Abstract
Objectives
To improve margin revision, this study characterizes the number, fragmentation, and orientation of tumor bed margins (TBM) in patients with pT1-2 pN0 squamous cell carcinoma (SCC) of the oral tongue.
Materials and Methods
Pathology reports (n = 346) were reviewed. TBM parameters were indexed. In Group 1 patients all margins were obtained from the glossectomy specimen and there were no TBM. In Revision Group/Group 2 (n = 103), tumor bed was sampled to revise suboptimal margins identified by examination of the glossectomy specimen. In Group 3 (n = 124), TBM were obtained before examination of the glossectomy specimen.
Results and Conclusions
Fewer TBMs were obtained per patient in Group 2 compared to Group 3 (57/103, 55% of patients with < 3 vs. 117/124, 94%, ≥3 TBMs, respectively). The new margin surface was more frequently indicated in Group 2 compared to Group 3 (59/103, 57%, vs. 19/124, 15%, p < .001). If glossectomy specimen margins are accepted as the reference standard, then the TBM was 15% sensitive in Group 2 (95% confidence interval [CI], 7–29) and 32% sensitive in Group 3 (95% CI, 15–55). TBM fragmentation (23/103, 22% vs. 42/124, 34%) and frozen vs. permanent discrepancies (8/103, 3% vs. 3/124, 2%) were similar between Groups 2 and 3. The new margin surface was not indicated in 6 of 11 cases with discrepant frozen vs. permanent pathology findings, precluding judgment on final margin status. To facilitate the assessment of final margins, TBM should be represented by one tissue fragment with a marked new margin surface.
Keywords: Tumor bed margin, Specimen margin, Margin orientation, Margin fragmentation, Revision, Squamous cell carcinoma, Oral tongue
Introduction
Margin evaluation is performed to assess the adequacy of tumor removal [1]. One of the quality initiatives introduced by the American Head and Neck Society deals with the management of patients with oral squamous cell carcinoma (SCC) extending to margins [2]. There are two major approaches to the sampling of margins [3–7]. In the specimen-driven approach, margin clearance is assessed from en bloc resection specimens. Studies have shown that the most relevant margins are those derived from the resection specimen [8–16]. In the defect-driven approach, the tumor bed is sampled after the primary resection.
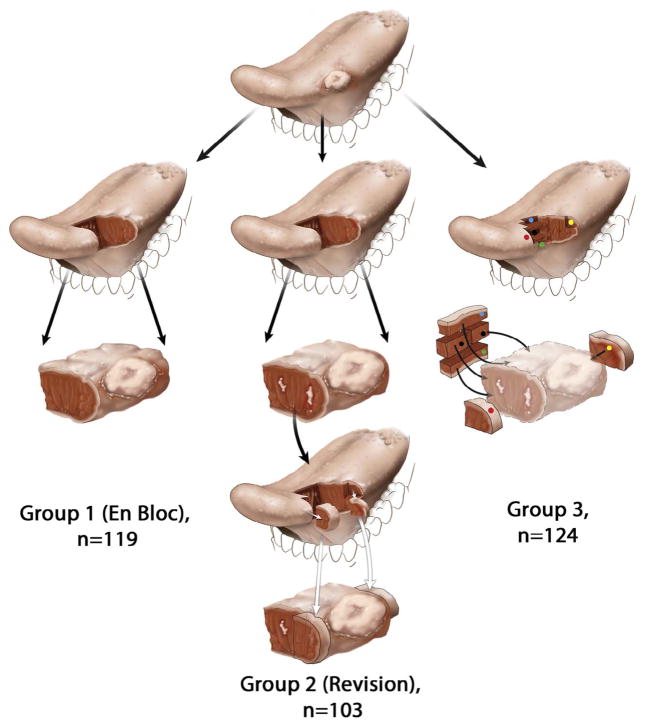
Tumor bed margins are obtained in two distinct scenarios (Fig. 1): (1) Upfront, without review of the resection specimen, and (2) To revise an inadequate margin identified by the review of the resection specimen. Margin revision is unavoidable in a subset of patients. However, as performed currently, margin revision does not improve clinical outcome. [8,11,12,17–19] A better understanding of the technical characteristics of tumor bed margins (i.e., number, fragmentation, orientation as to the new margin surface) may improve the quality of margin revision. The objective of this project was to characterize these technical characteristics of tumor bed margins in patients with pathological (p)T1-2 pN0 SCC of the oral tongue.
Fig. 1.
