Abstract
Introduction
The neonatal period is a highly vulnerable time for an infant completing many of the physiologic adjustments required for life outside the uterus. As a result, there are high rates of morbidity and mortality. The three major causes of mortality in developing countries include prematurity, infection, and perinatal asphyxia. The aim of this study was to identify the patterns of neonatal admission and factors associated with mortality among neonates admitted at the Neonatal Intensive Care Unit (NICU) of University of Gondar Hospital.
Materials and methods
A retrospective cross-sectional study was conducted among all admitted neonates in the NICU of University of Gondar referral hospital from December 1, 2015 to August 31, 2016. Information was extracted retrospectively during admission from patient records and death certificates, using a pretested questionnaire. The data were entered and analyzed using SPSS version 20, and p-values <0.05 were considered statistically significant.
Results
A total of 769 neonates was included in the study. There were 448 (58.3%) male neonates, and 398 (51.8%) neonates were rural residents. More than two-thirds of the 587 deliveries (76.3%) were performed in tertiary hospitals. Neonatal morbidity included hypothermia 546 (71%), sepsis 522 (67.9%), prematurity 250 (34.9%), polycythemia 242 (31.5%), hypoglycemia 142 (18.5), meconium aspiration syndrome 113 (14.7%), and perinatal asphyxia 96 (12.5%). The overall mortality was 110 (14.3%; 95% confidence interval [CI]: 11.9–16.9) of which 69 (62.7%) deaths occurred in the first 24 hours of age. In the multivariate analysis, mortality was associated with perinatal asphyxia (adjusted odds ratio [AOR]: 5.97; 95% CI: 3.06–11.64), instrumental delivery (AOR: 2.99; 95% CI: 1.08–8.31), and early onset neonatal sepsis (AOR: 2.66; 95% CI: 1.62–6.11).
Conclusion
Hypothermia, sepsis, and prematurity were the main reasons for NICU admission. Neonates often died within the first 24 hours of age. Implementing a better referral link and timely intervention could decrease neonatal mortality and morbidities in Gondar, Ethiopia.
Keywords: neonatal sepsis, hypothermia, neonatal mortality, neonatal admission
Introduction
In developed countries, the main cause of neonatal mortality is non-preventable, such as congenital abnormalities, whereas in developing countries majority of the diseases are preventable.1 The perinatal period is recognized as the most crucial period of life because of various problems faced by neonates. According to the World Health Organization (WHO) estimates, a significant proportion (40%) of all under-5 deaths occur in the neonatal or perinatal period. Of the estimated 130 million infants born each year worldwide, 4 million die in the first 28 days of life. Three quarters of neonatal deaths occur in the first week, and more than one quarter of deaths occur in the first 24 hours. These large fractions of deaths are preventable.2,3 Worldwide, the most common causes of neonatal deaths are preterm birth, birth asphyxia, sepsis, and pneumonia.4,5 Trends in mortality worldwide show that perinatal and neonatal mortality are declining less rapidly compared to infant and under-5 mortality. The share of neonatal deaths increased from 37% in 2000 to 41% in 2008.4 The slow decline in neonatal mortality as compared to postneonatal mortality calls for attention and efforts to reverse this trend.6
The Ethiopian population grows at a rate of 2.6% per annum and the majority of people (84%) reside in rural areas.7,8 According to the 2011 Ethiopia Demographic and Health Surveys, the country is experiencing a high neonatal mortality rate at 37 per 1,000 live births, comparable to the average rate of 35.9 per 1,000 live births for the African region.9 In the past decade, however, the country witnessed a significant decline in under-5 mortality rates from 166 per 1,000 in 2000 to 88 per 1,000 live births in 2011.10 The causes of neonatal mortality are not well documented in Ethiopia; however, reports from previous studies identified sepsis, asphyxia, birth injury, tetanus, preterm birth, congenital malformations, and “unknown causes” as major reasons for neonatal mortality.7
Regular neonatal auditing is vital as disease patterns and contributors to neonatal deaths vary from between regions. This study assessed the pattern of neonatal morbidity, including factors associated with neonatal mortality.
Materials and methods
Study design
A hospital-based retrospective cross-sectional study was conducted among all neonates admitted to the Neonatal Intensive Care Unit (NICU) at the University of Gondar referral hospital from December 1, 2015 to August 31, 2016.
