (Figure 4B). The monomeric and oligomeric DNA molecules were dramatically reduced to a hardly detectable level in atrnh1c compared with Col-0 (Figure 4B). We also checked DNA rearrangement and genome integrity in the atrnh1c mutant background, and our data revealed a dramatic increase in genome instability in atrnh1c (Figures 4C and 12; also see below). These results indicated that accumulation of R-loops indeed triggers the chloroplast genome instability, and underlines the essential role of AtRNH1C in chloroplast R-loop resolution and genome stabilization.
Figure 12.
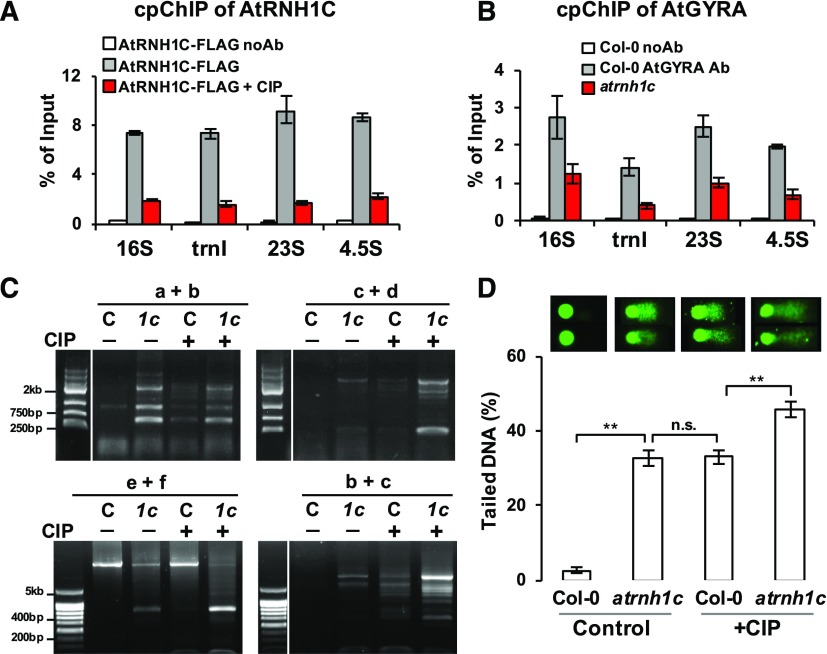
Both AtRNH1C and AtGyrases Are Necessary for cpDNA Integrity Maintenance.
(A) cpChIP-qPCR showing enrichment of AtRNH1C on chloroplast rDNA regions is significantly decreased when AtGyrases activity was inhibited by CIP.
(B) Anti-AtGyrA cpChIP-qPCR revealed the association of AtGyrases and the rDNA region and that this association is reduced in atrnh1c mutant. Values in (A) and (B) are the means ± se of three biological replicates.
(C) PCR-based detection of chloroplast DNA rearrangement events in Col-0 (C) and atrnh1c (1c) plants with or without CIP treatment. Information on primers (a to f) used for the PCR reactions is shown in Figure 4C.
(D) Comet assay analysis of genome integrity in Col-0 and atrnh1c plants, with or without CIP treatment. Two representative chloroplasts from different backgrounds/treatments are shown on top, and the average tail lengths ±se are from analysis of Supplemental Figure 11. More details can be found in Supplemental Data Set 1. Asterisks indicate significant difference (P < 0.001).
Multiple DNA and RNA Metabolism-Related Proteins Are Coimmunoprecipitated with AtRNH1C
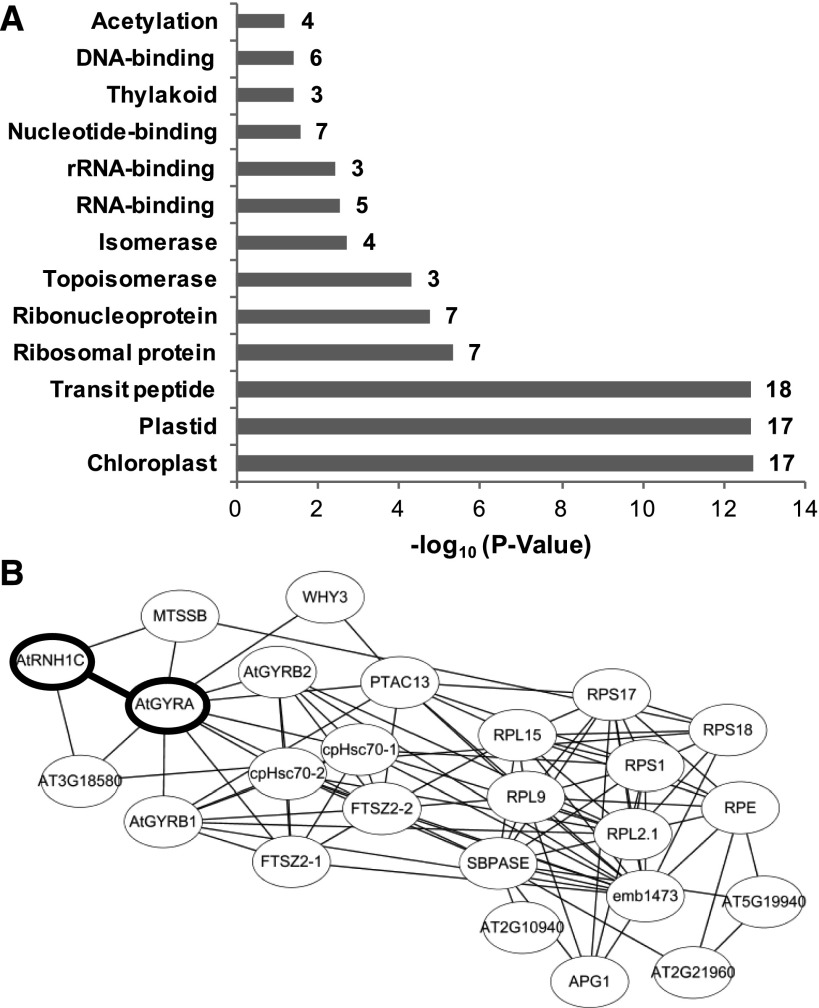
To further explore the molecular basis of AtRNH1C-mediated regulation of chloroplast R-loop levels and its impact on genome integrity and chloroplast development, we analyzed the AtRNH1C interactome through affinity purification of FLAG-tagged AtRNH1C from isolated chloroplasts of complemented plants (Figure 2C; Supplemental Figure 1D). The copurified proteins were subjected to mass spectrometry analysis (see Methods). Most of the copurified proteins were involved in DNA and RNA metabolism (Table 1, Figure 5), and the most abundant interactors were Arabidopsis DNA gyrase proteins (AtGyrases), including AtGyrA and AtGyrBs (Table 1). DNA gyrases, also known as type II DNA topoisomerases, are evolutionarily conserved (Supplemental Figure 5). They are necessary for introducing negative supercoils and relaxing positive supercoils during transcription and DNA replication in prokaryotes (Aldred et al., 2014; Chang et al., 2013; Higgins, 2014). Plant DNA gyrases have been proposed to have similar functions in the chloroplast (Cho et al., 2004; Evans-Roberts et al., 2016; Wall et al., 2004). The exact functions and regulatory mechanisms of DNA gyrases in plant organelles are not fully understood, although there is evidence that plant organelle-localized DNA gyrases are essential for plant development, as knockout mutations of either AtGyrA or AtGyrBs are lethal (Wall et al., 2004).
Table 1. Proteins That Coimmunoprecipitated with AtRNH1C.
| Name | Locus | Description | Unique Peptidesa | Percentage of Coverage |
|---|---|---|---|---|
| AtRNH1C | At1G24090 | RNase H domain-containing protein | 22-10-15 | 67-46-60 |
| AtGyrA | At3G10690 | DNA gyrase subunit A | 4-2-1 | 5.9-1.7-0.9 |
| AtGyrB1b | At3G10270 | DNA gyrase subunit B | 2-0-0 | 12-3.1-10.5 |
| AtGyrB2b | At5G04130 | DNA gyrase subunit B | 3-3-9 | 10.8-9-16.5 |
| cpHsc70-1 | At4G24280 | Heat shock 70 kD protein 6 | 0-7-1 | 0-12.3-1.4 |
| cpHsc70-2 | At5G49910 | Heat shock 70 kD protein 7 | 0-9-1 | 0-14.9-1.4 |
| pTAC13 | At3G09210 | Plastid transcriptionally active 13 | 1-6-4 | 3.6-22.2-13.5 |
| MTSSB1/2 | At3G18580/At4G11060 | Single-stranded DNA binding protein/DnaA-like | 2-4-0 | 9.7-22.1-0 |
| WHY1/WHY3 | At2G02740/At1G14410 | Single-stranded DNA-binding protein | 0-3-2 | 0-14.2-10.8 |
| At1G66260 | At1G66260 | THO complex subunit 4C | 0-1-4 | 0-5.4-18 |
| FTSZ2-2 | At3G52750 | Cell division protein FtsZ | 0-3-1 | 0-9.9-3.8 |
| FTSZ2-1 | At2G36250 | Cell division protein FtsZ homolog 2-1 | 0-4-0 | 0-16.1-0 |
| emb1473 | At1G78630 | 50S ribosomal protein L13 | 2-2-2 | 12-17-7 |
| RPL9 | At3G44890 | 50S ribosomal protein L9 | 0-3-6 | 0-14.2-33 |
| RPS17 | At1G79850 | 30S ribosomal protein S17 | 0-1-4 | 0-5.4-26.9 |
| RPL2.1 | AtCG00830 | 50S ribosomal protein L2 | 0-1-2 | 0-2.9-12.4 |
| RPS18 | AtCG00650 | 30S ribosomal protein S18 | 0-1-2 | 0-6.9-18.8 |
| RPL15 | At3G25920 | 50S ribosomal protein L15 | 0-1-2 | 0-4-9.4 |
| RPS1 | At5G30510 | Putative ribosomal protein S1 | 0-7-2 | 0-18-5.5 |
| At2G21960 | At2G21960 | Unknown protein | 0-2-3 | 0-7.2-10.5 |
| RPE | At5G61410 | d-ribulose-5-phosphate-3-epimerase | 0-4-2 | 0-26.3-12.5 |
| At5G19940 | At5G19940 | Plastid-lipid associated protein PAP/fibrillin family protein | 0-3-3 | 0-17.8-17 |
| APG1 | At3G63410 | 2-Methyl-6-phytyl-1,4-hydroquinone methyltransferase | 0-1-3 | 0-5-14.5 |
| SBPASE | At3G55800 | Sedoheptulose-1,7-bisphosphatase | 0-1-4 | 0-3-12 |
| At2G10940 | At2G10940 | Lipid binding | 0-2-3 | 0-11-7.9 |
Peptides from three biological replicates of protein mass spectrometry analysis. Background from the Col-0 plants was omitted, and the proteins listed here are unique to AtRNH1C.
