SCID is a heterogeneous group of genetic disorders resulting in severe T- and B-cell immunodeficiency with susceptibility to life-threatening infections.1,2 Because most patients lack the optimal donor for hematopoietic cell transplantation (HCT), an HLA-matched sibling donor (MSD),1 many SCID HCTs employ alternate stem cell sources: T-cell-depleted mismatched related donors (MMRDs); closely-HLA-matched unrelated adult donors (URDs); or unrelated umbilical cord blood (UCB). Because SCID-affected recipients are unable to reject HLA-matched allogeneic cells, MSD bone marrow transplantations (BMTs) are typically done without conditioning.1,2 This principle also applies to MMRD HCT with the exception that natural killer (NK) cells in certain SCID genotypes may resist donor engraftment.3,4 However, most URD and UCB HCTs have been performed using alkylator-chemotherapy conditioning, reflecting experience with HCT for non-SCID conditions.1,5
In a previously reported retrospective multicenter study, comparing unconditioned URD to MSD HCT for SCID, despite similar T-cell engraftment, 5-year survival was inferior in URD recipients.6 However, pre-HCT serotherapy in the URD group may have improved survival. Compared to MSD recipients, the URD group also had higher rates of acute GvHD. The genotypes of the unconditioned URD group were IL2RG (n = 27) and ADA (n = 7), both associated with lack of NK cells (NK−); RAG2 (n = 1) and DCLRE1C/Artemis (n = 1), both NK+; and unknown SCID genotypes (n = 7), with variable NK cells. The Artemis SCID patient engrafted, but died on day +145 from respiratory syncytial virus (RSV) and chronic GvHD. Thus, it remains unclear whether unconditioned URD HCT could provide T-cell reconstitution for NK+ SCID if infections and GvHD could be controlled. We report here successful unconditioned URD HCTs in NK+ IL7R and Artemis SCID.
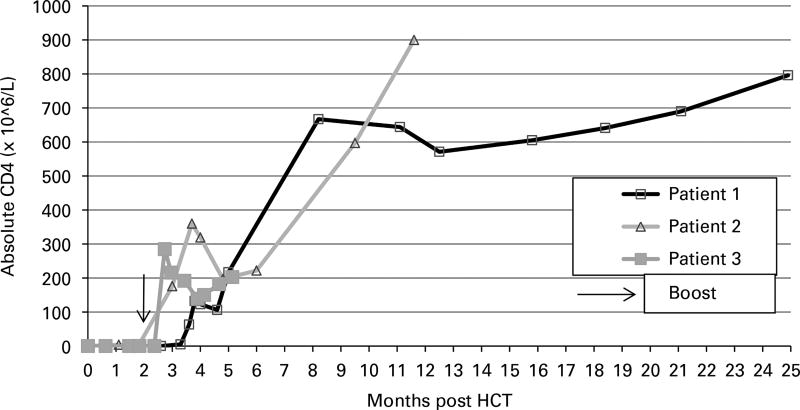
Patient 1, a 4-week-old, healthy Caucasian female, diagnosed with SCID following newborn screening (NBS) had IL7R compound heterozygous mutations c.361dupA, p.I121NfsX8; c.616C>T, p.R206X (reference sequences NM_002185.3, NP_002176, respectively). She had transplacental maternal engraftment (TME) of 14% of her CD3+ cells, however, her mother was medically unable to donate stem cells. An URD BMT was administered with alemtuzumab-only conditioning, which temporarily ablated her peripheral lymphocyte count (Table 1). She had an uneventful course post HCT without GvHD. Donor cells were detected (6% whole blood) by day +23, and host B cells rebounded promptly. However, she had slow T-cell recovery, with <10 × 106 CD3+ cells/L until day +100. After this, her CD4+ and CD8+ cells steadily increased, with 100% donor CD3+ cells. She achieved a CD4+ cell count of >200 × 106/L with a normal proliferative response to PHA by 5 months post HCT, at which point all prophylactic medications other than IgG infusions were discontinued and precautions were lifted (Figure 1). Her T-cell immunity at 40 months post BMT was excellent (Table 1). Despite no appreciable donor myeloid or B cells, IgG infusions were stopped at 21 months post HCT when she demonstrated normal IgA and IgM levels and class-switched memory B cells (CD19+/CD27+/IgM−/IgD−). She has made specific Abs to all inactivated and live vaccines.
