Synopsis
This article provides a brief review of recent investigations concerning the structure and properties of the tooth. The last decade has brought a greater emphasis on the “durability” of the tooth, an improved understanding of the fatigue and fracture behavior of the principal tissues and their importance to tooth failures. The primary contributions to tooth durability are discussed, including the process of placing a restoration, the impact of aging, and challenges posed by the oral environment. The significance of these findings to the dental community, and their importance to the pursuit of lifelong oral health are highlighted.
Keywords: aging, dentin, durability, enamel, fatigue, fracture, tubules
Introduction
It seems appropriate to begin this review of the structure and properties of the tooth from the foundation provided by previous reviews of relevance. For instance, Pashley [1] reviewed the microstructure of dentin, and the variations in tubule density and dimensions from the deep to the peripheral regions. He discussed their influence on fluid movement within the lumens, i.e. the fluid dynamics, as well as contributions of the smear layer developed during cutting to dentin permeability. Results from that body of work established the importance of the smear layer to adhesive dentistry, and have guided the development of products to treat dentin sensitivity.
Marshall et al. [2] also reviewed the structure and properties of dentin, and placed emphasis on adhesive bonding due to the transition taking place in restorative materials. That review provided a comprehensive discussion of sclerotic and transparent dentin, as well as demineralized, remineralized, and hypermineralized forms. These “altered forms” exhibit distinct microstructures, which are important to acid etching and bonding [e.g. 3,4]. The importance of location and tubule orientation to the mechanical behavior of dentin were also highlighted, as well as the need for adopting site-specific descriptions of properties that could be developed using instrumented indentation.
Kinney et al., [5] presented a comprehensive review of the structure and mechanical behavior of dentin, the first after decades. That review serves as the bible on the mechanical behavior of dentin and now guides the way we think about the tooth as a load bearing structure. More emphasis was placed on the importance of the collagen, and its contribution to the elastic properties and viscoelastic behavior of dentin. This review also started the discussion of flaws, including their contributions to the strength, and fatigue behavior. Kinney et al [5] proposed that a fracture mechanics approach should be adopted to describe the strength of dentin, especially when considering the importance of changes in microstructure associated with the “altered forms”.
The last decade has brought greater interest in, and understanding of, the tooth’s durability. Therefore, the objective of this review is to discuss the structure and properties most relevant to tooth durability, with an emphasis on their importance to clinical practice.
Damage and Flaws
The mechanical forms of tooth failure originate at defects, which could be intrinsic, or extrinsic, e.g. as a result of treatment or induced during function. Defects within the tooth structure reduce its capacity to bear loads, thereby reducing its resistance to the forces of mastication.
Cracks in teeth facilitate tooth fracture [6,7], but how do they develop? This question is being debated in the fields of endodontics and prosthodontics. In endodontics, the concern is whether flaws are introduced during instrumentation of the canal and if they are the cause of vertical root fractures. Studies have reported that damage is introduced during instrumentation of the canal [8–10], while others have shown there is no difference in the number of microcracks within instrumented teeth vs. controls (i.e. without instrumentation) [11–13]. This debate is ongoing and has yet to address the residual strength of a tooth with defects.
Recent investigations have explored if the introduction of cavity preparations and adhesive bonding reduce the tooth’s durability. For instance, laser cutting preparations were shown to introduce cracks in dentin [14] that reduce the strength. However, Sehy and Drummond [15] could not identify visible or microscopic cracking in dentin after performing bur treatments. Majd et al [16] evaluated the influence of bur cutting and an abrasive air jet treatment on the fatigue strength of coronal dentin. Despite an increase in surface roughness with respect to control surfaces and development of a smear layer, flaws or cracks were not evident in the prepared surfaces and there was no change to the static strength. However, both treatments caused a significant reduction in the fatigue strength of dentin, with bur cutting resulting in nearly 40% reduction in the fatigue limit.
More recently, Majd et al. [17] explored the influence of cutting direction on the fatigue strength of tooth structure. Treatments involved cutting of coronal dentin using a medium-grit diamond in parallel and transverse cutting directions with respect to the direction of cyclic tensile stress. A reduction in static strength of approximately 20% resulted from cutting in each direction. Results from cyclic loading (Fig. 1A) showed that the cutting caused a significant reduction in fatigue strength; the largest decrease (nearly 60%) resulted from cutting in the perpendicular direction. The messages from these studies are that: 1) the process of cutting dentin with either carbide or diamond abrasive burs introduces flaws within dentin, and 2) although these flaws are not visible, they undergo growth as a result of cyclic loading until reaching a length that enables failure. The results also indicate that the fatigue strength of dentin is very sensitive to the presence of flaws [18]. Due to the substantial reduction in fatigue strength by diamonds, they should not be used in the cutting of dentin. As diamonds are regularly used in the clinic, this deserves immediate attention.
Fig. 1.
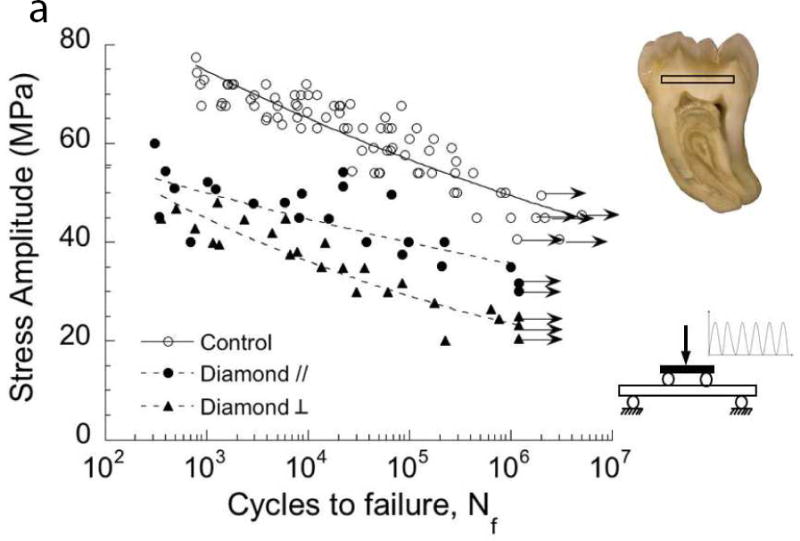
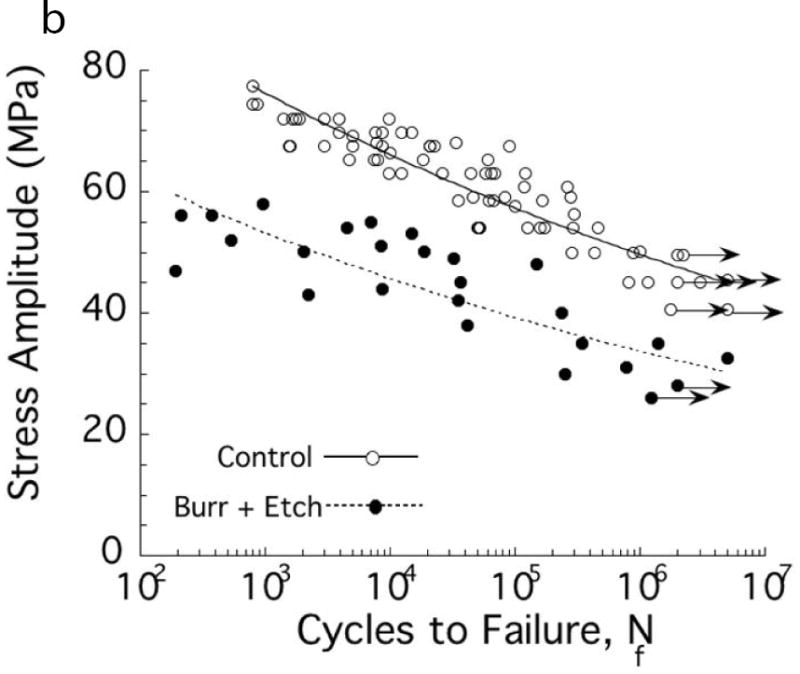
Importance of damage introduced during cavity preparations on the fatigue strength of coronal dentin. The “control” in these two diagrams consists of beams of coronal dentin prepared with diamond slicing wheels, and with average surface roughness (Ra) of less than 0.2 μm. (A) Comparison of the fatigue strength after cutting treatments with medium diamond abrasive and water spray irrigation. Results are shown for cutting parallel (//) and perpendicular (□) to the length of the beam, which is important to the orientation of damage. Data points with arrows represent beams that did not fail and the test was discontinued. (B) Comparison of the fatigue strength distribution of the control with dentin beams subjected to a bur cutting treatment followed by a 15 second etch with 37.5% gel. Cutting was performed with a 6-flute tungsten carbide straight fissure bur and commercial air turbine with water spray irrigation.
