The ALMT4 anion channel of Arabidopsis mediates Mal2− efflux from the vacuole during stomatal closure and its activity depends on phosphorylation.
Abstract
Stomatal pores are formed between a pair of guard cells and allow plant uptake of CO2 and water evaporation. Their aperture depends on changes in osmolyte concentration of guard cell vacuoles, specifically of K+ and Mal2−. Efflux of Mal2− from the vacuole is required for stomatal closure; however, it is not clear how the anion is released. Here, we report the identification of ALMT4 (ALUMINUM ACTIVATED MALATE TRANSPORTER4) as an Arabidopsis thaliana ion channel that can mediate Mal2− release from the vacuole and is required for stomatal closure in response to abscisic acid (ABA). Knockout mutants showed impaired stomatal closure in response to the drought stress hormone ABA and increased whole-plant wilting in response to drought and ABA. Electrophysiological data show that ALMT4 can mediate Mal2− efflux and that the channel activity is dependent on a phosphorylatable C-terminal serine. Dephosphomimetic mutants of ALMT4 S382 showed increased channel activity and Mal2− efflux. Reconstituting the active channel in almt4 mutants impaired growth and stomatal opening. Phosphomimetic mutants were electrically inactive and phenocopied the almt4 mutants. Surprisingly, S382 can be phosphorylated by mitogen-activated protein kinases in vitro. In brief, ALMT4 likely mediates Mal2− efflux during ABA-induced stomatal closure and its activity depends on phosphorylation.
INTRODUCTION
Plants are in constant interaction with their environment. They take up carbon dioxide from the atmosphere for photosynthesis and release oxygen and water vapor in return. This exchange is essential for human life on earth, not only for the oxygen we breathe but also for the biomass produced through photosynthesis, its role in food production, as well as for the global water cycle. Plant gas exchange with the environment is facilitated through stomata, pores formed between a pair of guard cells. Guard cells swell to open the stomata and shrink to close these pores. Swelling and shrinkage depends on cell turgor, which is largely controlled by fluxes of osmotically active solutes into and out of the guard cell vacuole.
Ion channels and transporters at the plasma membrane and the vacuolar membrane (tonoplast) regulate fluxes of these ions during stomatal movement. Over the past four decades a wealth of information has been gathered regarding solute fluxes across the guard cell plasma membrane. However, in part due to the technical challenge of accessing an intracellular organelle for electrophysiological measurements, our knowledge of the transport processes across the vacuolar membrane during stomatal movement is still limited, especially regarding anion transport (Eisenach and De Angeli, 2017).
Vacuolar proton pumps acidify the vacuolar lumen and generate a proton gradient. The concomitant separation of charge establishes an electric potential gradient across the vacuolar membrane, i.e., the vacuolar membrane potential. The membrane potential reported in the literature is generally between 0 and −30 mV (Eisenach and De Angeli, 2017). In Arabidopsis thaliana, NHX-type transporters exploit the proton gradient to take up K+ into the vacuole in exchange for protons (Andrés et al., 2014). During stomatal opening, guard cell K+ content increases by nearly one order of magnitude, and 90% of this K+ is stored in the vacuole (Roelfsema and Hedrich, 2005). Movement of only K+ across the tonoplast would cause dramatic changes in membrane potential. Accordingly, K+ is charge-balanced by counter ions, such as Cl−, NO3−, and Mal2−, which also act as osmotica (Roelfsema and Hedrich, 2005). Control of the vacuolar membrane potential is important since the activity of several ion channels depends on membrane voltage.
Mal2− is the deprotonated form of malic acid, and the term malate refers to all protonation states of this dicarboxylic acid. With a pKa of 5.2 for Mal2−/MalH−, Mal2− is the dominant form in the cytosol and predominant in vacuoles of Arabidopsis guard cells (∼80% Mal2− at vacuolar pH 5.8). Early research highlighting the importance of Mal2− as counter ion over inorganic ions such as Cl− was performed in Vicia faba (Van Kirk and Raschke, 1978) and Commelina communis (MacRobbie and Lettau, 1980). In Arabidopsis, the guard cell malate concentration increases 2- to 3-fold during stomatal opening (Ding et al., 2014; Takahashi et al., 2015). The higher guard cell malate content of some Arabidopsis ecotypes corresponds with higher stomatal aperture and reduced stomatal closure (Monda et al. 2011, 2016; Takahashi et al., 2015) and the higher stomatal conductance in the anion channel knockout mutant almt12 corresponds with higher accumulation of malate and other organic acids (Medeiros et al., 2016). Knockout mutants of ABCB14, which transports malate into guard cells, have altered stomatal response to CO2 (Lee et al., 2008).
Given its weak acid properties, Mal2− not only acts as an osmoticum, but its free cytoplasmic concentration should also influence cytoplasmic pH. Mal2− might therefore have a role in regulating guard cell cytosolic pH, which is known to affect guard cell ion channels (for example, Gobert et al., 2007). Recently, it was shown that Mal2− can also function as a signaling molecule at low cytosolic concentrations; Mal2− activates the vacuolar Cl− inward rectifying channel ALMT9 and causes enhanced Cl− uptake during stomatal opening (De Angeli et al., 2013; Baetz et al., 2016).
In C3 plants, stomata open in response to light during the day to allow for CO2 uptake and its light-dependent photosynthetic assimilation. When the plant faces drought, however, stomata close in the light. Drought is signaled to the guard cells via the phytohormone abscisic acid (ABA), which causes rapid stomatal closure (Roelfsema and Hedrich, 2005). This response is crucial to prevent wilting and ensure plant survival. While we know how efflux of K+ from the vacuole is achieved during stomatal closure in Arabidopsis (Gobert et al., 2007), our information on vacuolar anion efflux in general is sparse. Specifically, we do not know how Mal2− efflux during stomatal closure is achieved. The anion H+-antiporters CLCc and CLCa are important for uptake of Cl− and NO3− during stomatal opening (Jossier et al., 2010; Wege et al., 2014), but knockout mutants also show impaired stomatal closure. For CLCa, this might be due to a stimulation of NO3− efflux activity in response to ABA (Wege et al., 2014).
Large and fast changes in ion concentration, such as those likely to occur during stomatal closure (MacRobbie, 1995b, 2006), are often mediated by ion channels (Hille, 2001; Chrispeels et al., 1999). Hence, it has been suggested that anion efflux from the vacuole could occur through vacuolar ALMT channels (Kollist et al., 2014). At the plasma membrane, ALMT12/ QUICK ANION CHANNEL1 (QUAC1) mediates anion current out of the cell and is required for stomatal closure (Meyer et al., 2010; Sasaki et al., 2010). ALMTs are a small gene family of anion channels. They were originally denoted as ALUMINUM ACTIVATED MALATE TRANSPORTERs (ALMTs) and identified as malate exporters conferring aluminum resistance. In Arabidopsis, this gene family comprises 13 members that cluster in four distinct phylogenetic clades (Dreyer et al., 2012). Clade II contains five members, ALMT3, ALMT4, ALMT5, ALMT6, and ALMT9, with ALMT9 and ALMT6 localized to the vacuole (Kovermann et al., 2007; Meyer et al., 2011). ALMT9 mediates voltage-dependent, inward Mal2− currents, i.e., Mal2− fluxes directed into the vacuole (Kovermann et al., 2007; Zhang et al., 2013; Bertl et al., 1992; Hafke et al., 2003). Surprisingly patch-clamp studies revealed that ALMT9 also acts as a guard cell, Mal2−-activated Cl− channel, and vacuolar Cl− uptake through ALMT9 is required for stomatal opening (De Angeli et al., 2013). ALMT6 was identified as a potential vacuolar ion channel candidate to be involved in stomatal movement (Meyer et al., 2011). The channel is specifically expressed in guard cells of Arabidopsis, and patch-clamp analysis of guard cell vacuoles revealed that ALMT6 mediated Ca2+- and voltage-dependent Mal2− inward currents, mediating vacuolar Mal2− uptake. Moreover, outward currents were detected at low vacuolar pH. Despite its guard cell-specific expression, no visible phenotype regarding stomatal aperture or whole-plant water use was found in almt6 knockout plants. Therefore, the physiological role of ALMT6 in planta is still unclear.
Here, we report the identification of ALMT4 as a vacuolar anion channel in guard cells. Knockout mutants of almt4 are impaired in stomatal movement in response to ABA, which suggests that this protein might be involved in solute loss from the vacuole. Indeed, ALMT4 is able to mediate Mal2− efflux from the vacuole, and genetic and biochemical data strongly indicate that ALMT4’s activity is controlled by phosphorylation.
RESULTS
Mutants of ALMT4 Show Impaired Stomatal Closure and Increased Water Loss in Response to ABA, and Are More Susceptible to Drought
To investigate vacuolar ALMT channels potentially involved in stomatal movement, we focused on ALMT4, the clade II ALMT member most closely related to guard cell-specific ALMT6. We identified two knockout alleles (in the Col-0 and No-0 ecotypes) and corresponding wild-type-like outcrosses (referred to as the wild type). All plants were from the same seed batch and blind-analyzed.
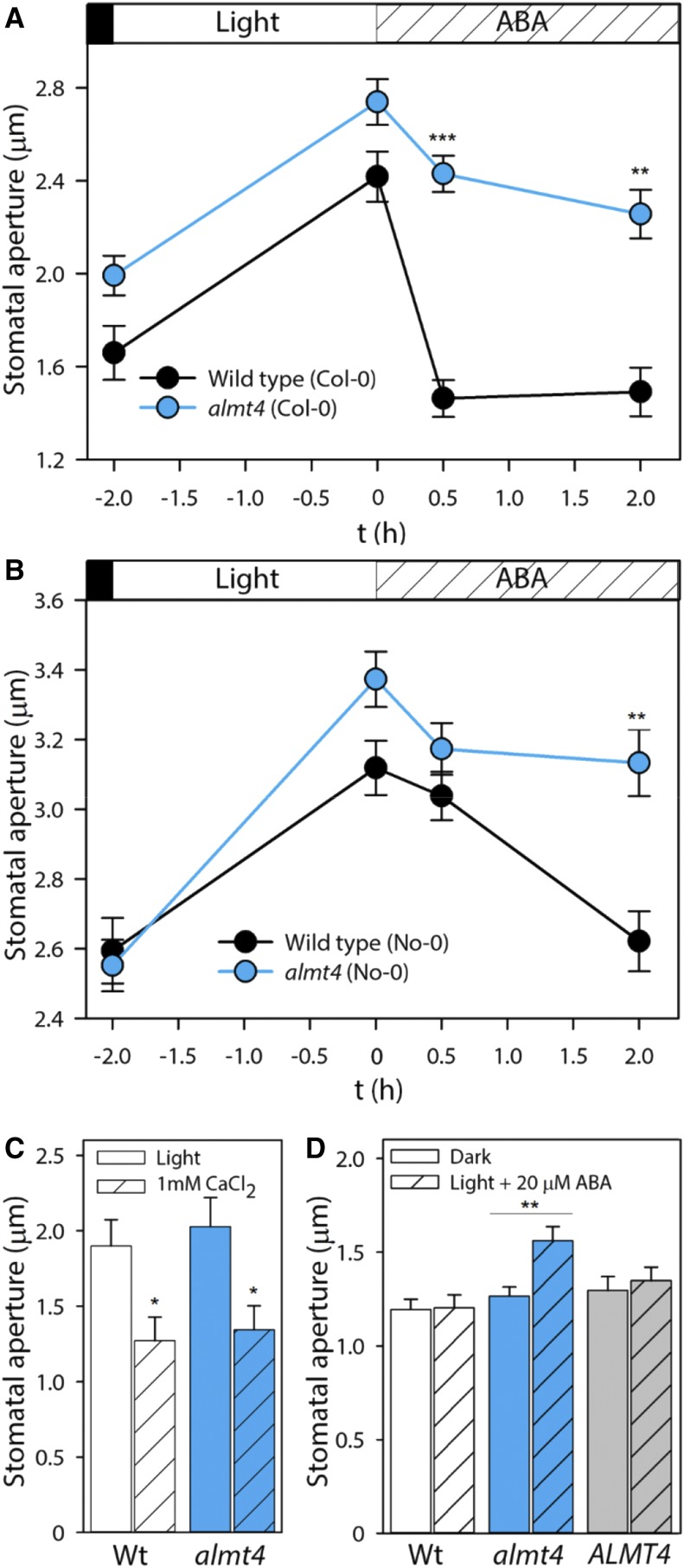
Stomatal apertures were measured from epidermal peels of dark-adapted plants, after a 2-h light period and during a 0.5 and 2 h incubation with ABA (Figures 1A and B). Stomatal opening was not impaired in almt4 mutants; however, stomatal closure in response to ABA was significantly slowed and incomplete (Figures 1A and B). After 0.5 h of ABA treatment, the Col-0 wild-type stomata had closed completely. When the difference between the dark-adapted and the light-adapted aperture was normalized to 100%, the wild-type aperture decreased in response to ABA by over 100%, indicating complete closure with respect to the dark state. When the same normalization was applied to almt4, the mutant closed by only ∼40%. This difference was statistically significant, as was the difference in stomatal aperture after 2 h of ABA. In the No-0 background, differences between almt4 knockout and wild type were more pronounced after 2 h of ABA treatment. Whereas the wild type displayed a stomatal aperture decreased by 95% compared with the initial dark value, the almt4 mutant aperture decreased by only ∼30%; this difference in degree of closure was statistically significant. The stomatal phenotype of both almt4 mutant alleles is comparable to those of guard cell plasma membrane ion channel mutants involved in anion loss (almt12/quac1; Meyer et al., 2010) and K+ loss (gork; Hosy et al., 2003).
Figure 1.
Responses of Stomatal Aperture to ABA and Ca2+.
Stomatal aperture was measured on epidermal peels from the wild type, almt4, or an ALMT4 complementation line.
(A) and (B) Aperture was measured from dark-adapted plants, after a 2-h incubation under white light and subsequent 0.5 and 2 h incubation with 20 µM ABA for the Col-0 (A) and the No-0 (B) allele. Significant differences were compared between lines within one time point.
(C) Aperture was measured after a 2-h incubation under white light (simple bars) and subsequent 2-h incubation with 1 mM CaCl2 (shaded bars).