Two protein sequences of At3G10270 and At5G04130 have high similarity; see Supplemental Figure 5C for details.
Figure 5.
Analysis of AtRNH1C Coimmunoprecipitated Proteins.
(A) The mass spectrometry data were analyzed by DAVID Bioinformatics Resources 6.8 (Huang et al., 2009), which can cluster functional-related genes.
(B) The protein interaction was predicted by STRING (Protein-Protein Interaction Networks), and the network was made by Cytoscape (Shannon et al., 2003). Bold oval shapes are the newly validated interaction in this research.
Besides AtGyrases, we have also found several other DNA and RNA metabolism-related proteins copurified with AtRNH1C. These include AtWHY1/3, which is important for maintaining cpDNA (Maréchal et al., 2009; Zampini et al., 2015); the single-stranded DNA binding, DnaA-like protein MTSSB1 and -2, homologs of which were previously copurified from the soybean (Glycine max) chloroplast replication origin complex (Lassen et al., 2011); and several heat shock proteins including cpHsc70, transcriptionally active protein pTAC13, RNA processing complex protein THOC4, ribosomal binding proteins, and FtsZ proteins involved in chloroplast division and others (Table 1, Figure 5). These results suggest that AtRNH1C may form multiple complexes with different proteins and have diverse ways to restrict R-loop levels in the chloroplast nucleoid. The direct interactions of these proteins with AtRNAH1C need to be further investigated through other approaches.
AtRNH1C Interacts with DNA Gyrases to Regulate Chloroplast Development
To gain insight into the mechanisms of AtRNH1C-regulated R-loop and chloroplast genome stability, we tested biological relevance of AtRNH1C interaction with AtGyrases complex. We chose to explore the AtGyrases for two reasons: (1) the quinolone drug ciprofloxacin (CIP), which is the specific inhibitor of AtGyrA (Evans-Roberts et al., 2016), induced cpDNA damage and genome instability in chloroplast (Cappadocia et al., 2010; Evans-Roberts et al., 2016; Lepage et al., 2013; Maréchal et al., 2009; Rowan et al., 2010); and (2) although AtGyrases were reported to localized in both chloroplast and mitochondria, their inhibition seriously affects chloroplast morphology, but does not result in obvious defects in mitochondria (Evans-Roberts et al., 2016), indicating a specific function for AtGyrases in the chloroplast.
To validate the interaction between AtRNH1C and AtGyrases, we generated a specific antibody against AtGyrA (Supplemental Figures 5A and 6A). AtGyrA protein levels were not altered in either atrnh1c mutants or upon CIP treatment (Supplemental Figure 6A), indicating that expression of AtGyrases was not affected by AtRNH1C mutation or CIP treatment. We confirmed the interaction between AtRNH1C and AtGyrases by in vivo coimmunoprecipitation (co-IP) assays (Figure 6A).
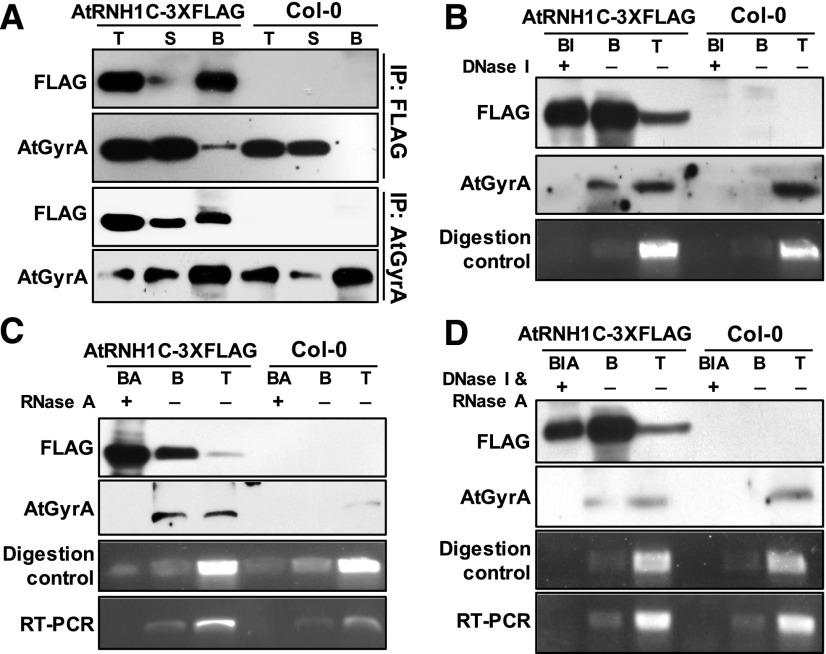
Figure 6.
AtRNH1C Coimmunoprecipitates with AtGyrases in a DNA- and RNA-Dependent Manner.
(A) AtRNH1C coimmunoprecipitates with AtGyrA. After immunoprecipitation, anti-FLAG and anti-AtGyrA antibodies were used to detect AtRNH1C-3XFLAG and AtGyrA proteins, respectively. T, total protein; S, supernatant; B, beads with copurified proteins.
(B) to (D) AtRNH1C interacts with AtGyrases mediated by DNA/RNA. After immunoprecipitation with anti-FLAG M2 beads, anti-AtGyrA antibody was used to detect AtGyrA proteins. RT-PCR and digestion control were performed by amplifying a 16S rRNA fragment from cDNA reverse transcribed from RNA or DNA isolated from beads with or without nucleases treatment. Primers are listed in Supplemental Table 2. T, total protein; S, supernatant; B, immunoprecipitated beads with copurified proteins; BI, immunoprecipitated beads with DNase I treatment; BA, immunoprecipitated beads with RNase A treatment; BIA, immunoprecipitated beads with DNase I and RNase A treatment.
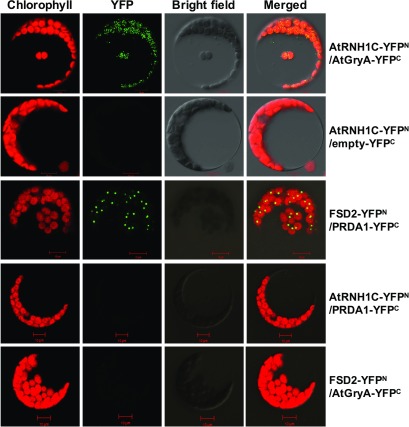
As both DNA gyrases and RNase H1 are involved in DNA and/or RNA metabolism, we assumed that their interaction may depends on DNA and/or RNA. We then performed the co-IP assay with DNase I and/or RNase A treatment (Figures 6B to 6D). Interaction between AtRNH1C and AtGyrases was disrupted by either DNase I or RNase A treatments or by combination of the two enzymes, after co-IP (Figures 6B to 6D). These results clearly indicated that the interaction between AtRNH1C and AtGyrases is mainly on through a DNA and/or RNA molecule, possibly through R-loop structures. To further determine the association between AtRNH1C and AtGyrases in vivo, bimolecular fluorescence complementation (BiFC) assay was performed. YFP signal was detected in protoplasts cotransformed with AtRNH1C-YFPN and AtGyrA-YFPC, but not detected with other tested YFP fused protein combinations (Figure 7), suggesting the in vivo interaction between these two proteins (Figure 7).
Figure 7.
BiFC Analysis of AtRNH1C and AtGyrA Interaction in Chloroplast.
In vivo interaction of AtRNH1C and AtGyrA examined by BiFC. YFP signals was observed and imaged by confocal microscopy. Cotransformation of AtRNH1C-YFPN and AtGyrA-YFPC shows reconstitution of YFP signals as an indicator of interaction. AtRNH1C-YFPN, empty-YFPC, RNH1C-YFPN, PRDA1-YFPC, FSD2-YFPN, and AtGryA-YFPC transiently coexpressed in protoplasts as negative control, showing no YFP signal. FSD2-YFPN and PRDA1-YFPC were used as a positive control. Bar = 10 µm.
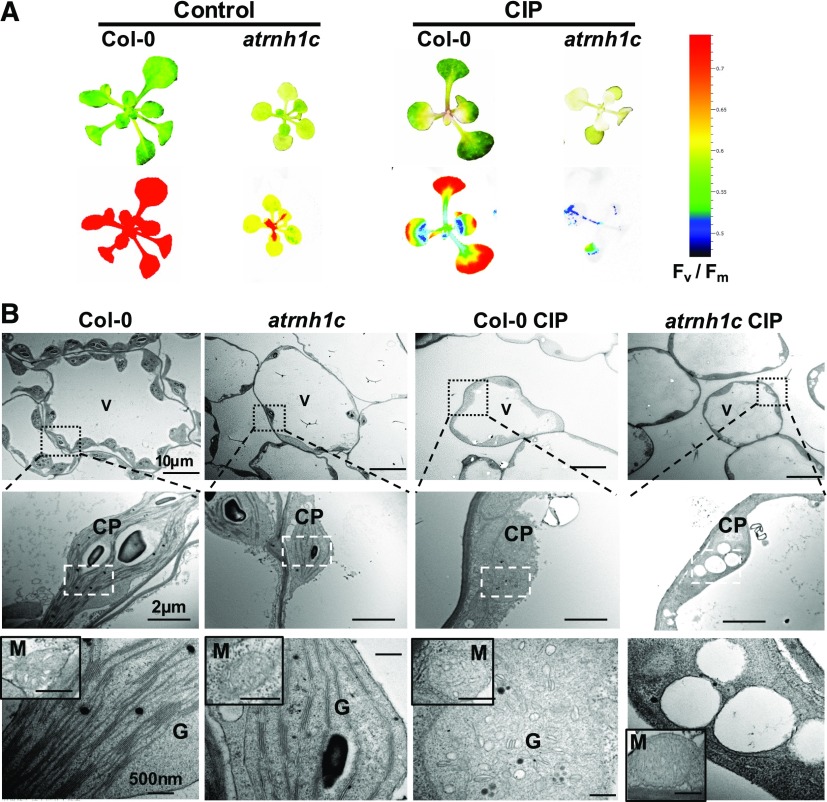
Application of CIP to Col-0 plants resulted in growth inhibition and leaf chlorosis (Figure 8A; Evans-Roberts et al., 2016; Wall et al., 2004). Applying CIP treatment to atrnh1c enhanced the effect of the CIP treatment observed in Col-0 (Figure 8A). Electron microscopy observations revealed that chloroplasts were smaller, and the size, number, and shape of thylakoids were altered in atrnh1c (Figure 8B). These deleterious morphologies were enhanced when plants were treated with CIP, as thylakoid stacks were replaced by large, round vesicles especially in atrnh1c (Figure 8B), indicating that plastid development was severely compromised. The additive effect of atrnh1c and CIP treatment indicate that AtRNH1C and AtGyrases might prevent R-loops using different mechanisms, apart from their shared function. Interestingly, similarly to the previous observations (Evans-Roberts et al., 2016), little or no defects of mitochondrial morphology were seen in either the atrnh1c mutant or CIP-treated plants (Figure 8B). This finding further suggests that AtGyrases, and their association with AtRNH1C, may have a specific function in the maintenance of R-loop homeostasis and genome integrity in chloroplasts.