A: Adapted from Majd B, Majd H, Porter JA, et al. Degradation in the fatigue strength of dentin by diamond bur preparations: Importance of cutting direction. J Biomed Mater Res B Appl Biomater 2016;104(1):39–49; with permission.
B: Adapted from Lee HH, Majd H, Orrego S, et al. Degradation in the fatigue strength of dentin by cutting, etching and adhesive bonding. Dent Mater 2014;30(9):1061–72; with permission.
Etching generally follows cutting of the cavity preparation. That process could alter the surface generated by cutting. Lee et al., [19] explored whether etching and adhesive bonding improved the durability of dentin after cutting. The fatigue strength distribution of dentin subjected to bur treatment followed by etching for 15 sec, with that of “flaw-free” controls is shown in Fig. 1B. Etching does not improve the fatigue strength after bur cutting. That study also showed that etching alone, sans cutting, caused a reduction in fatigue strength and that application of a resin adhesive afterwards did not improve the fatigue strength [19]. Hence, the processes used in placing bonded restorations introduce flaws that reduce the tooth’s durability.
Damage may also be introduced in enamel as a result of cutting and the placement of restorations. The microstructure of enamel resists the growth of this damage and will be discussed later. Cyclic contact is also a source of damage. Indeed, due to the improvements in clinical success of dental ceramics, contact between these “engineered” materials and natural tooth structure is increasingly common. Damage to the opposing natural tooth enamel is a relevant concern.
Gao et al [20] recently evaluated the damage resulting from contact fatigue of enamel with ceramic restorations. Results from cyclic loading experiments are presented in terms of a contact load-life diagram in Fig. 2A. As expected, there is a decrease in number of cycles to failure (i.e. life) with increasing contact load. This outcome reflects the importance of occlusal adjustment to avoid concentration of contact stress and its magnitude. Furthermore, enamel does not exhibit a “fatigue limit”, i.e. all cyclic loads caused damage and there is no minimum load that the enamel can bear an infinite number of cycles. An example of the contact fatigue damage due to cyclic loading at a maximum load of 400 N is shown in Fig. 2B. The indentations exhibited two separate families of cracks, including cylindrical cracks within the contact area and radial cracks extending outside the contact region. Both the length and number of radial cracks increased with the magnitude of contact loads. The cracks also developed at contact stresses well below those necessary for damage under static indentations [21]. The changes in surface roughness and damage could facilitate bacterial adhesion, bacterial penetration, or even tooth fracture. Contact fatigue damage in the opposing enamel should be considered in the future development of materials for crown replacement. Clearly, greater clinical focus on opposing tooth structure to crowns and their prognosis is warranted.
Fig. 2.
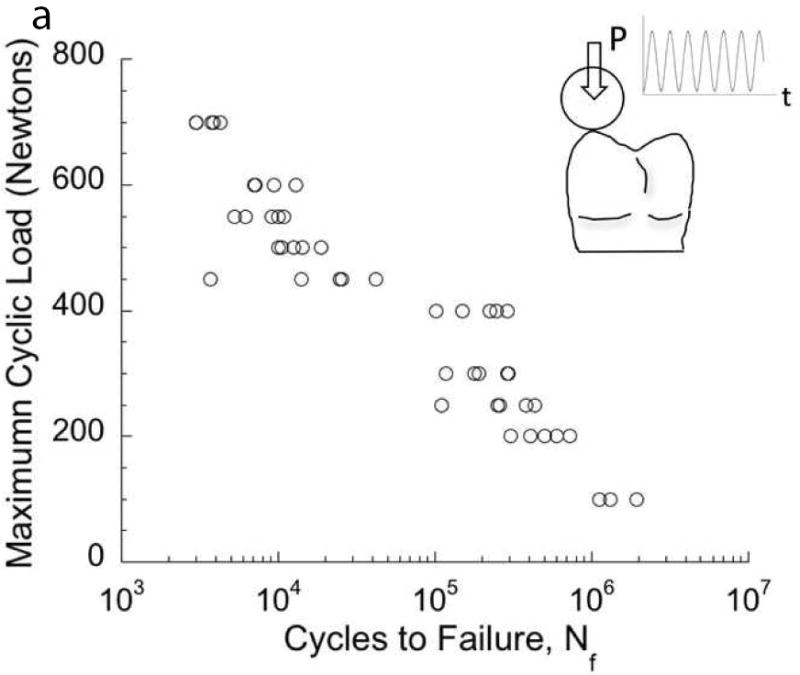
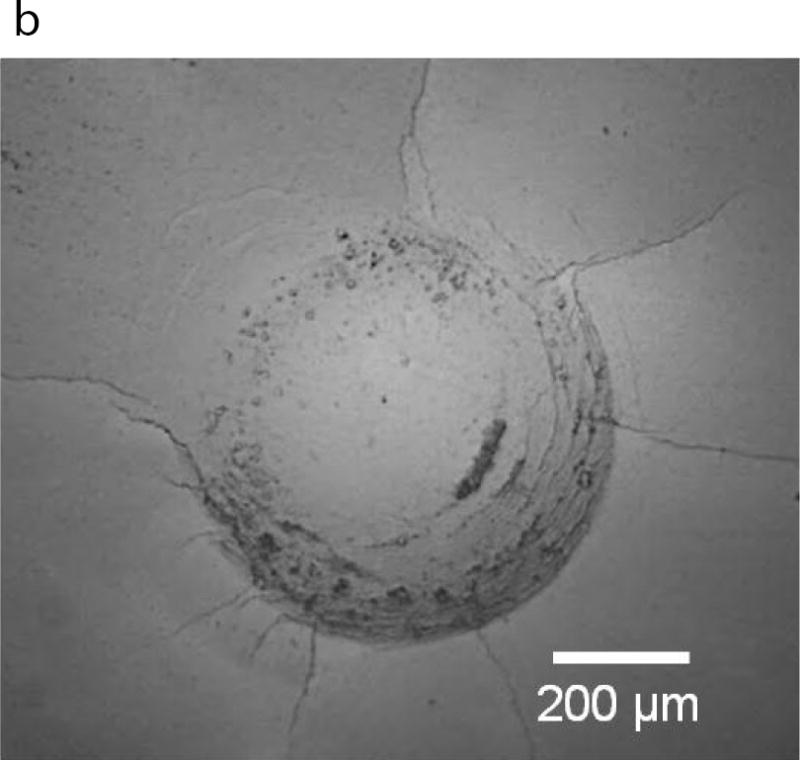
Contact damage of enamel resulting from cyclic loading. (A) Load-life diagram for cyclic contact of cuspal enamel described in terms of the maximum contact load and the number of cycles to failure. Failure was defined by an increase in maximum indentation depth that exceeded that in the first cycle by 15 μm. (B) Typical contact damage pattern resulting from cyclic loading. Note the large number of circumferential cracks inside the contact zone, and the radial cracks extending from the contact periphery. This particular damage pattern resulted from cyclic contact with maximum load of 400N and after a total of 160k cycles.