Setting
The health sector in Ethiopia has a three-tier health care delivery system for ~100 million people. This includes primary hospitals, health centers, and satellite health posts. A primary hospital, health center, and health post form a “primary health care unit” with each health center having five satellite health posts. The second level in the tier is a general hospital with a population coverage of 1–1.5 million people, and the third a specialized hospital that covers a population of 3.5–5 million people. According to a 2010 report, Ethiopia has 14,416 health posts, 2,689 health centers, and 195 hospitals.11 This study was conducted in the University of Gondar Hospital (UoGH), which is one of the largest referral hospitals with easy access tô5 million people and also one of the oldest academic institutions in Ethiopia. UoGH is located at the heart of Gondar city in the northwestern part of the country. Pediatrics and child health department provides services for both rural and urban populations and includes outpatient clinics, an emergency department, pediatric and malnutrition wards, and NICU. The NICU was established 20 years ago and serves as a tertiary and a referral unit for the region. The UoGH NICU receives high risk babies delivered within the institution, referrals from other health facilities, and referrals from home deliveries. The number of admitted neonates varies from time to time; the average annual admission rate being 1,140. The NICU has a 32-bed capacity. It has radiant warmers to keep the room warm and nine incubators for premature neonates. The unit does not have a mechanical ventilator or continuous positive airway pressure (CPAP) machine but uses bubble CPAP locally developed for neonates with respiratory distress syndrome (RDS). Additionally, there are three phototherapy machines and four incubators for term neonates. The babies receive oxygen through nasal prongs or a nasal catheter from oxygen cylinders or oxygen concentrators. Ampicillin and gentamicin are commonly used antibiotics for the treatment of sepsis empirically. Medications are administered via peripheral vein and in few cases the umbilical vain is used. The NICU has four rooms, one for preterm babies, one for term babies, one isolation room for communicable diseases, and one on the maternity side where relatively stable neonates and those who need kangaroo mother care are kept. The NICU is staffed with seven medical interns, two pediatric residents, one pediatrician, and 17 nurses.
Participants
All neonates admitted to the NICU from December 1, 2015 to August 31, 2016 were prospective participants of this study.
Neonatal mortality was the dependent variable, and the independent variables included sex of the patient, age at admission, gestational age, birth weight, place of birth, antenatal care (ANC) follow-up, maternal age, parity, duration of hospital stay, address, and mode of delivery.
Exclusion criteria
Neonates, who were kept under observation, including those referred from other health facilities with suspected disease but labeled healthy after evaluation by residents or attending physicians in the NICU were excluded from the study.
Data collection procedure
Data were collected retrospectively from admission/discharge registration books and death certificates using a pretested, structured questionnaire prepared in English. Important variables were extracted by pediatric residents practicing in the neonatal ward. Prior to actual data collection, training was provided to data collectors on the data collection techniques in order to familiarize data collectors with the tool.
Operational definition
In this setting, disease diagnosis was based on clinical presentation and supportive laboratory results. Prematurity was described as live born neonates delivered before 37 completed weeks. For mothers who did not know dates of their last normal menstrual period, the new Ballard score was used to estimate the gestational age.12 Birth weight was classified using WHO weight classification.13 Sepsis and meningitis were diagnosed after isolating the pathogenic organism from the blood or cerebral spinal fluid whenever possible; otherwise, most of the other diagnoses depended on history, physical examination, and other supportive investigations. Birth asphyxia was diagnosed whenever a neonate had an Apgar score <6 in the fifth minute and/or was unresponsive to stimuli or convulsion not explained by other causes.14 for babies born outside health facilities with unknown Apgar scores, details were obtained from the mother about the neonate: if he/she did not cry immediately after birth; had respiratory distress, floppiness, loss of consciousness, presence of convulsion, and loss of neonatal reflexes. In the present study, radiologic examination including X-ray was performed inconsistently; however, for the diagnosis of RDS, clinical criteria were used and risk factors like premature infant with signs and symptoms of rapid labored, grunting type of breathing manifesting immediately or within a few hours after delivery and with subcostal retraction, cyanosis, and decreased air entry in bilateral lung field or those who had chest X-ray examination with characteristic findings for RDS were also included. Venous hematocrit level >65% or a venous hemoglobin concentration in excess of 22 g/dL 6 hours after delivery was considered as polycythemia.15 Both early onset neonatal sepsis (EONS) and late onset neonatal sepsis (LONS) were defined after assessing the risk factors for infection including prematurity, maternal infection during labor, and clinical signs and symptoms suggestive of infection. Neonates who presented to the NICU with a diagnosis of sepsis within 72 hours of birth are labeled as EONS, while those who came in after 72 hours of birth are labeled LONS. Anthropometric assessment was carried out using Lubchenco curve.16 All other assessments were based on physician judgment as written in the patient card.
Sample size
All neonates admitted to the NICU in the study period were included.
