Abstract
Objectives
Benzodiazepines are used for treating anxiety, epilepsy, muscle spasm, alcohol withdrawal, palliation, insomnia, and sedation as they allosterically modulate γ-amino-butyric acid type A (GABAA) receptors. Despite widespread use, the importance and mechanism of their immune side-effects are poorly understood. Herein we sought to elucidate the impact and mechanism of benzodiazepine-induced susceptibility to infection at anxiolytic doses in mice.
Design
Animal randomized controlled trial.
Setting
Laboratory.
Subjects
Adult female C57BL/6 and BALB/c mice.
Interventions
The effect of a subsedative, anxiolytic dose of diazepam (2 mg kg−1 intraperitoneal) was investigated in a murine Streptococcus pneumoniae pneumonia model.
Measurement and Main Results
Mortality, bacterial and cytokine load, cell recruitment, and intracellular pH were measured. Diazepam treatment did not affect immune homeostasis in the lung. However, diazepam increased mortality and bacterial load from S. pneumoniae pneumonia. The increases in mortality and bacterial load were reversed by a GABAA antagonist, bicuculline, indicating dependence on GABAA receptor signaling. While cell recruitment was unaltered by diazepam, the cytokine response to infection was affected, suggesting that local responses to the pathogen were perturbed. Macrophage and monocytes expressed benzodiazepine sensitive (α1-γ2) GABAA receptors. Interestingly macrophage GABAA receptor expression was regulated by bacterial toll-like receptor agonists and cytokines indicating an endogenous role in the immune response. Functionally diazepam appeared to counteract the endogenous down-regulation of GABAA signaling during infection. Consistent with augmented GABAA signaling, diazepam provoked intracellular acidosis in macrophage, leading to impaired cytokine production, bacterial phagocytosis and killing. In contrast, selective benzodiazepines that do not target the α1 GABAA subunit did not affect macrophage function ex vivo or increase susceptibility to pneumonia in vivo.
Conclusions
Our data highlight the regulation of macrophage function by GABAA receptor signaling and the potential harm of benzodiazepine exposure during pneumonia. Therapeutically, selective drugs may improve the safety profile of benzodiazepines.
Keywords: γ-amino-butyric acid type A receptor, benzodiazepine, pneumonia
Benzodiazepines are World Health Organization essential medicines, used for treating anxiety, epilepsy, muscle spasm, alcohol withdrawal, palliation, insomnia, and sedation. In the United States and the United Kingdom, approximately 2% of the general population has taken benzodiazepines for 12 months or more (1), and their use is even more prevalent in elderly patients (up to 10%) (2). Furthermore, they are the most common sedative used in critically ill patients (3). To produce their clinical effects, benzodiazepines allosterically modulate γ-amino-butyric acid type A (GABAA) receptors sensitizing them to GABA (4–8). GABAA receptors are pentameric, ligand-gated channels that conduct chloride and bicarbonate anions (9). The most prevalent GABAA receptor in the brain is the benzodiazepine-sensitive α1β2γ2 receptor with the benzodiazepine recognition site formed by the α-γ2 subunit interface (4, 8).
GABAA receptors are also expressed on immune cells. Macrophage and monocytes express α-subunits (10, 11) and β-subunits (10, 12, 13) but studies of lymphocytes are contradictory (10, 12, 14). Currently, it is unclear whether any immune cell expresses the γ2 subunit and thus whether their GABAA receptors are benzodiazepine sensitive. Macrophages synthesize GABA (12, 15), upregulate the expression of the synthetic enzyme of GABA, glutamic acid decarboxylase, on immune challenge (12), and serum GABA becomes detectable in experimental sepsis (16). Therefore, it is conceivable that GABA signaling acts to regulate immune responses. Consistent with an immune regulatory role, preliminary evidence suggests that GABAA receptor expression is also influenced by inflammatory stimuli as a form of biological feedback (11, 17). Therefore we investigated the immune-regulated expression of the GABAAγ2 subunit on immune cells.
Activation of GABAA receptors leads to a measurable electrophysiological response (12) and suppression of cytokine release from macrophage ex vivo (11, 12) and improves experimental autoimmune encephalitis in vivo (12), implying immunomodulatory activity. Of course, perturbing appropriate inflammation can be detrimental, and in human sepsis this has been correlated with increased mortality (3, 18). Consistent with this premise, avoiding benzodiazepine sedation in septic critically ill patients reduced mortality by 70% (19, 20). In the Safety and Efficacy of Dexmedetomidine Compared with Midazolam study, sedation with midazolam appeared to double the risk of secondary infections in critically ill patients compared with sedation with dexmedetomidine (21). However, a more recent study did not observe the difference in infection rates (22). Several studies have shown that benzodiazepines increase mortality from infection including those from Klebisella pneumoniae (23), Mycobacterium bovis (24), Salmonella typhimurium (25), and Vaccinia (26). In particular, the prototypical benzodiazepine, diazepam, increases mortality from intraperitoneal K. pneumonia at subsedative doses (23). However, the mechanism of this effect and the relevance to clinical routes of infection, such as pneumonia, are unknown. Our objective was to clarify the mechanism of this effect using a pneumonia infection model with exposure to anxiolytic doses of diazepam. Indeed, we have recently observed in a cohort of 4,964 patients that benzodiazepine administration was associated with increased 30-day (adjusted hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.06–1.39) and long-term mortality (adjusted HR, 1.32; 95% CI, 1.19–1.47) from community-acquired pneumonia (27). Using a case-control design, comparing the 4,964 cases with 29,697 controls, we have also recently shown that benzodiazepines increase the odds of developing pneumonia (adjusted odds ratio, 1.54; 95% CI, 1.42–1.67) (27). In these analyses, we adjusted for age, social deprivation, previous pneumonia, pulmonary disease, ischemic heart disease or other comorbidity, psychiatric disease, smoking, and alcohol use. Thus, despite accounting for multiple confounders a significant signal was still noted from benzodiazepine exposure. Further, understanding of the biological plausibility for this effect is still required, hence our interest in further preclinical mechanistic work.
As a class, GABAA modulators including benzodiazepines, appear to exert similar effects on immune responses ex vivo (3). However, dependence on GABAA receptor signaling ex vivo or in vivo has not been tested using pharmacological antagonists. We hypothesized that the mechanism of benzodiazepine-induced susceptibility to pneumonia would involve GABAA receptor activation.