Schematic representation of the glossectomy workflow. For simplicity, a predominantly exophytic tumor at the lateral edge of the oral tongue is illustrated, with the row representing the next step in the workflow. In Group 1 (left column), tumor bed margins were not sampled. In Group 2 (middle column), margins were examined from the glossectomy specimen and found to be positive or otherwise suboptimal. The surgeon revised margins by obtaining additional tissue from the tumor bed. White irregular areas in the anterior aspect of glossectomy specimen represent residual carcinoma at the initial anterior margin (third row). The surgeon revised anterior margin by obtaining additional tissue from the tumor bed, representing a new anterior margin (fourth row). To imagine the relationship between the actual glossectomy margins and additional tissue, the two types of margins are superimposed in the fifth row. Due to the challenges of relocating the exact aspect of the relevant anterior margin in the tumor bed, size discrepancy, and uncertain orientation of the additional tissue, it is conceivable that in some cases the revised margin may not actually cover the entire residual tumor at the anterior glossectomy margin. In Group 3 (right column), five margins are primarily sampled from the tumor bed (red, green, yellow, blue, and black dots), without prior examination of the glossectomy specimen (displayed in lighter colors in the third row) by the pathologist.
Material and methods
Study population
Surgical pathology reports were reviewed for oral tongue SCC treated from 1986 to 2014. The studied patients satisfied the following inclusion criteria:
pT1 or pT2 primary SCC of the oral tongue;
Elective neck dissection with histologically proven benign lymph nodes (pN0).
Conventional SCC morphology. (4) Lack of pre-operative treatment.
This work was approved by the Total Quality Council, University of Pittsburgh Medical Center. Surgical pathology reports were reviewed for patients’ age, gender, type of glossectomy, pT as per the 7th edition of the American Joint Committee on Cancer (AJCC) [20], status of the glossectomy specimen margins, and characteristics of tumor bed margins (orientation, labeling, fragmentation, positive versus negative). The presence of invasive or in situ carcinoma at the margin was considered a positive margin. The status of both glossectomy and tumor bed margins was examined as a binary variable (positive versus negative). Margins assessed from the glossectomy specimen were referred to as “glossectomy margins”. Margins obtained from the tumor bed (also known as margins form wound, cavity, defect, or patient) were referred to as “tumor bed margins”.
Only patients with glossectomy that included sampling of tumor bed are in the current study. Therefore, 119 patients from the prior study by Maxwell et al. [12] were not included in the current study. Two institutions who participated in the Maxwell et al. study could not provide additional technical details (e.g., pathology reports were in German) or could contribute fewer than 10 cases and were excluded. Five new institutions took part in the current study, contributing data on 139 new patients with early SCC of the oral tongue (with surgery that included sampling of the tumor bed). Overall, of the 227 patients in Groups 2 and 3, only 89 patients were part of the prior study by Maxwell et al. Of note, the technical data on tumor bed margins presented in this manuscript were not previously described in the prior study.
Margin sampling and surgical workflow analysis
The surgical workflow for all patients included in this study was categorized according to the surgeon’s preferred approach to margin sampling and surgical intra-operative findings (Fig. 1) [12].
Briefly, in Group 1, all margins were obtained from the glossectomy specimen. This scenario reflects an en bloc glossectomy with clinically and/or pathologically satisfactory margins as determined by surgeons or pathologists at the time of surgery. Patients from Group 1 were not included in this study since tumor bed margins were not sampled.
In Group 2 (revision group), glossectomy specimens were intraoperatively examined, found to be positive or otherwise suboptimal (i.e., distorted, ulcerated, or lacking normal mucosa at the mucosal margin), and the surgeon revised the margins by obtaining additional tissue from the tumor bed. Revision was performed as a second procedure only in 6 cases. The intent to revise was reflected in the labeling: such tumor bed margins were labeled as “new”, “additional”, “#2”, “revised”, “supplemental”, “repeat”, or “re-resection”.
In Group 3, glossectomy specimens were not intraoperatively examined by a pathologist and margins were primarily sampled from the tumor bed. In some cases (n = 7), surgeons’ reliance on tumor bed margins was reflected in labeling main resection specimen as “non-marginal”.
Statistical analysis
Differences between Groups 2 and 3 were analyzed with Fisher’s exact test for categorical data. All statistical analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
Tumor bed margins were obtained in 227 of 346 (66%) patients with pT1-2 pN0 SCC of the oral tongue (Fig. 1). There was no difference in basic clinicopathologic parameters among patients in Groups 2 and 3 (Table 1). Tumor bed margin characteristics are summarized in Table 2.