Data compilation and analysis
Data were entered, cleaned, checked for completeness, compiled, and analyzed using SPSS version 20 (IBM Corporation, Armonk, NY, USA). Descriptive statistics and logistic regression were computed. Crude odds ratio and adjusted odds ratio (AOR) were analyzed with a 95% confidence interval (CI) and p-value <0.05 was considered a statistically significant association. All variables were used in the bivariate logistic regression and variables with significant associations (p-value <0.05) were further considered for multivariate logistic regression to determine independent associations of each variable.
Ethical statement
Ethical approval was obtained from the institutional review board of the University of Gondar before conducting the research. Patient consent was not required for retrospective study, as the institution reserves the right to own the medical record of patients.
Results
Neonatal and maternal characteristics
Out of 797 admitted neonates, 28 (3.5%) were excluded from the study for incomplete recordings. In all, 769 neonates were eligible for the study, out of which 448 (58.3%) were male neonates making the male to female ratio 1.4:1. The number of neonates born at UoGH was 587 (76.3%); the rest were born at surrounding health centers 109 (14.2%), home 33 (4.3%), and primary hospital 9 (1.2%). Among 717 (93.2%) neonates with known gestational age, more than half (456, 63.6%) were term and 250 (34.9%) were preterm. Birth weight was documented for 655 (85.2%) neonates, of which 388 (59.2%) had normal birth weight and 232 (35.4%) had low birth weight (LBW). With respect to anthropometry, 570 (74.1%) neonates were appropriate for their gestational age and 41 small for gestational age.
Majority (670, 87.1%) of the study neonates were singleton, and 94 (12.2%) and 5 (0.7%) were twins and triplets, respectively. Out of 611 (79.5%) neonates who had their maternal age recorded, in 552 (90.3%) the age ranged from 18 to 35 years followed by 36 (5.9%) neonates with maternal age >35 years and 23 (3.8%) with maternal age <18 years. There were 430 (55.9%) primipara mothers and 339 (44.1%) multiparous women. Seven hundred and thirty (94.9%) mothers had an ANC follow-up and 32 (4.2%) were positive for human immunodeficiency virus (HIV) of which 23 (6.2%) were from urban areas. There were 4.2% HIV-exposed neonates. Mode of delivery was spontaneous vaginal delivery in 463 (60.2%) and cesarean section in 259 (33.7%) study participants (Tables 1 and 2).
Table 1.
Demographic characteristics of neonates admitted to the University of Gondar Hospital, Ethiopia from December 1, 2015 to August 31, 2016
| Variables | Male, n (%) N=448 | Female, n (%) N=321 | Total, n (%) N=769 |
|---|---|---|---|
| Residency | |||
| Urban | 216 (48.2) | 155 (48.3) | 371 (48.2) |
| Rural | 232 (51.8) | 166 (51.7) | 398 (51.8) |
| Place of delivery | |||
| Tertiary hospital | 338 (75.5) | 249 (77.6) | 587 (76.3) |
| Health center | 68 (15.2) | 41 (12.8) | 109 (14.2) |
| Primary hospital | 6 (1.3) | 3 (0.9) | 9 (1.2) |
| Home | 22 (4.9) | 11 (3.4) | 33 (4.3) |
| Othersa | 14 (3.1) | 17 (5.3) | 31 (4.0) |
| Gestational age (n=717) | |||
| <34 weeks | 53 (15.4) | 89 (23.9) | 142 (19.8) |
| 34–36 weeks | 54 (15.7) | 54 (14.5) | 108 (15.1) |
| 37–41 weeks | 232 (67.4) | 224 (60.1) | 456 (63.6) |
| ≥42 weeks | 5 (1.5) | 6 (1.6) | 11 (1.5) |
| Birth weight (n=655) | |||
| <2,500 g | 98 (31.7) | 134 (38.7) | 232 (35.4) |
| 2,500–3,999 g | 188 (60.8) | 200 (57.8) | 388 (59.2) |
| >4,000 g | 23 (7.4) | 12 (3.5) | 35 (5.3) |
| Anthropometry | |||
| Appropriate for gestational age | 334 (74.6) | 236 (73.5) | 570 (74.1) |
| Large for gestational age | 24 (5.4) | 9 (2.8) | 33 (4.2) |
| Small for gestational age | 15 (3.4) | 26 (8.1) | 41 (5.3) |
| Not applicableb | 75 (16.7) | 50 (15.6) | 125 (16.3) |
Notes:
On the way to a health facility and in the ambulance.
Neonates who came to the hospital after 7 days of postpartal age.
Table 2.