Materials and Methods
Drugs
For the in vivo work, clinical grade diazepam (Hameln Pharmaceuticals, Gloucester, UK) was diluted in phosphate buffered saline (PBS); the vehicle control was 4% ethanol in PBS. Diazepam was given at 2 mg kg−1 as this dose provides anxiolysis but not sedation in mice (6, 7, 28, 29); 2 mg kg−1 is a large dose of diazepam for humans, but in mice this produces the behavioral response we were attempting to model: anxiolysis but not sedation in mice (6, 7, 28, 29); 2 mg kg−1 is a large dose of diazepam for humans, but in mice this produces the behavioral response we were attempting to model: anxiolysis at subsedative levels. The α2/3 GABAA subunit selective benzodiazepine NS11394 was obtained from Neurosearch, Ballerup, Denmark. NS11394 was diluted in 4% ethanol in PBS similar to diazepam and given at 2 mg kg−1 as it has comparable anxiolytic efficacy (28). The GABAA antagonist, bicuculline methiodide (Tocris, Bristol, UK), was diluted in PBS and also given at 2 mg kg−1 based on previous work (30). All drugs were given twice daily in a volume of 200 μL by intraperitoneal injection. Additionally, for the ex vivo work diazepam, the α2/3 GABAA subunit selective benzodiazepine, L-818-437, and the nonselective non-benzodiazepine GABAA agonists, GABA and muscimol, were obtained from Tocris.
Animal Models
All protocols were approved by the Home Office (UK), conforming to the United Kingdom Animals (Scientific Procedures) Act of 1986. All animals used were C57BL/6 mice (weighing 17–19 g) unless stated. In the single infection animal model, mice were infected intranasally with 1 × 106 colony forming units (CFUs) of Streptococcus pneumoniae (serotype 2), strain D39 (NCTC 7466, London, UK). In the coinfection model, mice were infected with 50 hemagglutination units of A/X31 (H3N2) influenza seven days before 1 × 104 (lethal) or 2 × 103 (sublethal) CFUs of S. pneumoniae. Allergic pulmonary disease was induced by intranasal 15 μg house dust mite extract (Dermatophagoides pteronyssinus, Greer Laboratories, Lenoir, NC) in BALB/c mice on alternate days for 3 weeks. Survival was assessed according to Home Office rules limiting the severity of animal illness. If three or more of the following criteria were achieved, the animal was culled: piloerection, increased docility or aggression, immobility, hunched posture, sunken eyes, respiratory distress, dehydration, and loss of more than 25% of body weight.
Recovery of Samples
Mice were sacrificed by administration of pentobarbitone and exsanguination. In separate cohorts, bronchoalveolar lavage (BAL) fluid was obtained by inflation of the lung four times with 1.5 mL of 5 mM EDTA in Hank's Balanced Salt Solution (HBSS) via an intratracheal cannula; 100 μL was used for bacterial CFU counts, the remainder was centrifuged, and the supernatant was stored at −80°C. Lung tissue was disrupted through a 100-μM sieve (BD Labware, Franklin Lakes, NJ) with 100 μL then set aside for bacterial CFU counts. The remainder was then spun and red blood cells lysed by adding ammonium-chloride-potassium buffer (0.15 M ammonium chloride, 1 M potassium hydrogen carbonate and 0.01 mM EDTA, pH 7.2) and washed with RPMI containing 10% fetal calf serum. BAL and lung cell viability was assessed by trypan blue exclusion and cells resuspended in RPMI containing 10% fetal calf serum and 2 mM l-glutamine at 1 × 106 cells mL−1.
Bacterial Load
CFU counts were used to assess bacterial load in the airway (BAL) and lung and for the ex vivo killing assay. Serial dilutions were made in PBS and plated onto Columbia agar supplemented with 5% defibrinated horse blood and counted after incubation at 37°C in 5% carbon dioxide for 16 hours.
Cell Staining
Cells were identified by antibody purchased from BD Pharmingen, (Heidelberg, Germany). For receptor staining of mouse cells, cells were selected by forward/side scatter and the following surface markers: alveolar macrophage (CD11c+ F480+ CD11b+), splenic and peritoneal macrophage (F480+ CD11b+), monocyte (CD11c− CD11b+ F480− Ly6G−), neutrophil (CD11c− CD11b+ Ly6G+), CD4 positive lymphocyte (CD4+ CD3+ CD8−), CD8 positive lymphocyte (CD4− CD3+ CD8+) and B cell (CD19+ CD3−). The antibodies for GABAA subunits (α1–4, β2, γ2, and δ) came from Abcam (Cambridge, UK). The glutamic acid decarboxylase 65/67 antibody came from Millipore (Billerica, MA), and the secondary allophycocyanin-conjugated antibody was from ebioscience (Hatfield, UK). The isotype antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Positive staining was defined as within 1% of the upper limit of the isotype control. Cells were washed in phosphate-buffered azide and data were acquired on a BD FACS LSR II; 30,000 lymphocyte or myeloid events were analyzed with the FlowJo analysis program.
Interleukin-6 and Tumor Necrosis Factor-alpha Enzyme-Linked Immunosorbant Assay
Cytokine quantification was performed by enzyme-linked immunosorbant assay or luminex according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Ex Vivo Toll-Like Receptor and Cytokine Stimulation
Alveolar macrophages were treated for 16 hours with different Toll-like receptor (TLR) agonists (Invivogen, San Diego, CA) or cytokines (IL-4, 100 ng mL−1; IL-10, 100 ng mL−1; IL-13, 100 ng mL−1; IL-33, 30 ng mL−1; IFN-ϒ, 100 ng mL−1; TNF-α, 100 ng mL−1; IL-6, 100 ng mL−1; IL-1β, 1 ng mL−1). Lipopolysaccharide (LPS) stimulations were also done at 100 ng mL−1. Cells were then harvested and stained as previously described. For the ex vivo experiments, diazepam and L838-417 were obtained from Tocris.