Table 1.
Patient Clinical and Pathologic Characteristics.
| Total, n = 227 | Group 2, Revision Group (n = 103) | Group 3, Tumor Bed Margin Group (n = 124) | |
|---|---|---|---|
| Age, median (range), years | 58 (23–83) | 59 (24–85) | |
| Sex, n (%) | Men | 50 (49) | 67 (54) |
| Women | 53 (52) | 57 (46) | |
| Procedure, n (%) | Partial glossectomy | 89 (86) | 111 (90) |
| Hemiglossectomy | 11 (11) | 13 (11) | |
| Subtotal glossectomy | 3 (3) | 0 (0) | |
| pT, n (%) | T1 | 59 (57) | 72 (58) |
| T2 | 44 (43) | 52 (42) |
Table 2.
Tumor Bed Margin Characteristics.
| Total, n = 227 | Revision Group (n = 103) | Tumor Bed Margin Group (n = 124) | P value | ||
|---|---|---|---|---|---|
| Cases with positive glossectomy margin, n (%) | 52 (50) | 22 (18) | < 0.001 | ||
| Cases with positive tumor bed margin, n (%) | 8 (8) | 7 (6) | 0.6 | ||
| Fragmentation, n (%) | 23 (22) | 42 (34) | 0.07 | ||
| Oriented Tumor Bed Margins, n (%) | 59 (57) | 19 (15) | < 0.001 | ||
| True margin indicated | Yes | For all margins (in cases with > 1 tumor bed margin), n (%) | 46 (45) | 5 (4) | < 0.001 |
| For some margins (in cases with > 1 tumor bed margin), n (%) | 13 (13) | 14 (11) | |||
| Frozen versus permanent sampling discrepancy, n (%) | 8 (8) | 3 (2) | 0.07 | ||
Primary reliance on tumor bed margins is associated with sampling of ≥3 tumor bed margins per patient
Fewer tumor bed margins per patient (or procedure) were obtained in the revision group/Group 2 compared to Group 3 (Fig. 2). For Group 2 patients, most commonly one tumor bed margin was revised per procedure (41/103, 40%).
Fig. 2.
Approach to margin assessment and number of tumor bed margins per patient. For more than half of Group 2 patients (57/103, 55%) < 3 tumor bed margins were taken. In Group 3, ≥3 tumor bed margins were obtained in all but 7 patients (117/124, 94%).
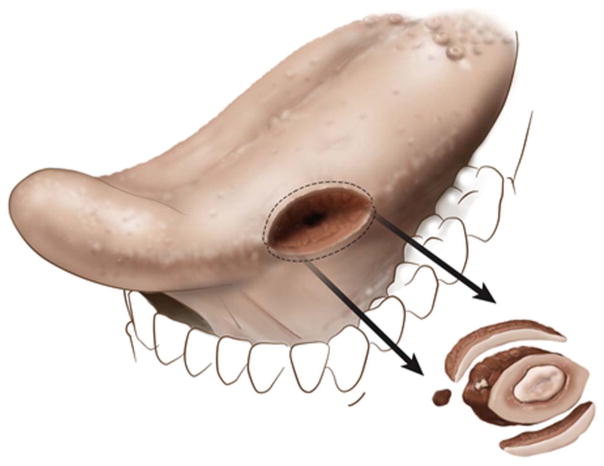
In contrast, 5 tumor bed margins per patient were commonly 52/124 (42%) obtained in Group 3 cases. The smallest carcinomas in Group 3 (n = 9, 16.5 mm, median) were accompanied by 3 tumor bed margins (Fig. 3).
Fig. 3.
An illustration of how tumor bed margins are likely sampled for smaller tumors (partial glossectomy with 3 tumor bed margins).
Examination of the resection specimen does not improve localization of positive tumor bed margins
As expected, the number of cases with at least one positive glossectomy specimen margin was higher in the revision group/Group 2 (Table 2). However, the number of positive tumor bed margins was equal in Groups 2 and 3. This finding is somewhat counterintuitive as in the revision scenario tumor bed sampling was guided by the results of margins’ assessment from the resection specimen. This finding confirms the challenges of re-localizing the area of concern in the tumor bed [21].
Low sensitivity of tumor bed samples for positive glossectomy specimen margins
Sensitivity of the tumor bed margin was defined as “the ability of the tumor bed biopsy to predict positive glossectomy specimen margin”. When margins obtained from the glossectomy specimen are accepted as the reference standard, then the tumor bed margin was 15% sensitive (95% confidence interval [CI], 7–29) in the revision group/Group 2 and 32% sensitive (95 CI, 15–55) in Group 3.