A and B: Adapted from Gao SS, An BB, Yahyazadehfar M, et al. Contact fatigue of human enamel: Experiments, mechanisms and modeling. J Mech Behav Biomed Mater 2016;60:438–50; with permission.
Important Components of Structure
The durability of the tooth depends on the structure and properties of the individual tissues. Shahmoradi et al. [22] recently presented a very nice review of the structure and properties of the cementum, dentin and enamel. The present effort is focused on the dentin and enamel, in essence the “load bearing” tissues, and aspects important to their durability.
Dentin and enamel are regarded as hierarchical materials due to the multiple length scales of the microstructural elements. At the largest length scale, the most distinct feature of the structure of dentin is the tubules. The tubules serve many functions, including hydration of the tooth, a conduit for transduction of physical signals to sensory responses, and as an anchor in adhesive bonding [23]. This network of channels extends radially outward from the pulp towards the Dentin Enamel Junction (DEJ) and cementum. The tubule density and diameter are lowest at the DEJ and increase with proximity to the pulp [1,2]. Although this basic microstructure is well-recognized, the potential importance of factors such as ethnic background, environment, diet, etc. are generally ignored.
A recent study addressed this concern by comparing the microstructure of coronal dentin from age-matched pairs of donor teeth from the United States and Colombia, South America [24]. There was a significant reduction in the lumen density with depth (p≤0.05) for both donor groups, and no significant difference (p>0.05) in the tubule density between the two. However, there were differences evident in the diameter of the lumens. Fig. 3A compares representative micrographs of peripheral dentin from the teeth of donors living in the US and Colombia (CO). The average lumen diameters of the coronal dentin within the deep, middle and peripheral regions of the two donor groups are compared in Fig. 3B. For the US group, there was a significant decrease in lumen diameter from the deep to the peripheral dentin, with average lumen diameters decreasing from approximately 1.8 μm to ≤1 μm. However, there was no significant change in the lumen diameter with depth for the CO donors. The largest difference between the two groups was evident in the peripheral dentin (Fig. 3A), where the average lumen diameter of tissue from the CO donors was over 40% greater.
Figure 3.
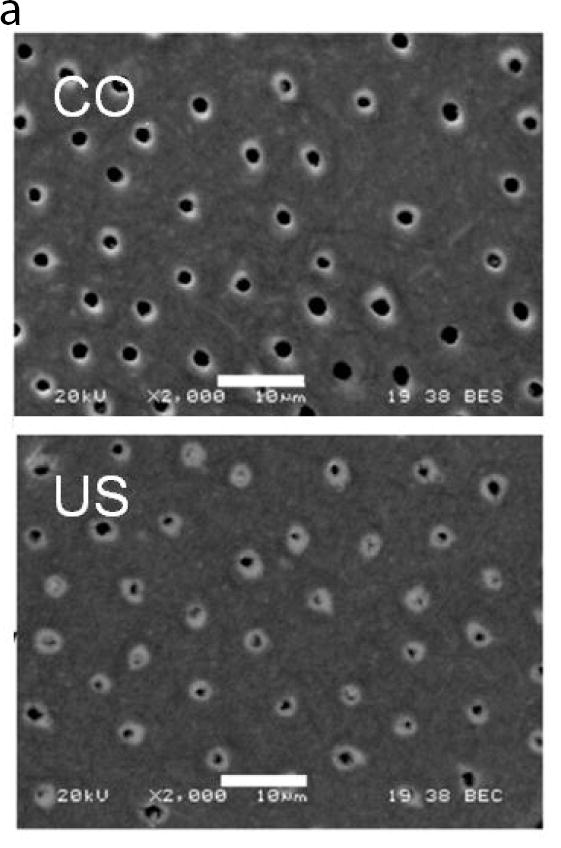
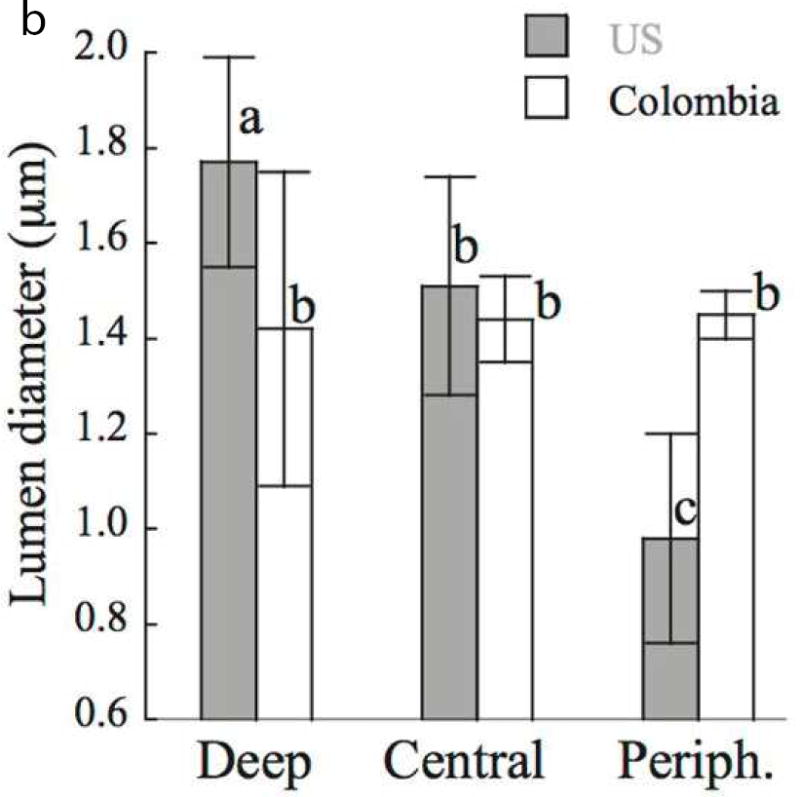
A comparison of the structure of coronal dentin obtained from donor teeth of residents from Colombia (CO) and the United States (US). The two groups consist of age matched young donors with 18≤age≤35 yrs. (A) Micrographs obtained from scanning electron microscopy of peripheral dentin obtained from representative donor teeth of residents from the US and CO. (B) The distribution of tubule lumen diameter of coronal dentin in the deep, central and peripheral regions. The column height represents the average and the caps indicate the standard deviation. Columns with different letters are significantly different (p≤0.05). Note the consistent diameter amongst the three locations of the Colombian teeth.