(D) Aperture was measured from dark-adapted plants (simple bars) and after 2-h incubation under white light and 20 µM ABA (shaded bars).
Data points are means of four different plants and for each plant, 60 stomata were analyzed (error bars denote the se). Significance was analyzed by Student’s t test and is indicated by asterisks: *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
To confirm that impaired stomatal closure is indeed a function of the ALMT4 gene product, we transformed almt4 (Col-0) with a construct harboring the genomic ALMT4 sequence fused to its native promoter (denoted: ALMT4). A complemented line was confirmed by RT-PCR (ALMT4-1; Supplemental Figure 1A) and investigated together with almt4 mutant and the wild type in a second, independent experiment (Supplemental Figure 2). The impaired stomatal closure phenotype of almt4 mutants was confirmed in this second experiment and was rescued in the complementation line. This result indicated that the almt4 stomatal phenotype is associated with ALMT4 function in guard cells.
A rise in the cytosolic free Ca2+ concentration is a signaling intermediate in ABA-induced stomatal closure (Roelfsema and Hedrich, 2010). However, Ca2+-induced closure was not affected in the almt4 mutant (Figure 1C). ABA not only induces closure but also inhibits stomatal opening. We tested whether ABA inhibition of stomatal opening was affected and, indeed, when simultaneously incubated with ABA, almt4 mutant stomata opened in response to light, whereas stomata of the wild type and complementation line did not (Figure 1D).
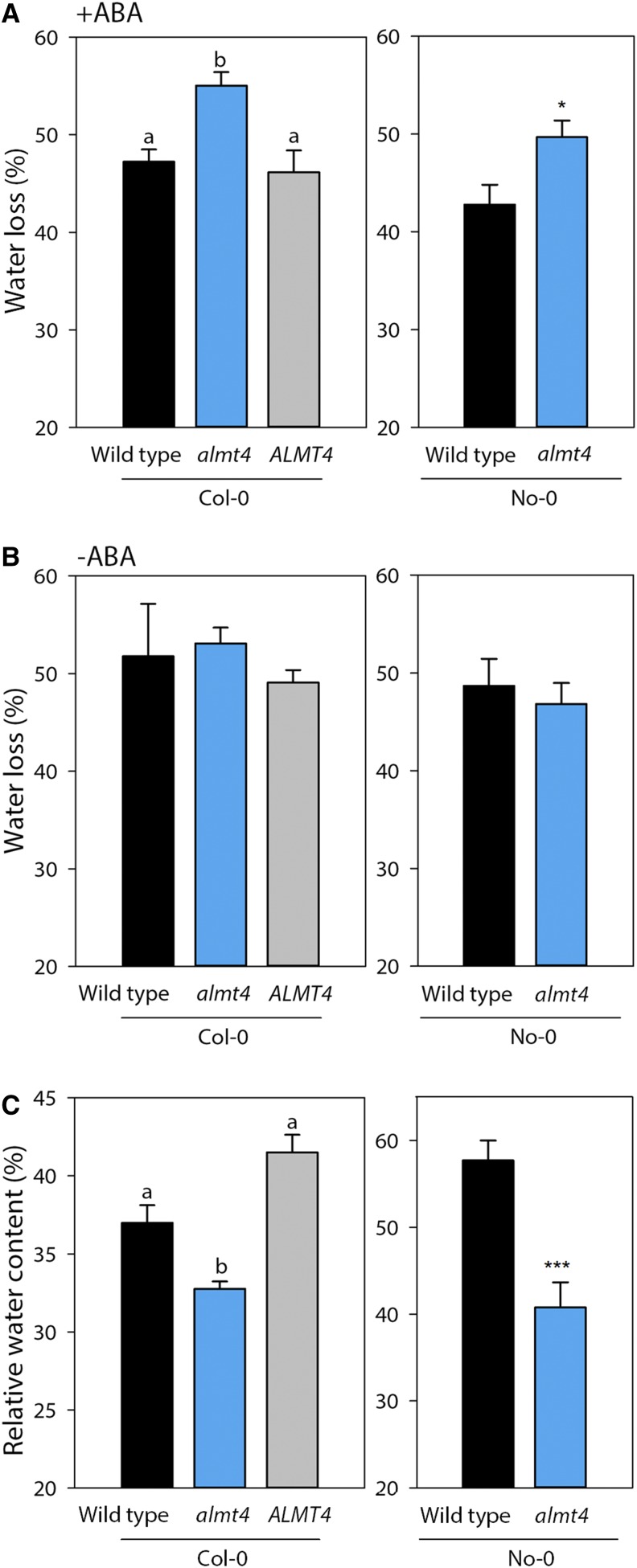
The observed mutant stomatal phenotypes suggested a function for ALMT4 in whole-plant water balance. We therefore performed gas exchange experiments to analyze whole-plant ensemble stomatal behavior in response to dark and light stimuli (Supplemental Figures 3A and 3B). We found that there were no consistent differences in dark- or light-adapted transpiration rates between the wild type and almt4 mutants of both ecotypes. Therefore, the altered stomatal behavior in almt4 might be specific to ABA. To test whether the impaired ABA response in the mutant has an effect on the whole-plant level, we performed a water loss (Figures 2A and 2B) and a drought stress (Figure 2C) experiment. For the first experiment, petioles of rosettes leaves were incubated in ABA (Figure 2A) or a control solution (Figure 2B) for 1 h and then left to air dry. The fresh weight was monitored before and after drying, and the difference in fresh weight was interpreted as the percentage of water loss. To a large degree this water loss is due to stomatal transpiration, and we found that indeed almt4 mutants of Col-0 and No-0 lost 55% and 50% of their water, respectively (Figure 2A). By contrast, corresponding wild types lost only 47% and 42% of water, respectively, and the mutant phenotype was rescued in the complementation line (46% water loss). When leaves were not pretreated with ABA, that is, stomata were not allowed to close before drying, no significant differences in water loss were detected between the mutant, wild type, and complementation line (Figure 2B).
Figure 2.
Whole-Plant Stomatal Phenotype in Response to ABA and Drought Stress.
(A) and (B) Water loss of air-dried rosette leaves of Col-0 and No-0 lines (n = 6). ABA ([A]; 20 µM in double-distilled water, final concentration of 0.2% ethanol) or a control solution ([B]; double-distilled water, final concentration of 0.2% ethanol) was fed to leaves via petioles for an hour in the light, and leaves were left to air-dry in the light for 7 h. Fresh weight was recorded before and after drying and water loss was calculated.
(C) RWC of rosette leaves from 10 individual, soil-grown plants per line after the plants had been exposed to drought stress.
Data are means ± se and were analyzed either by ANOVA followed by multiple pairwise comparison or Student’s t test. Significances by ANOVA are indicated by lettering with different letters denoting significant differences (P ≤ 0.05) and significance for t test by asterisks (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
During drought stress, whole plants synthesize ABA to aid stomatal closure and prevent wilting. To analyze if almt4 mutants show an effect under physiological conditions, we performed a drought stress experiment. We withheld watering for 2 weeks, until plants showed obvious signs of wilting, and determined relative water content (RWC) of drought-stressed rosette leaves. After drought stress, almt4 mutants had a significantly lower RWC (32 and 41% Col-0 and No-0, respectively) when compared with the wild type (37 and 58%, Col-0 and No-0, respectively) and the complementation line (42%; Figure 2C).
The observed phenotypes did not result from pleiotropic effects caused by differential expression of other members of the clade II ALMT family. Expression levels of all clade II ALMT mRNAs were not significantly different between the wild type and almt4 (Supplemental Figure 1B).
ALMT4 Is Localized to the Vacuolar Membrane and Is Expressed in Leaf Mesophyll and Guard Cells
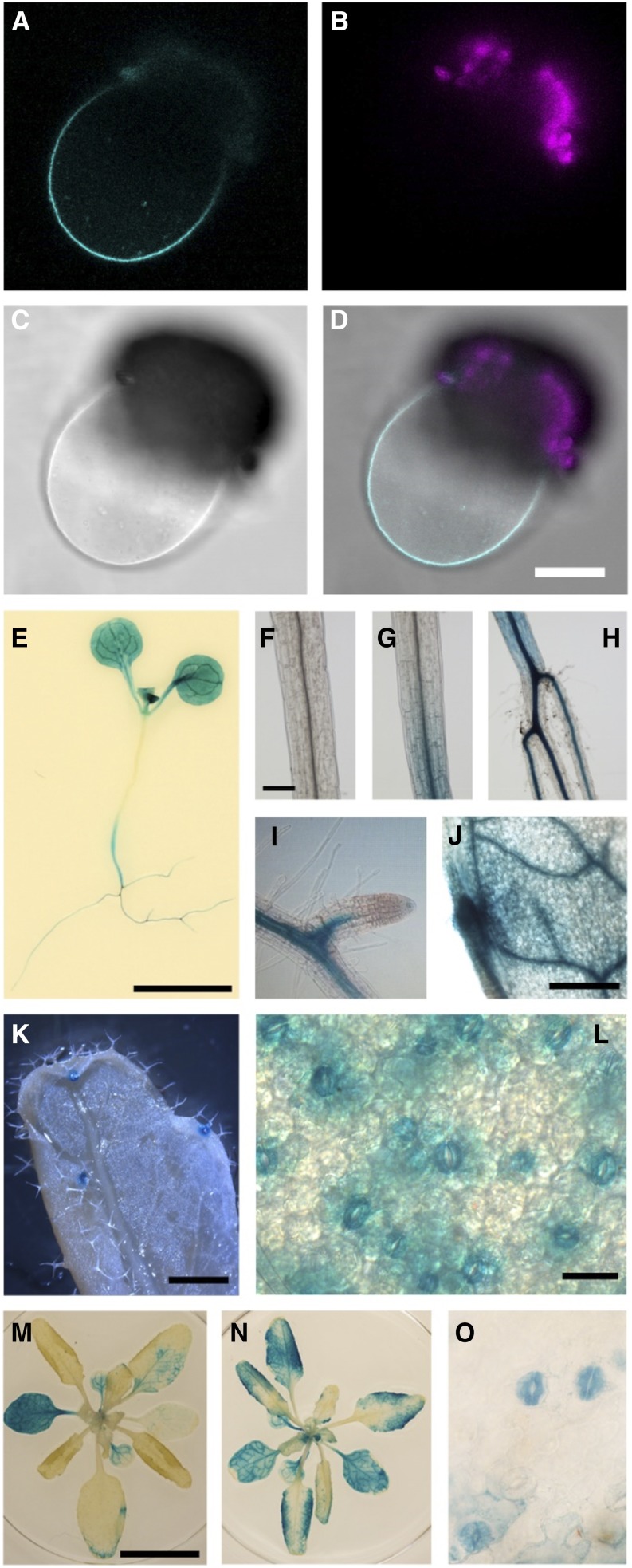
Next, we examined intracellular, organ, and tissue-specific localization of ALMT4. ALMT4 was cloned from Arabidopsis rosette leaf cDNA, and transiently expressed in Nicotiana benthamiana with a C-terminal fusion to GFP. Fluorescence was observed intracellularly in intact epidermis cells. When we isolated protoplasts and lysed them using an osmotic shock treatment to release the central vacuole, GFP fluorescence was clearly visible at the vacuolar membrane (Figures 3A to 3D).
Figure 3.
Localization and Expression of ALMT4.
(A) to (D) ALMT4-GFP transiently expressed in N. benthamiana localizes to the vacuolar membrane of a lysed protoplast ([A] to [D]). GFP fluorescence (cyan; [A]), chlorophyll fluorescence from protoplast debris (magenta; [B]), bright field (C), and overlay (D). Bar = 25 µm.
(E) to (O) An ALMT4Pro:GUS construct causes reporter gene expression in seedlings ([E]; bar = 5 mm), specifically in the hypocotyl ([F], shoot; [G], shoot-hypocotyl transition; [H], hypocotyl-root transition; bar = 200 µm), in the vasculature of roots ([I]; bar = 50 µm) and leaves ([J]; bar = 500 µm), and in hydathodes ([K]; bar = 1 mm), stomata and mesophyll cells ([L]; bar = 20 µm). Reporter gene expression in mature whole rosettes ([M]; bar = 1 cm) increased when rosettes were incubated in 20 µM ABA for 2 h at room temperature ([N]; scale as in [M]), and ABA caused expression in guard cells without concurrent mesophyll staining ([O]; scale as in [L]).
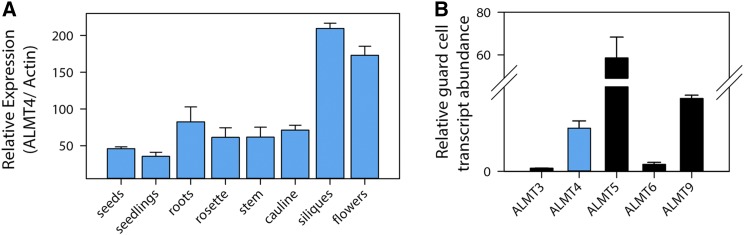
We investigated organ-specific expression by qRT-PCR analysis (Figure 4A). ALMT4 was expressed throughout the plant at similar levels in roots, rosette leaves, cauline leaves, and stem. Expression was overall lower in seeds and seedlings and highest in siliques and flowers. The almt4 mutant phenotypes suggested ALMT4 expression in guard cells. To analyze tissue-specific expression, we fused a 1.8-kb promoter fragment of ALMT4 to the GUS reporter gene (ALMT4Pro:GUS). GUS reporter activity was found throughout the plant in cotyledons and leaves (Figures 3E and 3L to 3N), in the vasculature and cortex of the hypocotyl (Figures 3F to 3H), and in the root vasculature (Figure 3I). In leaves, GUS activity was high in the leaf vasculature (Figure 3J), the mesophyll (Figures 3E, 3M, and 3N), hydathodes (Figure 3K), and guard cells (Figures 3L and 3O). Furthermore, GUS reporter gene activity increased when rosettes were preincubated in ABA (Figure 3N). Usually, staining was observed in mesophyll and guard cells, but after ABA treatment, guard cell staining increased compared with mesophyll staining (Figure 3O).
Figure 4.
Expression of ALMT4 throughout the Plant and in Guard Cells.