Ex Vivo Phagocytosis and Bacterial Killing Assays
Alveolar macrophage phagocytosis was assessed by incubation for 1 hour with phrodo-labeled Staphylococcus aureus (Invitrogen, Paisely, UK). Bacterial killing by alveolar macrophage was assessed following pretreatment of cells for 16 hours with IFN-ϒ (100 ng mL−1) at 37°C. S. pneumoniae were preopsonized with mouse serum at 37°C for 20 minutes and then mixed in a 1:1 ratio with cells. After incubation for 60 minutes at 37°C, cells were lysed, and the supernatant was plated in serial dilutions for bacterial load. Neutrophil assays were conducted with human neutrophils. S. aureus strains (MM85T, Oxford strain, NCTC 6571/ATCC 8144, TCS Bioscience Buckingham, UK) were preopsonized with human IgG for 20 minutes and then mixed with neutrophils at a 1:1 ratio for 20 minutes (at 37°C). Cells were then lysed, plated, and counted after incubation overnight. Neutrophil respiratory burst was measured by Amplex assay (Invitrogen) following stimulation with Phorbol 12-myristate 13-acetate. Alveolar macrophage pH was assessed using a 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) probe (Invitrogen), in 10 mM HEPES, 8 mM MES, 140 mM NaCL, 5 mM KCL, and 5 mM glucose, measured on an Omega Fluorostar plate reader (Buckingham, UK).
Statistical Analysis
Data are presented as mean ± sd in the text. Data in the figures are presented as mean ± sem. Survival data were analyzed by log-rank test. Bacterial load were analyzed by Kruskal-Wallis and post hoc Dunn’s multiple comparison test for multiple comparisons or Mann-Whitney when there were only two groups to compare. Cell counts, cytokine levels, and ex vivo assays were analyzed by Mann-Whitney U test or analysis of variance and post-hoc Tukey test. Significance was set at a p value of less than 0.05.
Results
We hypothesized that that diazepam would increase susceptibility to pneumonia through inappropriate augmentation of GABAA immune regulation, and this was tested by investigating the immune effects of an anxiolytic, subsedative dose of diazepam (6) that has previously been shown to increase susceptibility to infection in mice (23). We initially established that pretreatment of C57BL/6 mice for seven days with twice daily diazepam did not affect immune homeostasis in the healthy lung. Cell populations in the lung and surface receptors on the resident immune cells and alveolar macrophages were unchanged (Supplementary Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCM/A604).
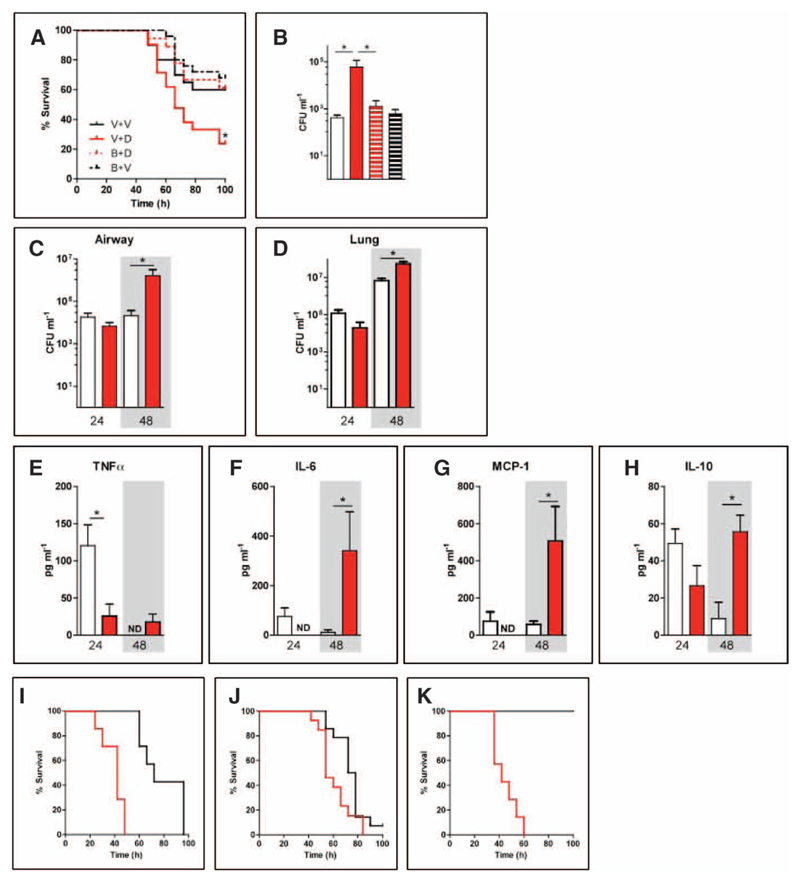
When given on day 7 of the drug-treatment protocol, intranasal S. pneumonia (1 × 106 CFUs) produced an aggressive pneumonia in C57BL/6 mice. Despite the lack of changes to the baseline state, treatment with diazepam increased mortality from S. pneumoniae pneumonia (HR, 2.52 [1.08–5.90]; p = 0.033)—an effect attenuated by the GABAA receptor antagonist, bicuculline, indicating a critical role of GABAA receptor activation (Fig. 1A). Forty-eight hours into the infection, diazepam also increased airway bacterial counts. This was again dependent on GABAA receptor activation (Fig. 1B). To ascertain whether diazepam exerted effects before 48 hours, we harvested animals that were treated with vehicle or diazepam at 24 and 48 hours. Unlike at 48 hours into the infection, bacterial counts were unaffected by diazepam at 24 hours (Fig. 1, C and D). Diazepam also did not affect cell recruitment at either 24 or 48 hours (Supplementary Figs. S2 and S3, Supplemental Digital Content 1, http://links.lww.com/CCM/A604), suggesting that it perturbs local responses to the infection. Consistent with this, diazepam inhibited the TNF-α, IL-6, and MCP-1 airway inflammatory response at 24 hours postinfection (Fig. 1E–h). This was followed by an exacerbated cytokine response at 48 hours with high levels of proinflammatory and anti-inflammatory mediators (Fig. 1e–h; Supplementary Fig. S4, Supplemental Digital Content 1, http://links.lww.com/CCM/A604), likely attributable to the increased bacterial load (Fig. 1, c and D).
Figure 1. Benzodiazepines increase mortality from pneumonia in C57BL/6 mice through GABAA signaling.