Sociodemographic and clinical characteristics of mothers who gave birth to neonates admitted to the University of Gondar Hospital, Ethiopia from December 1, 2015 to August 31, 2016
| Variables | Urban, n (%) N=371 | Rural, n (%) N=398 | Total, n (%) N=769 |
|---|---|---|---|
| Number of gestations | |||
| Singleton | 333 (89.8) | 337 (84.7) | 670 (87.1) |
| Twins | 34 (9.2) | 60 (15.1) | 94 (12.2) |
| Triplets | 4 (1.1) | 1 (0.3) | 5 (0.7) |
| Maternal age (n=611) | |||
| <18 years | 14 (4.7) | 9 (2.9) | 23 (3.8) |
| 18–35 years | 265 (88.9) | 287 (91.7) | 552 (90.3) |
| >35 years | 19 (6.4) | 17 (5.4) | 36 (5.9) |
| Parity | |||
| Para I | 222 (59.8) | 208 (52.3) | 430 (55.9) |
| Para II–IV | 137 (36.9) | 132 (33.2) | 269 (34.9) |
| ≥Para V | 12 (3.2) | 58 (14.6) | 70 (9.1) |
| ANC follow-up | 355 (95.7) | 375 (94.2) | 730 (94.9) |
| HIV positive | 23 (6.2) | 9 (2.3) | 32 (4.2) |
| VDRL reactive | 4 (1.1) | 4 (1.0) | 8 (1.0) |
| Mode of delivery | |||
| Vaginal delivery | 226 (60.9) | 237 (59.6) | 463 (60.2) |
| Assisted breech | 2 (0.5) | 8 (2.0) | 10 (1.3) |
| Instrumental | 14 (3.8) | 23 (5.8) | 37 (4.8) |
| Cesarean section | 129 (34.8) | 130 (32.7) | 259 (33.7) |
Abbreviations: ANC, antenatal care; VDRL, venereal disease research laboratory.
Clinical characteristics of the study neonates
Two thirds of the neonates (531, 69.1%) were admitted within 24 hours of age and the median age at admission was 1 hour with an interquartile range of 29.7 hours. The median hospital stay was 72 hours with an interquartile range of 92 hours. The major cause of morbidity among all neonates was hypothermia (546, 71%) of which 111 (14.4%) had mild hypothermia (cold stress), 428 (55.7%) had moderate hypothermia, and 7 (0.9%) had severe hypothermia. Neonatal sepsis was identified in 522 (67.9%) newborns among which 458 (59.6%) were EONS and 64 (8.3%) were LONS; both types of neonatal sepsis were treated by ampicillin and gentamycin. Other common neonatal problems included polycythemia (242, 31.5%), hypoglycemia (142, 18.5%), perinatal asphyxia (96, 12.5%), hyperbilirubinemia (91, 11.8%), and RDS (84, 10.9%). The mean CPAP treatment period for RDS was 3.9 days with standard deviation (±) 3.35 days. All neonates with a diagnosis of RDS were treated by CPAP. Congenital heart disease was identified in 6 (0.8%) neonates and other forms of congenital malformations including cleft lip, cleft palate, polydactyle, and spinal dysraphism were identified in 10 (1.3%) admitted neonates (Table 3).
Table 3.
Clinical characteristics of neonates admitted to the University of Gondar Hospital, Ethiopia from December 1, 2015 to August 31, 2016
| Variables | Urban, n (%) N=448 | Rural, n (%) N=321 | Total, n (%) N=769 |
|---|---|---|---|
| Hospital stay | |||
| 0–24 hours | 107 (23.9) | 77 (23.9) | 184 (23.9) |
| 24.1–72 hours | 176 (39.3) | 128 (39.9) | 304 (39.5) |
| 72.1–168 hours | 108 (24.1) | 72 (22.4) | 180 (23.4) |
| >7 days | 57 (12.7) | 44 (13.7) | 101 (13.1) |
| Body temperature | |||
| Normal | 105 (23.4) | 53 (16.5) | 158 (20.6) |
| Cold stress | 59 (13.2) | 52 (16.2) | 111 (14.4) |
| Moderate hypothermia | 242 (54.0) | 186 (57.9) | 428 (55.7) |
| Severe hypothermia | 6 (1.3) | 1 (0.3) | 7 (0.9) |
| Fever | 36 (8.0) | 29 (9.0) | 65 (8.5) |
| Hypoglycemia | 79 (17.6) | 63 (19.6) | 142 (18.5) |
| Sepsis | |||
| Early onset neonatal sepsis | 264 (58.9) | 194 (60.4) | 458 (59.6) |
| Late onset neonatal sepsis | 39 (8.7) | 25 (7.8) | 64 (8.3) |
| Hyperbilirubinemia | 57 (12.7) | 34 (10.6) | 91 (11.8) |
| Perinatal asphyxia | 56 (12.5) | 40 (12.5) | 96 (12.5) |
| Respiratory distress syndrome | 50 (11.2) | 34 (10.6) | 84 (10.9) |
| Meconium aspiration syndrome | 63 (14.1) | 50 (15.6) | 113 (14.7) |
| Polycythemia | 143 (31.9) | 99 (30.8) | 242 (31.5) |
Factors associated with poor neonatal outcome
Of all admitted neonates, 629 (81.8%) were discharged after they showed improvement and the overall mortality was 110 (14.3%). All selected variables including residency, place of birth, gestational age, birth weight, ANC followup, temperature, hypoglycemia, sepsis, perinatal asphyxia, and HMD were used in the bivariate logistic regression and variables which had significant associations were further considered for multivariate logistic regression to determine the independent association of each variable. Results of the bivariate analysis revealed that rural residency, delivery at a health center, gestational age <34 weeks, LBW, and lack of ANC follow-up were significantly associated with neonatal death. In addition to these, newborns with morbidity such as moderate hypothermia, EONS, hypoglycemia, and perinatal asphyxia were significantly associated with neonatal death.