(A) ALMT4 expression was quantified by qRT-PCR across a range of plant organs. Data are shown as mean ± sd (n = 2 independent experiments with five plants and three technical replicates each); ACTIN2 served as a reference gene; expression was normalized to ALMT4 mRNA levels in seeds.
(B) Abundance of clade II ALMT mRNAs in guard cells, relative to guard-cell-specific ALMT6. Data were taken from Obulareddy et al. (2013), and originally reported as fragments per kilobase of exon per million fragments mapped. Data are means ± sd from two samples.
In the absence of public expression data, further evidence for guard cell expression of ALMT4 came from a study on RNA isolation from guard cells (Obulareddy et al., 2013). The released mRNA expression data for all ALMT members of clade II are visualized in Figure 4B. The data show that ALMT4 is expressed at a level similar to ALMT9 in guard cells and that the expression of ALMT6 is about 10 times lower compared with ALMT9.
ALMT4 Macroscopic Currents Resemble Those of ALMT9 but Show Distinct Kinetics and Rectification
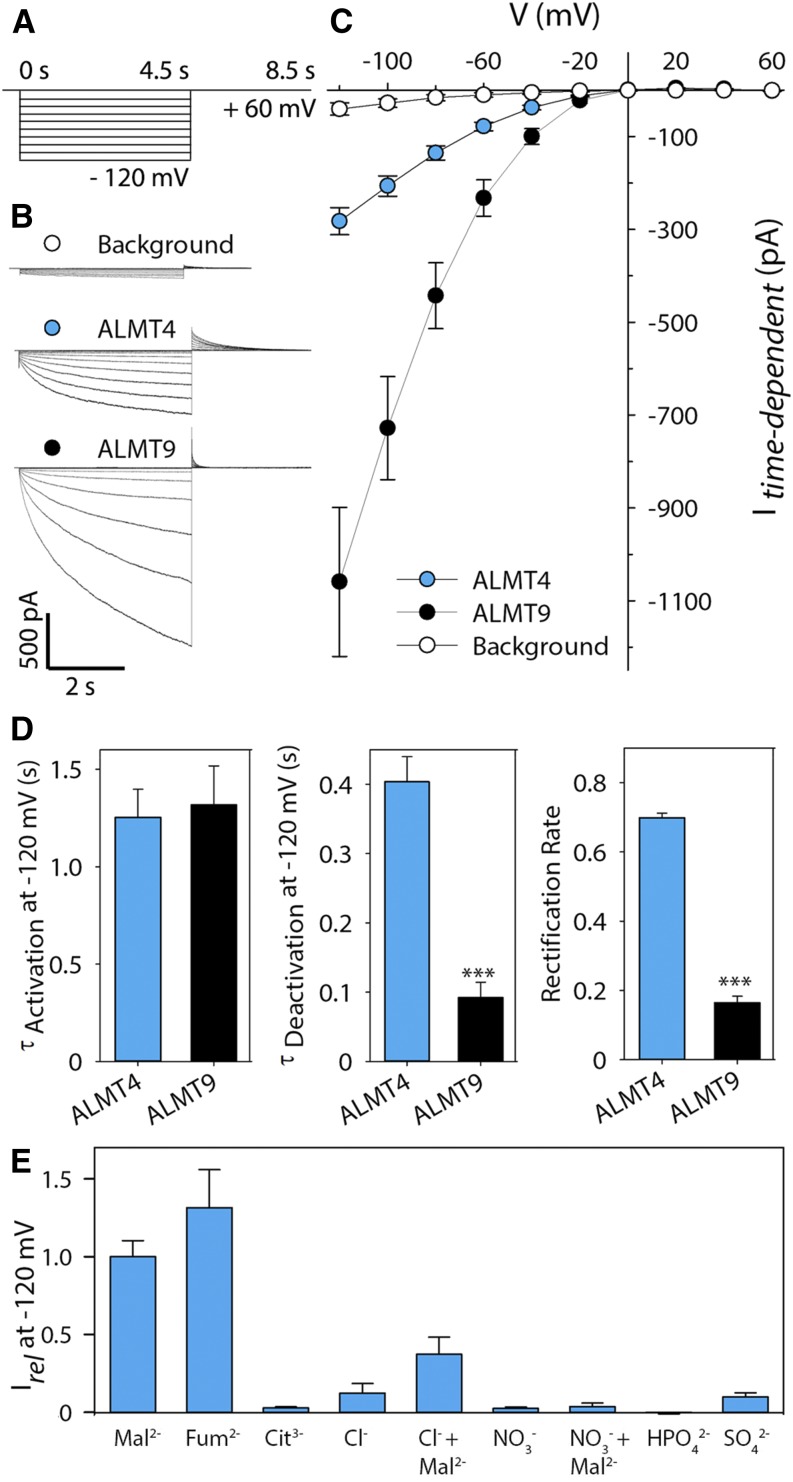
Taken together, the above data suggest a function for ALMT4 at the guard cell vacuolar membrane, necessary for stomatal closing. By contrast, ALMT9 functions at the guard cell vacuolar membrane but is necessary for stomatal opening, mediating Cl− uptake into the vacuole (De Angeli et al., 2013). The opposite mutant phenotypes suggested a mechanism for ALMT4 opposite to that of ALMT9, i.e., that ALMT4 might carry anion currents in the opposite direction, from the vacuole to the cytoplasm. We used ALMT4-GFP expression in the vacuolar membrane of N. benthamiana to study the channel’s electrophysiological characteristics by patch-clamp (Figure 5). We obtained excised outside-out patches and used symmetric Mal2− conditions, with 100 mM Mal2− in the pipette, representing the vacuolar side (Mal2−vac), and 100 mM Mal2− in the bath, representing the cytoplasmic side (Mal2−cyt). To analyze the current-voltage (I-V) relationship, we applied a voltage clamp protocol as shown in Figure 5A. For comparison, we also measured background currents and currents through ALMT9 (Figures 5B and 5C). ALMT9 displayed typical inwardly directed Mal2− currents that activated between 0 mV and −20 mV (Figures 5B and 5C), as has been reported previously (Kovermann et al., 2007; De Angeli et al., 2013; Zhang et al., 2013, 2014). Surprisingly, 30% of all patches obtained for ALMT4 displayed current with mean amplitudes smaller than 100 pA at −120 mV, i.e., they were indistinguishable from background currents. This finding suggested that the data were pools of two different populations. Indeed, a normality test of current distribution at −120 mV show that data were not normally distributed, and a histogram showed that the data cluster in two groups (Supplemental Figure 4). One group overlapped with the distribution of background current data and probably represents current from largely inactive ALMT4 channels. We therefore grouped all data with mean amplitudes that overlapped with background currents, i.e., below 100 pA at −120 mV, and excluded them from any further analysis. Active ALMT4 currents were inwardly directed and yielded a mean steady state amplitude of −282 pA ± 30 pA at −120 mV, about 4 times lower than that of ALMT9 (Figure 5C), despite similar GFP fluorescence at the tonoplast.
Figure 5.
Comparison of ALMT4- and ALMT9-Mediated Currents.
(A) Schematic representation of the voltage protocol used. Activating currents were evoked by successive voltage pulses ranging from a holding voltage of +60 mV to −120 mV and recorded for 4.5 s, with successive voltage pulses increasing by 20 mV. Deactivating tail currents were recorded for 4 s by a subsequent step to holding voltage.
(B) Representative current traces of background current from untransformed vacuoles (top, white), ALMT4 (middle, blue), and ALMT9 (bottom, black).
(C) I-V curve of background (white circles, n = 11), ALMT4 (blue circles, n = 11), and ALMT9 (black circles, n = 12) time-dependent currents at steady state in symmetric Mal2− conditions (100 mM Mal2−vac/100 mM Mal2−cyt). Currents were measured on excised outside-out patches of N. benthamiana vacuoles, either untransformed or confirmed to express ALMT9-GFP or ALMT4-GFP. Data points are means of current means at steady state. Error bars denote the se.
(D) Time constants for activating (left) and deactivating (middle) time-dependent current at −120 mV and rectification rate (right). For analysis methods, see Supplemental Figure 5 and Methods. Data are means ± se.
(E) Current means for different substrates relative to Mal2− current at −120 mV (n = 3) ± se. In case of Cl− and NO3−, 1 mM Mal2− was added to investigate potential current activation.
We tested whether ALMT4-mediated currents might be low because Mal2− is not the preferred permeating substrate by substitution of the cytosolic side solution with buffers based on different anions (Figure 5E). Among the organic ions, ALMT4 was permeable to Mal2− and fumarate (Fum2−) but not to citrate (Cit3−). Using reversal potential analysis, we found that ALMT4 was 1.8 times more permeable to Fum2− compared with Mal2−. Among the anions, ALMT4 was not permeable to Cl−, NO3−, HPO42−, and SO42−, but we detected an activation of Cl− current by 1 mM Mal2−, similar to what was previously reported for ALMT9. Although Arabidopsis contains high fumarate levels compared with many other species (Chia et al., 2000; Araújo et al., 2011), a role for fumarate in Arabidopsis guard cell physiology is hardly documented in the literature. Although, like ALMT9, ALMT4 can transport Cl− into the vacuole when activated by Mal2−, the stomatal phenotypes are contrary to this notion, so that at least for guard cells, we exclude a role of ALMT4 in Cl− uptake. Therefore, we concentrated on Mal2− as a substrate in our electrophysiological experiments.
Besides differences in channel activity and macroscopic current amplitude, ALMT4 macroscopic current kinetics differed significantly from those of ALMT9. Activating and deactivating current kinetics reflect transition reactions between the open and the closed state of ion channels. They can be fitted by exponential functions to derive time constants (τ) that inform on the rates of the state transitions (Supplemental Figure 5; see Methods). While the time constant for current activation (τActivation) at −120 mV was similar between both channels (Figure 5D, Table 1), the time constant for deactivation (τDeactivation) of ALMT4 was about 4 times larger than that of ALMT9. This finding indicated that channel closing in response to a deactivating voltage took longer in ALMT4 channels. Furthermore, we examined the open channel rectification of ALMT9 and ALMT4 by determining the rectification rate (Figure 5D). The rectification rate is the ratio of activated channel steady state conductance at −120 mV (Gact-120) and open channel conductance at the deactivating tail voltage (+60 mV; Gtail+60; see Supplemental Figure 5). It denotes the preference of the permeation pathway for inward or outward rectification. We found that ALMT4 displayed a rectification rate significantly increased by more than 4-fold when compared with that of ALMT9 (Table 1, compared with Zhang et al., 2013). The large rectification rate of ALMT4, compared with ALMT9, indicates that its propensity to conduct inward ion fluxes is similar to its propensity to conduct outward current fluxes; thus, ALMT4 might be more prone to mediate Mal2− outward currents.
Table 1. Macroscopic Open Channel Currents (Itail0) of Vacuolar Excised Outside-Out Patches Expressing ALMT9, ALMT4 S382A, and ALMT4 Wild Type Were Fitted by a Boltzman Function (Equation 3).
| Parameter of Gating and Current Kinetics | ALMT9 (n = 7) | ALMT4 Wild Type (n = 8) | ALMT4 S382A (n = 8) |
|---|---|---|---|
| Itail0 max (pA) | 121 ± 38a | 142 ± 16a | 256 ± 18b |
| V1/2 (mV) | −90 ± 10a | −63 ± 4b | −67 ± 1b |
| zg | 0.7 ± 0.1 | 0.7 ± 0.02 | 0.7 ± 0.01 |
| τActivation | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.4 |
| τDeactivation | 0.1 ± 0.02a | 0.4 ± 0.04b | 0.4 ± 0.1b |
| R | 0.2 ± 0.02a | 0.7 ± 0.01b | 0.8 ± 0.1b |
From the fit, the maximal Itail0 (Itail0 max), the half-maximal activation voltage (V1/2), and the gating charge (zg) were derived. The time-dependent current proportion of activating currents and deactivating tail currents were fitted by standard exponential functions to derive activating (τActivation) and deactivating (τDeactivation) time constants. The rectification rates (R) were derived by dividing the steady state time-dependent conductance at the deactivating tail voltage of +60 mV by the conductance at the activating voltage of −120 mV. Data are averages ± se. Significant differences were tested for each parameter by ANOVA followed by Holm-Sidak multiple comparison (P ≤ 0.001) and are indicated by different letters.
ALMT4 Is Phosphorylated at S382 and Currents Are Activated by Dephosphomimetic Mutation
ALMT4 current generally displayed a low macroscopic steady state current amplitude and activity. ALMT12/QUAC1 also displayed a low basal activity (Meyer et al., 2010) but was markedly activated by OST1-mediated phosphorylation in response to ABA (Imes et al., 2013).
The ABA-related almt4 phenotypes led us to test recombinant OST1 on ALMT4. We patched vacuoles expressing ALMT4 in experiments analogous to those shown in Figure 5. Once stable ALMT4 currents were recorded, we perfused the membrane patch with a bath solution containing recombinant OST1 together with ATP, constantly monitoring current activity. However, no changes in current activity or I-V relations could be detected upon application of the kinase (data not shown). ATP on its own had no effect on the current.