A: Adapted from Ivancik J, Naranjo M, Correa S, et al. Differences in the microstructure and fatigue properties of dentin between residents of North and South America. Arch Oral Biol 2014;59(10):1001–1012; with permission.
B: From Ivancik J, Naranjo M, Correa S, et al. Differences in the microstructure and fatigue properties of dentin between residents of North and South America. Arch Oral Biol 2014;59(10):1001–1012; with permission.
For both donor groups, the measures of microstructure were within the ranges previously reported for tubule density [e.g. 25] and tubule diameter [e.g. 26]. Nevertheless, a similar study of teeth from Brazilian donors reported a constant lumen diameter over the crown depth [27]. There are two consequences of these findings to consider. Dentin bond strength is a function of both tubule density and tubule dimensions [28,29]. Thus, reported spatial variations in bond strength related to dentin microstructure, and the choice of products based on this expectation, may not be applicable to all patient groups. Secondly, based on the correlations between microstructure and properties of dentin [30–32], the differences in microstructure could be relevant to tooth durability.
The investigation performed by Ivancik et al [24] also compared the fatigue crack growth resistance of dentin for the US and CO groups. For the US donor teeth, the fatigue crack growth resistance decreased significantly from the peripheral to the deep dentin. In contrast, there were no significant differences in the crack growth resistance of dentin between the three depths for the CO donors. These results are highly relevant to the propensity for fracture of restored teeth. For instance, for shallow restorations extending only into peripheral dentin, there is greater probability for fatigue crack growth to facilitate tooth fractures in the teeth of CO patients due to the lower resistance to cyclic crack growth. For restorations extending into the deep dentin, there is a greater probability of fatigue crack growth and consequent tooth fracture in the patients of the US. In essence, the concept of “extension for prevention” would be more detrimental in the teeth of US patients! A previous investigation reported significant differences in the fatigue crack growth resistance of dentin obtained from donor teeth of residents of the US and China but did not included a detailed assessment of the microstructure [33]. Based on the implications to tooth durability, this topic should be explored in further detail. It should also be considered in the future development of biomimetic restorative materials.
The most dominant feature of the enamel at the microscopic scale is the enamel rods. Each rod consists of an assembly of apatite nanocrystalline needle-like structures that are aligned parallel to one another and maintained bound together by the non-collagenous proteins. This description is a simplification, and more detailed treatments are presented elsewhere [34–36]. The enamel rods extend from the DEJ to the occlusal surface of the tooth. Adjacent to the occlusal surface within the “outer” enamel, the rods extend inwards in a nearly parallel arrangement. In the inner enamel (approaching the DEJ), the rods are assembled in sub-unit bands. Each band of rods follows a slightly opposed path that results in a complex decussating structure [37], and is responsible for the “Hunter-Schreger bands” evident in visual inspections of the enamel that results from the variations in reflected light with rod orientation [23].
The discovery of Hunter-Schreger Bands (HSB)s in tooth enamel was reported over two centuries ago. However, Lynch et al. [38] recently presented a very nice evaluation of Hunter-Schreger Band (HSB) distributions in human teeth, which showed that the packing densities were greatest in teeth and areas where the functional/occlusal loads were largest. They implicated that the HSB packing densities and patterns are important to adhesive bond strengths to enamel, abfraction lesions and even the cracked tooth syndrome. That is consistent with recent evaluations concerning the fracture resistance of enamel [39–42] discussed in the following section.
Over the past decade there has been an increasing emphasis on the importance of the organic phase, which operates at the nanoscale to contribute to the properties of dentin and enamel. Recent work has provided a better understanding of the proteoglycans (PGs) and their contributions to the mechanical behavior of dentin [43–45]. These matrix proteins are non-collagenous structures that have in the past been largely overlooked. The PGs serve as linkages between the collagens fibrils [46] and secure the collagenous network together. Recent work on the mechanical behavior of enamel has been focused on the importance of the matrix proteins. The proteins constitute between 1 to 2% of the total composition and are primarily located at the interface of the enamel rods. The proteins are important in modulating stress in the enamel of the tooth crown [35] and play roles in the elastic and viscoelastic behavior [22].
The proteins, and their viscoelastic behavior, are considered to be responsible for the toughness of mineralized tissues [47]. Thus, damage or denaturation of the non-collagenous proteins in dentin and enamel are expected to decrease tooth durability. Indeed, the loss of enamel proteins by tooth whitening [48,49] or by treatment with potassium hydroxide [50] causes a significant reduction of the fracture toughness. Considering that both whitening treatments [51–53] and radiation therapy for oral cancers [54] cause degradation of the enamel proteins, this is an important issue to the field of dentistry, and one that requires further investigation.
Properties of Importance
The properties of primary relevance to tooth durability are those defining the fatigue and fracture behavior. There have been several reviews in the last decade concerning the fatigue behavior of hard tissues of the tooth [33,55,56]. Therefore, the emphasis here is placed on recent findings and their importance to the practitioner.
If flaws are introduced within dentin as a result of the cavity preparation as discussed in Section 2, the largest degradations in properties will be realized in the resistance to cyclic loading and fatigue. Dentin exhibits anisotropy, with lower resistance to fatigue failure when the cyclic stresses are directed parallel to the axis of the tubules. Cracks within coronal dentin prefer to grow perpendicular to the tubules [57]. This characteristic of the fatigue behavior is one of the primary contributions to cusp fractures in restored teeth, i.e. cracks initiate near stress concentrations (e.g. the line angles of the preparation) and then extend perpendicular to the tubules. The anisotropy in fatigue resistance is actually due to the orientation of the collagen fibrils in the intertubular dentin and their ability to resist crack growth via extrinsic toughening.
Another recent finding concerning the durability of dentin is the importance of tubule density. A comparison of the fatigue crack growth resistance of coronal dentin from the deep, central and peripheral regions is shown in Fig. 4A. There is a significant decrease in the fatigue crack growth resistance with increasing proximity to the pulp cavity. Specifically, cyclic crack growth in deep dentin will occur at stresses approximately 40% lower than those required for peripheral dentin [58]. In addition, cracks in deep dentin undergo cyclic extension at incremental rates of between 100 to 1000 times faster than in peripheral dentin. There are equivalent spatial variations in the fracture toughness of coronal dentin with depth [32,59,60]. Overall, these findings indicate that the probability of restoration failure by tooth fracture increases with penetration of the caries beyond the DEJ, and also significantly with the depth of the preparation into dentin. Early detection is critical for many reasons.
Fig. 4.