Fragmentation of tumor bed margins
In both groups, tumor bed margins are commonly fragmented (Table 2).
Orientation of tumor bed margins: Uncertain new margin surface
In the revision group/Group 2, tumor bed margins are more likely to be oriented as to the new margin surface (Table 2, Fig. 4): in 45% of cases (46/103), the new margin surface was indicated for all revised margins.
Fig. 4.
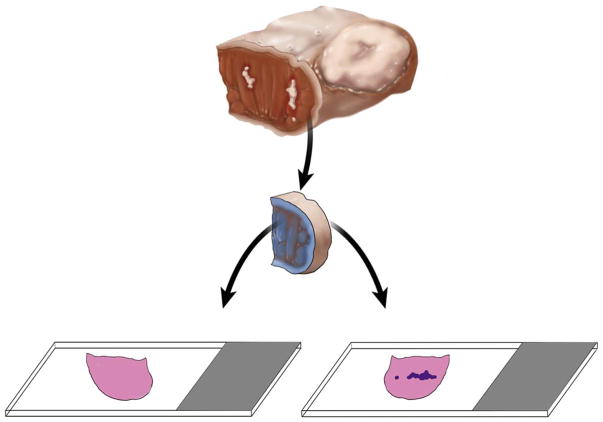
Unequivocal orientation of the revised tumor bed margin. The new margin surface of the revised anterior margin from left partial glossectomy is indicated by ink. The white irregular area in the anterior aspect of glossectomy specimen represents residual carcinoma at the initial anterior margin. The dark blue irregular area on the right glass slide represents tumor. Without orientation, the surface to be first examined intraoperatively is picked randomly. When indicated, the new margin surface will be examined first intraoperatively. If the frozen section is negative for tumor, but the permanent section of the frozen remnant reveals tumor, the overall margin status is “close, but negative”. Such determination is impossible if the tumor bed margin was fragmented or the new true margin surface was not indicated. In addition to ink, new margin surface can be indicated by a stitch, clip, or directly by surgeon. This revision margin is thin and is best processed as shave margin. Thicker margins can be processed as radial margins (new margin surface re-inked, cut into, and embedded on edge).
The new margin surface was identified by ink, stitch, or clip (Table 3, Figures 4 and 5). In 40% (31/78) of cases, only anatomic orientation (i.e., anterior) was provided, making it difficult to deduce the new margin surface, especially when the main resection specimen is not available intraoperatively (Fig. 5).
Table 3.
Methods of indicating new margin surface on tumor bed margins.
| Method | Number of cases (n = 78) |
|---|---|
| Tumor bed margin oriented as to anterior or other aspect, without unequivocal indication of the new margin (Fig. 4), n | 31 |
| Ink at new margin, n | 22 |
| Suture/stitch at new margin, n | 16 |
| New margin unstained, n | 4 |
| Oriented by surgeon, n | 3 |
| Not specified, n | 2 |
Fig. 5.
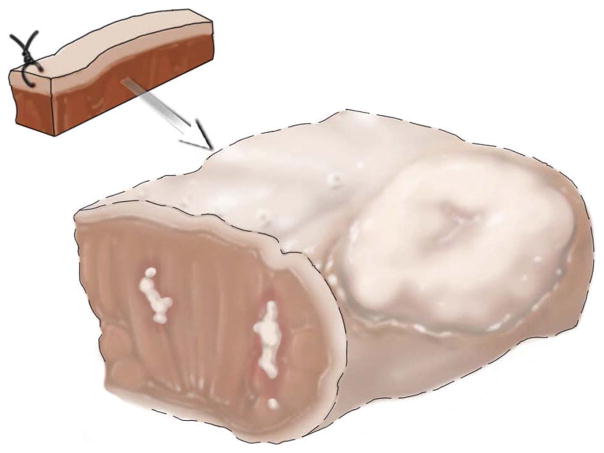
Indirect orientation of the revised tumor bed margin. The anterior aspect of the new margin is indicated by stitch. However, if the relationship between the tumor bed margin and main resection specimen is unknown, the new margin surface may be difficult to figure out.