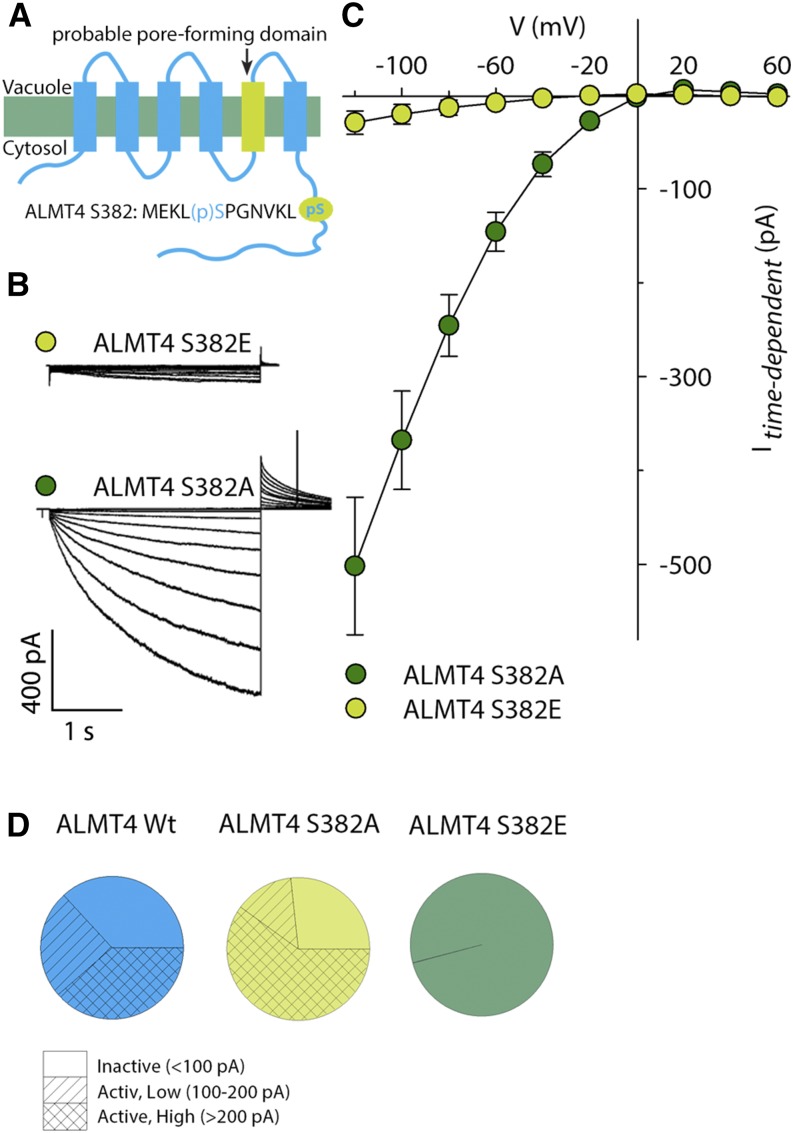
In the search for other putative phosphorylation sites, we mined the public PhosPhAt database (Heazlewood et al., 2008) and found a phosphoproteomic data set containing a phosphopeptide of ALMT4, showing phosphorylation at S382 [MEKL(p)SPGNVLK; Figure 6A]. S382 is located within the C-terminal moiety of the protein, which is predicted to be localized in the cytoplasm (Figure 6A). To test the significance of S382 phosphorylation for ALMT4 function, we generated ALMT4 S382E and ALMT4 S382A constructs, i.e., introduced phosphomimetic and dephosphomimetic mutations, respectively. Expression of both constructs at the tonoplast was confirmed by fluorescence microscopy before performing patch-clamp analysis as described above. We found a striking difference in Mal2− currents and I-V relations between both constructs (Figures 6B and 6C). In all patches expressing ALMT4 S382E-GFP, we detected only background current activity (compared with Figures 6C and 5C). ALMT4 S382E yielded a mean current amplitude of −30 pA ± 12 pA at −120 mV (Figure 6C). However, patches expressing ALMT4 S382A-GFP yielded a mean current amplitude of −502 pA ± 73 pA at −120 mV, nearly double that of the wild-type channel (compared with Figures 6C and 5C). Furthermore, the percentage of patches showing background current activity was decreased (Figure 6D). In summary, these data show that mutation of ALMT4 S382 to glutamate causes background current amplitudes, suggesting that phosphorylation is required and sufficient for channel deactivation. Mutation to alanine causes an increase in channel activity as well as an increase in macroscopic Mal2− current amplitude, suggesting that dephosphorylation is required for channel activation, but other factors might also contribute.
Figure 6.
Identification of a Phosphorylation Site in the ALMT4 C Terminus and Comparison of Site-Directed Mutant Currents.
(A) Schematic representation of the presumed ALMT4 topology showing the probable pore-forming domain as determined by Zhang et al. (2013) and the approximate position of a phosphopeptide retrieved from the PhosPhAt database.
(B) Representative current traces of ALMT4 S382E (light green) and ALMT4 S382A (dark green).
(C) I-V curve of ALMT4 S382A (dark green circles, n = 11) and ALMT4 S382E (light green circles, n = 9) time-dependent currents at steady state in symmetric Mal2− conditions (100 mM Mal2−vac/100 mM Mal2−cyt). Currents were measured on excised outside-out patches of N. benthamiana vacuoles confirmed to express ALMT4 S382E-GFP and ALMT4 S382A-GFP and were evoked by a voltage protocol as described in Figure 5.
(D) Distribution of wild-type and mutant ALMT4 current amplitudes at −120 mV in ALMT4 wild type (blue), ALMT4 S382A (light green), and ALMT4 S382E (dark green). Current amplitudes below 100 pA were classified as inactive (empty), between 100 and 200 pA as low amplitude active (dashed), and above 200 pA as high amplitude active (crossed).
The almt4 Mutants Transformed with Active ALMT4 S382A or Inactive ALMT4 S382E Display Distinct Growth and Stomatal Phenotypes
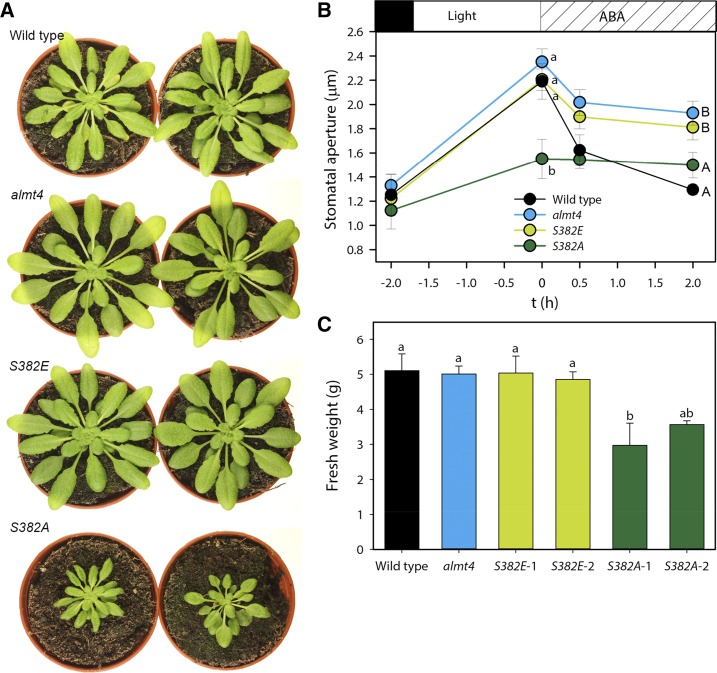
To investigate whether S382 of ALMT4 does play a role in vivo and whether phosphorylation at this residue might underpin the observed almt4 stomatal phenotype, we transformed the almt4 mutant (Col-0) with ALMT4 S382A and S382E constructs, termed S382A and S382E, respectively. Surprisingly, S382A lines showed a strongly reduced rosette area and fresh weight (Figures 7A and 7C). Evidently, expression of constitutively active ALMT4 causes severe growth defects. Epidermal peels of the transformants, almt4 mutant, and the wild type were examined in an experiment akin to that shown in Figure 1. As found in the previous experiments, the almt4 mutant stomata opened during a 2-h light treatment but showed delayed and incomplete closure upon treatment with 20 µM ABA (Figure 7B, compared with Figure 1A). Strikingly, S382A expressing the constitutively active channel displayed a marked impairment in stomatal opening, showing ∼42 and 46% less opening than almt4 and the wild type, respectively. However, stomata of almt4 transformed with the constitutively inactive channel (S382E) displayed wild-type-like opening behavior (Figure 7B). Stomatal closure in S382E was comparable to almt4, with stomata closing by 40% (compared with the dark value) in both cases, whereas wild-type stomata closed by 96% (Figure 7B). Stomata of S382A closed by only 12%. Comparable results were obtained with lines from independent transformation events (Supplemental Figure 6).
Figure 7.
Stomatal Aperture Response and Rosette Growth of almt4 Knockout Lines Transformed with Full-Length ALMT4 S382A (S382A) and S382E (S382E).
(A) Representative images of wild-type, almt4, S382E, and S382A plants.
(B) Epidermal peels of wild-type (black), almt4 (blue), S382E (light green), and S382A (dark green) plants. Stomatal aperture was measured from dark-adapted plants, after a 2-h incubation under white light and subsequent 0.5- and 2-h incubations with 20 µM ABA. Data points are means of five different plants using 60 stomata per plant and significance of differences was tested between lines within one time point.
(C) Fresh weight of 6-week-old wild type, almt4, and two independent lines transformed with full-length ALMT4 S382A (S382A-1 and S382A-2) and S382E (S382E-1 and S382E-2), respectively (n = 6).
Data are means ± se. Significant differences were determined using ANOVA followed by multiple comparison (at least P ≤ 0.05) and are indicated by different letters.
These results show that mutating S382 has a dramatic effect on plant growth and stomatal movement and suggest that the S382A mutation prevents guard cell solute accumulation and efficient stomatal opening. This conclusion suggested that active ALMT4 might be involved in solute loss from the vacuole and be able to mediate Mal2− efflux at the prevailing membrane potential.
Active ALMT4 S382A Mediates Mal2− Outward Currents
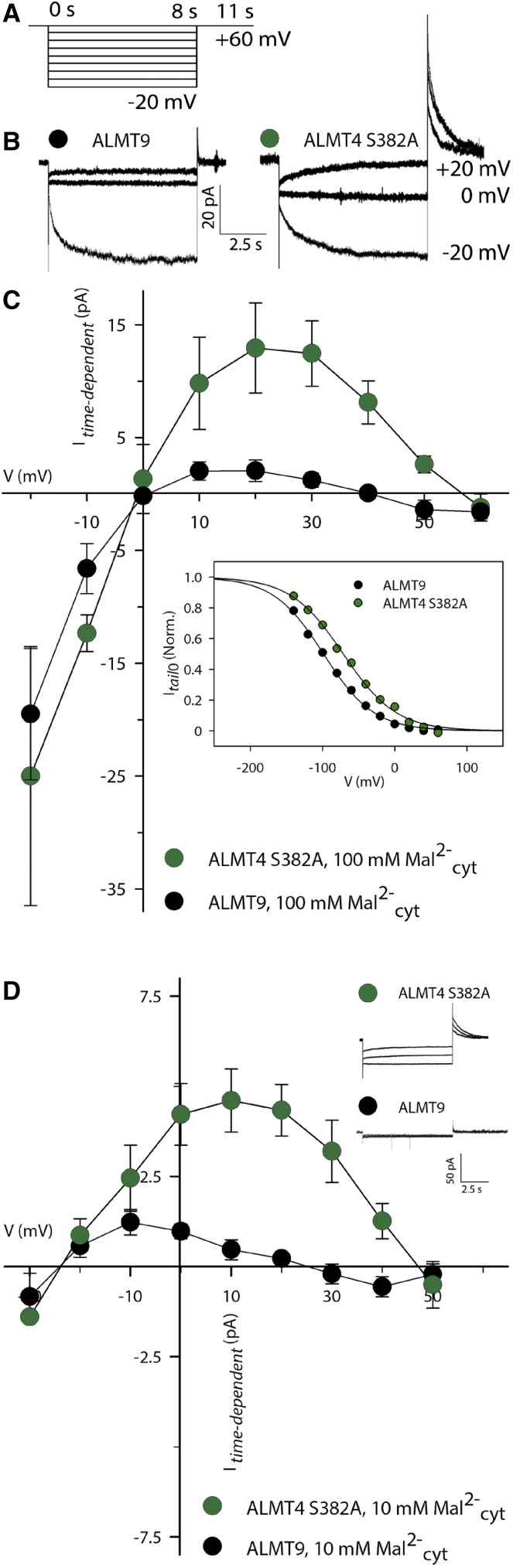
To test whether ALMT4 S382A can mediate Mal2− efflux from the vacuole, we devised a slow voltage-clamp protocol with longer-lasting voltage steps increasing by 10 mV, in order to resolve such a current (Figure 8A). Indeed, the I-V relation displayed positive outward currents at voltages positive of the reversal potential for Mal2− (ERevMal2− = 0 mV in symmetric ionic conditions; Figures 8B and 8C). The maximal steady state mean current amplitude of active ALMT4 S382A was 13 pA ± 4 pA at +20 mV. Within the same set of experiments, we also investigated ALMT9 and found only very small outward currents at positive voltages, with a mean steady state current amplitude of 2 pA ± 1 pA at +20 mV (Figures 8B and 8C). To examine the reason for the difference in ability to conduct outward current between ALMT4 S382A and ALMT9, we compared the relative open probabilities (Porel) of both channels (Supplemental Figure 5; see Methods). Porel is proportional to Itail0 according to equation 2 (see Methods) and Boltzman analysis of Itail0 (Figure 8C, inset; equation 3; see Methods) indicated that, compared with ALMT9, ALMT4 S382A displayed a marked and statistically significant shift of the V1/2 by 22 mV to more positive voltages (Table 1). ALMT4 S382A was not significantly different from wild-type ALMT4 in its gating characteristics, macroscopic current kinetics, and rectification ratio (Table 1). Considering that Mal2− current typically activates between 0 mV and −20 mV in ALMT9 in symmetric Mal2− conditions, the positive-going shift in V1/2 explains why ALMT4 S382A is open at voltages positive of the reversal potential for Mal2− (here, 0 mV) and able to mediate outward current along the electric gradient.
Figure 8.
Analysis of ALMT4 S382A Outward Mal2− Current.
(A) Schematic representation of the voltage protocol used. Activating currents were evoked by successive voltage pulses ranging from a holding voltage of +60 mV to −20 mV and recorded for 8 s, with successive voltage pulses increasing by 10 mV. Deactivating tail currents were recorded for 3 s by a subsequent step to holding voltage.
(B) Representative current traces of ALMT9 (black) and ALMT4 S382A (dark green) in symmetric Mal2− conditions. For clarity, currents at −20 mV, 0 mV, and +20 mV are shown.
(C) I-V curve of ALMT4 S382A (dark green circles, n = 7) and ALMT9 (black circles, n = 8) time-dependent currents at steady state in symmetric Mal2− conditions (green and black circles, 100 mM Mal2−vac/100 mM Mal2−cyt). Inset: Tail current amplitudes of open channel current (Itail0) normalized to the respective maximum Itail0 of ALMT9 (black circles) and active ALMT4 S382A (dark green circles). Data are representative and were fitted with the Boltzman function (black lines; Supplemental Figure 5; see Methods). Averages of derived gating parameters are shown in Table 1.