Table 1.
Patient characteristics and T-cell immunity following unconditioned unrelated donor HCT
| Transplant characteristics | T-cell immunity at last F/U | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Gene | NK cells (×106 /L) |
Age at URD HCT |
Conditioning | HLA match | BM cell dose (×108 /kg) |
GvHD prophylaxis |
Boost | F/U (mo) |
CD4 (×106/L) |
CD8 (×106/L) |
Naive CD4/ CD45RA (×106/L) |
TRECs (per uL) |
PHA (% control) |
TCR spectratyping |
|
| Normal values | >870 | >472 | >138 | >25 | >50 | ≥20 | |||||||||
| Patient 1 | IL7R | 179 | 3.5 mo | Alema | 11/12 (HLA-DP) | 5 | Tacro/MTX | NA | 40 | 1044 | 1253 | 229 | 84 | 100 | 22/24 Polyclonal families |
| Patient 2 | DCLREC1 | 902 | 4.5 mo | Alema | 10/12 (HLA-B allele and -DP) | #1:7 #2:4.5 | CSA/MP | Day +64 | 28 | 900 | 378 | 567 | 29 | 100 | ND |
| Patient 3 | IL7R | 350 | 3 mo | Alema | 11/12 (HLA-DP) | #1:2.1 #2:2.1 | Tacro/MTX | Day +65 | 15 | 1315 | 1151 | 789 | 64 | 100 | 22/24 Polyclonal families |
Abbreviations: BM=bone marrow; CSA= cyclosporin; F/U =follow-up; HCT =hematopoietic cell transplant; mo=months; MP =methylprednisolone; MTX =methotrexate; NA =not applicable; ND= not done; NK =natural killer; Tacro =tacrolimus; TRECs =TCR excision circles; URD=unrelated donor.
Alemtuzumab given on days − 12 to − 10 (total dose 1.5 mg/kg).
Figure 1.
CD4+ cell recovery post HCT.
Patient 2, a 1-week-old, Navajo female, diagnosed with SCID following NBS was homozygous for the Navajo founder Artemis SCID mutation (c.597C>A, NM_001033855.2, rs121908157; p.Tyr199X). Oral ulcers typical of this genotype were present,7 but there was no detectable TME. At 1.8 months of age, she received an unconditioned maternal haploidentical CD34-selected PBSC transplantation4 following rabbit anti-thymocyte globulin (total dose of 3.5 mg/kg) on days −5 to − 1 in an attempt to eliminate host NK cells.8 Subsequently, she developed norovirus. When no donor cells were detected by day +41, she was considered non-engrafted. Therefore, an URD BMT was administered using alemtuzumab-only conditioning (Table 1). Following the URD BMT, she had no GvHD, but asymptomatic adenovirus viremia was detected, promptly clearing after cidofovir was initiated on day +49. Donor cells (6% whole blood) were detected by day +13; however T cells again became undetectable at day +56, and she received an unconditioned bone marrow boost (4.5 × 108 TNC/kg) on day +64 with cyclosporine and methotrexate as GvHD prophylaxis. By day +27 post boost, her CD4+ and CD8+ counts rose to 176 × 106/L and 51 × 106/L, respectively, with 100% donor CD3+ cells. She achieved a CD4+ cell count of >200 × 106/L with a proliferative response to PHA of >50% of the lower limit of control by 5 months post URD HCT, at which point she was taken off all prophylactic medications (other than IgG infusions) and precautions (Figure 1). Her T-cell immunity at 28 months post BMT is excellent (Table 1). She has no donor myeloid chimerism, and absent B-cell reconstitution makes continued IgG infusions necessary. Her norovirus has clinically resolved and stool is negative by EM.