A, Mice were treated with the vehicle for bicuculline (phosphate-buffered saline [PBS]) and then the vehicle for diazepam (V+V; black solid line) or diazepam (2 mg kg-1) (V+D; red solid line). Two other groups received bicuculline followed by diazepam (B+D; red dotted line) or the vehicle for diazepam (4% ethanol in PBS; black dotted line) (B+V). Survival (n = 20 per group) after intranasal Streptococcus pneumoniae was assessed. B, Bacterial load in the airway at 48 hr (n = 8 per group) was also assessed. The groups are vehicle for bicuculline (PBS) and then the vehicle for diazepam (white bar) or diazepam (red bar); two other groups received bicuculline followed by diazepam (red striped bar) or the vehicle for diazepam (black striped bar).In a separate experiment, bacterial load in the airway (C) and lung (D) and airway lavage, tumor necrosis factor (TNF)-α (E), interleukin (IL)-6 (F), monocyte chemoattractant protein (MCP)-1 (G), and IL-10 (H) were assessed at 24 and 48 hr after S. pneumoniae infection (n = 5 mice per group). The two treatment groups for these experiments (C–H) were the vehicle for diazepam (white bar) or diazepam (2 mg kg−1; red bar). Survival was also analyzed in mice given S. pneumoniae 7 days after influenza (H3N2 X31/A) infection. I, Diazepam was administered prior to and during bacterial superinfection of influenza (n = 8 per group; red = diazepam; black = vehicle). In separate experiments, diazepam was administered twice daily following (J) 1 × 104 colony-forming units (n = 13 per group) or (K) 2 × 103 CFU (n = 7 per group) bacterial superinfection of influenza. *p < 0.05. Graphs show mean ± sem. ND = not detected..
Bacterial superinfection contributed to 26% to 38% of mortality in the recent H1N1 influenza pandemic (31) and occurred in 20% to 24% of critically ill influenza-infected patients (31) where benzodiazepines are typically administered (19). Therefore, we tested whether diazepam still increased mortality when animals were made vulnerable to S. pneumoniae by influenza infection seven days earlier. Diazepam treatment throughout the influenza and bacterial superinfection phases increased the risk of death HR, 22.4 [4.3–117.5]; p = 0.001; Fig. 1I). Furthermore diazepam, even when started after the bacterial superinfection of influenza, increased mortality rate from 1 × 104 CFU(HR, 3.1 [1.16–8.35]; p = 0.020; Fig. 1j) and 2 × 103 CFU (HR, 34.8 [6.32–191.7]; p = 0.001; Fig. 1k) of S. pneumoniae.
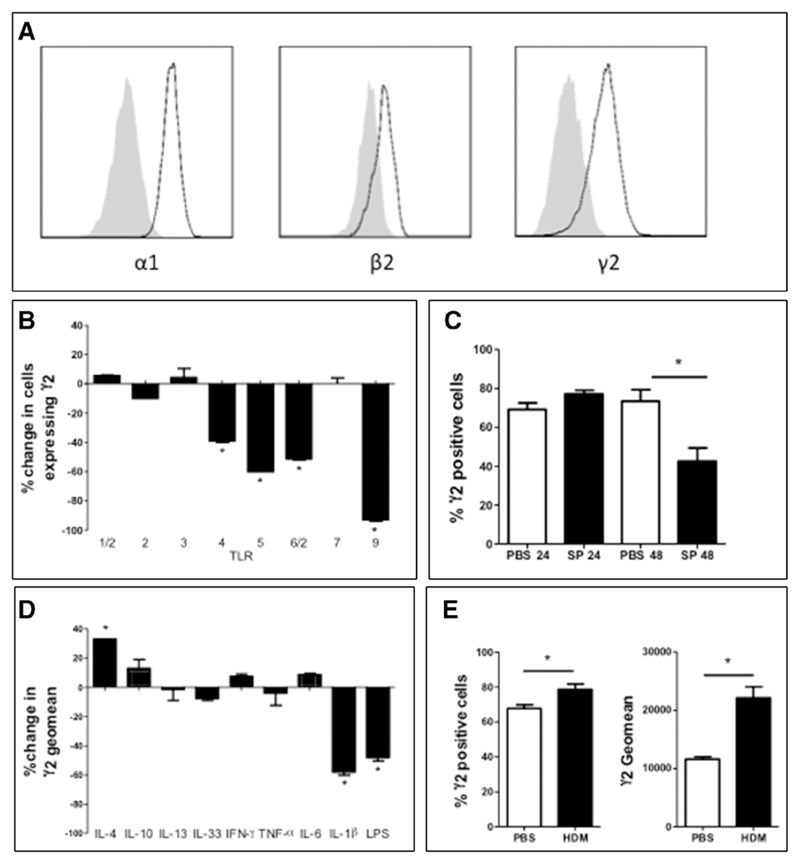
Next we profiled GABAA receptor expression on immune cells to identify which local responses may be affected by diazepam. We initially screened immune cells from naïve C57BL/6 mice for γ2 expression (as a marker of benzodiazepine-sensitive GABAA receptors). Alveolar macrophages abundantly expressed γ2 (64% ± 17% of cells) with evidence of α1β2γ2 receptors (Fig. 2A) (Table 1). Lower levels of γ2 were present on splenic macrophages (36% ± 5%; p < 0.05) and monocytes (20% ± 7%; p < 0.05) with very low levels on CD4 cells (6% ± 1%; p < 0.05). γ2 subunits were not detected on other cell types. As benzodiazepines that are selective for specific α subunits are under development (7, 28), we investigated the expression of other α subunits. While α1 subunits were highly expressed on macrophages and monocytes, α2–4 subunits were rarely detected or absent (Table 1). Greater than 80% of all immune cells expressed the synthetic enzyme for GABA (Table 1), consistent with data from macrophages (15).
Figure 2. Alveolar macrophage express benzodiazepine-sensitive, α1-γ2 subunit containing GABAA receptors.