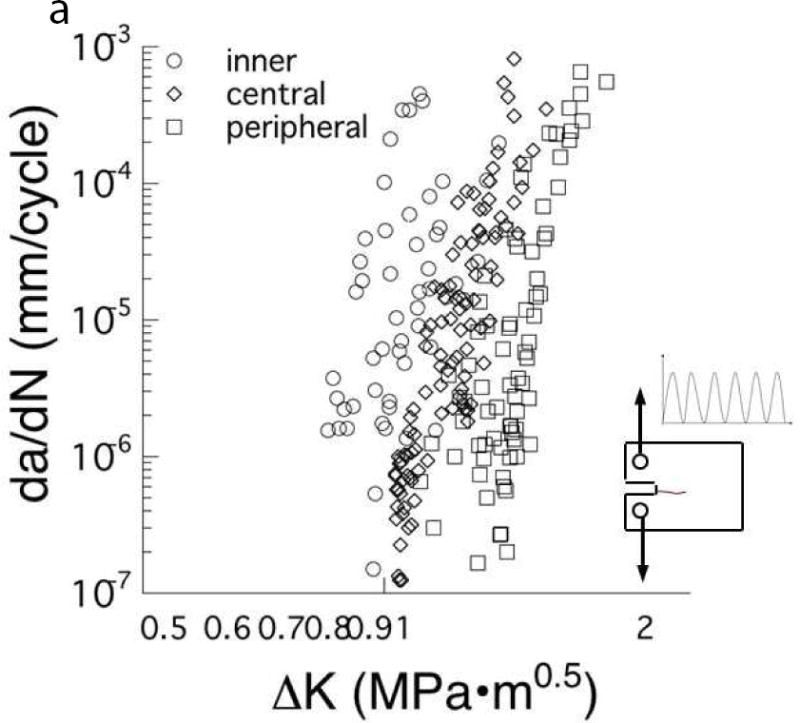
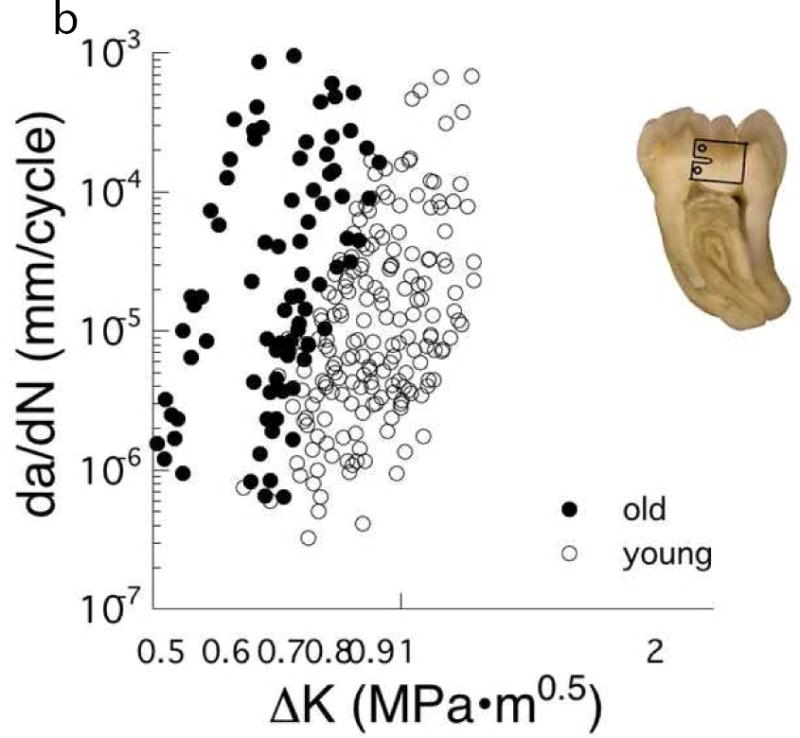
The importance of location and age on the fatigue crack growth resistance of coronal dentin. (A) Responses obtained for cyclic crack growth occurring “in-plane” with the dentin tubules. These responses are for young dentin (age ≤ 30 yrs) and stratified according to depth, including close to the pulp (inner), the mid–coronal region (central) and close to the DEJ (peripheral). (B) Comparison of cyclic crack growth in the dentin of teeth from young (age ≤ 30 yrs) and old (55 yrs ≤ age) donors. These responses are for cyclic crack growth occurring perpendicular to the dentin tubules.
A: Adapted from Ivancik J, Neerchal NK, Romberg E, et al. The reduction in fatigue crack growth resistance of dentin with depth. J Dent Res 2011;90(8):1031–1036; with permission.
B: Adapted from Ivancik J, Majd H, Bajaj D, et al. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomater 2012;8(7):2737–2746; with permission.
Fatigue is equally important to the enamel but has received limited attention. Results of contact fatigue suggest that the enamel is not resistant to cyclic loading [20]. An estimate of the fatigue limit of outer (near-occlusal) enamel was recently reported in an assessment of adhesive bond durability [61]. For cyclic tensile stresses directed perpendicular to the rods, the apparent fatigue limit of the enamel was approximately 9 MPa. That value is less than 25% of the fatigue limit of coronal dentin [62] and less than one tenth the apparent ultimate tensile strength of enamel [21]! Thus, cyclic loads that cause stresses transverse to the enamel rods are highly likely to induce fatigue cracks within the enamel.
Considering its low fatigue strength, why don’t all teeth with visible cracks in the tooth crown undergo fracture? While the DEJ has been credited with preventing cracks from continuing into the dentin [e.g. 64,65], recent studies concerning the crack growth resistance of enamel [40,41,66] support an alternative explanation. Specifically, these studies have shown that the fracture toughness of enamel increases with crack length. For cracks extending from the occlusal surface towards the DEJ, the crack growth resistance can undergo an increase by a factor of 3 or more (Fig 5A). Upon entering the decussated enamel, the crack encounters a concert of mechanisms operating at many length scales to resist crack growth. As such, cracks that reach the DEJ have limited energy to propagate further. Consequently, it is generally not necessary to restore a tooth with visible enamel cracks, as they have been arrested by the underlying microstructure. Perhaps contrary to previous views, the DEJ itself is actually the second line of defense against crack propagation from enamel into dentin [67]. There is also some evidence that cracks in the occlusal enamel can actually undergo healing as a result of crack closure forces promoted by the enamel proteins that tether the enamel rods [63].
Fig. 5.