In the multivariate analysis, mortality was significantly associated with perinatal asphyxia (AOR: 5.97; 95% CI: 3.06–11.64), instrumental delivery (AOR: 2.99; 95% CI: 1.08–8.31), and EONS (AOR: 2.66; 95% CI: 1.62–6.11) (Table 4).
Table 4.
Multivariable analysis of factors associated with neonatal death in the University of Gondar Hospital, Ethiopia from December 1, 2015 to August 31, 2016
| Variables | N (%) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|
| Residency | |||
| Urban | 39 (10.5) | 1.00 | |
| Rural | 71 (17.8) | 1.85 (1.22, 2.81) | 1.56 (0.88, 2.77) |
| Place of delivery | |||
| Hospital | 72 (12.1) | 1.00 | |
| Health center | 26 (23.9) | 2.28 (1.38, 3.78) | 1.52 (0.67, 3.48) |
| Othersa | 12 (18.8) | 1.68 (0.86, 3.29) | 0.92 (0.19, 4.46) |
| Gestational age in weeks | |||
| 37–42 weeks | 48 (10.5) | 1.00 | |
| <34 weeks | 41 (28.9) | 3.45 (2.16, 5.52) | 1.04 (0.37, 2.93) |
| 34–37 weeks | 8 (7.4) | 0.68 (0.31, 1.48) | 0.76 (0.28, 2.06) |
| >42 weeks | 1 (9.1) | 0.85 (0.11, 6.78) | 1.07 (0.11, 10.06) |
| Birth weight in grams | |||
| 2,500–3,990 | 42 (10.8) | 1 | |
| <2,500 | 49 (21.1) | 2.21 (1.41, 3.46) | 1.04 (0.43, 2.47) |
| >4,000 | 3 (8.6) | 0.77 (0.23, 2.63) | 1.62 (0.38, 7.03) |
| ANC follow-up | |||
| Yes | 98 (13.4) | 1.00 | |
| No | 12 (30.8) | 2.87 (1.41, 5.84) | 2.03 (0.68, 6.02) |
| Mode of delivery | |||
| Vaginal delivery | 69 (14.9) | 1.00 | |
| Assisted breech | 4 (40.0) | 3.81 (1.05, 13.84) | 3.69 (0.74, 18.41) |
| Instrumental | 8 (21.6) | 1.58 (0.69, 3.59) | 2.99 (1.07, 8.31)* |
| Cesarean section | 29 (11.2) | 0.72 (0.45, 1.14) | 0.87 (0.46, 1.64) |
| Body temperature | |||
| Normal temperature | 8 (5.1) | 1.00 | |
| Cold stress | 11 (9.9) | 2.06 (0.80, 5.31) | 1.52 (0.43, 5.36) |
| Moderate hypothermia | 78 (18.2) | 4.18 (1.97, 8.87) | 1.56 (0.53, 4.61) |
| Severe hypothermia | 5 (71.2) | 46.88 (7.84, 280.02) | 10.45 (1.04, 104.66)* |
| Fever | 8 (12.3) | 2.63 (0.94, 7.34) | 1.69 (0.37, 7.81) |
| Hypoglycemia | |||
| No | 82 (13.1) | 1.00 | |
| Yes | 28 (19.7) | 1.63 (1.02, 2.62) | 0.95 (0.48, 1.85) |
| Neonatal sepsis | |||
| No sepsis | 11 (4.5) | 1.00 | |
| Early onset | 88 (19.2) | 5.10 (2.67, 9.75) | 2.66 (1.16, 6.11)* |
| Late onset | 11 (17.2) | 4.45 (1.83, 10.81) | 13.51 (2.64, 69.00)* |
| Perinatal asphyxia | |||
| No | 77 (11.4) | 1.00 | |
| Yes | 33 (34.4) | 4.05 (2.50, 6.58) | 5.97 (3.06, 11.64)* |
| RDS | |||
| No | 67 (9.8) | 1.00 | |
| Yes | 43 (51.2) | 9.67 (5.89, 15.89) | 12.97 (5.37, 31.30)* |
Notes:
Home or on the way to a health facility.
p-value <0.05.