Figure 8.
Both AtRNH1C and AtGyrases Are Important for Chloroplast Morphology and Plant Development.
(A) Effects of the Gyrase A-specific drug CIP on 3-week-old Col-0 and atrnh1c seedlings. Upper panel, Seedlings after CIP treatment; lower panel, chlorophyll fluorescence after CIP treatment. Color scale represents Fv/Fm.
(B) Chloroplast ultrastructure of Col-0 and atrnh1c plants from their first true leaves analyzed by electron microscopy. CP, chloroplast; G, grana; V, vacuole; M, mitochondrion.
AtRNH1C and AtGyrases Are Important for Chloroplast Genome Replication and rDNA Cluster Transcription
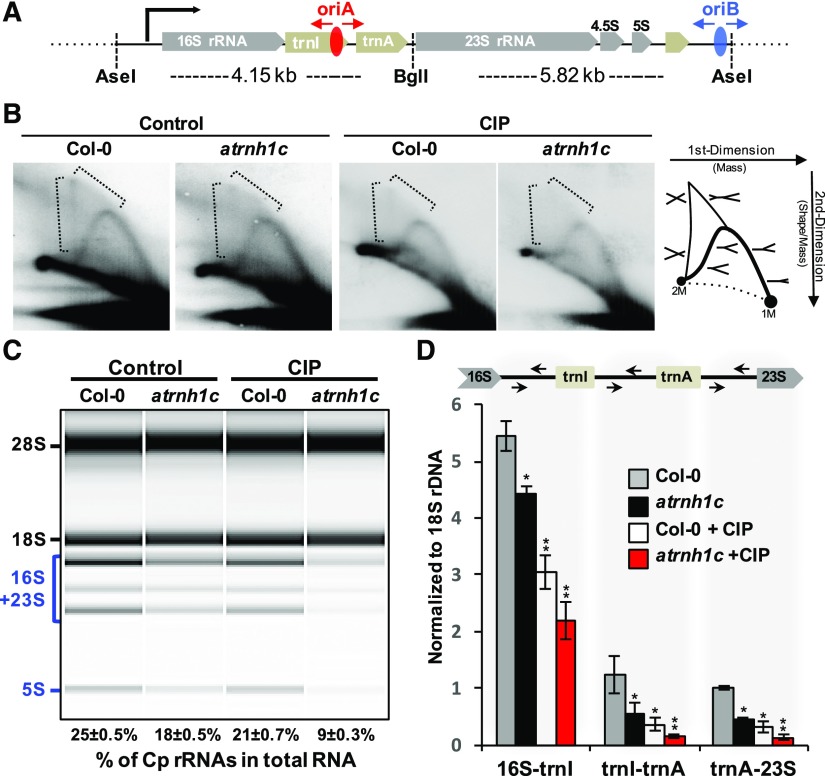
The essential role of DNA gyrases in transcription and replication had been revealed in prokaryotes (Aldred et al., 2014; Chang et al., 2013; Higgins, 2014), but the regulatory mechanisms of AtGyrases in chloroplasts are not fully understood. Two replication origins (OriA and OriB) have been previously found in the tobacco (Nicotiana tabacum) chloroplast rDNA region (Kunnimalaiyaan and Nielsen, 1997). There are two rDNA clusters in the chloroplast genome, presenting as inverted repeat regions thus form the head-on collision region with transcription direction (IR regions; Figures 9A; Supplemental Figures 7B to 7D). IR regions have high GC content sequence properties, are highly transcribed, and contain replication origins OriA and OriB, which prompted us to examine the regulation between transcription and replication within this region. Although neither OriA nor OriB is essential for plastid DNA replication in tobacco (Scharff and Koop, 2007), the sequences of OriA and OriB are highly conserved in most plant chloroplast rDNAs (Figure 9A; Supplemental Figure 7A). This suggests a conserved role for OriA- and OriB-initiated chloroplast DNA replication in green plants. We explored the potential roles of AtGyrases and AtRNH1C in cpDNA replication using 2D gel electrophoresis (Friedman and Brewer, 1995). Replication intermediates of the rDNA region (Figure 9B; Supplemental Figure 7E, dashed brackets) were decreased in atrnh1c compared with Col-0. This indicates that replication progress is problematic in atrnh1c and suggests that resolution of R-loops by AtRNH1C is necessary for DNA replication. Reduce of replication intermediates was also observed upon inhibition of AtGyrases activity in Col-0 by CIP treatment (Figure 9B; Supplemental Figure 7E), suggesting the importance of AtGyrases in cpDNA replication. The “X” replication intermediates almost completely disappeared when AtGyrases activity was inhibited in atrnh1c mutant (Figure 9B; Supplemental Figure 7E). These results suggested that both AtRNH1C and AtGyrases, and their association, play important roles in DNA replication. Interestingly, however, different from replication blockage patterns in nuclear genes caused by R-loop accumulation mutants in other organisms (Alzu et al., 2012; Gómez-González et al., 2009; Stuckey et al., 2015), we did not see replication blockage in the R-loop-accumulating atrnh1c and CIP-treated plants (Figure 9B; Supplemental Figure 7E). The lack of observed replication blockage might be attributable to the fact that most of the genome is fragmented (Figure 4B).
Figure 9.
AtRNH1C and AtGyrases Are Essential in Releasing Transcription and Replication Conflict.
(A) Illustration of the rDNA region in the Arabidopsis chloroplast genome and the 2D gel restriction strategy. Replication origins OriA and OriB located within the rDNA region are shown. The red and blue ellipses represent OriA and OriB, respectively. AseI and BglI were used for cpDNA digestion in DNA replication analysis.
(B) Two-dimensional gel analysis detected DNA replication intermediates in the chloroplast 16S rDNA region in 2-week-old Col-0 and atrnh1c plants with or without CIP treatment. DNA replication intermediates are illustrated in the left.
(C) Bioanalyzer (Agilent 2100) results showing total RNAs isolated from 2-week-old Col-0 and atrnh1c plants leaves, with or without CIP treatment. Calculation of percentage of cp-rRNAs in total RNAs are shown at the bottom.
(D) RT-qPCR of nascent chloroplast rRNA abundance. Arrows indicate primer positions used for the RT-qPCR. The same RNA samples were used as in (C). Values in (C) and (D) are the means ± se of three biological replicates. Expression levels in (D) were normalized to nucleus-encoded 18S rRNA and presented as the relative value to Col-0 5S rRNA. Asterisks indicate significant difference to Col-0: **P < 0.001 and *P < 0.05. Expression levels normalized to a chloroplast gene atpI and quantitative analyses of mature cp-rRNAs and nuclear 25S rRNA are shown in Supplemental Figures 8B to 8E.
Next, we analyzed the transcriptional regulation by AtGyrases and AtRNH1C around the replication origins within the rDNA region; apart from the most abundant nuclear rRNAs, natural chloroplast rRNAs are the second largest proportion of Arabidopsis total RNAs (Figure 9C; Supplemental Figure 8A). Transcription of cp-rRNAs is similar to the ancestral operon-like system (Stern et al., 2010) and starts from the 16S rDNA (Figure 9C; Supplemental Figure 7C). As the rRNA transcription initiation site is head-on to the replication initiation IR regions (Figure 9A; Supplemental Figure 7C), we tested whether AtRNH1C and AtGyrases are essential for maintenance of cpRNA levels. We found that the mature rRNAs from chloroplast (cp-rRNAs) were dramatically decreased in atrnh1c mutant compared with Col-0 (Figure 9C; Supplemental Figure 8A). From analysis of the unprocessed rRNA, which reflects the rDNA transcription states, we found that the transcription of the rDNA cluster was decreased in the atrnh1c mutant compared with Col-0 when either take a nuclear gene 18S or a chloroplast gene atpI as references (Figure 9D; Supplemental Figure 8E). The levels of both mature cp-rRNAs and unprocessed rRNA decreased upon CIP treatment in the atrnh1c mutant (Figures 9C and 9D; Supplemental Figure 8). These data indicate that AtRNH1C, together with AtGyrases, is important for regulation of the rRNA cluster transcription, as well as DNA replication, in the head-on collision rDNA region.