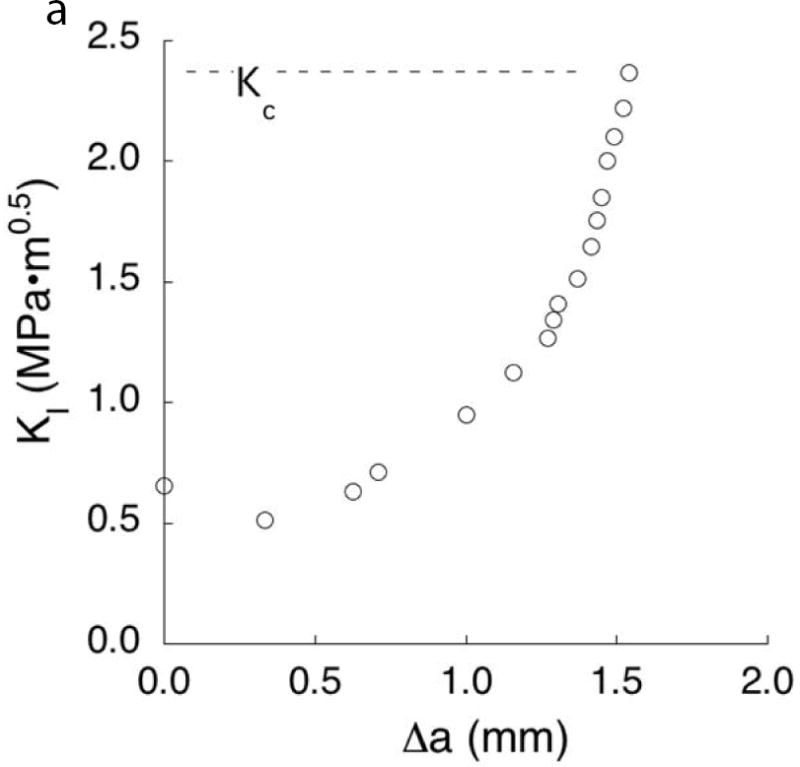
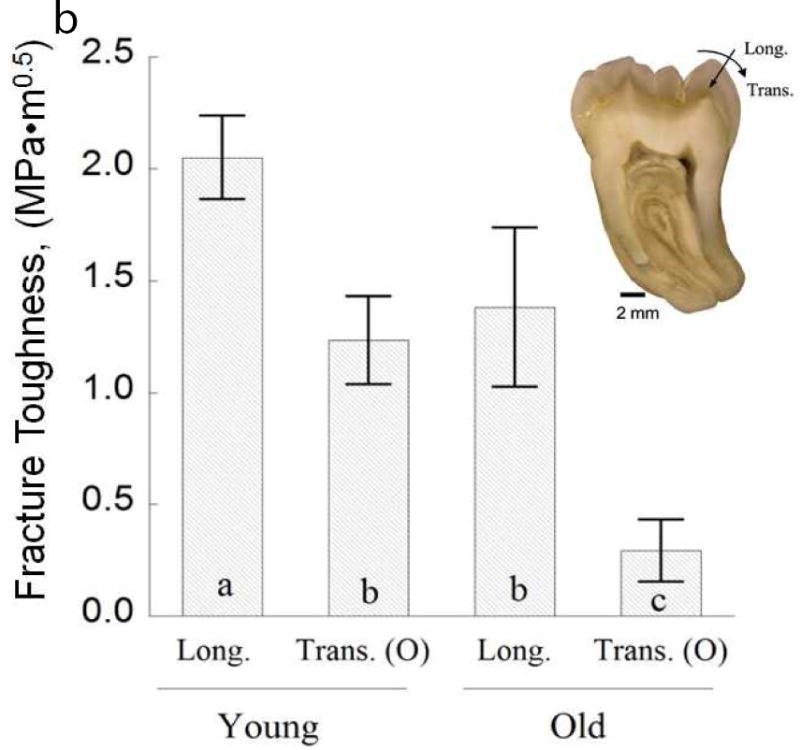
Characteristics of the crack growth resistance of enamel. (A) The increase in resistance to fracture of enamel with crack extension. This data was obtained from crack growth in the longitudinal direction of cuspal enamel, i.e. from the occlusal surface towards the DEJ. The “fracture toughness” of the specimen (Kc), was identified from the last point of stable crack extension preceding bulk fracture. (B) The fracture toughness of the enamel from 3rd molars for cracks extending from the occlusal surface to the DEJ (longitudinal) vs growth with buccal-lingual orientation (transverse). The columns represent the average values with standard deviations. Columns with different letters are significantly different.
A: Data from Bajaj D, Arola D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater 2009;5(8):3045–56.
B: Adapted from Yahyazadehfar M, Zhang D, Arola D. On the importance of aging to the crack growth resistance of human enamel. Acta Biomater 2016;32:269; and Bajaj D, Arola D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater 2009;5(8):3046; with permission.
There has been some debate whether cracks in teeth initiate from the occlusal surface or from the enamel tufts at the DEJ [68,69]. Recent work has shown that cracks extending from the DEJ undergo a limited increase in toughness (~30%), in comparison to nearly a 400% increase for cracks extending from the occlusal surface [40]. It appears that the microstructure of enamel has evolved to be most effective at resisting crack growth from the occlusal surface. This is an exciting observation and could potentially inspire the design of the next generation of materials for tooth crown replacement! The design of enamel suggests that materials for crown replacement should be designed with greater toughness, and perhaps with graded microstructure to resist crack growth from both the occlusal surface and interior, rather than with uniform properties throughout.
Cracks on the tooth’s surface must undergo a combination of growth towards the DEJ (i.e. longitudinally along the rods) and about the occlusal surface (i.e. transverse to the rods). Using bovine incisors, Bechtle et al. [41] found that the resistance to fracture is greatest in the transverse orientation. But in a similar evaluation of human enamel [42] the crack growth resistance was essentially the same in both directions. Results showed that the most important contributor to the crack growth resistance was the degree of decussation that the crack encountered. Inner enamel, which possesses the highest percentage of decussation, achieved the largest increase in crack growth resistance with extension. These results compliment those of Lynch et al. [38] concerning the importance of HSBs in enamel, and convey that regions with the largest packing density (i.e. complexity in the decussation pattern) exhibit the largest resistance to crack growth. Clearly, these results indicate that preserving the decussated enamel is critical to maintaining the tooth’s durability.
Aging
There are changes in the structure of dentin and enamel with age that are relevant to the tooth’s durability. In dentin, there is a graduate reduction in the diameter of the tubule lumens with age due to their progressive filling with mineral [70]. This process begins in the third decade of life, and continues until the lumens become completely filled [55] at which point the tissue is considered sclerotic [23]. As a consequence, there is an increase in the mineral content of dentin with age [59].
The changes in microstructure of dentin with age cause a degradation in the fatigue and fracture properties [71]. For example, there is a substantial reduction in the fatigue strength. Over a span of age from loose definitions of young (age ≤ 30) to old (55 ≤ age), the decrease in fatigue strength of coronal dentin is approximately 50% [61]. The reduction appears to be less extensive for the mid-coronal third of the root [71], which suggests that there are spatial variations in aging. One limitation to this interpretation is that few studies have explored aging of radicular dentin. Nevertheless, this decrease in fatigue strength increases the sensitivity to defects introduced during cavity preparations.
If a crack is introduced in dentin as a result of the cavity preparation, then the fatigue crack growth resistance and fracture toughness become critical to tooth durability. A comparison of cyclic crack growth in coronal dentin from a selection of young and old donor teeth [57, 72] is shown in Fig. 4B. These results show that there is a decrease in resistance to crack growth with age, and an increase in the incremental rate of growth by nearly 100 times. Regardless of age, the direction of lowest fatigue crack growth resistance is perpendicular to the lumens and the degradation by aging is most severe in the peripheral dentin [57]. Consistent with the changes in fatigue crack growth resistance, there is a reduction in the fracture toughness of dentin with aging [59, 73, 74]. Thus, the occlusal force borne by the restoration in teeth of senior patients should be reduced, or the contact area increased, to reduce the stress from mastication to prevent fracture.
The principal cause for the age-related degradation in properties of dentin is still unclear. In a recent multi-variable analysis of the reduction in flexure strength of dentin with age, Shinno et al., [75] reported that the changes were correlated with increasing Advanced Glycation End (AGE) product content, and increasing mineral density. AGEs are intra- and interfibrillar nonenzymatic crosslinks that develop through glycation [76]. There is an accumulation of AGEs in dentin collagen with age and the density of AGEs is greatest in collagen near the dentin tubules [77]. As this process causes a decrease in strength and fracture resistance of human bone with age, an equivalent response would be expected in dentin. Further exploration of the mechanisms of aging in dentin is needed.