Patient 3, a 2-week-old, Navajo female, diagnosed with SCID following NBS was homozygous for a novel homozygous damaging mutation in IL7R (J Puck, personal communication). She had thrush and diaper dermatitis, which responded to fluconazole and topical therapy. TME was negative, and an URD BMT was administered with alemtuzumab-only conditioning (Table 1). She had an uneventful course post HCT without GvHD. Donor cells were detected (2% whole blood) by day +20, and host B cells rebounded promptly. Slow T-cell recovery was observed, with <10 × 106 CD3+ cells/L through day +55, so she received an unconditioned bone marrow boost (2.1 × 108 TNC/kg) on day +65 with tacrolimus as GvHD prophylaxis. By day +17 post boost, her CD4+ and CD8+ cell counts rose to 285 × 106/L and 44 × 106/L, respectively, with 100% donor CD3+ cells. She achieved a CD4+ cell count of >200 × 106/L with a proliferative response to PHA of >50% of the lower limit of control by 3.5 months post initial BMT, at which point prophylactic medications and precautions (other than IgG infusions) were stopped (Figure 1). Her T-cell immunity at 15 months post BMT is excellent (Table 1). Despite no appreciable donor myeloid or B cells, by 5 months post HCT, her IgA and IgM levels normalized. She remained on IVIg while awaiting B-cell phenotyping.
These cases illustrate the ability of unconditioned well-matched URD BMT to achieve excellent T-cell reconstitution (and thymopoiesis as indicated by naive T cells and TRECs) in NK+ SCID. A previous report demonstrated high GvHD rates, and that the use of serotherapy pre-HCT showed a trend toward improved overall survival, therefore we used pre-HCT alemtuzumab for all three patients. This has also been hypothesized to prevent NK-mediated rejection in patients with NK+ SCID.9 We were successful in preventing GvHD; however, the optimal dosage in young children is not established, and serotherapy may significantly delay homeostatic memory T-cell expansion by eliminating infused donor T cells.10 Therefore, patience must be utilized with this approach, allowing sufficient time to pass before naive thymopoiesis can be expected, though low-level donor chimerism can be seen within 2–3 weeks post HCT. This approach was facilitated by the fact that all three patients were diagnosed by NBS and were relatively free of serious infections at the time of HCT; it may not be appropriate for actively infected patients. While we performed boosts on two patients, ultimately their T-cell recovery kinetics mirrored that of the non-boosted patient, suggesting that this may not have been necessary. In the absence of infection, we now advocate waiting at least 3 months to allow time for de novo T-cell production to occur.
IL7R deficiency is an ideal SCID genotype for this approach, since host B cells typically function normally when adequate donor T-cell support is achieved.5,11 Unconditioned URD HCT is also attractive for patients with Artemis deficiency to avoid longterm adverse effects of alkylating-agent chemotherapy, though these patients are likely to remain B-cell deficient.7 After allowing time for additional physical and neurocognitive development, a second HCT from the original donor may be considered, conditioned with a marrow space-making agent.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley R. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: The Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133:1092–1098. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak C, Hung G, Horn B, Dunn E, Oon C, Cowan M. Megadose CD34(+) cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2008;14:1125–1133. doi: 10.1016/j.bbmt.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Haddad E, Leroy S, Buckley R. B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131:994–1000. doi: 10.1016/j.jaci.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorak C, Hassan A, Slatter M, Hönig M, Lankester A, Buckley R, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134:935–943. doi: 10.1016/j.jaci.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz C, Neven B, Dvorak C, Leroy S, Ege M, Pannicke U, et al. SCID patients with ARTEMIS versus RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS deficient SCID. Blood. 2013;123:281–289. doi: 10.1182/blood-2013-01-476432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stauch D, Dernier A, Sarmiento Marchese E, Kunert K, Volk H-D, Pratschke J, et al. Targeting of natural killer cells by rabbit antithymocyte globulin and campath-1H: similar effects independent of specificity. PLoS ONE. 2009;4:e4709. doi: 10.1371/journal.pone.0004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak C, Horn B, Puck J, Adams S, Veys P, Czechowicz A, et al. A trial of alemtuzumab adjunctive therapy in allogeneic hematopoietic cell transplantation with minimal conditioning for severe combined immunodeficiency. Pediatr Transplant. 2014;18:609–616. doi: 10.1111/petr.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willemsen L, Jol-van der Zijde C, Admiraal R, Putter H, Jansen-Hoogendijk A, Ostaijen-Ten Dam M, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant. 2015;21:473–482. doi: 10.1016/j.bbmt.2014.11.674. [DOI] [PubMed] [Google Scholar]
- 11.Buckley R, Win C, Moser B, Parrott R, Sajaroff E, Sarzotti-Kelsoe M. Post-transplantation B cell function in different molecular types of SCID. J Clin Immunol. 2013;33:96–110. doi: 10.1007/s10875-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]