A, Alveolar macrophage, purified by lavage of C57BL/6 mice, were assessed for α1, β2, and γ2 GABAA subunit expression by flow cytometry. Gray shading = isotype. Black outline = GABAA subunit. Alveolar macrophage from C57BL/6 mice were incubated with (B) toll-like receptor (TLR) agonists or (D) cytokines (and lipopolysaccharide [LPS]) and γ2 subunit expression determined by flow cytometry (n = 3 wells of 100,000 cells/treatment) at 16 hr. C, Mice were given Streptococcus pneumoniae (SP) or phosphate-buffered saline (PBS) intranasally, alveolar macrophage γ2 subunit expression was assessed at 24 and 48 hr (n = 5C57BL/6 mice/group). E, Alveolar macrophage γ2 subunit expression in response to 3 wk of house dust mite (HDM) treatment (n = 5 BALB/c mice/group) assessed by flow cytometry. *p < 0.05 versus control or vehicle. Graphs show mean ± sem. IL = interleukin; TNF-α = tumor necrosis factor-α; IFN = interferon.
Table 1. Percentage Expression of GABAA Subunits and the Synthetic Enzyme for GABA, Glutamic Acid Decarboxylase, on Cells From C57BL/6 Mice.
| GABAA Signaling Proteins | ||||||||
|---|---|---|---|---|---|---|---|---|
| α1 | α2 | α3 | α4 | β2 | γ2 | σ | Glutamic Acid Decarboxylase | |
| Alveolar macrophage | 99 (1) | 4 (1) | 4 (2) | ND | 42 (19) | 71 (11) | ND | 93 (5) |
| Splenic macrophage | 37 (7)a | 5 (2) | 7 (3) | NT | NT | 36 (5)a | NT | NT |
| Peritoneal macrophage | 21 (8)a | NT | NT | NT | NT | 13 (4)a | NT | NT |
| Monocyte | 34 (12)a | 6 (2) | 8 (3) | ND | NT | 20 (7)a | ND | 90 (5) |
| Neutrophil | 5 (1)a | ND | ND | ND | NT | ND | ND | 94 (3) |
| CD4 | 10 (2)a | NT | NT | ND | NT | 6 (1)a | ND | 93 (3) |
| CD8 | 4 (1)a | NT | NT | ND | NT | ND | ND | 91 (1) |
| B Cell | 8 (3)a | NT | NT | ND | NT | ND | ND | 84 (4) |
| NK cell | 9 (2)a | NT | NT | ND | NT | ND | ND | 89 (3) |
ND = not detected; NT = not tested.
Data are presented as mean (sd).
p < 0.05 versus alveolar macrophage.
While alveolar and splenic macrophages abundantly expressed the γ2 subunit, peritoneal macrophages did not (Table 1). Given this evidence for GABAA receptor regulation, we investigated what factors modulated alveolar macrophage γ2 subunit expression. Ex vivo stimulation with bacterial TLRs (TLR-4, -5, -6/2, and -9), but not viral TLRs (TLR-3 and -7), reduced γ2 subunit expression (Fig. 2B). In vivo, 48 hours following S. pneumoniae infection, γ2 subunit expression decreased by 46% (Fig. 2C). Endogenous reduction of GABAA receptor expression during infection explains why bicuculline did not act antithetically to diazepam (Fig. 1, A and B) and protect against S. pneumoniae. Indeed bicuculline alone exerted no effect on the response to infection, perhaps due to the endogenous (physiological) antagonism of GABAA receptor signaling induced by receptor down-regulation. This could be expected to reduce the effect of the pharmacological antagonist. Nonetheless, bicuculline was able to attenuate the effect of diazepam confirming that the benzodiazepine augmented GABAA receptor activity was responsible for diazepam’s immune effects. We also investigated the effects of cytokine signaling on alveolar macrophage GABAA γ2 subunit expression ex vivo. IL-1β reduced, while IL-4 increased, expression (Fig. 2D). In vivo, treatment of BALB/c mice with the allergen house dust mite, promoting a Th2 cytokine environment (including IL-4), also caused an increase in γ2 expression (Fig. 2E).
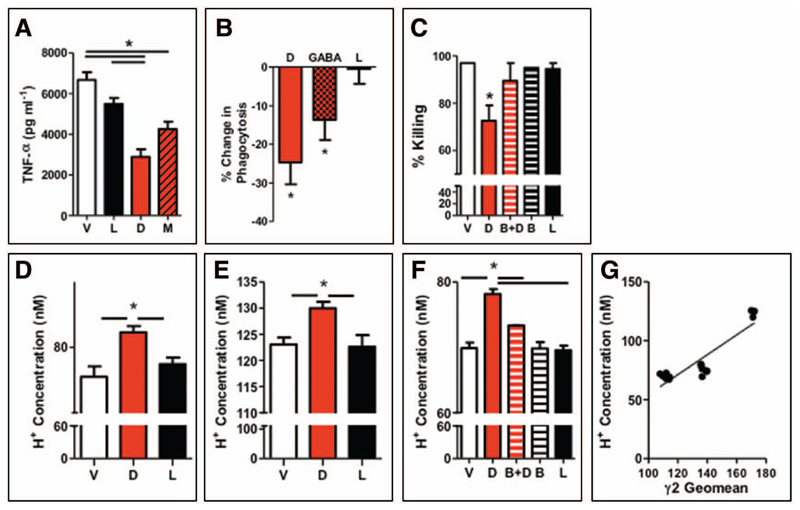
To further enhance the plausibility of our findings, we investigated the ex vivo effects of augmenting GABAA receptor signaling. GABAA receptor activation inhibited LPS-induced alveolar macrophage TNF-α (Fig. 3A) and IL-6 release (Supplementary Fig. S5, Supplemental Digital Content 1, http://links.lww.com/CCM/A604). To functionally confirm our finding that alveolar macrophages express α1 containing GABAA receptors, we used a benzodiazepine that is selective for α2/3, but lacks activity at α1, containing GABAA receptors (7): L-838-417. L-838-417 did not affect cytokine release, suggesting a critical role for α1 containing GABAA receptors (Fig. 3A). GABAA receptor activation, with diazepam or GABA, also reduced phagocytosis of phrodo-labeled S. aureus (Fig. 3B). Again L-838-417 lacked effect. IFN-γ-stimulated killing of S. pneumoniae was inhibited by diazepam by 28%, a defect antagonized by bicuculline and not mimicked by L-838-417 (Fig. 3C). Consistent with a lack of GABAA receptor expression (10), GABAergic drugs did not modify neutrophil bacterial killing or respiratory burst ex vivo (Supplementary Fig. S6, Supplemental Digital Content 1, http://links.lww.com/CCM/A604).