There are also changes to the structure and properties of enamel with age. Anecdotal evidence shows that the density of cracks and craze lines in the enamel increases with age. The hardness and elastic modulus of enamel increase with age [78,79] and both contribute to an increase in the indentation brittleness [80]. That suggests that there is greater propensity for cracks and contact damage to develop in the teeth of senior patients.
Once cracks are identified in the surface of teeth, it is common to question whether they warrant treatment. From a mechanical point of view, the answer depends on the crack length and the relative crack growth resistance of the tissue. A comparison of the fracture toughness in two directions of cuspal enamel from the teeth of young (age ≤ 25) and old (55 ≤ age) donor teeth are shown in Fig. 5B [81]. For the longitudinal direction, the fracture toughness decreased by approximately 35% over the age span from the young and old groups. For the transverse direction of crack extension, the fracture toughness of the old enamel (0.25 MPa•m0.5) was nearly 70% lower than that obtained from the young teeth! Contrary to the crack growth toughening observed in young enamel (Fig. 5A), the process is negligible in old enamel [81]. This finding provides further evidence of why cracks are more frequently identified in the surfaces of teeth of seniors. Due to the reduction in fracture toughness, cracks are much more detrimental in the teeth of seniors and may warrant greater attention.
What are the mechanisms contributing to the changes in mechanical properties? There is an increase in the mineral density of enamel with aging [82,83], and a decrease in the volume of protein matrix. This reduction can occur progressively as a result of the variations in oral cavity pH [23]. It can also result from restorative or cosmetic dental procedures such as teeth whitening [e.g. 51, 84,85], which damage or denature the enamel proteins. Indeed, a recent evaluation concerning the mechanical behavior of enamel after whitening showed that that bleaching resulted in an average 40% decrease in the fracture toughness after treatment by carbamide- or hydrogen-peroxide treatments [49]. These results suggest that whitening treatments could cause an “accelerated” aging of the enamel, which could be tremendously detrimental at later stages of life or after accumulated treatments.
Oral Environment
A recurrence of caries at the tooth margin may result from the acid production of biofilms and is considered the primary reason for the replacement of restorations [86,87]. Acidic environments resulting from biofilm activity could also increase the probability of tooth fractures due to synergism between chemical and mechanical mechanisms of degradation. This is a relatively new area of exploration.
The importance of pH variations in the oral environment on the fatigue strength of dentin were recently evaluated by Do et al [88]. A comparison of fatigue life diagrams for coronal dentin evaluated in neutral and a lactic acid environment with pH=5 is shown in Fig. 6A. Exposure to the acidic conditions caused a significant reduction in fatigue strength and nearly 30% reduction in the fatigue limit. Surprisingly, the reduction in fatigue strength began after only 4 hours of exposure to the lower pH.
Fig. 6.
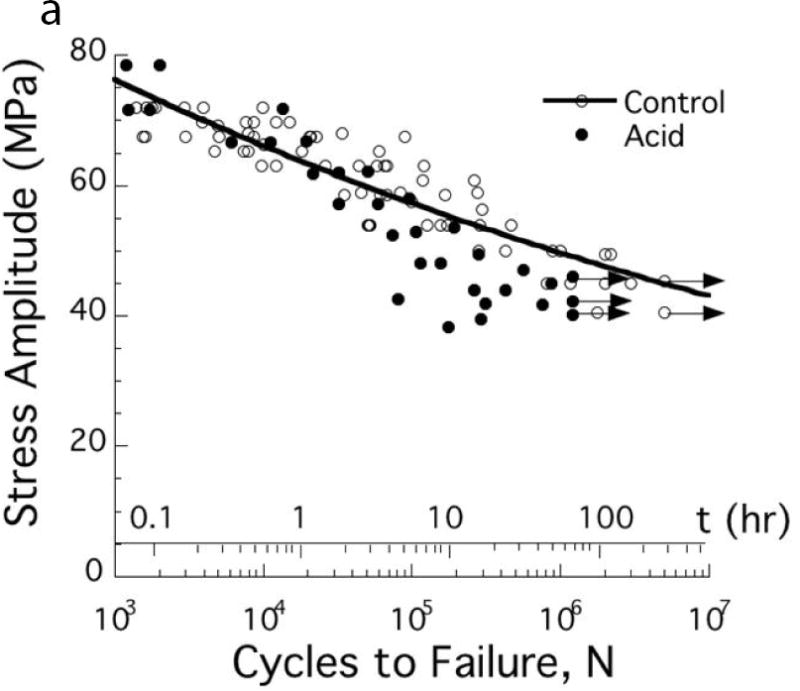
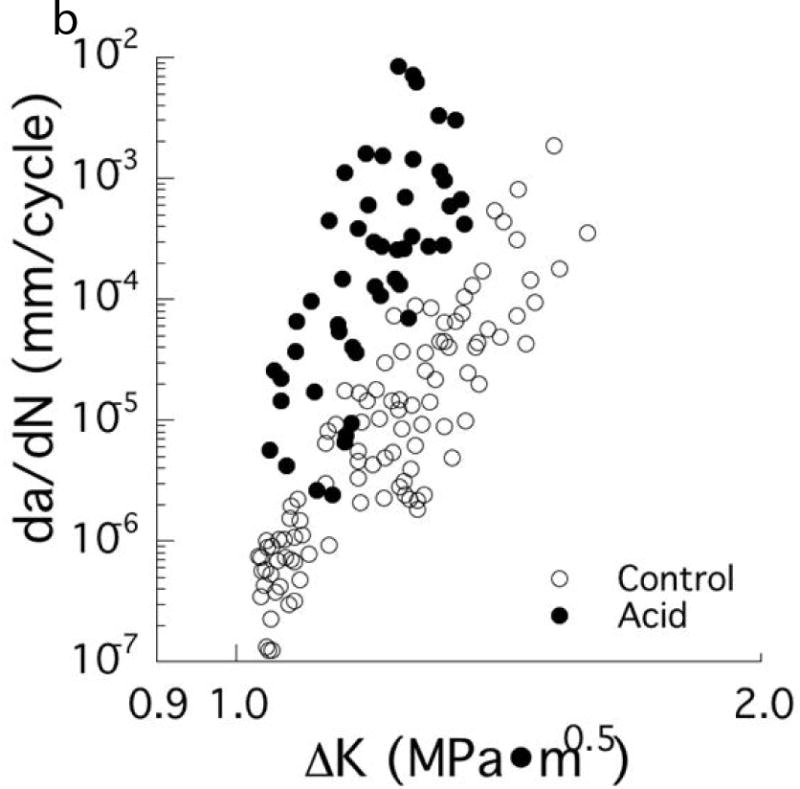
Degradation in the fatigue resistance of coronal dentin with exposure to an acidic environment. (A) Stress life fatigue behavior. The data points with arrows represent beams that did not fail and the test was discontinued. Note that a significant degradation in fatigue strength occurs after only 4 hours of acid exposure. (B) Fatigue crack growth resistance of mid-coronal dentin. (A, B) The control was evaluated in a neutral environment (pH=7) and the acid condition consisted of exposure to a lactic acid solution with pH = 5.