(D) I-V curve of ALMT4 S382A (dark-green circles, n = 7) and ALMT9 (black circles, n = 5) time-dependent currents at steady state in asymmetric Mal2− conditions (100 mM Mal2−vac/10 mM Mal2−cyt) and representative current traces (inset).
Currents were measured on excised outside-out patches of N. benthamiana vacuoles, confirmed to express ALMT4 S382A-GFP and ALMT9-GFP. Data points are means of current means at steady state. Error bars denote the se.
In planta, the Mal2− concentration gradient is directed out of the vacuole, with lower cytosolic and higher vacuolar malate concentrations (Gerhardt and Heldt, 1984). We applied a 10-fold Mal2− concentration gradient and observed a shift in outward current to more negative voltages, consistent with the concomitant shift in reversal potential (Figure 8D). Also, the current amplitude was diminished, with the highest outward current amplitude of active ALMT4 S382A at 5 pA ± 1 pA. This was also true for ALMT9, which nonetheless showed negligible outward current, consistent with the results displayed in Figure 8C. The decrease in current amplitude could reflect a sensitivity of the channel gate to the cytoplasmic Mal2− concentration, to avoid Mal2− efflux from the vacuole. However, it is not the case for ALMT4 S382A, since we did not observe a shift in V1/2 between 10 and 100 mM Mal2− (Supplemental Table 1). This lack of a shift shows that the channel is open to allow Mal2− efflux at 10 mM Mal2−cyt. Furthermore, ALMT4 S382A displayed defined tail currents at outward current voltages (Figure 8D, inset; Supplemental Table 1, Itail deact), confirming that the channel is open and able to mediate Mal2− efflux from the vacuole at 10 mM Mal2−cyt. However, the time constant for activation was larger at 10 mM Mal2−cyt (Supplemental Table 1, τActivation), indicating that it takes longer for macroscopic currents to reach maximal outward amplitudes. This finding could explain the decrease in outward current amplitude at 10 mM Mal2−cyt, although other factors likely contribute to this phenomenon.
ALMT4 Is Phosphorylated by MAP Kinases in Vitro
The accumulated data suggest that ALMT4 is phosphorylated in vivo and that this phosphorylation causes inactivation of vacuolar Mal2− fluxes. We asked which type of kinase might be responsible for this phosphorylation. Thus, we screened the amino acid sequence containing the phosphorylated S382 for motifs of canonical kinases implicated in abiotic stress signaling in guard cells, such as Ca2+-dependent kinases or OST1, albeit to no avail. We noticed that S382 is followed by a proline, indicating that it might be the target of a proline-directed kinase.
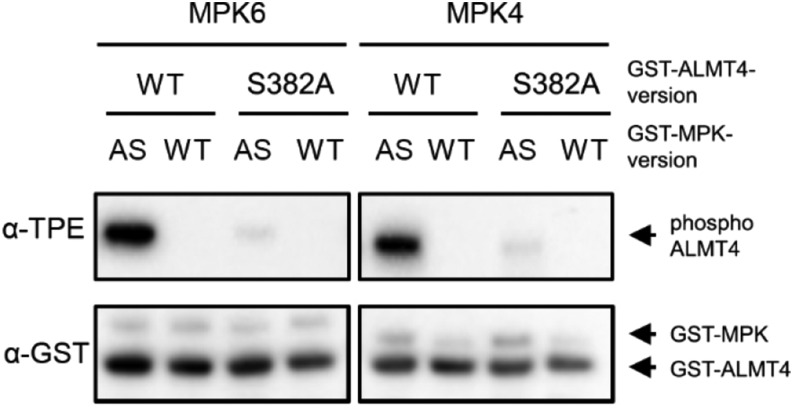
Mitogen-activated protein kinases (MPKs) are proline-directed serine/threonine kinases. To test whether the S382-bearing C terminus of ALMT4 could indeed be phosphorylated by an MPK, we conducted a kinase assay. We GST-tagged the cytosolic C-terminal part of wild-type ALMT4 (GST-ALMT4WT) and used site-directed mutagenesis to exchange S382 to Ala (GST-ALMT4S382A). Along with GST-ALMT4WT and GST-ALMT4S382A, we also prepared recombinant MPK4 and MPK6 to test whether these kinases can phosphorylate S382 of ALMT4 because the role of these kinases in ABA-mediated stomatal closure had been investigated previously (Hettenhausen et al., 2012; Xing et al., 2008; Montillet et al., 2013). We performed a recently established kinase assay that allows the detection of in vitro-phosphorylated MPK substrates by immunoblotting analyses (Leissing et al., 2016). As shown in Figure 9, activated recombinant MPK4 and MPK6 strongly phosphorylated GST-ALMT4WT. By contrast, when S382 was mutated to Ala (GST-ALMT4S382A), neither of the two MPKs was able to phosphorylate the protein (Figure 9). These results indicate that MPK4 and MPK6 can phosphorylate the ALMT4 C terminus at position S382 in vitro.
Figure 9.
In Vitro Phosphorylation of ALMT4 S382 by MPK6 and MPK4.
Thiophosphorylation assays of GST-tagged native and phosphosite S382A mutant C-terminal ALMT4. GST-ALMT4WT (WT) and GST- ALMT4S382A (S382A) were incubated in the presence of Bn-ATPγS as cofactor with GST-MPK4 or GST-MPK6 (see Methods). The GST-MPK recombinant proteins used were sensitive to Bn-ATPγS as a thiophosphate donor (analog-sensitive [AS]) or were wild type, as a negative control. Phosphorylated ALMT4 (phosphoALMT4) was visualized by an anti-thiophosphate ester-specific antibody (α-TPE), and the GST fusion proteins were visualized by an anti-GST antibody (α-GST) to test for equal loading.
DISCUSSION
Stomatal closure requires retrieval of solutes from the guard cell vacuole, including Mal2−, which can act as an osmoticum and charge-balances K+. However, to date it has been unclear how Mal2− is released from the vacuole during stomatal closure and the identity of the transport proteins has been obscure.
Here, we provide genetic and physiological evidence that ALMT4, a clade II member of Arabidopsis ALMT channels, mediates vacuolar Mal2− efflux during drought stress-induced stomatal closure and that the channel is regulated by phosphorylation. Three key findings lead us to this conclusion. First, loss of function of ALMT4 causes normal stomatal opening but delayed and incomplete stomatal closure in response to ABA, as well as increased water loss during drought and ABA treatment. Second, electrophysiological measurements show that ALMT4 is a voltage-dependent Mal2− channel with distinct gating properties and rectification that allow Mal2− efflux through it. Third, ALMT4 was deactivated by a S382E phosphomimetic mutation in patch-clamp studies and S382 could be phosphorylated by MPKs in vitro. In planta, the inactive channel (S382E) mimicked the almt4 mutant, whereas the constitutively active channel (S382A) showed impaired stomatal opening and stunted growth.
Function of ALMT4 in the Plant
Our electrophysiological data show that ALMT4 is permeable to Mal2−, Fum2−, and Cl− when activated by cytosolic Mal2−. Although ALMT4 can mediate vacuolar uptake of these substrates in electrophysiological experiments, our phenotypic data argue against an anion uptake function for ALMT4, at least in guard cells. This is further supported by the fact that mutants transformed with the active ALMT4 S382A showed impaired stomatal opening. Therefore, we propose that ALMT4 is responsible for Mal2− efflux during stomatal closure, a function that has not been characterized so far. ALMT4 might also mediate efflux of Fum2− but there is little evidence for a role for Fum2− in guard cells.
Although we have identified a role in guard cells, we cannot exclude that ALMT4 has additional functions in other cell types and organs since ALMT4 is expressed throughout the plant. The S382A growth phenotype is likely a result of the permanently reduced stomatal aperture causing reduced photosynthetic carbon assimilation and consequently growth. In addition, it might be caused by a reduced capability to store Mal2− and Fum2− in mesophyll vacuoles and, hence, generally impaired turgor-driven expansion growth. The growth phenotype could also be a result of reduced root growth due to impaired dicarboxylate storage or release for respiration. Both Mal2− and Fum2− are tricarboxylic acid cycle intermediates that accumulate during the day and are used for respiration during the night (Gago et al., 2016). Hence, in mesophyll tissue, release of Mal2− and Fum2− from the vacuole for respiration during the night might be a further important function of ALMT4. Although we could rule out an ALMT4 function in germination (Supplemental Figures 7A and 7B), one could envisage that ALMT4 is important during fertilization. Given its expression in flowers, it may regulate turgor or intracellular pH during pollen tube growth. Although we have focused on the role of Mal2− as a charge-balancing counter ion and osmoticum during stomatal regulation, ALMT4-mediated Mal2− fluxes might contribute to maintaining cytoplasmic pH, which is crucial during pollen tube growth (Michard et al., 2017; Lovy-Wheeler et al., 2006). Furthermore, Mal2− can act as a signaling molecule, activating Cl− uptake through ALMT9 (De Angeli et al., 2013). A deregulation of cytosolic Mal2− concentration might hamper vacuolar Cl− uptake, causing toxicity effects or altering the vacuolar membrane potential throughout the plant.
Potential Mechanism of ALMT4 Function during Stomatal Closure
In this study, ALMT4 is implicated in stomatal closure in response to ABA and drought stress, but not in response to darkness. This pattern hints that ALMT4 is part of an ABA-specific signaling pathway. Unlike ALMT6, ALMT4 does not depend on cytosolic Ca2+ and stomata of the almt4 mutant closed normally in response to external Ca2+. ABA-induced closure comprises a Ca2+-dependent and a Ca2+-independent pathway (Munemasa et al., 2015). Our data suggest that ALMT4 is either a target of Ca2+-independent ABA signaling or that this channel is an upstream component in Ca2+-dependent signaling. The latter could be the case if ALMT4 function leads to a change in tonoplast membrane voltage, causing Ca2+ efflux from the vacuole to generate cytosolic Ca2+ signals. As part of Ca2+-independent signaling, ALMT4 could be the target of Ca2+-independent kinases or phosphatases. However, there is also no indication of involvement of the core ABA-kinase OST1 in ALMT4 regulation. Instead, ALMT4 was inactivated when an in vivo phosphorylated serine was mutated to a phosphomimetic glutamate. This suggests that ALMT4 is regulated by ABA and de/phosphorylation through as yet unknown signaling pathways.
The sequence of events allowing net solute loss from the guard cell in response to ABA has been well established mainly as regards events at the plasma membrane (Munemasa et al., 2015); however, little is known about how ABA regulates solute loss from the vacuole. K+ efflux has been suggested to represent an early step in ABA-mediated changes at the vacuolar membrane (MacRobbie, 1995a, 1995b, 2000; Kollist et al., 2014), and it is mediated by channels of the two pore K+ (TPK) channel family, in particular TPK1 (Gobert et al., 2007). K+ efflux from the vacuole would lead to a depolarization of the vacuolar membrane potential. Depolarization would favor anion efflux, along their cytosol-directed concentration gradient. Despite the low vacuolar pH (∼5.8 in Arabidopsis; Andrés et al., 2014), the largest part of malate is present as Mal2− and hence gives rise to an outwardly directed Mal2− concentration gradient (Meyer et al., 2011; Gerhardt and Heldt, 1984). If ALMT4 were open at all voltages, the direction of Mal2− flux would depend solely on its electro-chemical gradient. The Nernst potential resulting from that gradient indicates the voltage at which the direction of flux reverses, the reversal potential. Based on Mal2− concentration measurements and simulations (Martinoia and Rentsch, 1994; Gerhardt and Heldt, 1984; Chen et al., 2012; Hills et al., 2012), the physiological reversal potential for Mal2− will be negative, probably around −30 mV. Hence, Mal2− efflux can be expected for voltages positive of this potential if the channel is open at more positive voltages. The shift in ALMT4 open probability by more than 20 mV to more positive voltages compared with ALMT9 can therefore explain its ability to mediate Mal2− efflux. Furthermore, the higher Mal2− outward currents in ALMT4 compared with ALMT9, and the lower respective inward currents can be explained by the drastically increased ALMT4 rectification rate. This is illustrated in our working model (Figure 10): In response to a shift in vacuolar membrane voltage, ALMT9 closes thereby ceasing anion uptake. However, ALMT4 is still open and, since at more depolarized voltages the Mal2− reversal potential favors efflux, ALMT4 mediates anion loss from the vacuole. In this study, we show that ALMT4 is also involved in inhibition by ABA of stomatal opening. Presumably ABA leads to ALMT4 activation, leading to Mal2− efflux, counteracting Mal2− influx by other transporters. Possibly, ALMT4 carries more than one function and it might additionally halt uptake of anions through other ALMT channels via subunit interaction (Hille, 2001; Zhang et al., 2013; Geiger et al., 2009; Zheng, 2013).