Abbreviations: ANC, antenatal care; AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; RDS, respiratory distress syndrome.
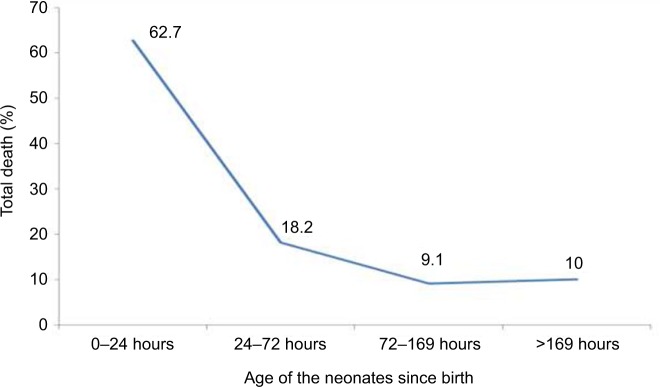
Patterns of neonatal mortality
More than half of the deaths (69, 62.7%) occurred within the first 24 hours of age and 80.9% died within 72 hours of age (Figure 1). The death pattern declined as the age of the child crossed 72 hours.
Figure 1.
Percentage of mortality among neonates who died at the Gondar University Hospital, Ethiopia from December 1, 2015 to August 31, 2016.
Discussion
Among many neonatal conditions, the three major contributors to the global burden of neonatal disease are premature birth, birth asphyxia, and neonatal infections.17,18 The aim of this study was to identify the patterns of neonatal admissions and factors associated with mortality among neonates admitted to UoGH. In all, 769 neonates were included during the study period and male predominance was noted in 58.3% of the study participants, which is in line with studies carried out in other developing countries: 63% in Pakistan, 57.8% in South Africa, 63.3% in India, and 61.1% in St Paul’s Hospital Millennium Medical College (SPHMMC), Ethiopia.19–22 In some studies, the discrepancy between the number of boys and girls born has been interpreted as a natural selection response to differential survival prospects.23 Additionally, cultural and social factors could contribute to male babies getting more attention by parents than females.
The proportion of preterm admission was 34.8%, consistent with the range of 25.8%–50.4% reported from other developing countries.20–23,25 The biology that affects the fetus in utero is related to maternal physiology including maternal illness and infection during pregnancy.26 Thirty five percent of neonates in this study had LBW which is comparable with studies from Pakistan (37.7%) and Tanzania (29%), but lower than South Africa (52.5%) and India (60%).19–22 Although there are numerous factors associated with LBW and prematurity, the major contributors are low socioeconomic status, maternal infection, maternal undernutrition, and anemia.27 In this study, twin birth was more common among mothers from rural residence compared to urban areas. Since there are reports showing association between grand multiparity and twin birth,28 the finding could be explained by the higher proportion of grand multiparity of the mothers from rural area (14.6%) compared to those from the urban setting (3.2%) (Table 2).
Previous studies have identified an increased occurrence of prematurity among male newborns compared to female newborns.29 Contrary to those reports, the present study showed a high rate of prematurity among female (23.9%) newborns than their male (15.4%) counterparts. Since the study site is a teaching referral hospital for a large number of primary and midlevel health facilities in its area, most of the preterm male neonates, known to be susceptible to death compared to females,30 are more likely not to make it alive to the referral hospital when referred to the study site from a distant facility. Moreover, there is a report of higher proportion of male stillbirth compared to female stillbirths.31 Therefore, the high occurrence of prematurity among female than male newborns in this study has a plausible explanation.
The two most common problems identified were hypothermia (71%) and neonatal sepsis (67.9%). These findings were significantly high as compared to the studies performed elsewhere; for instance, neonatal infection was 20.3% in Pakistan and 21% in South African studies,19,20 whereas neonatal sepsis was 16.9% in Nigeria, 34.5% in Nepal, and 22.7% in an SPHMMC Ethiopian study.22,25,32 This high discrepancy of prevalence of neonatal sepsis in developing countries could be explained by the variation of diagnostic criteria used for sepsis and sample size difference. Infection in developing countries is high as compared to the developed world, which could be explained by low socioeconomic status and the presence of other risks including prematurity, LBW, prolonged labor, and rupture of membrane.33 This study implied that there is a clear gap in the management and prevention of sepsis and hypothermia.