Figure 3. Only benzodiazepines that activate α1 subunit containing GABAA receptors affect macrophage function.
A, Alveolar macrophages, purified from C57BL/6 mice, were incubated with vehicle (V), diazepam (D) (10 μM), muscimol (M) (100 μM) or L-838-417 (L) (567nM; an equivalent Ki multiple to diazepam6), and lipopolysaccharide (LPS) (100 ng ml−1), tumor necrosis factor (TNF)-α release was measured by ELISA (n = 3–7 per group). B, Change in phagocytosis of phrodo-labeled Staphylococcus aureus (n = 3–6 per group) assessed at 1 hr with diazepam, GABA (100 μM), or L. C, Alveolar macrophages were incubated with V, D, bicuculline (B), and B and D (B+D), or L, and killing of Streptococcus pneumoniae was assessed by counting bacterial colonies (n = 3 wells/group). Cytoplasmic pH was measured by BCECF probe in untreated (D), interleukin-4–treated (E), and LPS-treated alveolar macrophage (F) (n = 10 wells per treatment). G, H+ concentration correlates with expression of γ2 subunit expression as assessed by geomean.
Activation of neuronal GABAA receptors leads to cytoplasmic acidification through bicarbonate efflux (9). As acidification of the cytoplasm is known to reduce TNF-α production (32) and bacterial phagocytosis and killing (33) by alveolar macrophage, we hypothesized that GABAA receptor stimulation perturbed immune responses by altering intracellular pH. Augmenting GABAA signaling with diazepam (or another GABAA agonist muscimol [100 μM, unpublished observations]) increased the intracellular H+ concentration by 10% (baseline pH 7.13 ± 0.03 vs. diazepam pH 7.08 ± 0.01; p = 0.004; Fig. 3D). α1 containing GABAA receptors were again implicated as L-838-417 and did not alter pH (pH 7.12 ± 0.02; Fig. 3D). Increasing GABAA receptor expression with IL-4 pretreatment increased H+ concentration by 167% (pH 6.91 ± 0.01; p < 0.001 vs. baseline pH; Fig. 3E); this was further augmented by diazepam (Fig. 3E). In contrast, reducing GABAA receptor expression with LPS led to cytoplasmic alkalosis (pH 7.16 ± 0.01; p < 0.001 vs. baseline; Fig. 3F). Despite reduced receptor expression by LPS, diazepam still increased the cytoplasmic H+ concentration (pH 7.11 ± 0.01; p < 0.001), an effect antagonized by bicuculline (pH 7.13 ± 0.01; p < 0.001; Fig. 3F). In contrast, L-838-417 (pH 7.17 ± 0.01) and bicuculline (pH 7.16 + 0.02) exerted no effect alone. Furthermore, H+ concentration correlated with changes in GABAA subunit expression (r2 = 0.80; p < 0.001; Fig. 3G). To summarize, augmenting GABAA receptor activity acidifies the cytoplasm of alveolar macrophage through α1-γ2 containing GABAA receptors.
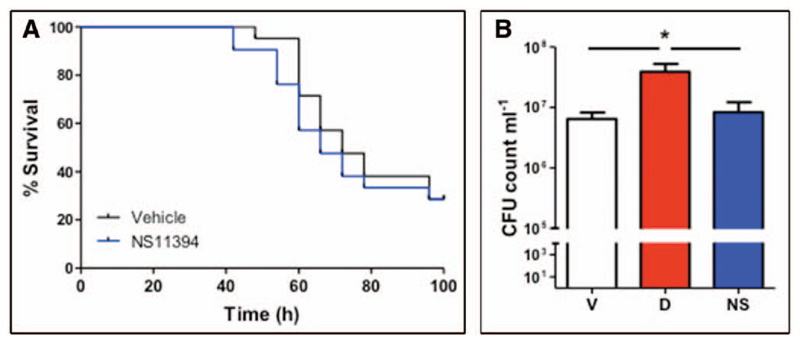
To show whether α1 subunit containing GABAA receptors were responsible for the benzodiazepine-induced increase in susceptibility to infection, we compared diazepam and a subunit selective benzodiazepine in vivo. We did not choose to use L-838-417 in vivo due to its extremely short half-life (7). Rather, we selected NS11394, as its pharmacokinetics are compatible with twice daily dosing in mice (28), and it produces anxiolysis at comparable doses to diazepam (6, 28). This is possible as anxiolysis is mediated by α2/3-, not α1-, containing GABAA receptors (5, 6, 34). Unlike diazepam, NS11394 did not increase mortality from infection (HR, 1.23 [0.56–2.67]; p = 0.6; Fig. 4A) nor were bacterial counts higher than vehicle-treated controls (Fig. 4B); again cell recruitment was unaffected (data not shown).
Figure 4. A selective benzodiazepine that does not activate α1 subunit containing GABAA receptors does not affect outcomes from pneumonia.
A, Mice were treated with NS11394 (NS) (2 mg·kg−1; blue line) or vehicle (black line) for seven days prior to and during Streptococcus pneumoniae infection and survival assessed (n = 20/group). B, Bacterial load in lung homogenates at 48 hr was monitored comparing treatments of vehicle (V) (black bar), NS11394 (NS) 2 mg·kg−1; blue bar) or diazepam (D) (2 mg·kg−1; red bar) (n = 16 per group). *p < 0.05 versus control unless otherwise specified. Graphs show mean ± sem.
Discussion
In summary, we provide evidence that benzodiazepines increase mortality from pneumonia in mice. Mechanistically, our results suggest that benzodiazepines increase susceptibility to S. pneumoniae by augmenting GABAA receptor activity. These in-vivo changes were subsequently correlated with ex vivo changes in macrophage function. Indeed GABAA receptor activation leads to a fundamental change in macrophage physiology via cytoplasmic acidification. To fine tune alveolar macrophage responses, GABAA signaling is tightly controlled through immune regulation. During infection, administration of benzodiazepines opposes the endogenous drive to reduce GABAA signaling, increasing susceptibility to pneumonia. In contrast, treatment with IL-4 ex vivo or house dust mite in vivo increased GABAA subunit expression, and this led to cytoplasmic acidification. This intracellular pH change is consistent with the “alternatively activated” macrophage phenotype induced by IL-4: reduced TNF-α production and bacterial phagocytosis and killing (35).