Frozen versus final pathology sampling issues
There were 11 patients with frozen versus permanent pathology sampling discrepancies. In 9 tumor bed margins, the carcinoma was seen on permanent section of the frozen section remnant only (i.e., negative “frozen” and positive “permanent”). In 2 cases, the carcinoma was seen on frozen section only. In 6 of 11 cases with discrepant frozen and final pathology findings, the new margin surface was not indicated. There was one diagnostic interpretive error (Group 3).
Discussion
It has been previously shown that reliance on tumor bed margins alone (Group 3 in this study) correlates with worse local control and should no longer be considered a viable method of margin assessment [6,7,9,11–13,15,16]. Prognostically relevant margin information is best derived from the resection specimen. The specimen-driven approach to margin sampling is now endorsed by the 8th edition of American Joint Cancer Committee staging manual. Similarly, the oral cavity cancer checklist by the College of American Pathologists (CAP) prompts reporting of margins from the resection specimen.
In this retrospective multi-institutional study, surgeons primarily relied on tumor bed margins in 124/346 (36%) of patients. Some resected specimens were labeled as “non-marginal tissue”. This discourages pathologists from reporting specimen-derived margins. Tumor bed margins have a sensitivity of 15 to 32% for identifying positive margins from resection specimens, indicating that tumor bed biopsies are a sub-optimal method for “mapping out” a tumor [22].
As performed currently, margin revision does not improve clinical outcome (e.g., local recurrence rate). [8,11,13,17–19] In this study, we highlighted some of the technical parameters that may help to assess the quality of the revision. In Group 3, in 94% of cases there were ≥3 tumor bed biopsies, 85% of which were not oriented as to the margin surface and 34% of which were fragmented.
Any methods of unequivocally indicating new margin surface – by stitch, ink, clip, or directly by surgeon – are most helpful. The lack of orientation as to the new margin surface (Fig. 4) and fragmentation make it uncertain which surface of the tumor bed margin should be examined first intraoperatively. The lack of orientation also precludes adequate resolution of frozen versus permanent pathology sampling issues. In the absence of tumor bed margin orientation (Fig. 4), the assumption that frozen section results are always representative of margin status is baseless. When the new margin surface is not indicated, it is unclear whether it is represented on frozen or permanent section. It is also unclear whether, by cutting deeper into the tissue block, one gets closer to or further from the actual margin.
Fragmentation of the tumor bed margins complicates the understanding of the spatial relationship between the main resection specimen and revised margin. Without reconstructing the relationship between the tumor bed margin and resected specimen, pathologists cannot determine whether the new margin actually adequately fits (in terms of the size and shape) to the revised aspect of the resected specimen.
This retrospective study of tumor bed margins is based on review of surgical pathology reports and can reliably address only such margins’ parameters as number, orientation, sensitivity, sampling discrepancies, and fragmentation. This study is limited in its ability to reliably assess size and site of tumor bed margins. Empirically, many tumor bed margins are rather small and thin [3] (e.g., < 3 mm). Thus while tumor bed margins may be technically “negative,” the overall final margin may actually still be “close.” While revisiting the definition of a “close” margin was not an aim of this study, margin clearance has been shown to inversely correlate with recurrence rate [11]. Suboptimal re-localization of the area of concern in the tumor bed is a known limitation: relocation of the site of interest in a tumor bed may be off target by about 1 cm in 1/3 of cases [21].
The details of tumor bed margins described here are critical for deciding on the final margin status in cases with discrepant tumor bed and resection specimen margins. In this study, in 44 of 52 cases with attempted margin revision, the new tumor bed margin was negative while the corresponding initial resection specimen margin was positive. To reconcile such margins, one has to consider the shape, tissue type, size, and orientation of the tumor bed margin. Practically, pathologists have the following options [7]:
Acknowledge adequate revision and state that the final overall margin is negative;
State that the final margin status cannot be determined when the revised margin is fragmented or un-oriented as to the new margin surface;
State the inadequacy of margin revision and report margin status based on the resection specimen only. The latter scenario is most appropriate if the revised margin is unoriented, fragmented, and too small to “cover” the carcinoma at the resection specimen margin.
In summary, the additional tissue obtained from the tumor bed should be represented by one oriented tissue fragment; this facilitates the assessment of revised margins by pathologists. When tumor bed sampling is not guided by the examination of the glossectomy specimen, the number of tumor bed margins is higher. The common lack of orientation of the tumor bed margins prevents resolution of frozen versus permanent discrepancies.
Acknowledgments
The authors wish to thank Laura Sesto for medical illustrations. A subset of reports was retrieved using the Text Information Extraction System, a system supported by Grant Number R01 CA132672 from the National Cancer Institute (NCI) and Grant Number 2ULRR024153 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Conflict of Interest and Disclosure
None.