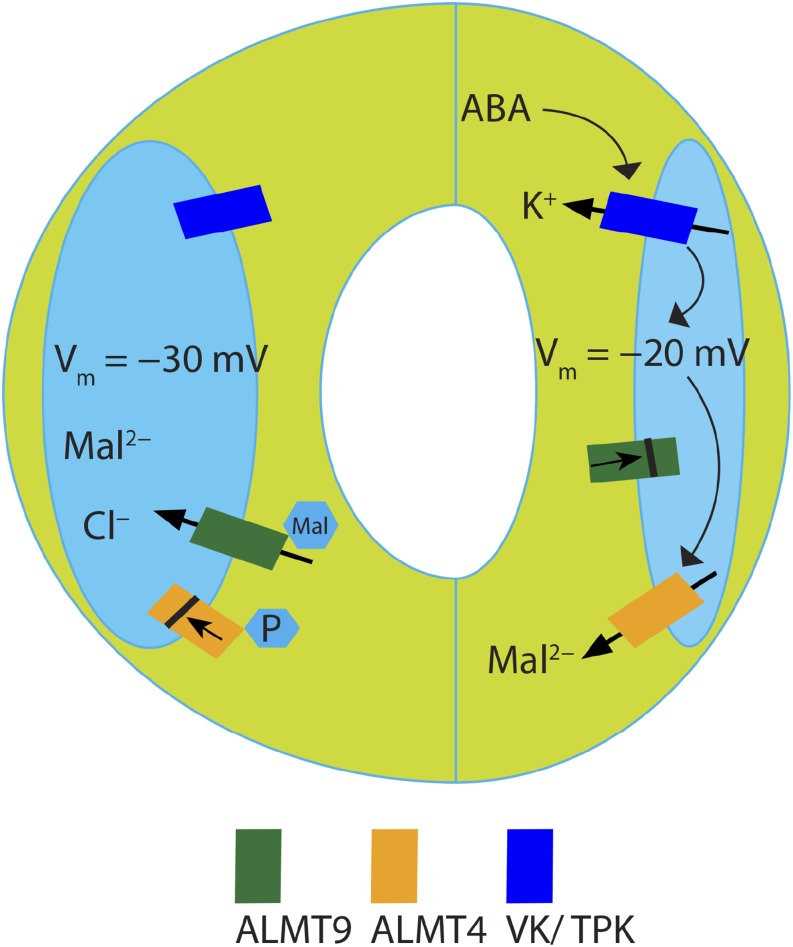
Figure 10.
Model of ALMT4 Function in Stomatal Closure.
Other (not all) identified conductances at the vacuolar membrane that contribute to stomatal opening and closing are also depicted (VK/TPK = vacuolar K+ channel/two-pore K+ channel). Events hypothetically taking place in response to ABA are depicted by black arrows. During stomatal opening (left), vacuolar uptake of Cl− is facilitated through ALMT9, while ALMT4 is phosphorylated and inactive. During stomatal closure (right) in response to ABA, the vacuolar K+ conductance is activated causing a depolarization of the vacuolar membrane potential, which leads to ALMT9 channel closure and cessation of solute uptake. ALMT4 is dephosphorylated by an unknown phosphatase and active. Since active ALMT4 is open at more positive voltages than ALMT9 it can mediate Mal2− efflux at more depolarized tonoplast potentials.
Although current amplitudes decreased when an outward-directed Mal2− concentration gradient was applied in patch clamp experiments, the difference in outward current between ALMT4 and ALMT9 was consistent. The amplitude decrease could not be explained by a Mal2− dependence of channel open probability, as is the case for some K+ channels (Blatt, 1988; Johansson et al., 2006). Although larger time constants for current activation might in part explain the decreased outward current amplitudes, interacting ions or proteins might also influence ALMT4 activity. However, in our experiments, the vacuolar membrane is isolated from any cytosolic and vacuolar contents and the resulting absence of potential interacting or modulating factors might explain the observed current decrease.
Regulation of ALMT4 by Phosphorylation
Regarding ALMT4 function in stomata, our results with phosphomimic and dephosphomimic mutants suggest that the resting state of the channel is phosphorylated and that it becomes active through dephosphorylation when ABA-dependent stomatal closure occurs. According to our electrophysiological results, channels in the S382E lines would be constitutively inactive. This would explain why these lines display almt4 mutant-like stomatal behavior. The transformants expressing a constitutively active channel (S382A) show drastically impaired opening. A phosphorylation-induced inactivation of the channel during stomatal opening would explain why ALMT4, which is present in the almt9 mutant that displays impaired stomatal opening, cannot compensate for the lack of ALMT9. One could speculate that S382 is dephosphorylated only during short, specific times, maybe in response to drought stress, whereas phosphorylation might represent a more constitute deactivation, preventing efflux during stomatal opening.
Here, we provide in vitro data indicating that ALMT4 might be phosphorylated and deactivated by MPKs. Typically, MAP kinase signaling comprises a cascade of three kinases (Lee et al., 2016). In 2011, it was shown that VIK of Arabidopsis, a MAP kinase kinase, also directly interacts with a vacuolar transport protein (Wingenter et al., 2011). The authors showed that VIK interacted with and stimulated the glucose uptake activity of the TONOPLAST MONOSACCHARIDE TRANSPORTER1. MPKs are not known to be part of the core ABA-signaling network, but more and more evidence is emerging that implicates MAP kinases in biotic and abiotic stress signaling during stomatal movement (Lee et al., 2016; de Zelicourt et al., 2016; Jakobson et al., 2016; Hõrak et al., 2016; Su et al., 2017). However, no MAP kinase ion channel substrates involved in stomatal movement have been identified. Here, we show that ALMT4 might be an in vivo substrate of MPKs. To date, MPK9 and MPK12 are known to be required for ABA-induced stomatal closure through activation of the SLAC1 anion efflux channel (Jammes et al., 2009; Lee et al., 2016). Also, Nicotiana attenuata deficient in NaMPK4 was impaired in ABA-induced stomatal closure (Hettenhausen et al., 2012). In addition, MPK12 and MPK4 of Arabidopsis interact with and inhibit the CO2-responsive kinase HT1, causing SLAC1 activation and stomatal closure in response to high CO2 (Jakobson et al., 2016; Hõrak et al., 2016). Therefore, MPK4 and MPK12 are unlikely to be required for ALMT4 inactivation during stomatal opening. Instead, our data suggest that a potential MAP kinase acting on ALMT4 would counteract closure and that it might be inactivated by ABA. Despite many studies showing mpk mutants to be impaired in stomatal closure, MPK6 is an example where a MAP kinase is NOT required for stomatal closure in response to ABA (Montillet et al., 2013); conversely, a dominant-negative MPK6 mutant was hypersensitive to ABA (Leung et al., 2006). However, MPK6 is required for stomatal closure in response to the bacterial elicitor flagellin (Montillet et al., 2013; Su et al., 2017) and it is required, in redundance with MPK3, for stomatal immunity involving altered organic acid metabolism (Su et al., 2017). In line with the results on ABA response in mpk6 mutants, it was recently shown that ABA inactivated MPK6 (Mine et al., 2017). MPK6 is activated upon bacterial infection, during both pattern-triggered and effector-triggered immunity. Some pathogens, such as Pseudomonas syringae pv tomato (Pto) DC3000, counteract immunity by activating, among others, ABA signaling, and ABA has been known to negatively impact immunity against Pto DC3000 (de Torres Zabala et al., 2009). Mine et al. (2017) showed that ABA induced the expression of HAI phosphatases of the PP2C (protein phosphatase 2C)-type, which in turn inactivate MPK6 through dephosphorylation. Thus, one could speculate that active MPK6 shuts down ALMT4 in the ground state but that this kinase is inactivated in response to ABA, thus ceasing ALMT4 phosphorylation, causing Mal2− efflux. However, while our data show that MPK6 can phosphorylate ALMT4 in vitro, it cannot be excluded that another MPK is involved in this process, and it is a challenge for the future to pinpoint the exact kinases and phosphatases involved in ALMT4 regulation in vivo.
Conservation of ALMT4 S382 among ALMT Channels
Mutation of S382 has a striking effect on ALMT4 current activity and plant growth, and phosphorylation of this site seems to act in a deactivating manner. To investigate if this site is conserved among clade II ALMTs, we performed an amino acid sequence alignment (Supplemental Figure 8). Interestingly, S382 is conserved only in ALMT5, but here the serine is not followed by proline. Therefore, proline-directed phosphorylation of S382 in clade II ALMTs seems to be unique to ALMT4. This is in line with our observation that ALMT9, which does not feature a phosphorylatable serine in the vicinity of the identified motif, did not show discernible outward Mal2− currents and almt9 knockout mutants showed wild-type-like stomatal closure in response to ABA (De Angeli et al., 2013). Interestingly, however, the SP dipeptide motif was conserved in ALMT12. ALMT12, like ALMT4, is involved in stomatal closure and mediates anion efflux from the guard cell. A site-directed mutagenesis screen of ALMT12 electrophysiological properties had been conducted previously to identify putative regulatory C-terminal serines (Mumm et al., 2013). In this screen ALMT12 C-terminal serines were mutated to alanine with the reasoning that if they were phosphorylated, the S-to-A mutation would deactivate the channel. However, these mutations did not shut down channel activity. In light of our results, these serines and the ALMT12 S382A homolog might bear importance nonetheless but phosphorylation might deactivate the channel rather than activate it. It is therefore tempting to speculate that ALMT4 and ALMT12 act in concert during drought stress-induced stomatal closure and that this relies on dephosphorylation, rather than phosphorylation. Although, in guard cells, many ion channels and transporters are activated by phosphorylation, there are some exceptions such as the inward-rectifying K+-channel and the plasma membrane H+-ATPase (Fuglsang et al., 2007; Sato et al., 2009). Phosphorylation can also target proteins for degradation; however, our GFP-tagged phosphomimetic channel mutant construct yielded strong fluorescence at the vacuolar membrane, comparable to that of the wild type. Therefore, in light of our results, the localization and stability of ALMT channels involved in stomatal closure are not likely to be modulated by phosphorylation/dephosphorylation but the channel might be activated by dephosphorylation.
METHODS
Molecular Biology
ALMT4 (At1g25480) cDNA was amplified from Col-0 rosette leaf cDNA and cloned into the binary expression vector pART27 (Gleave, 1992) using primers including overhangs for XhoI and KpnI restriction sites at the 5′ and the 3′ end, respectively, as well as excluding the stop codon and including a 3′ seven-amino acid linker (sense, 5′-TCAGATCGCTCGAGATGGCTGACCAAACTAGAG-3′; antisense, 5′-CGGTACCAGATCCAGCAGCACCTCCAGATATCCGTGTCTCGCTAAC-3′). The pART27 construct contained GFP at the 3′ end and in frame with ALMT4 and was used for localization and patch-clamp experiments. ALMT4 complementation constructs were cloned using ligation-independent cloning (LIC) into pPLV22 (De Rybel et al., 2011), which contained a tandem-tomato fluorescent marker in frame at the 3′ end of the inserted complementation construct. For this, a fragment containing a 1837-bp sequence upstream of the ALMT4 start codon as well as the complete genomic DNA excluding the stop codon was amplified from Col-0 leaf genomic DNA. Primers with LIC-compatible overhangs were used (sense, 5′-TAGTTGGAATGGGTTCGAAGTTTCTCTCACAGGATAA-3′; antisense, 5′-TAGTTGGAATGGGTTCGAAGTTTCTCTCACAGGATAA-3′). To generate the ALMT4Pro:GUS construct, the 1837-bp promoter sequence upstream of the start codon of ALMT4 was cloned into pMDC163 (Curtis and Grossniklaus, 2003). The primers that were used to amplify the ALMT4 promoter region were as follows: sense, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTAGTTTCTCTCACAGGATAA-3′; and antisense, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGTTGCGTTGATTTCTTCTTCC-3′.
Site-directed mutagenesis was performed on the pART27 expression and pPLV22 complementation constructs according to Zhang et al. (2013) using primers for the S382A mutation (sense, 5′-AGAAGCTAGCTCCAGGGAACGTT-3′; antisense, 5′-TTCCCTGGAGCTAGCTTCTCCAT-3′) and for the S382E mutation (sense, 5′-AGAAGCTAGAGCCAGGGAACGTT-3′; antisense, 5′-TTCCCTGGCTCTAGCTTCTCCAT-3′), respectively.
For expression of GST-ALMT4WT in Escherichia coli, ALMT4 was amplified from cDNA by PCR using oligonucleotide primers ALMT4320 (sense, 5′-GGATCCCCGCTGGAGAAGACCTCCATAAAC-3′) and ALMT4548 (antisense, 5′-GAATTCCTATATCCGTGTCTCGCTAACTTG-3′). Next, the PCR-product was digested with BamHI and EcoRI and ligated in plasmid pGEX-5X-3 (GE Healthcare). To generate GST-ALMT4S382A, site-directed mutagenesis was performed according to the instructions of the QuickChangeII site-directed mutagenesis kit (Stratagene).
Knockout lines for ALMT4 (see section below) were genotyped using T-DNA left border primers (SALK, 5′-ATTTTGCCGATTTCGGAAC-3′; RIKEN, 5′-TACCTCGGGTTCGAAATCGAT-3′) and gene-specific primers for SALK_086236 (left primer, 5′-TTCGATCAAATCTTCCGGATTT-3′; right primer, 5′-CTTCAAGTGAATTGGCCACAC-3′), and for RATM13-0411-1_G/PST (left primer, 5′-GCATTCAGGGTGTTTTTGTTG-3′; right primer, 5′-AAACATCTTTTATGGACGGCC-3′). Genotyping was performed using the KAPA3G plant PCR kit (KAPA Biosystems) according to the manufacturer’s instructions.
Plant Growth, Transformation, and Selection
Arabidopsis thaliana plants were grown in controlled environment chambers (HIROSS; Klimatec) at short-day (8 h light/16 h dark, 60% relative humidity (RH), 21°C) or long-day (16 h light/8 h dark, 60% RH, 21°C) conditions for physiological experiments. Plants were grown under a white light spectrum generated by four fluorescent lamps, three Osram Biolux 36W/965 or 58W/965 lamps, and one Osram Fluora 36W/77 or 58W/77 lamp. For propagation, plants were grown in the greenhouse. Data from plant lines that were compared in any one experiment were always grown from seeds that were harvested together from plants grown at the same time, under the same conditions. Seeds were sown onto moist soil (Einheitserdewerke Patzer) and vernalized at 4°C for 48 h. After 10 to 14 d, seedlings were transplanted into individual pots.
Arabidopsis was transformed using the floral dip method (Clough and Bent, 1998) according to Zhang et al. (2006). Transformants with pPLV22 constructs carried the bar gene and seeds were sown onto soil in a lawn-like fashion and treated with BASTA (150 µM; Omya) three times every 2 to 3 d, 14 d after germination. Resistant seedlings were transferred into individual pots and propagated.
Two independent knockout lines of ALMT4 were ordered from NASC (The European Arabidopsis Stock Centre, Loughborough, UK): a T-DNA insertion line in the Columbia-0 (Col-0) background (SALK_086236) with the flanking sequence in the third exon (Alonso et al., 2003), and a Ds transposon mutant in the Nossen-0 (No-0) background (RATM13-0411-1_G/PST) with the flanking sequence in the first exon (Ito et al., 2002). From heterozygous parents, individuals of each line were genotyped as described above and a homozygous knockout as well as homozygous wild type outcrosses were selected for each line. Thus, in case of any undetected T-DNA or transposon insertions, the wild type will have a corresponding genetic background to the mutant.