AtRNH1C and AtGyrases Prevent R-Loop Overaccumulation on Chloroplast rDNA to Release Transcription-Replication Conflict and Maintain cpDNA Integrity
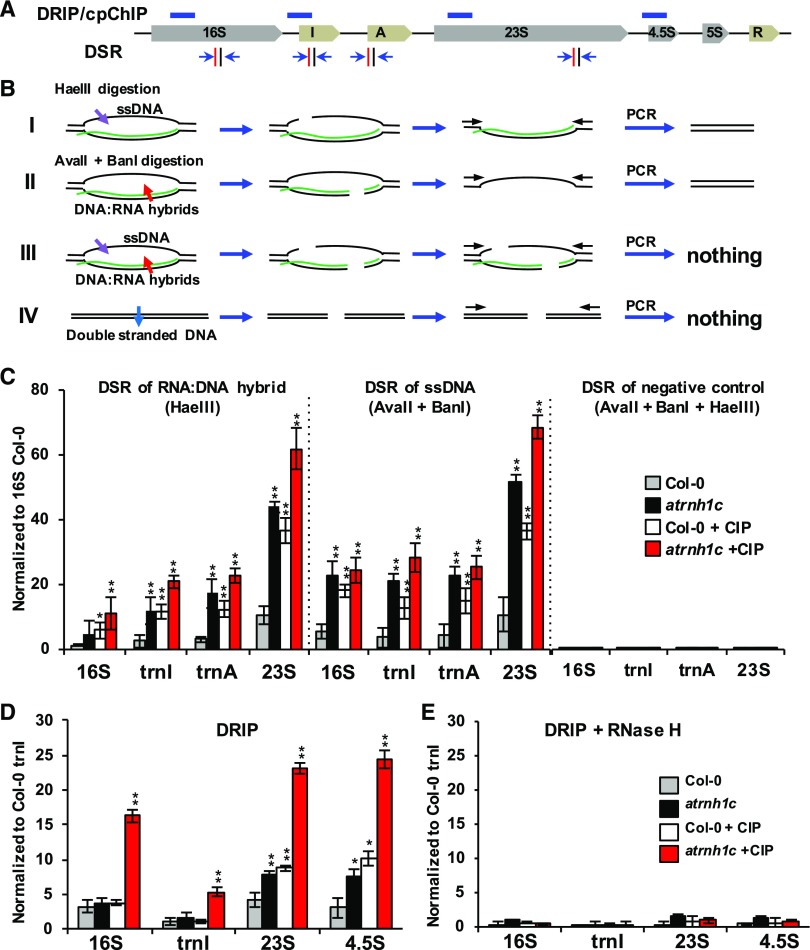
We next explored whether R-loops form in IR regions and whether AtRNH1C and AtGyrases, and their interactions, are responsible for R-loop level regulation that ultimately may impact both replication and transcription of IR. It has been reported that nuclear rDNA is highly enriched with R-loops (El Hage et al., 2014; Wahba et al., 2016); the IR regions have the highest GC content in the chloroplast genome (Supplemental Figure 7B), suggesting that R-loops might form around IR regions. To check this, we applied DNA:RNA immunoprecipitation (DRIP; Figures 10D and 10E; Ginno et al., 2012; Skourti-Stathaki et al., 2011; Sun et al., 2013) and AtRNH1C-M pull-down assays (Supplemental Figure 9). In addition, we developed a new approach that we termed the digestion-sensitive R-loop (DSR) method (Figures 10A to 10C). Each of these analyses detected R-loops in the cp-rDNA regions. R-loop levels increased in both atrnh1c mutants and plants in which AtGyrases activity was inhibited by CIP. The effect was additive when AtGyrase activity was inhibited in the atrnh1c mutant (Figures 10A to 10E; Supplemental Figure 9). Similar trends can be detected in the treatment of another AtGyrases inhibitor Novobiocin (NOV), which was shown in inhibition of the AtGyrase but not introduce double-strand breaks (Cappadocia et al., 2010). We then applied R-loop footprinting (native bisulfite conversion) to map the position and boundaries of the single-stranded DNA within the cp-rDNA R-loops (Sun et al., 2013; Yu et al., 2003). We found that the nontemplated rDNA strand (regarding the direction of rRNA transcription) has single-stranded status (Figures 11A and 11B). Interestingly, the borders of the R-loop were clearly extended in atrnh1c compared with Col-0, and AtGyrases-inhibited Col-0 plants had a similar effect to that of atrnh1c (Figure 11B). In AtGyrase-inhibited atrnh1c plants, the borders of the R-loop were moderately extended compared with either atrnh1c or CIP treated Col-0, again demonstrating that R-loops accumulate in atrnh1c and AtGyrase-inhibited plants. Together, these results suggested that AtRNH1C, in collaboration with AtGyrases, is important for restricting R-loop levels in the IR region.
Figure 10.
AtRNH1C and AtGyrases Prevent R-Loop Overaccumulation on Chloroplast rDNA IR Regions.
(A) Schematic representation of amplicon locations of CHIP/DRIP-qPCR. Amplicon locations of ChIP/DRIP-qPCR experiments and primer pairs for DSR analysis within the chloroplast rDNA region are indicated as blue bars and blue arrows, respectively. Vertical red and black bars represent HaeIII and AvaII/BanI restriction sites, respectively.
(B) Strategy used for DSR. HaeIII can digest both dsDNA and ssDNA substrates but not the DNA strand of DNA:RNA hybrid (I); AvaII and BanI can digest dsDNA and DNA:RNA hybrid substrates but not ssDNA (II); when combined, these two class of enzymes will cut the R-loops (III). The enzymes can digest all double-stranded DNA (IV). See supplemental experimental procedures for more information.
(C) R-loop accumulation over the chloroplast rDNA region detected by DSR-qPCR. R-loops cannot be detected by DSR-qPCR if the DNAs were treated with HaeIII, AvaII, and BanI together (case III in [B]), as the combination of these enzymes can digest every form of DNA substrates. Values are the means ± se of three biological replicates.
(D) DRIP-qPCR indicating R-loop accumulation over the chloroplast rDNA region is AtRNH1C and AtGyrase dependent.
(E) DRIP results of RNase H-treated DNA samples that were used as a negative control of (D). Data are the means ± se of three biological replicates. Percentage of input was calculated and normalized to Col-0. Asterisks indicate significant difference to Col-0: **P < 0.001 and *P < 0.05.
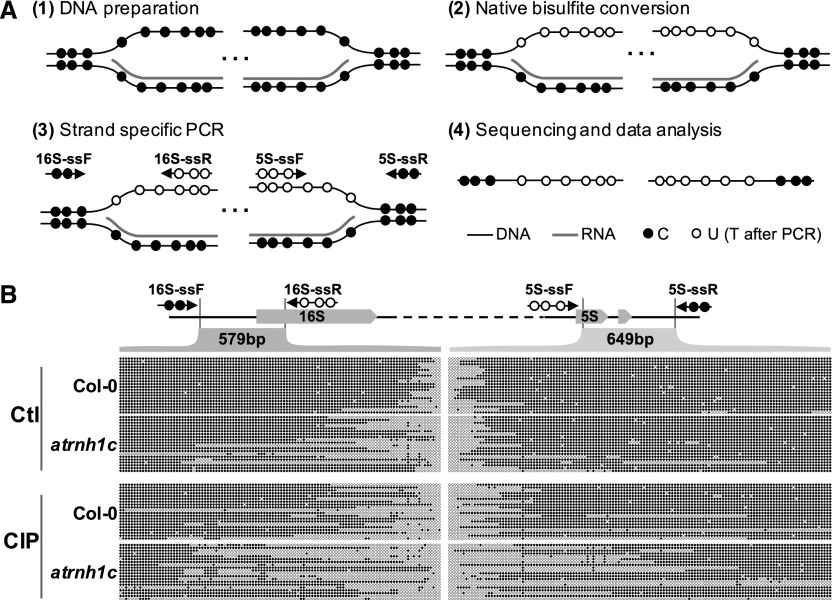
Figure 11.
R-Loop Footprinting in the cp-rDNA Region.
(A) Schematic steps of the R-loop footprinting method. C’s (solid circles) can be converted to U’s (empty circles) by bisulfite only in single-stranded DNA. The length of the R-loop is revealed after strand-specific PCR and sequencing.
(B) R-loop footprinting comparison of cp-rDNA R-loops in Col-0 and atrnh1c with (CIP) and without CIP treatment (Ctl). The beginning (16S rDNA, in light green) and the end (5S rDNA, in light purple) of the cp-rDNA regions were analyzed. Converted (gray arrows) and nonconverted (black arrows) primers are paired for PCR after samples were treated by the native bisulfite conversion method. Twenty individual colonies from PCR of each sample were sent for sequencing. The empty dots indicate unprotected cytosines during native bisulfite conversion, which represent the single-stranded DNA regions of R-loops. Results shown are from one of the two biological replicates. The raw data of sequencing can be found in Supplemental File 2.
As AtRNH1C is localized to the chloroplast thylakoid/nucleoid (Figure 3), and interacted with AtGyrases (Figures 5 to 7) to modulate rDNA replication (Figure 9), R-loop resolution (Figure 10), and cp-rRNA transcription (Figure 9), we tested the direct association of AtRNH1C and AtGyrases with the chloroplast IR regions by chloroplast chromatin immunoprecipitation (cpChIP; Yagi et al., 2012). Our data showed that AtRNH1C was associated with chloroplast IR regions (Figure 12A), and interestingly, this association was dependent on the activity of AtGyrases, as CIP treatment reduced the association of AtRNH1C with chloroplast rDNA regions (Figure 12A). In addition, cpChIP results using anti-AtGyrA antibody revealed that the AtGyrases were associated with rDNA region as well in an AtRNH1C-dependent manner (Figure 12B).
In summary, we have shown that AtRNH1C- and AtGyrase-mediated regulation of R-loops in the highly transcribed rDNA regions is essential for chloroplast DNA replication and transcription (Figures 9 and 10). AtGyrases are involved in R-loop regulation during transcription and/or replication, probably by coordinating cotranscriptional RNA processing in the chloroplast (Table 1, Figure 5). Mutation of AtRNH1C or chemical inhibition of AtGyrases results in the accumulation of R-loops that subsequently reduces the efficiency of both transcription and replication in the head-on collision IR region and causing transcription-replication conflicts, which could lead to genome instability (Figure 4) (Costantino and Koshland, 2015; Gaillard and Aguilera, 2016; García-Muse and Aguilera, 2016; Santos-Pereira and Aguilera, 2015; Skourti-Stathaki and Proudfoot, 2014; Sollier and Cimprich, 2015). Chloroplast DNA rearrangements and defects in genome integrity in the atrnh1c were enhanced upon CIP and NOV treatments (Figure 12C; Supplemental Figure 10B) and comet assays also showed that chloroplast genomic DNA was more fragmented in atrnh1c with AtGyrases inhibited (Figure 12D; Supplemental Figure 11). These results suggest AtRNH1C and AtGyrases cooperate to restrict R-loops and maintain genome integrity in Arabidopsis chloroplasts, and loss function of either results in R-loop accumulation and triggers chloroplast genome instability.
DISCUSSION
In plants, R-loops have been found to modulate the expression of the nuclear genes, thereby affecting plant development, such as flowering time regulation in Arabidopsis (Sun et al., 2013), and root development in rice (Shafiq et al., 2017). However, little is known about R-loop involvement in other biological processes in plants. Here, by analyzing the function of the chloroplast-localized RNase H1 protein AtRNH1C in Arabidopsis, we found that R-loop levels are essential to chloroplast genome stability. We have revealed that AtRNH1C is responsible for connecting R-loop homeostasis and genome stability in chloroplast and is necessary for chloroplast development. Furthermore, we uncovered the regulation mechanisms of RNase H1 and DNA gyrases involved chloroplast genome integrity maintenance. Our findings extend the basic understanding of R-loop functions, especially in eukaryotic DNA-containing organelles.