References
- 1.Weinstock YE, Alava I, 3rd, Dierks EJ. Pitfalls in determining head and neck surgical margins. Oral Maxillofac Surg Clin North Am. 2014;26:151–62. doi: 10.1016/j.coms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY. Quality initiatives in head and neck cancer. Curr Oncol Rep. 2010;12:109–14. doi: 10.1007/s11912-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 3.Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107:2792–800. doi: 10.1002/cncr.22347. [DOI] [PubMed] [Google Scholar]
- 4.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27:952–8. doi: 10.1002/hed.20269. [DOI] [PubMed] [Google Scholar]
- 5.Hinni ML, Ferlito A, Brandwein-Gensler MS, Takes RP, Silver CE, Westra WH, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35:1362–70. doi: 10.1002/hed.23110. [DOI] [PubMed] [Google Scholar]
- 6.Varvares MA, Walker RJ, Chiosea S. Does a specimen-based margin analysis of early tongue cancer better predict local control? Laryngoscope. 2016;126:2426–7. doi: 10.1002/lary.26081. [DOI] [PubMed] [Google Scholar]
- 7.Chiosea SI. Intraoperative margin assessment in early oral squamous cell carcinoma. Surg Pathol Clin. 2017;10:1–14. doi: 10.1016/j.path.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Patel RS, Goldstein DP, Guillemaud J, Bruch GA, Brown D, Gilbert RW, et al. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32:1444–51. doi: 10.1002/hed.21334. [DOI] [PubMed] [Google Scholar]
- 9.Yahalom R, Dobriyan A, Vered M, Talmi YP, Teicher S, Bedrin L. A prospective study of surgical margin status in oral squamous cell carcinoma: a preliminary report. J Surg Oncol. 2008;98:572–8. doi: 10.1002/jso.21034. [DOI] [PubMed] [Google Scholar]
- 10.Amit M, Na’ara S, Leider-Trejo L, Akrish S, Cohen JT, Billan S, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck. 2016;38(Suppl 1):E1803–9. doi: 10.1002/hed.24320. [DOI] [PubMed] [Google Scholar]
- 11.Chang AM, Kim SW, Duvvuri U, Johnson JT, Myers EN, Ferris RL, et al. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49:1077–82. doi: 10.1016/j.oraloncology.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell JH, Thompson LD, Brandwein-Gensler MS, Weiss BG, Canis M, Purgina B, et al. Early Oral Tongue Squamous Cell Carcinoma: Sampling of Margins From Tumor Bed and Worse Local Control. JAMA Otolaryngol Head Neck Surg. 2015;141:1104–10. doi: 10.1001/jamaoto.2015.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125:2298–307. doi: 10.1002/lary.25397. [DOI] [PubMed] [Google Scholar]
- 14.Baddour HM, Magliocca KR, Chen AY. The importance of margins in head and neck cancer. J Surg Oncol. 2016;113:248–55. doi: 10.1002/jso.24134. [DOI] [PubMed] [Google Scholar]
- 15.Ettl T, El-Gindi A, Hautmann M, Gosau M, Weber F, Rohrmeier C, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17–23. doi: 10.1016/j.oraloncology.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142:1191–8. doi: 10.1001/jamaoto.2016.2329. [DOI] [PubMed] [Google Scholar]
- 17.Guillemaud JP, Patel RS, Goldstein DP, Higgins KM, Enepekides DJ. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2010;39:370–7. [PubMed] [Google Scholar]
- 18.Kwok P, Gleich O, Hubner G, Strutz J. Prognostic importance of “clear versus revised margins” in oral and pharyngeal cancer. Head Neck. 2010;32:1479–84. doi: 10.1002/hed.21349. [DOI] [PubMed] [Google Scholar]
- 19.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg. 1986;152:354–60. doi: 10.1016/0002-9610(86)90304-1. [DOI] [PubMed] [Google Scholar]
- 20.Edge SBDRB, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. New York: Springer; 2009. [Google Scholar]
- 21.Kerawala CJ, Ong TK. Relocating the site of frozen sections–is there room for improvement? Head Neck. 2001;23:230–2. doi: 10.1002/1097-0347(200103)23:3<230::aid-hed1023>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Hinni ML, Zarka MA, Hoxworth JM. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope. 2013;123:1190–8. doi: 10.1002/lary.23900. [DOI] [PubMed] [Google Scholar]