The site-specific regulation of macrophage is also of interest. Macrophage GABAA receptor expression was highest in the lung (a mucosal site) and was lower in the nonmucosal sites (spleen and peritoneal cavity). Future studies should address the source of GABA, possibly from epithelial (36) or immune cell (12) sources. Another possibility is that GABA is released from bacterial pathogens (37, 38), especially as serum GABA becomes detectable in sepsis (16). In this scenario, pathogen-produced GABA may act to disable the host immune response. In contrast, down-regulation of host GABAA receptors on macrophages may reveal an attempt to block this pathogen–host interaction. Finally, macrophage GABAA channels may have tonic activity that does not require GABA for activation (39).
Macrophages and monocytes expressed α1 subunit containing GABAA receptors. Consistent with this, benzodiazepines that lack activity at α1 subunits lack the immunosuppression of nonselective drugs; thus enhanced benzodiazepine selectivity may improve the safety profile of this widely used class of drug. This is achievable for endpoints such as anxiolysis, which are mediated by α2/3 subunit containing GABAA receptors (34). Despite studying a subsedative, anxiolytic dose of a benzodiazepine, our results may also have relevance to sedative doses of the drugs. The sedative actions of GABAergic drugs are mediated by α1β2γ2 GABAA receptors (5, 34), and the same receptors are expressed by macrophages and monocytes. Therefore, it is unclear whether the sedative actions of GABAergic drugs such as benzodiazepines can be separated from their immunological effects. Further studies are required to compare the immune effects of GABAergic and non-GABAergic sedatives (19), to further build on investigations into their central nervous system side-effects (19, 20).
We are confident that the immune effects of diazepam are mediated by the GABAA receptor rather than other targets such as the mitochondrial translocator protein (also known as the peripheral benzodiazepine receptor [3]), as the diazepam effect is reversed by the GABAA antagonist bicuculline, diazepam does not effect neutrophil function (where GABAA receptor expression is absent but the translocator protein is present), and the selective benzodiazepine, NS11394 (that activates the translocator protein but not α1 containing GABAA receptors) did not cause immune suppression. Furthermore, activation of translocator protein is thought to stimulate chemotaxis in macrophage and monocytes ex vivo; we, and others (40), found no effect on chemotaxis in vivo. Finally, the immune function of the translocator protein is variably reported ex vivo (41, 42) with a lack of in vivo studies conducted, making any immune function obscure.
An important caveat to this work is that we are unable to definitively demonstrate that the benzodiazepine effect is mediated by a specific cell type. We suggest that our findings may be explained by perturbed function of alveolar macrophage based on their abundant expression of GABAA receptors, the immune regulation of their expression, and our ex vivo functional studies. Studies with genetically modified mice are complex due to the critical role of the γ2 subunit in neurodevelopment (8, 43). Future studies should genetically manipulate γ2 subunit expression in individual immune cell populations to definitively identify the cell type responsible for the benzodiazepine effect. However, from a drug development perspective, the exact immune cellular target may not be critical given that the drugs need to be able to penetrate the blood-brain barrier to have the clinically desired effect and so will be able to access the lung and that therapeutically, selective benzodiazepines that do not target the α1 GABAA subunit did not increase susceptibility to infection.
Conclusions
In our studies, diazepam appeared to oppose the endogenous regulation of GABAA receptor activity to increase susceptibility to infection via activation of α1-subunit–containing GABAA receptors. Activation of GABAA receptors on macrophages leads to cytoplasmic acidification and impaired antipathogen responses. Our data highlight the regulation of macrophage function by GABAA receptor signaling and the potential harm of benzodiazepine exposure during pneumonia. These data are supported by our parallel epidemiological study, suggesting that benzodiazepines increase susceptibility to pneumonia in humans (27). Nonetheless, it would be imprudent to immediately extrapolate these findings to clinical care, and further prospective cohort studies and randomized controlled trials are required to understand the impact of benzodiazepines on outcomes from infection. Our data also suggest that selective benzodiazepines may have an improved safety profile compared with their nonselective counterparts. Further preclinical study is required to develop these drugs.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Acknowledgments
We thank Dr. Vimal Grover, Dr. Stefan Gurney, Dr. Suveer Singh, Ms. Maryam Habibzay, Dr. Stephen Brickley, and Prof. Bill Wisden, (Imperial College London), Dr. Philippe Behe and Prof. Anthony Segal (University College London), and Dr. John Atack (Johnson & Johnson) for helpful advice. We also thank Dr. Max Mirza and Dr. Gordon Munro (Neurosearch) for their advice.
This work was supported, in part, by grant G0802353 to Dr. Sanders, grant G0802752 to Dr. Hussell, and grant G0802392 to Dr. Ma from the Medical Research Council, Swindon, United Kingdom, and grant 095707/Z/11/Z to Dr. Sanders from the Wellcome Trust. Dr. Sanders has received an honorarium for speaking on behalf of Hospira about immune effects of sedatives at the Canadian Society of Anesthesiology meeting. Hospira had no input into the design of the talk or this manuscript. There is no on-going relationship. Prof. Mervyn Maze has acted as a consultant for Hospira and Orion on the development of dexmedetomidine and received research funding from these organizations. These companies had no role in the work in this manuscript. Neurosearch supplied NS11394 at no cost.
Drs. Snelgrove and Ma received grant support from Wellcome Trust. Dr. Maze received grant support from MRC and the National Institutes of Health, receives royalties from Xenon Neuroprotection, and has patents with Dexmeditomidine (Stanford University).. Dr. Godlee received funding support from Wellcome Trust. Dr. Hussell received grant support from the Medical Research Council (Clinical Fellowship to Dr. Sanders). Dr. Fujimori is employed by the Imperial College London. Dr. Sanders received grant support, consulted for, and received honoraria from Hospira. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
See also Benzodiazepines: Revealing of the Secrets of GABAA-Mediated Immunosuppression: GABAA Subtype–Specific Benzodiazepines are the Hope: http://journals.lww.com/ccmjournal/Citation/2013/07000/Benzodiazepines___Revealing_of_the_Secrets_of.28.aspx
Dr. Sanders developed the hypothesis with Drs. Maze and Hussell. Drs. Sanders, Godlee, Fujimori, Goulding, Xin, Salek-Ardakani, Snelgrove, Ma, Maze, and Hussell designed and/or conducted the animal experiments. Dr. Sanders wrote the manuscript with input from all authors.