From analysis of AtRNH1C coimmunoprecipitated proteins, we identified several proteins that are involved in RNA and DNA metabolism (Table 1, Figure 5). Although in this study we focused on the AtGyrase complex as the most relevant interactor of AtRNH1C, our coimmunoprecipitation data suggest that there are multiple ways to restrict and resolve R-loop formation. Notably, it seems that AtRNH1C plays a central role with these components to restrict R-loops, although AtRNH1C knockout plants are not lethal (Figure 2; Supplemental Figure 1), thus reducing the risk of genome instability during different biological processes in the plant (Figure 5). The destabilization of the genome in chloroplasts is not always associated with the obvious chloroplast defects that lead to yellowish leaves. For example, mutation of RecA1 did not cause any leaf phenotype in the first three generations (Rowan et al., 2010). Similar findings have been reported for why1, why3, and reca1 polIb mutants (Maréchal et al., 2009; Zampini et al., 2015). By contrast, a strong yellowish phenotype can be seen in why1 why3 reca1 triple mutants (Zampini et al., 2015). In this work, when the single AtRNH1C gene was mutated, we observed dwarfism and yellowish (pale-green) leaves (Figure 2; Supplemental Figure 1), phenotypes almost as strong as those in the why1 why3 reca1 triple mutants, but different from the white variegation phenotype of the why1 why3 double mutant (Zampini et al., 2015). RecA1, the homolog of eukaryotic Rad51, is proposed to have a DNA repair function during chloroplast DNA damage (Rowan et al., 2010; Zampini et al., 2015). Our mass spectrometry results showed WHY1/3 proteins, but no RecA1 protein, in the AtRNH1C-related protein complexes. In addition, the reca1 mutant had a synergistic effect with why1 why3 (Zampini et al., 2015), implying that RecA1-mediated DNA repair could represent a different pathway from that of WHY1/3 or AtRNH1C. Interestingly, a recent study revealed another nuclear ssDNA binding protein Replication Protein A in association with RNase H1 and proposed its requirement in R-loop homeostasis and nuclear genome stability (Nguyen et al., 2017). Further analysis of the biological meaning of WHY1/3-AtRNH1C and MTSSB1/2-AtRNH1C interactions may uncover the similarities and differences between organellar and nuclear ssDNA binding proteins and RNase H1 interactions.
Our data suggest important roles for AtRNH1C in transcription-replication conflict release and genome maintenance; however, there is a strong possibility that AtRNH1C also promotes cpDNA repair. The enhanced effect on cpDNA stabilization in CIP-treated atrnh1c compared with CIP-treated Col-0 (Figures 12C and 12D) implies that AtRNH1C helped to repair DNA breaks that were induced by CIP treatment in Col-0 chloroplast, probably with the contribution of other interactors (Table 1, Figure 5). Consistently with this, recent studies in yeast revealed that RNase H proteins promoted DNA repair to maintain nuclear genome integrity (Amon and Koshland, 2016; Ohle et al., 2016).
RNA processing is one of the major mechanisms to regulate R-loop formation in the eukaryotic nucleus (reviewed in Santos-Pereira and Aguilera, 2015). Our coimmunoprecipitation data (Table 1, Figure 5) suggest that chloroplast RNA processing and transcription-related components are also involved in the regulation of R-loop levels, thus possibly regulating chloroplast genome stability. Consistent with this, transcription and RNA processing were shown to be important for chloroplast DNA replication in Chlamydomonas reinhardtii (Chang and Wu, 2000). Similarly, proteomic results from maize (Zea mays) chloroplasts revealed multiple RNA processing and transcription factors associated with WHY1 in nucleoids (Majeran et al., 2012). AtRNH1C, together with AtGyrases, could possibly be the connection between transcription and cotranscriptional RNA processing to resolve R-loops in the highly transcribed IR regions. However, this hypothesis needs to be confirmed via further investigation.
Different from other organisms, plants such as Arabidopsis contain more than one RNase H1-like protein (Figure 1A). Some eukaryotes, like human, encode one RNase H1 protein with dual localizations in both nucleus and mitochondria (Cerritelli and Crouch, 2009), with mitochondrion-localized RNase H1 being essential for organelle DNA replication. Recent research points to a long R-loop resolving role for RNase H1 and its critical physiological function in mammalian mitochondria (Akman et al., 2016; Holmes et al., 2015). The chloroplast-localized AtRNH1C interacts with the AtGyrases complex, which was demonstrated to be localized in both chloroplasts and mitochondria (Wall et al., 2004). However, the morphology of mitochondria was normal both upon inhibition of AtGyrases activity by CIP (Figure 8B; Evans-Roberts et al., 2016) and in the atrnh1c mutant, suggesting that AtGyrases have essential and specific functions in chloroplasts. The potential function of AtGyrases in mitochondria and whether they interact with AtRNH1B remain to be explored.
In summary, we found that genome stability in the plant-specific organelle, the chloroplast, is regulated by the cooperative activities of AtRNH1C and AtGyrases. Mutation of AtRNH1C, or inhibition of AtGyrases activity, results in R-loop accumulation and defects in transcription and replication, triggering DNA breaks and rearrangements, and destabilization of the genome. Our data suggest that the RNase H activity of AtRNH1C in the chloroplast allows AtGyrase-mediated DNA replication to proceed through the R-loops frequently formed in the highly transcribed genomic regions (such as the rDNA cluster), thus easing the head-on conflict between transcription and replication and helping to maintain genome integrity (Figure 13). Our findings lead to a new way of understanding how the integrity of the chloroplast genome is maintained during development and environmental changes, and how it influences plant development. These results have broad applicability, as many aspects of chloroplast genome organization and regulation are similar to those in both prokaryotic cells and other eukaryotic DNA-containing organelles, such as mitochondria.
Figure 13.
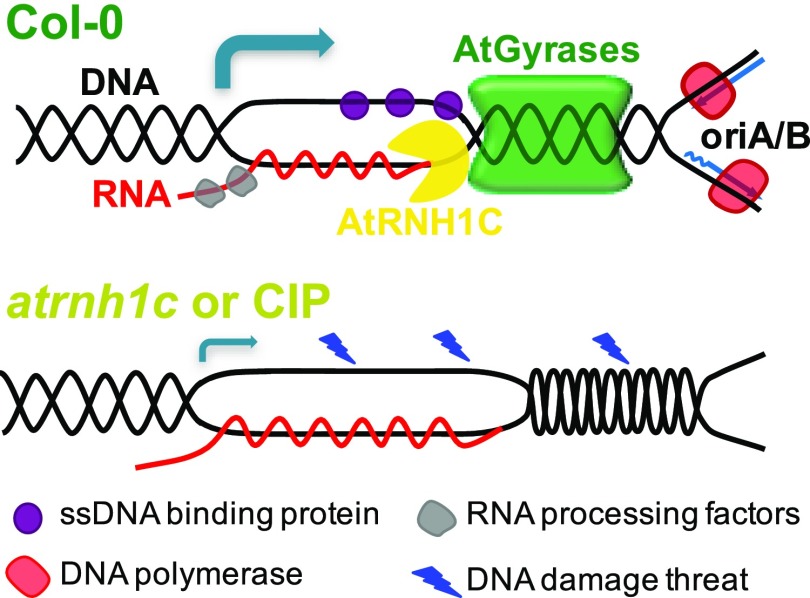
Working Model of AtRNH1C and AtGyrases Activities in Influencing Chloroplast Genome Stability.
In wild-type Col-0, AtRNH1C interacts with the AtGyrase complex to resolve collisions in the highly transcribed and DNA replication initiated-rDNA regions on the chloroplast genome. Transcription colliding head-on with replication will result in replication fork stalling; AtRNH1C removes accumulated R-loops to help AtGyrases remove the supercoiling, thereby enabling forward movement of DNA replication fork, as well as transcription of cp-rRNAs. Affecting the function of any components (in atrnh1c or CIP treatment) may lead to R-loop accumulation, DNA topological stresses, and transcription blockage, and thus hypersensitivity to DNA damage threatens, leading to genome instability. This defect may also influence the activity of ssDNA binding proteins and RNA processing factors (bottom panel). The arrows indicate the transcriptional direction of cp-rDNA regions, and the size of the arrow represents the transcription rate.
METHODS
Plant Growth and Drug Treatments
Seeds of wild-type Arabidopsis thaliana ecotype Col-0 (Columbia-0) and T-DNA insertion mutant of atrnh1c (SAIL_97_E11) were obtained from the Nottingham Arabidopsis Stock Centre. Sterilized seeds were germinated on 0.5× Murashige and Skoog (MS) medium and grown in a chamber under long-day conditions (day/night cycle of 16/8 h) at 22°C in white light of ∼120 to 150 μE m−2 s−1 and 18°C in dark. For CIP treatment, 7-d-old seedlings were transferred onto fresh 0.5× MS medium supplemented with 1 μM CIP for 1 week, and 0.5× MS medium supplemented with DMSO was used as a control. NOV treatment (50 mM) was performed according to Cappadocia et al. (2010). Plant materials were harvested and were used immediately for further experiments.
Comparison of RNase H1 Protein Sequences and Phylogenetic Tree Construction
The human RNase H1 (NP_002927) protein sequence was used as a query to search against the Arabidopsis genome in NCBI with BLASTP. After obtaining a protein (AT1G24090) annotated as “RNase H domain-containing protein,” we used it as a query to search protein database again in TAIR. This resulted in the identification of another two proteins (AT3G01410 and AT5G51080) that both harbor an RNase H domain. The human RNase H1, Escherichia coli (WP_054191752), and Arabidopsis AT1G24090 protein sequences were also used as queries for obtaining RNase H1 proteins in other species from NCBI. Multiple sequence alignments of obtained RNase H protein sequences from various species were performed using the ClustalW program and MEGA7. The alignment can be downloaded as Supplemental File 1. The phylogenetic tree was constructed in MEGA7 using the neighbor-joining method, and the bootstrap test was replicated 500 times. All protein information is shown in Figure 1A.
CRISPR/Cas9-Mediated AtRNH1C Genomic Deletion
The plant CRISPR/Cas9 system (Wang et al., 2015) was used to generate the genomic deletion of AtRNH1C gene (atrnh1cdel). The sequences of two sgRNAs are listed in Supplemental Table 1, and the locations of these sgRNAs are indicated in Figure 2A. More than 10 individual transgenic plants from T2 generation showed the similar yellowish leaf phenotype to atrnh1c mutant, and sequencing analysis of seven individual mutations of atrnh1cdel is shown in Supplemental Figure 1E.
Expression and Purification of the Recombinant Proteins
AtRNH1C coding sequence (or with site mutation D222N) lacking the chloroplast targeting sequence was amplified using Phusion (M0530S; NEB) and then PCR product was cloned into pGEX4T-1 vector and expressed in BL21 (DE3) cells. Briefly, BL21 (DE3) cells were grown at 37°C until OD600 reached to 0.6 to 0.8 and then IPTG was added to a final concentration of 0.2 mM. The cells were transferred to 16°C and incubated overnight with gentle shaking. Cells were collected and resuspended in 1× PBS buffer. Sonication was immediately performed until the suspension became transparent. The supernatant was collected and the protein was purified using GST beads (88822; Thermo) at 4°C for 2 h. The beads were washed with 1× PBS buffer for three times and proteins were eluted with elution buffer (50 mM reduced glutathione in 1× PBS buffer).