Intracellular Localization
Nicotiana benthamiana were transformed with the ALMT4-GFP construct. Three to five days after transformation, protoplasts and isolated vacuoles were obtained as described in the “Patch-Clamp Experiments” section. Samples were imaged using a Leica TCS SP5 confocal microscope (Leica Microsystems). GFP fluorescence was excited using a 488-nm laser, with GFP and chlorophyll fluorescence being collected between 500 to 600 nm and 698 to 787 nm, respectively.
GUS Staining
ALMT4 promoter activity was analyzed in three independent transgenic lines expressing ALMT4Pro:GUS. Plants were grown on soil in short-day conditions, for 3 weeks. For GUS staining, whole plantlets were vacuum infiltrated and incubated for 3 h at room temperature with a staining solution containing 1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronide) in 50 mM sodium phosphate buffer, pH 7.15, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, and 0.05% Triton X-100. The tissue was washed three times in 50 mM sodium phosphate buffer, pH 7.15, and cleared overnight at 37°C with 70% ethanol.
Expression Analysis
For the expression analysis of ALMT4, plants were grown under long-day conditions, either on plates for mRNA level determination in seedlings or in sterile cylinders for all other tissue. The plates and cylinders contained 0.5× Murashige and Skoog medium, pH 5.6, and 1% phytoagar. Seedlings were 7 d old when harvested. In all other tissue, the expression levels were determined in 7-week-old plants. In addition, dry seeds were used for analysis of ALMT4 transcript abundance. Two biological replicates were performed, in each of which material of five plants was pooled.
For expression analysis of clade II ALMT members, almt4 (Col-0) mutants and the appropriate wild-type plants were grown in a hydroponic culture for four weeks at 16 h light/8 h dark, 60% RH, 21°C. The 0.5× Murashige and Skoog medium, pH 5.6, was used as nutrient solution. For each genotype, a whole rosette was harvested, and two separate replicate experiments were performed.
Total RNA was extracted using the SV Total RNA Isolation System (Promega) and reverse transcribed using the M-MLV reverse transcriptase (Promega) according to the manufacturer’s instructions. Transcript levels were determined by qRT-PCR using the 7500 Fast Real-Time PCR System (Applied Biosystems) and a SYBR Green PCR Master Mix (Applied Biosystems). Transcript levels were calculated with the standard curve method as described by Pfaffl (2001) and were normalized against the expression of the actin gene ACT2. We used primers for ACT2 (sense, 5′-TGGAATCCACGAGACAACCTA-3′; antisense, 5′-TTCTGTGAACGATTCCTGGAC-3′), ALMT9 (sense, 5′-ACCTAATCCGGATCTTAGTCGATACT-3′; antisense, 5′-TCACCGAATAAAGTGGAAAGCTCAG-3′), ALMT6 (sense, 5′-CCGTTGCATGATGCTAGTAAATAC-3′; antisense, 5′-TGATGATGGTTTGCTCGAAA-3′), ALMT5 (sense, 5′-GAGCCGCTTCAAGATGCTAGTA-3′; antisense, 5′-ATGACTTCTTCAAACTCTCCTGCT-3′), ALMT4 (sense, 5′-TGACGCTAGCAAGTATGCTGTT-3′; antisense, 5′-CTTCAAATTCTCCAGCTGAAACAGA-3′), and ALMT3 (sense, 5′-GGCTTATCCTACAGAGCAGAGGCT-3′; antisense, 5′-TCAGAGCCAAACCCATCTTC-3′). All reactions were performed in technical triplicates that were averaged to generate a biological replicate.
Stomatal Experiments
Plants were grown under short-day conditions for 6 weeks and then transferred to an LED-controlled environment chamber (Fytoscope FS130; Photon Systems Instruments) and adapted at 250 µmol m−2 s−1 white light (12 h light/12 h dark) for 3 d. Before the experiment, the plant genotypes were blinded to avoid experimenter bias. Epidermal peels were obtained 1 h before the start of the light period in green light from young but fully expanded rosette leaves. Peels from at least four different plants per genotype were incubated in opening buffer (10 mM MES-KOH, pH 6.1, 0.1 mM CaCl2, and 30 mM KCl) in custom-built imaging chambers. To avoid chamber effects, each incubation chamber contained peels from all genotypes compared in any one experiment. After imaging in the dark, peels were incubated in 250 µmol m−2 s−1 white light for 2 h and imaged again. If required, the opening buffer was supplemented with 20 µM ABA to investigate ABA-mediated inhibition of stomatal opening. To induce stomatal closure, peels were treated with 20 µM ABA or 1 mM CaCl2 in opening buffer, returned to the light, and imaged over a 2-h time course at indicated times. Three images were obtained from each peel at each time point using an inverted Eclipse TS 100 microscope fitted with a Plan Fluor 40×/0.75 objective and a Digital Sight DS-Fi1 camera (Nikon). Finally, recorded images were blind-analyzed using ImageJ (Abràmoff et al., 2004), and the apertures of 60 stomata were measured per leaf. Mean apertures of a single leaf were averaged over at least four leaves measured per genotype.
Gas Exchange Measurements
Plants were grown in short-day conditions for 4 to 5 weeks and measured using an LI-6400XT infrared gas analyzer fitted with an LI-6400-17 whole-plant Arabidopsis chamber (LI-COR Environmental). Plants were adapted to chamber conditions (22°C block temperature, 55% RH, 400 ppm CO2) in the dark for 1 h prior to measurement.
Drought Stress and RWC Measurements
Plants were germinated in short-day conditions for 14 d and transplanted into individual pots, filled with 75 g ± 1 g sieved soil, and soaked in water overnight. Genotypes were randomly distributed on growth trays and plants were grown for 2 weeks before watering was stopped and the soil sealed with black plastic wrap to avoid evaporation. After 6 weeks, plants showed obvious signs of wilting, and from each plant the 12 youngest but fully expanded rosette leaves were harvested and sealed in a preweighed plastic zipper bag before fresh weight determination. Harvesting occurred from all genotypes within 1 h of solar noon. Subsequently, 10 mL of 5 mM CaCl2 in double-distilled water was added to the zipper bags to allow leaves to rehydrate overnight in the dark. Rehydrated leaves were patted dry and their turgid weight was determined. They were then dried at 60°C for 72 h before dry weight determination and RWC was calculated according to Equation 1:
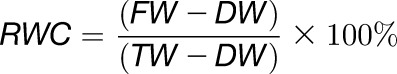 |
(1) |
where FW is the fresh weight, DW is the dry weight, and TW is the turgid weight.
Water Loss Experiments
Fully expanded rosette leaves were harvested, their fresh weight was recorded, and their petioles were incubated either in 20 µM ABA or in a control solution (double distilled water with 0.2% ethanol) in white light for 1 h. They were then left to air-dry for 7 h, their fresh weight was recorded and normalized to the initial value, and the difference was expressed as percentage of water loss.
Patch-Clamp Experiments
For patch-clamp analysis, we transiently transformed N. benthamiana with Agrobacterium tumefaciens harboring ALMT4-GFP, ALMT4 S382A-GFP, ALMT4 S382E-GFP, and ALMT9-GFP constructs using the agroinfiltration method (Sparkes et al., 2006). Experiments were performed 4 to 7 d after transformation, and expression of each fusion protein at the vacuolar membrane was confirmed before each patch (see below for microscope description).
Transformed N. benthamiana leaves were digested in digest solution (10 mM MES, pH 5.3 adjusted with KOH, 1 mM CaCl2, 500 mM sorbitol, 0.3% [w/v] Cellulase “Onozuka R-10” [Serva], 0.03% (w/v) Pectolyase Y-23 [Kyowa Chemical Industry]) for 35 min at 30°C. Protoplasts were washed twice in digest solution without enzymes and were allowed to settle by gravity for 10 to 20 min on ice. Protoplasts were kept on ice for 2 to 3 h, during which time aliquots were transferred onto a custom-built measuring chamber and perfused with standard Mal2− bath solution (100 mM malic acid, 1 mM CaCl2, pH adjusted with Bis-Tris propane to pH 7.5, osmotic content adjusted to 500 mosmol/ kg with sorbitol) in order to release vacuoles. Borosilicate thin-wall capillaries (30-0062) for patch pipettes were obtained from Harvard Apparatus. Capillaries were internally coated with Sigmacoat overnight at 60°C and patch pipettes were prepared using a P-30 Micropipette Puller (Sutter Instrument Company) to give a resistance of 5.5 MΩ ± 0.3 MΩ with standard Mal2−-based bath and pipette solutions. Finally, pipette tips were coated with silicone (Sylgard 184 Silicone Elastomer Kit; Dow Corning) to reduce pipette capacitance.
For measurements, pipettes were filled with standard pipette solution (112 mM malic acid, 10 mM HCl, 1 mM CaCl2, adjusted with Bis-Tris propane to pH 6, osmotic content adjusted to 550 mosmol/ kg with sorbitol). Pipettes were mounted onto a HEKA amplifier headstage connected to a HEKA EPC 10 USB amplifier (HEKA Elektronik Dr. Schulze). A 3 M KCl/0.1% agarose bridge served as a reference electrode. The measuring chamber and micromanipulator were fitted onto an Eclipse Ti inverted microscope fitted with Plan Fluor 20×/0.45 and 40×/0.6 objectives (Nikon). GFP fluorescence was excited using a CoolLED p-100 and emission collected using a F36-525 EGFP HC filter set (Semrock; AHF). Seals between vacuolar membrane and the pipette were created and, if they were larger than 2 GΩ, access was established by application of a 0.5 ms 700 mV pulse and parallel application of underpressure through suction. After break-in, an outside-out membrane patch was excised. The membrane patch was perfused with standard bath solutions, referred to as cytoplasmic solutions, and were exchanged for different bath solutions as required. When the Mal2− concentration was changed, the bath solution osmotic content was adjusted accordingly, and the ionic strength was adjusted with MES. For measurements with other substrates, the bath and pipette solutions were as described for standard solution but the charge carrying or activating ion was replaced at the indicated concentrations. In all experiments, the measured liquid junction potential was below 1 mV and therefore no correction was applied. Currents were recorded with a 1-kHz sampling interval, filtered at 0.3 kHz using the Patchmaster software (HEKA), and analyzed using Fitmaster (HEKA).
In Vitro Kinase Assay
Substrate-labeling reactions were performed essentially as described (Leissing et al., 2016). In brief, 100 ng activated recombinant wild-type or analog-sensitive GST-MPKs were mixed with 1 µg of GST-ALMT4WT or GST-ALMT4S382A in kinase reaction buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 mM DTT) including 1 mM N6-Bn-ATPγS (Biolog). Each 30-μL reaction was stopped by adding 20 mM EDTA after 1 h and the thiophosphorylated substrate was alkylated with 2.5 mM p-nitrobenzyl mesylate (Abcam) in 5% (v/v) DMSO for 2 h. The alkylation reaction was mixed with SDS loading buffer before samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane (Carl Roth). Immunodetection was performed using the rabbit monoclonal antibodies against the thiophosphate ester (α-TPE; Abcam) to detect thiophosphorylated kinase substrates. Rabbit α-GST (CST) served to check for equal gel loading. Antigen-antibody complexes were detected with horseradish peroxidase-coupled anti-rabbit secondary antibodies (CST) followed by chemiluminescence detection with Luminata Crescendo HRP substrate (Millipore). Using independent protein preparations, all substrate thiophosphorylation reactions were repeated at least twice with similar results.
Analysis of State Transition Rate Time Constants
Macroscopic voltage-dependent current kinetics can be derived from voltage-clamp experiments (Supplemental Figure 5) where each activating voltage step (e.g., from + 60 mV to –100mV) is followed by a deactivating voltage step (e.g., from –100 mV to + 60 mV). The activation kinetics of the current in response to the voltage step informs on the reaction rate of the closed to open channel state transition. Conversely, the kinetics of the ionic currents in response to the deactivating pulse reflects the reaction rate of open to closed channel state transition. During this deactivating pulse until the channels are closed they conduct an outward current since the electrochemical gradient has reversed together with the transmembrane voltage. The currents measured during a deactivating voltage step are called tail currents. Since activating and deactivating current kinetics reflect state transition reactions, they can be fitted by exponential functions (Supplemental Figure 5) to derive time constants (τ) that inform on the rates of the state transitions.
Boltzman Analysis
In I-V curve recordings, the current at the start of each deactivating voltage step (Itail0; Supplemental Figure 5) is proportional to Porel of the channel at the end of the previous activation pulse (Equation 2). We analyzed the Porel-to-voltage relationship using a Boltzman function (Equation 3) and derived gating parameters, the half-maximal activation voltage (V1/2), and the gating charge (zg). The rational of the analysis is illustrated in Supplemental Figure 5.
| (2) |
where Itail 0 is the tail current amplitude at the start of the deactivating voltage pulse, N is the number of channels contributing to macroscopic current, i is the single channel conductance, Porel is the relative open probability at the activation pulse, and Vm is the membrane voltage.
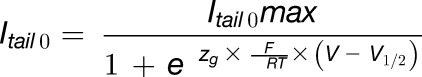 |
(3) |
where Itail 0 is the tail current amplitude at the start of the deactivating voltage pulse, V is the applied activation pulse voltage, V1/2 is the half-maximal activation voltage, zg is the gating charge, R is the universal gas constant, F is the Faraday constant, and T equaled 298.15K.
Statistics
The n numbers used for statistical analysis are given in the figures and tables. Data were tested for normal distribution. Normally distributed data were compared by Student’s t test or, if more than two groups were compared, by ANOVA and a post-hoc test. If normality test failed, data were tested by ANOVA on ranks. Tests were conducted using SigmaPlot (Systat Software). Values from statistical analyses can be found in Supplemental File 1.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemie, AppliChem, or Roth unless stated otherwise.
Accession Numbers
Sequence data from this article can be found in the GenBank data library under accession numbers At1g18420 (ALMT3), At1g25480 (ALMT4), At1g68600 (ALMT5), At2g17470 (ALMT6), At3g18440 (ALMT9), At4g17970 (ALMT12), and AT3G18780 (Actin2).