References
- 1.Woods JH, Katz JL, Winger G. Benzodiazepines: Use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 2.Gleason PP, Schulz R, Smith NL, et al. Correlates and prevalence of benzodiazepine use in community-dwelling elderly. J Gen Intern Med. 1998;13:243–250. doi: 10.1046/j.1525-1497.1998.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25:551–570. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Pritchett DB, Sontheimer H, Shivers BD, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 6.Löw K, Crestani F, Keist R, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 7.McKernan RM, Rosahl TW, Reynolds DS, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 8.Günther U, Benson J, Benke D, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330:163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- 10.Alam S, Laughton DL, Walding A, et al. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 2006;43:1432–1442. doi: 10.1016/j.molimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 11.Reyes-García MG, Hernández-Hernández F, Hernández-Téllez B, et al. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Bhat R, Axtell R, Mitra A, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler DW, Thompson AJ, Corletto F, et al. Anaesthetic impairment of immune function is mediated via GABA(A) receptors. PLoS ONE. 2011;6:e17152. doi: 10.1371/journal.pone.0017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J, Lu Y, Zhang H, et al. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 15.Stuckey DJ, Anthony DC, Lowe JP, et al. Detection of the inhibitory neurotransmitter GABA in macrophages by magnetic resonance spectroscopy. J Leukoc Biol. 2005;78:393–400. doi: 10.1189/jlb.1203604. [DOI] [PubMed] [Google Scholar]
- 16.Winder TR, Minuk GY, Sargeant EJ, et al. gamma-Aminobutyric acid (GABA) and sepsis-related encephalopathy. Can J Neurol Sci. 1988;15:23–25. doi: 10.1017/s0317167100027128. [DOI] [PubMed] [Google Scholar]
- 17.Serantes R, Arnalich F, Figueroa M, et al. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: Relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–14643. doi: 10.1074/jbc.M512489200. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 19.Pandharipande PP, Sanders RD, Girard TD, et al. MENDS investigators Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 21.Riker RR, Shehabi Y, Bokesch PM, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 22.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine for Long-Term Sedation Investigators Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 23.Laschi A, Descotes J, Tachon P, et al. Adverse influence of diazepam upon resistance to Klebsiella pneumoniae infection in mice. Toxicol Lett. 1983;16:281–284. doi: 10.1016/0378-4274(83)90188-1. [DOI] [PubMed] [Google Scholar]
- 24.Domingues-Junior M, Pinheiro SR, Guerra JL, et al. Effects of treatment with amphetamine and diazepam on Mycobacterium bovis-induced infection in hamsters. Immunopharmacol Immunotoxicol. 2000;22:555–574. doi: 10.3109/08923970009026012. [DOI] [PubMed] [Google Scholar]
- 25.Galdiero F, Bentivoglio C, Nuzzo I, et al. Effects of benzodiazepines on immunodeficiency and resistance in mice. Life Sci. 1995;57:2413–2423. doi: 10.1016/0024-3205(95)02199-0. [DOI] [PubMed] [Google Scholar]
- 26.Huemer HP, Lassnig C, Nowotny N, et al. Diazepam leads to enhanced severity of orthopoxvirus infection and immune suppression. Vaccine. 2010;28:6152–6158. doi: 10.1016/j.vaccine.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: A nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68:163–170. doi: 10.1136/thoraxjnl-2012-202374. [DOI] [PubMed] [Google Scholar]
- 28.Mirza NR, Larsen JS, Mathiasen C, et al. NS11394 [3’-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: In vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- 29.Griebel G, Belzung C, Perrault G, et al. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 30.Elsersy H, Mixco J, Sheng H, et al. Selective gamma-aminobutyric acid type A receptor antagonism reverses isoflurane ischemic neuroprotection. Anesthesiology. 2006;105:81–90. doi: 10.1097/00000542-200607000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 32.Bidani A, Wang CZ, Saggi SJ, et al. Evidence for pH sensitivity of tumor necrosis factor-alpha release by alveolar macrophages. Lung. 1998;176:111–121. doi: 10.1007/pl00007593. [DOI] [PubMed] [Google Scholar]
- 33.Bidani A, Reisner BS, Haque AK, et al. Bactericidal activity of alveolar macrophages is suppressed by V-ATPase inhibition. Lung. 2000;178:91–104. doi: 10.1007/s004080000012. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Xiang YY, Wang S, Liu M, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 37.Yurdaydin C, Walsh TJ, Engler HD, et al. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 1995;679:42–48. doi: 10.1016/0006-8993(95)00241-h. [DOI] [PubMed] [Google Scholar]
- 38.Yang SY, Lü FX, Lu ZX, et al. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under sub-merged fermentation. Amino Acids. 2008;34:473–478. doi: 10.1007/s00726-007-0544-x. [DOI] [PubMed] [Google Scholar]
- 39.McCartney MR, Deeb TZ, Henderson TN, et al. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol. 2007;71:539–548. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- 40.Ruff MR, Pert CB, Weber RJ, et al. Benzodiazepine receptor-mediated chemotaxis of human monocytes. Science. 1985;229:1281–1283. doi: 10.1126/science.2994216. [DOI] [PubMed] [Google Scholar]
- 41.Finnerty M, Marczynski TJ, Amirault HJ, et al. Benzodiazepines inhibit neutrophil chemotaxis and superoxide production in a stimulus dependent manner; PK-11195 antagonizes these effects. Immunopharmacology. 1991;22:185–193. doi: 10.1016/0162-3109(91)90043-x. [DOI] [PubMed] [Google Scholar]
- 42.Marino F, Cattaneo S, Cosentino M, et al. Diazepam stimulates migration and phagocytosis of human neutrophils: Possible contribution of peripheral-type benzodiazepine receptors and intracellular calcium. Pharmacology. 2001;63:42–49. doi: 10.1159/000056111. [DOI] [PubMed] [Google Scholar]
- 43.Berry RB, Chandra D, Diaz-Granados JL, et al. Investigation of ethanol-induced impairment of spatial memory in gamma2 heterozygous knockout mice. Neurosci Lett. 2009;455:84–87. doi: 10.1016/j.neulet.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.