Detection of GFP-Fusion Proteins
Protoplasts were prepared from 4-week-old Arabidopsis leaves and were transformed with column-purified plasmid DNA (10 μg per 2 × 104 protoplasts) according to Yoo et al. (2007). After 18 h of incubation in the dark at room temperature, GFP fluorescence and chlorophyll autofluorescence were detected with a confocal microscope (Zeiss LSM780). Mitochondria were stained by MitoTracker Red CMXRos (Thermo Fisher; catalog no. M7512).
Antibodies
Anti-AtGyrA antibody was prepared using the synthetic peptide AGKRGDEQVKEESGC as the antigen (shown in Supplemental Figure 5A). The peptide was conjugated to keyhole limpet hemocyanin and injected into two rabbits. Immune serum was purified, and the mass spectrometry analysis after immunoprecipitation with purified anti-AtGyrA antibody was performed to demonstrate the quality of the antibody. The list of peptides of AtGyrA after mass spectrometry is shown in Supplemental Figure 6B.
Anti-R-loop (DNA:RNA hybrid) antibody S9.6 (ENH001) was either purchased from Kerafast or purified from the HB-8730 cell line (ATCC).
Monoclonal anti-FLAG M2 (F1804), anti-plant-actin rabbit polyclonal antibody (BE0027), anti-RbcL antibody for Rubisco large subunit, form I and form II (AS03037A), anti-psbO antibody (ab65563), and anti-Histone H3 antibody (nucleus loading, ab1791) were purchased from Sigma, Agrisera, EASYBio, and Abcam, respectively.
Chloroplast Isolation and Chloroplast DNA Extraction
Chloroplasts were isolated using a chloroplast isolation kit (product code CP-ISO; Sigma Aldrich) and following the instructions from the manufacturer. Briefly, sterile-grown plant tissue was homogenized in chloroplast isolation buffer (CIB): 10 mM HEPES-KOH (pH 8.0), 150 mM sorbitol, 2.5 mM EDTA (pH 8.0), 2.5 mM EGTA (pH 8.0), 2.5 mM MgCl2, 5 mM NaHCO3, and 0.1% BSA. The homogenate was filtered through three layers of Miracloth and centrifuged at 3000g for 10 min. The pellet was washed twice with CIB and was loaded on top of the Percoll gradient (30%:70% Percoll in CIB) and centrifuged for 30 min at 4°C 1500g. The intact chloroplasts were taken from the interface between the 30% and 70% Percoll layers and washed twice in CIB buffer without BSA. The isolated chloroplasts were used for either R-loop analysis or comet assay.
For chloroplast DNA extraction, the isolated chloroplasts were lysed with lysis solution (CIB with 1% SDS and proteinase K) at 37°C overnight. Proteinase K was deactivated by adding PMSF to a final concentration of 0.1 mM, and samples were incubated for 1 h at room temperature. Potassium acetate was added to a final concentration of 20 μM, followed by incubation for 10 min on ice and then centrifuge to remove the pelleted SDS; this step was repeated several times until SDS was completely removed. After two rounds of phenol/chloroform extraction, the solution was centrifuged (12,000g, 10 min at 4°C), and chloroplast DNA was precipitated with an equal volume of isopropyl alcohol, washed twice with 70% ethanol, air-dried, and dissolved in TE buffer (pH 8.0).
PFGE
Intact chloroplasts suspended in CIB were mixed 1:1 with 1% low-melting-point agarose (Promega V2111) dissolved in TE buffer (10 mM Tris, pH 8.0; 1 mM EDTA, pH 8.0). One hundred microliters of chloroplast/agarose mixture was used to prepare the plugs (plug molds from Bio-Rad) and allowed to solidify at 4°C. Plugs were placed into lysis buffer (2% sarkosyl, 0.45 M EDTA, and 10 mg/mL proteinase K) at 50°C, and buffer was exchanged three times in 30 h. Plugs were then washed in TE buffer six times at 4°C, with the first two washes containing 1 mM PMSF (Sigma-Aldrich). PFGE was performed in a 1% gel prepared in 1× TBE buffer, and Chef Mapper III (Bio-Rad) was used to run the gels in 0.5× TBE buffer. A Lambda Ladder (New England Biolabs; N0341) was used to indicate the molecular weight. Detail electrophoresis parameters were 5 to 120 s of pulse time at 4.5 V/cm, with a total run time of 36 h. Gels were treated by following the standard DNA gel blotting and DNA was transferred onto a Hybond N+ membrane (GE Amersham) according to the manufacturer’s manual with an extended blotting time of 36 h. A 505-bp rbcL gene fragment from chloroplast genome was used as the probe for hybridization.
Slot-Blot Hybridization Analysis of Chloroplast R-Loops
Different amount of chloroplast DNA treated with the indicated proteins (GST-RNH1C, RNase H, GST-RNH1C-M, and GST) or without treatment (control) were slotted on to nitrocellulose membrane (Hybond N+) and detected by R-loop (DNA:RNA hybrid) antibody S9.6.
Detection of cpDNA Rearrangement Events
Detection of cpDNA rearrangement events was performed according to Cappadocia et al. (2010). Briefly, outward- and inward-facing PCR primers spaced by ∼8 to 140 kb were designed (Supplemental Table 1) to amplify the rearranged cpDNA sequences. PCR was performed on DNA samples from Col-0 and atrnh1c seedlings with or without CIP treatment and analyzed by gel electrophoresis. Visible DNA bands were sequenced to confirm the PCR products were due to cpDNA rearrangement.
Protein Mass Spectrometry Analysis
Crude chloroplasts were isolated from 20 g samples of leaves of 4-week-old Col-0 and AtRNH1C-3×FLAG complemented plants with 50 mL of CIB. Chloroplast pellets were lysed with 20 mL protein extraction buffer (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 5 mM MgCl2, 10% [v/v] glycerol, 0.2% Nonidet P-40, and protease inhibitor [Roche]). FLAG M2 magnetic beads (50 μL, M8823; Sigma-Aldrich) were added to the samples, followed by incubation for 3 h with rotation at 4°C. The column was washed three times (10 min each) with protein extraction buffer, with new tubes used in the last wash. The proteins were eluted from beads with FLAG peptides, separated via SDS-PAGE, and submitted for mass spectrometry.
Protein Coimmunoprecipitation Analysis
Chloroplast total protein was added to the prepared beads (30 μL Dynabeads protein A [Life Technologies] washed with protein extraction buffer) and incubated with anti-FLAG antibody (Sigma-Aldrich) or anti-AtGyrA antibody for 3 h at 4°C with rotation. The beads were washed three times with TTBS buffer (17 mM Tris, 130 mM NaCl, pH 7.5, and 1% Triton X-100) and boiled in 100 μL SDS protein sample buffer. The immunoprecipitated proteins were separated by SDS-PAGE and transferred to a PVDF membrane using a Bio-Rad blotting system. Anti-AtGyrA antibody (see below) and anti-FLAG antibody (Sigma-Aldrich) were used to detect the immunoprecipitated proteins including AtRNH1C-3XFLAG and AtGyrA.
For analyzing the nuclease sensitivity of the protein-protein interactions, the immunoprecipitated beads were collected as described above, then the beads were divided into equal aliquots and incubated with DNase I (Life Technologies), RNase A (Thermo), or both in 1× TURBO DNase buffer for 1 h at 37°C. Half of the nuclease-treated beads were washed three times with TTBS buffer and the immunoprecipitated proteins were detected by protein gel blot. The remaining beads were eluted with elution buffer (100 mM glycine-HCl, pH 2.7) and neutralized with neutralization buffer (2 M Tris buffer, pH 7.5). The DNA and RNA were recovered and extracted by phenol/chloroform or TRIzol reagent (Life Technologies). The RNA was reverse transcribed to cDNA with 1× All-In-One RT MasterMix (catalog no. G486; abm). The digestion efficiency was determined by amplifying a 16S fragment (Supplemental Table 1).
BiFC Assay
BiFC assay was performed according to Walter et al. (2004) with minor modifications. Full-length AtRNH1C cDNA was fused with N-terminal coding sequence of YFP (1–155 amino acids), and AtGyrA cDNA was fused with C-terminal coding sequence of YFP (156–239 amino acids) and cloned into pUC19 plasmids. Plasmids were cotransformed into protoplasts. YFP was imaged using a confocal laser scanning microscope (Zeiss LSM780). PRDA1 and FSD2 from Qiao et al. (2013) were used as a control according to the instructions of BiFC analysis (Kudla and Bock, 2016). Primers are listed in Supplemental Table 1.
Two-Dimensional Gel Electrophoresis of Replication Intermediates
Two-dimensional agarose gel analysis of DNA replication intermediates was performed according to Friedman and Brewer (1995) with minor modifications. Briefly, 25 μg chloroplast DNA from each sample was digested with the appropriate restriction enzymes (Supplemental Figure 7D) and separated for the first dimension in a 0.35% agarose gel without ethidium bromide, in 1× TBE buffer at 0.7 V/cm for ∼36 h at room temperature. Electrophoresis in the second dimension was performed in a 1% agarose gel in 1× TBE containing 0.3 μg/mL ethidium bromide, at 6 V/cm for ∼5 h at 4°C. Gels were treated following standard DNA gel blotting methods and DNA was transferred onto Hybond N+ according to the manufacturer’s manual. Replication intermediates at the indicated regions were examined by hybridizing the membrane to a labeled specific probe shown in Supplemental Figure 7D.