Supplemental Material
Supplemental Figure 1. ALMT4 mRNA expression analysis.
Supplemental Figure 2. Response of stomatal aperture to ABA (second experiment).
Supplemental Figure 3. Transpiration rate response over time of dark-adapted plants to light and dark.
Supplemental Figure 4. Current amplitude distribution.
Supplemental Figure 5. Illustration of Boltzman and tail current analysis.
Supplemental Figure 6. Stomatal response to ABA of S382 mutants (second independent line).
Supplemental Figure 7. Germination with and without ABA.
Supplemental Figure 8. Alignment of ALMT4 with clade II ALMT channels and ALMT12 from Arabidopsis.
Supplemental Table 1. ALMT4 S382A gating parameters in 100 and 10 mM Mal2−.
Supplemental File 1. Tables of results from statistical analyses.
Acknowledgments
We acknowledge Karl Huwiler and Christian Frey for help with plant maintenance and Daniel Bollier for technical support. We thank Dietmar Geiger (Würzburg) for the provision of OST1 constructs. C.E. was supported by the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant agreement GA-2010-267243–PLANT FELLOWS and the University of Zurich Forschungskredit. U.B. and A.D.A. were supported by the Swiss National Foundation (31003A_141090/1) and University of Zurich Forschungskredit. G.J.M.B. was supported by the Deutsche Forschungsgemeinschaft (BE4054/2-1). J.Z. was a fellow of the Chinese Scholarship Council.
AUTHOR CONTRIBUTIONS
C.E., A.D.A., and E.M. designed the research. C.E., U.B., N.V.H., G.J.M.B., and J.Z. performed research. C.E., U.B., and G.J.M.B. analyzed data. C.E., A.D.A., G.J.M.B., and E.M. wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Abràmoff, M.D., Magalhães, P.J., and Ram, S.J. (2004). Image processing with ImageJ. Biophotonics International 11: 36–41.
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Andrés Z., Pérez-Hormaeche J., Leidi E.O., Schlücking K., Steinhorst L., McLachlan D.H., Schumacher K., Hetherington A.M., Kudla J., Cubero B., Pardo J.M. (2014). Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 111: E1806–E1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo W.L., Nunes-Nesi A., Fernie A.R. (2011). Fumarate: Multiple functions of a simple metabolite. Phytochemistry 72: 838–843. [DOI] [PubMed] [Google Scholar]
- Baetz U., Eisenach C., Tohge T., Martinoia E., De Angeli A. (2016). Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol. 172: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia D.W., Yoder T.J., Reiter W.-D., Gibson S.I. (2000). Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751. [DOI] [PubMed] [Google Scholar]
- Chrispeels M.J., Crawford N.M., Schroeder J.I. (1999). Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell 11: 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., et al. (1992). Electrical measurements on endomembranes. Science 258: 873–874. [DOI] [PubMed] [Google Scholar]
- Blatt M.R. (1988). Potassium-dependent, bipolar gating of K+ channels in guard cells. J. Membr. Biol. 102: 235–246. [Google Scholar]
- Chen Z.-H., Hills A., Bätz U., Amtmann A., Lew V.L., Blatt M.R. (2012). Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 159: 1235–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M.J., Crawford N.M., Schroeder J.I. (1999). Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell 11: 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis, M.D., and Grossniklaus, U. (2003). A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed]
- De Angeli A., Zhang J., Meyer S., Martinoia E. (2013). AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 4: 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B., van den Berg W., Lokerse A., Liao C.-Y., van Mourik H., Möller B., Peris C.L., Weijers D. (2011). A versatile set of ligation-independent cloning vectors for functional studies in plants. Plant Physiol. 156: 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres Zabala M., Bennett M.H., Truman W.H., Grant M.R. (2009). Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 59: 375–386. [DOI] [PubMed] [Google Scholar]
- de Zelicourt A., Colcombet J., Hirt H. (2016). The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 21: 677–685. [DOI] [PubMed] [Google Scholar]
- Ding Z.J., Yan J.Y., Xu X.Y., Yu D.Q., Li G.X., Zhang S.Q., Zheng S.J. (2014). Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. Plant J. 79: 13–27. [DOI] [PubMed] [Google Scholar]
- Dreyer I., Gomez-Porras J.L., Riaño-Pachón D.M., Hedrich R., Geiger D. (2012). Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front. Plant Sci. 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C., De Angeli A. (2017). Ion transport at the vacuole during stomatal movements. Plant Physiol. 174: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang A.T., Guo Y., Cuin T.A., Qiu Q., Song C., Kristiansen K.A., Bych K., Schulz A., Shabala S., Schumaker K.S., Palmgren M.G., Zhu J.K. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago J., Daloso D de M., Figueroa C.M., Flexas J., Fernie A.R., Nikoloski Z. (2016). Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 171: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Becker D., Vosloh D., Gambale F., Palme K., Rehers M., Anschuetz U., Dreyer I., Kudla J., Hedrich R. (2009). Heteromeric AtKC1middle dotAKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 284: 21288–21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H.W. (1984). Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 75: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave A.P. (1992). A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20: 1203–1207. [DOI] [PubMed] [Google Scholar]
- Gobert A., Isayenkov S., Voelker C., Czempinski K., Maathuis F.J.M. (2007). The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA 104: 10726–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafke J.B., Hafke Y., Smith J.A.C., Lüttge U., Thiel G. (2003). Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J. 35: 116–128. [DOI] [PubMed] [Google Scholar]
- Heazlewood J.L., Durek P., Hummel J., Selbig J., Weckwerth W., Walther D., Schulze W.X. (2008). PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res. 36: D1015–D1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettenhausen C., Baldwin I.T., Wu J. (2012). Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol. 158: 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (2001). Ion Channels of Excitable Membranes, 3rd ed. (Sunderland, MA: Sinauer Associates). [Google Scholar]
- Hills A., Chen Z.-H., Amtmann A., Blatt M.R., Lew V.L. (2012). OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol. 159: 1026–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak H., et al. (2016). A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28: 2493–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D., Mumm P., Böhm J., Al-Rasheid K.A.S., Marten I., Geiger D., Hedrich R. (2013). Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 74: 372–382. [DOI] [PubMed] [Google Scholar]
- Ito T., Motohashi R., Kuromori T., Mizukado S., Sakurai T., Kanahara H., Seki M., Shinozaki K. (2002). A new resource of locally transposed Dissociation elements for screening gene-knockout lines in silico on the Arabidopsis genome. Plant Physiol. 129: 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson L., et al. (2016). Natural variation in Arabidopsis Cvi-0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biol. 14: e2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 106: 20520–20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I., Wulfetange K., Porée F., Michard E., Gajdanowicz P., Lacombe B., Sentenac H., Thibaud J.-B., Mueller-Roeber B., Blatt M.R., Dreyer I. (2006). External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 46: 269–281. [DOI] [PubMed] [Google Scholar]
- Jossier M., Kroniewicz L., Dalmas F., Le Thiec D., Ephritikhine G., Thomine S., Barbier-Brygoo H., Vavasseur A., Filleur S., Leonhardt N. (2010). The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 64: 563–576. [DOI] [PubMed] [Google Scholar]
- Kollist H., Nuhkat M., Roelfsema M.R.G. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kovermann P., Meyer S., Hörtensteiner S., Picco C., Scholz-Starke J., Ravera S., Lee Y., Martinoia E. (2007). The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 52: 1169–1180. [DOI] [PubMed] [Google Scholar]
- Lee M., Choi Y., Burla B., Kim Y.-Y., Jeon B., Maeshima M., Yoo J.-Y., Martinoia E., Lee Y. (2008). The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 10: 1217–1223. [DOI] [PubMed] [Google Scholar]
- Lee Y., Kim Y.J., Kim M.-H., Kwak J.M. (2016). MAPK cascades in guard cell signal transduction. Front. Plant Sci. 7: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissing F., Nomoto M., Bocola M., Schwaneberg U., Tada Y., Conrath U., Beckers G.J.M. (2016). Substrate thiophosphorylation by Arabidopsis mitogen-activated protein kinases. BMC Plant Biol. 16: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Orfanidi S., Chefdor F., Mészaros T., Bolte S., Mizoguchi T., Shinozaki K., Giraudat J., Bögre L. (2006). Antagonistic interaction between MAP kinase and protein phosphatase 2C in stress recovery. Plant Sci. 171: 596–606. [Google Scholar]
- Lovy-Wheeler A., Kunkel J.G., Allwood E.G., Hussey P.J., Hepler P.K. (2006). Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell 18: 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A. (2000). ABA activates multiple Ca(2+) fluxes in stomatal guard cells, triggering vacuolar K(+)(Rb(+)) release. Proc. Natl. Acad. Sci. USA 97: 12361–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A.C. (1995a). ABA-induced ion efflux in stomatal guard cells: multiple actions of ABA inside and outside the cell. Plant J. 7: 565–576. [Google Scholar]
- MacRobbie E.A.C. (1995b). Effects of ABA on 86Rb+ fluxes at plasmalemma and tonoplast of stomatal guard cells. Plant J. 7: 835–843. [Google Scholar]
- MacRobbie E.A.C. (2006). Osmotic effects on vacuolar ion release in guard cells. Proc. Natl. Acad. Sci. USA 103: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A.C., Lettau J. (1980). Ion content and aperture in “isolated” guard cells of Commelina communis L. J. Membr. Biol. 53: 199–205. [Google Scholar]
- Martinoia E., Rentsch D. (1994). Malate compartmentation-responses to a complex metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 447–467. [Google Scholar]
- Medeiros D.B., Martins S.C.V., Cavalcanti J.H.F., Daloso D.M., Martinoia E., Nunes-Nesi A., DaMatta F.M., Fernie A.R., Araújo W.L. (2016). Enhanced photosynthesis and growth in atquac1 knockout mutants are due to altered organic acid accumulation and an increase in both stomatal and mesophyll conductance. Plant Physiol. 170: 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K.A., Geiger D., Marten I., Martinoia E., Hedrich R. (2010). AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63: 1054–1062. [DOI] [PubMed] [Google Scholar]
- Meyer S., Scholz-Starke J., De Angeli A., Kovermann P., Burla B., Gambale F., Martinoia E. (2011). Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 67: 247–257. [DOI] [PubMed] [Google Scholar]
- Michard E., Simon A.A., Tavares B., Wudick M.M., Feijó J.A. (2017). Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 173: 91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine A., Berens M.L., Nobori T., Anver S., Fukumoto K., Winkelmüller T.M., Takeda A., Becker D., Tsuda K. (2017). Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity. Proc. Natl. Acad. Sci. USA 114: 7456–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda K., Araki H., Kuhara S., Ishigaki G., Akashi R., Negi J., Kojima M., Sakakibara H., Takahashi S., Hashimoto-Sugimoto M., Goto N., Iba K. (2016). Enhanced stomatal conductance by a spontaneous Arabidopsis tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiol. 170: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda K., Negi J., Iio A., Kusumi K., Kojima M., Hashimoto M., Sakakibara H., Iba K. (2011). Environmental regulation of stomatal response in the Arabidopsis Cvi-0 ecotype. Planta 234: 555–563. [DOI] [PubMed] [Google Scholar]
- Montillet J.-L., et al. (2013). An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm P., Imes D., Martinoia E., Al-Rasheid K.A.S., Geiger D., Marten I., Hedrich R. (2013). C-terminus-mediated voltage gating of Arabidopsis guard cell anion channel QUAC1. Mol. Plant 6: 1550–1563. [DOI] [PubMed] [Google Scholar]
- Munemasa S., Hauser F., Park J., Waadt R., Brandt B., Schroeder J.I. (2015). Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 28: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulareddy N., Panchal S., Melotto M. (2013). Guard cell purification and RNA isolation suitable for high-throughput transcriptional analysis of cell-type responses to biotic stresses. Mol. Plant Microbe Interact. 26: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema M.R.G., Hedrich R. (2005). In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 167: 665–691. [DOI] [PubMed] [Google Scholar]
- Roelfsema M.R.G., Hedrich R. (2010). Making sense out of Ca(2+) signals: their role in regulating stomatal movements. Plant Cell Environ. 33: 305–321. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Mori I.C., Furuichi T., Munemasa S., Toyooka K., Matsuoka K., Murata Y., Yamamoto Y. (2010). Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 51: 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., Hibi T., Taniguchi M., Miyake H., Goto D.B., Uozumi N. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 424: 439–448. [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Su J., Zhang M., Zhang L., Sun T., Liu Y., Lukowitz W., Xu J., Zhang S. (2017). Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 29: 526–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S., et al. (2015). Natural variation in stomatal responses to environmental changes among Arabidopsis thaliana ecotypes. PLoS One 10: e0117449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk C.A., Raschke K. (1978). Presence of chloride reduces malate production in epidermis during stomatal opening. Plant Physiol. 61: 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege S., De Angeli A., Droillard M.-J., Kroniewicz L., Merlot S., Cornu D., Gambale F., Martinoia E., Barbier-Brygoo H., Thomine S., Leonhardt N., Filleur S. (2014). Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 7: ra65. [DOI] [PubMed] [Google Scholar]
- Wingenter K., Trentmann O., Winschuh I., Hörmiller I.I., Heyer A.G., Reinders J., Schulz A., Geiger D., Hedrich R., Neuhaus H.E. (2011). A member of the mitogen-activated protein 3-kinase family is involved in the regulation of plant vacuolar glucose uptake. Plant J. 68: 890–900. [DOI] [PubMed] [Google Scholar]
- Xing Y., Jia W., Zhang J. (2008). AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 54: 440–451. [DOI] [PubMed] [Google Scholar]
- Zhang J., Baetz U., Krügel U., Martinoia E., De Angeli A. (2013). Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol. 163: 830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Martinoia E., De Angeli A. (2014). Cytosolic nucleotides block and regulate the Arabidopsis vacuolar anion channel AtALMT9. J. Biol. Chem. 289: 25581–25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S.S., Niu Q.W., Chua N.H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1: 641–646. [DOI] [PubMed] [Google Scholar]
- Zheng J. (2013). Domain-domain interactions in ion channels. J. Gen. Physiol. 142: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]