In the present study, the overall neonatal mortality was 14.3% (95% CI: 11.9–16.9), which is in line with studies carried out in Nigeria (14.2%) and South Africa (13.8%) but lower than SPHMMC, Addis Ababa (23.2%) and Bangladesh (20.6%). And in contrary, lower mortalities of 6.2% and 4.6% were also reported from studies in Pakistan and Nepal, respectively.1,20,22,25,32,34 This difference in mortality pattern could be explained by the related factors for each study in addition to the quality of care delivered by the centers. More than half (62.7%) of the deaths occurred within 24 hours of admission, which was nearly comparable with the studies from Nigeria (55%) and Tanzania (56.7%).24,32 As the first few hours of postnatal age are crucial for new environment adaptation, very simple and inexpensive measures can significantly decrease neonatal mortality in the first few hours of life.
Predictors of mortality were identified from this study by multivariate analysis, including hypothermia, sepsis, and perinatal asphyxia. Similar reports were described in previous studies,22,35–38 indicating that the well-known contributors for neonatal deaths are treatable and preventable problems; meticulous evaluation and risk identification are the way forward to tackle and reduce neonatal mortality in developing countries.
Limitation of the study
As this is a retrospective cross-sectional study, cause–effect relation could not be analyzed and it could be also subjected to study design-related bias. Also, due to heavy rush in the neonatal ward, paper charts may not be complete for some of the cases. For the diagnosis of RDS, chest X-ray was not done consistently as recommended by experts. Another limitation is that some of the variables mentioned in the multivariate model had wide CIs and high odds ratio which may undermine the efficacy of this study.
Conclusion
Hypothermia, sepsis, and prematurity were the main reasons for NICU admission, and neonatal mortality was high in the first 24 hours of age. Implementing a better referral link and timely intervention could decrease neonatal mortality and morbidity in this setting.
Acknowledgments
The authors would like to acknowledge the assistance of the University of Gondar and all staffs working in the NICU. Thanks are also due to Dr Solomon Mekonnen and Dr Nebiyu Mesfin for reviewing the manuscript. The authors would like to thank Dr Rishi Mediratta and Alex Oldman who helped in editing the manuscript. No funding was provided for this study.
Footnotes
Author contributions
All authors were involved in research design, data analysis, and manuscript preparation and editing. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jehan I, Harris H, Salat S, et al. Neonatal mortality, risk factors and causes: a prospective population-based cohort study in urban Pakistan. Bull World Health Organ. 2009;87(2):130–138. doi: 10.2471/BLT.08.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews Z. World health report 2005: make every mother and child count. World Health. 2005;33(6):409–411. doi: 10.1080/14034940500217037. [DOI] [PubMed] [Google Scholar]
- 3.Aurangzeb B, Hameed A. Neonatal sepsis in hospital-born babies: bacterial isolates and antibiotic susceptibility patterns. J Coll Physicians Surg Pak. 2003;13(11):629–632. [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Bryce J, Boschi-Pinto C, Shibuya K, Black RE, WHO Child Health Epidemiology Reference Group WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 6.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths when ? where ? why ? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 7.The World Bank . World Development Indicators 2013. The World Bank; Washington DC, USA: 2013. [Google Scholar]
- 8.Central Statistical Agency . Population and Housing Census of Ethiopia. Ethiopia: Central Statistical Agency; 2007. [Google Scholar]
- 9.Oestergaard MZ, Inoue M, Yoshida S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8(8):e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Central Statistical Agency [Ethiopia] and ICF International . Ethiopia Demographic and Health Survey, 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International; 2012. [Google Scholar]
- 11.Federal Democratic Republic of Ethiopia Ministry of Health Health Sector Development Program IV 2010/11–2014/15. [Accessed September 15, 2016]. Available from: http://www.phe-ethiopia.org.