cpChIP
The Arabidopsis chloroplast ChIP assay was performed as described previously (Yagi et al., 2012). Specifically, Arabidopsis leaves (∼1 g) were grounded in 20 mL ice-cold CIB. The homogenate was filtrated with three layers of Miracloth and centrifuged at 1600g (4°C) for 7 min. For cross-linking, the pellet was suspended in 1 mL CIB containing 1% (v/v) formaldehyde and incubated at 25°C for 10 min with rotation. The cross-linking reaction was stopped by adding 150 μL 1 M glycine and incubated for 5 min. Cross-linked chloroplasts were pelleted by centrifugation and washed once with CIB, then suspended in lysis buffer containing 50 mM Tris-HCl (pH 7.6), 0.15 M NaCl, 1 mM EDTA (pH 8.0), 1% (v/v) Triton X-100, 0.1% (w/v) SDS, 0.1% (w/v) sodium deoxycholate, and protease inhibitor (Roche). Subsequently, chloroplast DNA was sheared by sonication (15 times, 30 s on/off; Biorupter Pico). The supernatant was collected by centrifugation at 12,000g (4°C) for 10 min and diluted 10 times with lysis buffer. The diluted extract was then added to the prepared beads (10 μL Dynabeads protein A [Life Technologies] washed with lysis buffer) and incubated with 5 μL anti-FLAG antibody (Sigma-Aldrich F7425) for 30 min at 4°C with rotation followed by washing three times with lysis buffer and incubation of 2 to ∼3 h at 4°C with rotation. The beads was washed once with lysis buffer, three times with low salt buffer (50 mM Tris-HCl, pH 7.5, 1% [v/v] Triton X-100, 0.1% [w/v] SDS, and 150 mM NaCl), three times with high-salt buffer (50 mM Tris-HCl, pH 7.5, 1% [v/v] Triton X-100, 0.1% [w/v] SDS, and 1 M NaCl), three times with LiCl buffer (20 mM Tris-HCl, pH 7.5, 1% [v/v] Nonidet P-40, 1% [w/v] sodium-deoxycholate, 1 mM EDTA, pH 8.0, and 250 mM LiCl), and three times with TE (10 mM Tris-HCl, pH 7.5, and 10 mM EDTA, pH 8.0). For reverse cross-linking, 100 μL 10% (g/mL) Chelex100 sodium form (Bio-Rad) was added to the washed beads and incubated at 95°C for 10 min under agitation (1300 rpm). Then, 2 μL 10 mg/mL Proteinase K (Millipore) was added, and samples were incubated at 45°C overnight followed by 95°C for 5 min to inactivate the Proteinase K. Immunoprecipitated DNA was purified with phenol:chloroform:isoamyl alcohol (25:24:1) and precipitated with 3 M sodium acetate (pH 5.2), glycogen, and ethanol. ChIP samples were quantified by real-time PCR (LightCycler 480; Roche). The results were calculated as percentage of input (%IP), representing the proportion of 100% input (total amount of chromatin used for each IP).
DRIP
DRIP analysis was performed as described previously (Ginno et al., 2012; Skourti-Stathaki et al., 2011; Sun et al., 2013). Briefly, chloroplast DNA was extracted as described above and fragmented into 300 to 500 bp with RsaI and SphI (New England Biolabs). Extracted DNA (12 μg) with or without RNase H (New England Biolabs) treatment was used for each DRIP assay. DNA was precleaned by Protein G beads (Invitrogen) before adding purified S9.6 antibody for an overnight incubation. Samples were incubated with Protein G beads, followed by washing and DNA recovery.
DSR Analysis
Most restriction enzymes have activity on double-strand DNA while a few restriction enzymes can digest single-strand DNA or DNA:RNA hybrids in addition (http://rebase.neb.com/cgi-bin/hybcombolist). Based on these enzyme properties, we developed a method to detect R-loop structures in specific genomic loci (Figure 10B). Specifically, HaeIII can digest dsDNA and ssDNA substrates, as well as RNA, but not DNA from DNA:RNA structure (Murray et al., 2010). The PCR amplicon from HaeIII-digested samples represents the levels of DNA from DNA:RNA hybrid (Figures 10B, I); AvaII and BanI can digest dsDNA and DNA:RNA hybrids but not ssDNA, and the PCR amplicon from AvaII- and BanI-digested samples represents the levels of DNA from single-stranded DNA (Figure 10B, II); when applying HaeIII, AvaII, and BanI, nothing will be amplified in a locus that has R-loops (Figure 10B-III).
For the experiments, 200 ng chloroplast DNA samples from different backgrounds or treatments was digested overnight in a final volume of 30 μL with either HaeIII, AvaII+BanI, or HaeIII+AvaII+BanI (New England Biolabs). The enzymes were deactivated at 65°C and the digested DNA samples were used as a template for qPCR as described below.
R-Loop Footprinting
R-loop footprinting was performed as previously described (Sun et al., 2013; Yu et al., 2003). Briefly, the purified cpDNA was treated with bisulfite from the EpiTect Plus DNA Bisulfite Kit (Qiagen 59124) at 37°C overnight, and purified DNA was used as template for PCR. Primers to detect the nontemplated strand were used to amplify the corresponding ssDNA, and PCR products were ligated into pGEM-T easy (Promega) and transformed into E. coli DH10B; 20 individual colonies from each sample were sent for sequencing, and sequence data can be found in Supplemental File 2. Primers used for PCR are listed in Supplemental Table 1.
qPCR of DSR, cpChIP, and DRIP Experiments
qPCR was performed using a LightCycler 480 (Roche). The reaction mixture (11 μL) contained 1 μL sample DNA, 5.5 μL of 480 SYBR Green I Master (Roche), and 2.5 pmol each forward and reverse primer of interest (see Supplemental Table 1 for primer sequences).
Comet Assay
Neutral comet assays were performed with purified chloroplasts by using a Single Cell Gel Electrophoresis Assay Kit (Trevigen) according to the manufacturer’s instructions. Briefly, 10 μL of chloroplasts suspension (1 × 106/mL) was combined with 90 μL LM Agarose (Trevigen), and 50 μL of each sample was spread onto the Comet Slide sample area. Slides were incubated at 4°C in dark for 20 min until a clear ring appeared at the edge of the CometSlide area, and then slides were incubated in lysis buffer overnight at 4°C in dark. Slides were further incubated in 1× neutral electrophoresis buffer (50 mM Tris and 150 mM sodium acetate, pH 9.0) for 30 min. For electrophoresis units, slides were placed into 1× neutral electrophoresis buffer and run at 17 V (1 V/cm) for 20 min. Slides were then incubated in DNA precipitation solution (1 M NH4Ac in 95% ethanol) for 30 min and 70% ethanol for another 30 min. Samples were dried at 37°C, then stained with SYBR Gold for 30 min followed by a wash of water. Samples were visualized by epifluorescence microscopy at 480 nm. The OpenComet tool (Gyori et al., 2014) launched from ImageJ software was used for analysis and quantification of the results.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the accession numbers listed in Figure 1. Additional sequence data from R-loop assays can be found in Supplemental File 2.
Supplemental Data
Supplemental Figure 1. Characterization of atrnh1c mutants.
Supplemental Figure 2. Morphologies of the protoplasts in atrnh1c mutants.
Supplemental Figure 3. AtRNH1C is a chloroplast-localized protein.
Supplemental Figure 4. Proteins and chloroplast DNA purification.
Supplemental Figure 5. Gyrase proteins are evolutionarily conserved in Arabidopsis thaliana and Escherichia coli.
Supplemental Figure 6. Validation of AtGyrA antibody.
Supplemental Figure 7. cp-rDNA replication needs both AtRNH1C and AtGyrases.
Supplemental Figure 8. AtRNH1C and AtGyrases influence chloroplast rRNA abundance.
Supplemental Figure 9. R-loop analysis in chloroplast IR regions.
Supplemental Figure 10. DNA rearrangement analysis in chloroplast IR regions.
Supplemental Figure 11. Mutation of AtRNH1C and treatment with an AtGyrase inhibitor result in chloroplast genome instability.
Supplemental Table 1. Oligonucleotides used in this study.
Supplemental Data Set 1. Quantification of chloroplast comet assay in Supplemental Figure 11 using OpenComet tool.
Supplemental File 1. FASTA file of alignment corresponding to the phylogenetic analysis in Figure 1.
Supplemental File 2. Sequence data for R-loop footprinting shown in Figure 11.
Acknowledgments
We thank all Sun Lab members for useful discussions and suggestions, Tsinghua Biochemical Analysis Center for help with mass spectrometry analysis, and the Nottingham Arabidopsis Stock Centre for the atrnh1c mutant (SAIL_97_E11). We also appreciate the suggestions and comments from five anonymous reviewers for improving the quality of this manuscript. Q.H. holds a postdoctoral fellowship from the Tsinghua University Postdoctoral Support Program. This work was supported by grants from the Tsinghua University Initiative Scientific Research Program, National Key R&D Program of China (2016YFA0500800), Tsinghua-Peking Joint Center for Life Sciences, and 1000 Young Talent Program of China (to Q.S.).
AUTHOR CONTRIBUTIONS
Q.S. initiated the project. Q.H. contributed the data for Figures 1A, 4B, 7, and 9, and Supplemental Figures 5, 7, 8, 9B, and 9C. L.C. contributed the data for Table 1, Figures 1B, 2B, 2G, 2H, and 12A, and Supplemental Figures 2 and 3C. W.X. contributed the data for Figure 11. Y.H. contributed the data for Figures 2D to 2F, and Supplemental Figures 1A to 1D and 3B. S.L. contributed the data for Figure 3B. Z.Y. contributed the remaining data. All authors analyzed the data. Q.S. wrote the article with assistance of Q.H. and input from all authors.