- 12.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Promoting Optimal Fetal Development: Report of a Technical Consultation. Geneva: World Health Organization; 2006. p. 3. [Google Scholar]
- 14.World Health Organization . Basic Newborn Resuscitation: A Practical Guide. Geneva: World Health Organization; 1997. [Google Scholar]
- 15.Ramamurthy RS, Brans WY. Neonatal polycythemia: I. Criteria for diagnosis and treatment. Pediatrics. 1981;68:168–174. [PubMed] [Google Scholar]
- 16.Lubchenco L, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from live born birth weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 17.World Health Organization . The Global Burden of Disease: 2004 Update. Geneva: WHO; 2008. [Google Scholar]
- 18.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali SR, Ahmed S, Lohana H. Disease patterns and outcomes of neonatal admissions at a secondary care hospital in Pakistan. Sultan Qaboos Univ Med J. 2013;13(3):418–421. [PMC free article] [PubMed] [Google Scholar]
- 20.Hoque M, Haaq S, Islam R. Causes of neonatal admissions and deaths at a rural hospital in KwaZulu-Natal, South Africa. South African J Epidemiol Infect. 2016;8782:26–29. [Google Scholar]
- 21.Anand K, Kant S, Kumar G, et al. Neonatal morbidity and mortality of sick newborns admitted in a teaching hospital of Uttarakhand. CHRISMED J Health Res. 2014;1(4):247–253. [Google Scholar]
- 22.Tekleab AM, Amaru GM, Tefera YA. Reasons for admission and neonatal outcome in the neonatal care unit of a tertiary care hospital in Addis Ababa : a prospective study. Res Reports Neonatol. 2016;201:17–23. [Google Scholar]
- 23.Crawford MA, Doyle W, Meadows N. Gender difference at birth and differences in fetal growth. Hum Reprod. 1987;2(6):517–520. doi: 10.1093/oxfordjournals.humrep.a136581. [DOI] [PubMed] [Google Scholar]
- 24.Mmbaga BT, Lie RT, Olomi R, Mahande MJ, Kvåle G, Daltveit AK. Cause-specific neonatal mortality in a neonatal care unit in Northern Tanzania : a registry based cohort study. BMC Pediatr. 2012;12(1):116–125. doi: 10.1186/1471-2431-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanodia P, Yadav SK, Bhatta NK, Singh RR. Disease profile and outcome of newborn admitted to neonatology unit of BPKIHS. J Coll Med Sci. 2015;11(3):20–24. [Google Scholar]
- 26.Lynch J, Kaplan G. Socioeconomic position. In: Berkman LF, Kawachi I, editors. Social Epidemiology. New York: Oxford University Press; 2000. pp. 13–35. [Google Scholar]
- 27.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep. 2007;55:1–32. [PubMed] [Google Scholar]
- 28.Smits J, Monden C. Twinning across the developing world. PLoS One. 2011;6(9):e25239. doi: 10.1371/journal.pone.0025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, et al. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17(10):2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]
- 30.Khoury MJ, Marks JS, McCarthy BJ. Factors affecting the sex differential in neonatal mortality. Am J Obstet Gynecol. 1985;151(6):777–782. doi: 10.1016/0002-9378(85)90518-6. [DOI] [PubMed] [Google Scholar]
- 31.Assefa N, Lakew Y, Belay B, et al. Neonatal mortality and causes of death in Kersa Health and Demographic Surveillance System (Kersa HDSS), Ethiopia, 2008–2013. Matern Health Neonatol Perinatol. 2016;2:7. doi: 10.1186/s40748-016-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekwochi U, Ndu IK, Nwokoye IC, Ezenwosu OU, Amadi OF, Osuorah DIC. Pattern of morbidity and mortality of newborns admitted into the sick and special care baby unit of Enugu State University Teaching Hospital, Enugu state. Niger J Clin Pract. 2014;17(3):346–351. doi: 10.4103/1119-3077.130238. [DOI] [PubMed] [Google Scholar]
- 33.Steer P. The epidemiology of preterm labor – a global perspective. J Perinat Med. 2005;33:273–276. doi: 10.1515/JPM.2005.053. [DOI] [PubMed] [Google Scholar]
- 34.Islam MN, Siddika M, Hossain MA, Bhuiyan MK, Ali MA. Morbidity pattern and mortality of neonates admitted in a tertiary level teaching hospital in Bangladesh. Mymensingh Med J. 2010;19(2):159–162. [PubMed] [Google Scholar]
- 35.Arafa MA, Alshehri MA. Predictors of neonatal mortality in the intensive care unit in Abha, Saudi Arabia. Saudi Med J. 2003;24(12):1374–1376. [PubMed] [Google Scholar]
- 36.Vaahtera M, Kulmala T, Ndekha M, et al. Antenatal and perinatal predictors of infant mortality in rural Malawi. Arch Dis Child Fetal Neonatal Ed. 2000;82:200–204. doi: 10.1136/fn.82.3.F200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worku B, Kassie A, Mekasha A, Tilahun B, Worku A. Predictors of early neonatal mortality at a neonatal intensive care unit of a specialized referral teaching hospital in Ethiopia. J Health Dev. 2012;26(3):200–207. [Google Scholar]
- 38.Shah S, Zemichael O, Meng HD. Factors associated with mortality and length of stay in hospitalised neonates in Eritrea, Africa: a cross-sectional study. BMJ Open. 2012;2(5):e000792. doi: 10.1136/bmjopen-2011-000792. [DOI] [PMC free article] [PubMed] [Google Scholar]