References
- Aguilera A., García-Muse T. (2013). Causes of genome instability. Annu. Rev. Genet. 47: 1–32. [DOI] [PubMed] [Google Scholar]
- Akman G., et al. (2016). Pathological ribonuclease H1 causes R-loop depletion and aberrant DNA segregation in mitochondria. Proc. Natl. Acad. Sci. USA 113: E4276–E4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldred K.J., Kerns R.J., Osheroff N. (2014). Mechanism of quinolone action and resistance. Biochemistry 53: 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.F., Raven J.A. (1996). Free-radical-induced mutation vs redox regulation: costs and benefits of genes in organelles. J. Mol. Evol. 42: 482–492. [DOI] [PubMed] [Google Scholar]
- Alzu A., Bermejo R., Begnis M., Lucca C., Piccini D., Carotenuto W., Saponaro M., Brambati A., Cocito A., Foiani M., Liberi G. (2012). Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell 151: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon J.D., Koshland D. (2016). RNase H enables efficient repair of R-loop induced DNA damage. eLife 5: e20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch P., Weber-Lotfi F., Ibrahim N., Tarasenko V., Cosset A., Paulus F., Lightowlers R.N., Dietrich A. (2011). DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim. Biophys. Acta 1813: 186–200. [DOI] [PubMed] [Google Scholar]
- Cappadocia L., Maréchal A., Parent J.S., Lepage E., Sygusch J., Brisson N. (2010). Crystal structures of DNA-Whirly complexes and their role in Arabidopsis organelle genome repair. Plant Cell 22: 1849–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli S.M., Crouch R.J. (2009). Ribonuclease H: the enzymes in eukaryotes. FEBS J. 276: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli S.M., Frolova E.G., Feng C., Grinberg A., Love P.E., Crouch R.J. (2003). Failure to produce mitochondrial DNA results in embryonic lethality in RNaseh1 null mice. Mol. Cell 11: 807–815. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Wang Y.R., Chen S.F., Wu C.C., Chan N.L. (2013). New insights into DNA-binding by type IIA topoisomerases. Curr. Opin. Struct. Biol. 23: 125–133. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Wu M. (2000). The effects of transcription and RNA processing on the initiation of chloroplast DNA replication in Chlamydomonas reinhardtii. Mol. Gen. Genet. 263: 320–327. [DOI] [PubMed] [Google Scholar]
- Chédin F. (2016). Nascent connections: R-loops and chromatin patterning. Trends Genet. 32: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., Lee S.S., Kim K.D., Hwang I., Lim J.S., Park Y.I., Pai H.S. (2004). DNA gyrase is involved in chloroplast nucleoid partitioning. Plant Cell 16: 2665–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino L., Koshland D. (2015). The Yin and Yang of R-loop biology. Curr. Opin. Cell Biol. 34: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.W., Wing R.A., Gruissem W. (1989). The chloroplast genome exists in multimeric forms. Proc. Natl. Acad. Sci. USA 86: 4156–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A., Webb S., Kerr A., Tollervey D. (2014). Genome-wide distribution of RNA-DNA hybrids identifies RNase H targets in tRNA genes, retrotransposons and mitochondria. PLoS Genet. 10: e1004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Roberts K.M., Mitchenall L.A., Wall M.K., Leroux J., Mylne J.S., Maxwell A. (2016). DNA gyrase is the target for the quinolone drug ciprofloxacin in Arabidopsis thaliana. J. Biol. Chem. 291: 3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman K.L., Brewer B.J. (1995). Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 262: 613–627. [DOI] [PubMed] [Google Scholar]
- Gaillard H., Aguilera A. (2016). Transcription as a threat to genome integrity. Annu. Rev. Biochem. 85: 291–317. [DOI] [PubMed] [Google Scholar]
- García-Muse T., Aguilera A. (2016). Transcription-replication conflicts: how they occur and how they are resolved. Nat. Rev. Mol. Cell Biol. 17: 553–563. [DOI] [PubMed] [Google Scholar]
- Ginno P.A., Lott P.L., Christensen H.C., Korf I., Chédin F. (2012). R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol. Cell 45: 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González B., Felipe-Abrio I., Aguilera A. (2009). The S-phase checkpoint is required to respond to R-loops accumulated in THO mutants. Mol. Cell. Biol. 29: 5203–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B.R. (2011). Chloroplast genomes of photosynthetic eukaryotes. Plant J. 66: 34–44. [DOI] [PubMed] [Google Scholar]
- Groh M., Gromak N. (2014). Out of balance: R-loops in human disease. PLoS Genet. 10: e1004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman B.L., Niyogi K.K. (2009). Evidence for base excision repair of oxidative DNA damage in chloroplasts of Arabidopsis thaliana. J. Biol. Chem. 284: 17006–17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyori B.M., Venkatachalam G., Thiagarajan P.S., Hsu D., Clement M.V. (2014). OpenComet: an automated tool for comet assay image analysis. Redox Biol. 2: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamperl S., Cimprich K.A. (2016). Conflict resolution in the genome: How transcription and replication make it work. Cell 167: 1455–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N.P. (2014). RNA polymerase: chromosome domain boundary maker and regulator of supercoil density. Curr. Opin. Microbiol. 22: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J.B., Akman G., Wood S.R., Sakhuja K., Cerritelli S.M., Moss C., Bowmaker M.R., Jacobs H.T., Crouch R.J., Holt I.J. (2015). Primer retention owing to the absence of RNase H1 is catastrophic for mitochondrial DNA replication. Proc. Natl. Acad. Sci. USA 112: 9334–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57. [DOI] [PubMed] [Google Scholar]
- Jarvis P., López-Juez E. (2013). Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14: 787–802. [DOI] [PubMed] [Google Scholar]
- Kudla J., Bock R. (2016). Lighting the way to protein-protein interactions: Recommendations on best practices for bimolecular fluorescence complementation analyses. Plant Cell 28: 1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.A., Oldenburg D.J., Bendich A.J. (2014). Changes in DNA damage, molecular integrity, and copy number for plastid DNA and mitochondrial DNA during maize development. J. Exp. Bot. 65: 6425–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnimalaiyaan M., Nielsen B.L. (1997). Fine mapping of replication origins (ori A and ori B) in Nicotiana tabacum chloroplast DNA. Nucleic Acids Res. 25: 3681–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen M.G., Kochhar S., Nielsen B.L. (2011). Identification of a soybean chloroplast DNA replication origin-binding protein. Plant Mol. Biol. 76: 463–471. Erratum. Plant Mol. Biol. 76 473. [DOI] [PubMed] [Google Scholar]
- Lepage É., Zampini É., Brisson N. (2013). Plastid genome instability leads to reactive oxygen species production and plastid-to-nucleus retrograde signaling in Arabidopsis. Plant Physiol. 163: 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W., Friso G., Asakura Y., Qu X., Huang M., Ponnala L., Watkins K.P., Barkan A., van Wijk K.J. (2012). Nucleoid-enriched proteomes in developing plastids and chloroplasts from maize leaves: a new conceptual framework for nucleoid functions. Plant Physiol. 158: 156–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal A., Brisson N. (2010). Recombination and the maintenance of plant organelle genome stability. New Phytol. 186: 299–317. [DOI] [PubMed] [Google Scholar]
- Maréchal A., Parent J.S., Véronneau-Lafortune F., Joyeux A., Lang B.F., Brisson N. (2009). Whirly proteins maintain plastid genome stability in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 14693–14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S.A., Nielsen B.L. (2016). Chloroplast DNA copy number changes during plant development in organelle DNA polymerase mutants. Front. Plant Sci. 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray I.A., Stickel S.K., Roberts R.J. (2010). Sequence-specific cleavage of RNA by Type II restriction enzymes. Nucleic Acids Res. 38: 8257–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.D., Yadav T., Giri S., Saez B., Graubert T.A., Zou L. (2017). Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol. Cell 65: 832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M., Gaidamakov S.A., Crouch R.J., Yang W. (2005). Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell 121: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Ohle C., Tesorero R., Schermann G., Dobrev N., Sinning I., Fischer T. (2016). Transient RNA-DNA hybrids are required for efficient double-strand break repair. Cell 167: 1001–1013. [DOI] [PubMed] [Google Scholar]
- Oldenburg D.J., Bendich A.J. (2015). DNA maintenance in plastids and mitochondria of plants. Front. Plant Sci. 6: 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powikrowska M., Oetke S., Jensen P.E., Krupinska K. (2014). Dynamic composition, shaping and organization of plastid nucleoids. Front. Plant Sci. 5: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Li J., Chu W., Luo M. (2013). PRDA1, a novel chloroplast nucleoid protein, is required for early chloroplast development and is involved in the regulation of plastid gene expression in Arabidopsis. Plant Cell Physiol. 54: 2071–2084. [DOI] [PubMed] [Google Scholar]
- Raven J.A. (2015). Implications of mutation of organelle genomes for organelle function and evolution. J. Exp. Bot. 66: 5639–5650. [DOI] [PubMed] [Google Scholar]
- Rowan B.A., Oldenburg D.J., Bendich A.J. (2010). RecA maintains the integrity of chloroplast DNA molecules in Arabidopsis. J. Exp. Bot. 61: 2575–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Pereira J.M., Aguilera A. (2015). R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 16: 583–597. [DOI] [PubMed] [Google Scholar]
- Sanz L.A., Hartono S.R., Lim Y.W., Steyaert S., Rajpurkar A., Ginno P.A., Xu X., Chédin F. (2016). Prevalent, dynamic, and conserved R-loop structures associate with specific epigenomic signatures in mammals. Mol. Cell 63: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff L.B., Koop H.U. (2007). Targeted inactivation of the tobacco plastome origins of replication A and B. Plant J. 50: 782–794. [DOI] [PubMed] [Google Scholar]
- Shafiq S., Chen C., Yang J., Cheng L., Ma F., Widemann E., Sun Q. (2017). DNA Topoisomerase 1 prevents R-loop accumulation to modulate auxin-regulated root development in rice. Mol. Plant 10: 821–833. [DOI] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver J.M., Oldenburg D.J., Bendich A.J. (2008). The structure of chloroplast DNA molecules and the effects of light on the amount of chloroplast DNA during development in Medicago truncatula. Plant Physiol. 146: 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K., Proudfoot N.J. (2014). A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 28: 1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K., Proudfoot N.J., Gromak N. (2011). Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell 42: 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J., Cimprich K.A. (2015). Breaking bad: R-loops and genome integrity. Trends Cell Biol. 25: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D.B., Goldschmidt-Clermont M., Hanson M.R. (2010). Chloroplast RNA metabolism. Annu. Rev. Plant Biol. 61: 125–155. [DOI] [PubMed] [Google Scholar]
- Stuckey R., García-Rodríguez N., Aguilera A., Wellinger R.E. (2015). Role for RNA:DNA hybrids in origin-independent replication priming in a eukaryotic system. Proc. Natl. Acad. Sci. USA 112: 5779–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Csorba T., Skourti-Stathaki K., Proudfoot N.J., Dean C. (2013). R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340: 619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L., Costantino L., Tan F.J., Zimmer A., Koshland D. (2016). S1-DRIP-seq identifies high expression and polyA tracts as major contributors to R-loop formation. Genes Dev. 30: 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M.K., Mitchenall L.A., Maxwell A. (2004). Arabidopsis thaliana DNA gyrase is targeted to chloroplasts and mitochondria. Proc. Natl. Acad. Sci. USA 101: 7821–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438. [DOI] [PubMed] [Google Scholar]
- Wang Z.P., Xing H.L., Dong L., Zhang H.Y., Han C.Y., Wang X.C., Chen Q.J. (2015). Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K.H., Li W.H., Sharp P.M. (1987). Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. USA 84: 9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Xu H., Li K., Fan Y., Liu Y., Yang X., Sun Q. (2017). The R-loop is a common chromatin feature of the Arabidopsis genome. Nat. Plants; 3: 704–714. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Ishizaki Y., Nakahira Y., Tozawa Y., Shiina T. (2012). Eukaryotic-type plastid nucleoid protein pTAC3 is essential for transcription by the bacterial-type plastid RNA polymerase. Proc. Natl. Acad. Sci. USA 109: 7541–7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Yu K., Chedin F., Hsieh C.L., Wilson T.E., Lieber M.R. (2003). R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat. Immunol. 4: 442–451. [DOI] [PubMed] [Google Scholar]
- Zampini É., Lepage É., Tremblay-Belzile S., Truche S., Brisson N. (2015). Organelle DNA rearrangement mapping reveals U-turn-like inversions as a major source of genomic instability in Arabidopsis and humans. Genome Res. 25: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]