A: Adapted from Do D, Orrego S, Majd H, et al. Accelerated fatigue of dentin with exposure to lactic acid. Biomaterials 2013;34(34):8650–8659; with permission.
B: From Orrego S, Xu H, Arola D. Degradation in the fatigue crack growth resistance of human dentin by lactic acid. Mater Sci Engr 2017;C 73:720; with permission.
The influence of lactic acid exposure on the fatigue crack growth resistance was also recently evaluated by Orrego et al. [89]. A comparison of the fatigue crack growth responses of mid-coronal dentin exposed to neutral and lactic acid conditions (pH=5) is shown in Fig. 6B. The acidic conditions caused a nearly ten-fold increase in the incremental rate of cyclic crack extension. The study also showed that the degradation by acid exposure increased from the peripheral to the mid-coronal dentin and that resin adhesive penetration within the lumens had no influence on the fatigue resistance. Therefore, exposing dentin to acidic environments contributes to the development of caries, but it also increases the chance of tooth fractures via fatigue-related failure and at lower mastication forces. The results of Lee et al [19] showed that even a 15 sec phosphoric acid etching treatment caused a reduction in the fatigue strength of dentin, which ultimately reduces the tooth’s durability. This new understanding suggests that it may be prudent to explore the use of less-aggressive acid etches for adhesives, adhesives with capacity to repair this damage. Alternatively, if bonding will continue to be the mainstay of restorative dentistry, the development of non-acidic adhesives could be a viable approach.
Summary
A review of the structure and properties of the tooth was presented with an emphasis on its durability. New findings indicate that the tooth’s durability is reduced by cracks and other forms of damage that are introduced during restorative processes and that result from cyclic contact fatigue with opposing teeth, and especially with ceramic crowns. The potential for growth of this damage depends on the fatigue and fracture resistance of the dentin and enamel, as well as their spatial variations in the tooth. Removal of the decussated enamel and the extension of restorations into the deep dentin are detrimental to durability, and not simply due to the reduction in tooth structure. Furthermore, there is greater understanding of the decreases in damage tolerance of dentin and enamel as a consequence of aging, and by exposure to acid conditions. The knowledge in this area is growing, and will be critical in making future improvements in restorative practices as well as in extending the definition of lifelong oral health.
Key Points.
The tooth’s durability is reduced by the introduction of damage in dentin or enamel, which can occur in the cutting of preparations and etching, and as a result of cyclic contact.
There is a substantial reduction in the fatigue and fracture resistance of both dentin and enamel with increasing patient age, which should be considered in the treatment plan.
Exposure of dentin to acidic environments reduces its fatigue strength and reduces the tooth’s durability. Exposure to biofilm and aggressive or over-etching should be avoided.
Acknowledgments
This work was supported in part by the National Institute of Dental & Craniofacial Research of the National Institutes of Health (NIDCR NIH) under award numbers R01DE016904 (PI D. Arola), and R01DE015306 (PI D. Pashley). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pashley DH. Dentin: a dynamic substrate-a review. Scanning Microsc. 1989;3(1):161–74. discussion 174–6. [PubMed] [Google Scholar]
- 2.Marshall GW, Jr, Marshall SJ, Kinney JH, et al. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–58. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 3.Perdigao J, Swift EJ, Jr, Denehy GE, et al. In vitro bond strengths and SEM evaluation of dentin bonding systems to different dentin substrates. J Dent Res. 1994;73(1):44–55. doi: 10.1177/00220345940730010601. [DOI] [PubMed] [Google Scholar]
- 4.Tay FR, Pashley DH. Resin bonding to cervical sclerotic dentin: a review. J Dent. 2004;32(3):173–96. doi: 10.1016/j.jdent.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14(1):13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 6.Arola D, Huang MP, Sultan MB. The failure of amalgam dental restorations due to cyclic fatigue crack growth. J Mater Sci Mater Med. 1999;10(6):319–27. doi: 10.1023/a:1026435821960. [DOI] [PubMed] [Google Scholar]
- 7.Lubisich EB, Hilton TJ, Ferracane J. Cracked teeth: a review of the literature. J Esthet Restor Dent. 2010;22(3):158–67. doi: 10.1111/j.1708-8240.2010.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shemesh H, Bier CA, Wu MK, et al. The effects of canal preparation and filling on the incidence of dentinal defects. Int Endod J. 2009;42(3):208–13. doi: 10.1111/j.1365-2591.2008.01502.x. [DOI] [PubMed] [Google Scholar]
- 9.Adorno CG, Yoshioka T, Jindan P, et al. The effect of endodontic procedures on apical crack initiation and propagation ex vivo. Int Endod J. 2013;46:763–8. doi: 10.1111/iej.12056. [DOI] [PubMed] [Google Scholar]
- 10.Bürklein S, Tsotsis P, Schäfer E. Incidence of dentinal defects after root canal preparation: reciprocating versus rotary instrumentation. J Endod. 2013;39(4):501–4. doi: 10.1016/j.joen.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Arias A, Lee YH, Peters CI, et al. Comparison of 2 canal preparation techniques in the induction of microcracks: A pilot study with cadaver mandibles. J Endod. 2014;40(7):982–5. doi: 10.1016/j.joen.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 12.De-Deus G, Silva EJ, Marins J, et al. Lack of causal relationship between dentinal microcracks and root canal preparation with reciprocation systems. J Endod. 2014;40(9):1447–50. doi: 10.1016/j.joen.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 13.De-Deus G, Belladonna FG, Souza EM, et al. Micro-computed tomographic assessment on the effect of proTaper next and twisted file adaptive systems on dentinal cracks. J Endod. 2015;41(7):1116–9. doi: 10.1016/j.joen.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Staninec M, Meshkin N, Manesh SK, et al. Weakening of dentin from cracks resulting from laser irradiation. Dent Mater. 2009;25(4):520–5. doi: 10.1016/j.dental.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehy C, Drummond JL. Micro-cracking of tooth structure. Am J Dent. 2004;17(5):378–80. [PubMed] [Google Scholar]
- 16.Majd H, Viray J, Porter JA, et al. Degradation in the fatigue resistance of dentin by bur and abrasive air-jet preparations. J Dent Res. 2012;91(9):894–9. doi: 10.1177/0022034512455800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majd B, Majd H, Porter JA, et al. Degradation in the fatigue strength of dentin by diamond bur preparations: Importance of cutting direction. J Biomed Mater Res B Appl Biomater. 2016;104(1):39–49. doi: 10.1002/jbm.b.33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arola D. Fatigue testing of biomaterials and their interfaces. Dent Mater. 2017 doi: 10.1016/j.dental.2017.01.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HH, Majd H, Orrego S, et al. Degradation in the fatigue strength of dentin by cutting, etching and adhesive bonding. Dent Mater. 2014;30(9):1061–72. doi: 10.1016/j.dental.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao SS, An BB, Yahyazadehfar M, Zhang D, Arola DD. Contact fatigue of human enamel: Experiments, mechanisms and modeling. J Mech Behav Biomed Mater. 2016;60:438–50. doi: 10.1016/j.jmbbm.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Chai H. On the mechanical properties of tooth enamel under spherical indentation. Acta Biomater. 2014;10(11):4852–60. doi: 10.1016/j.actbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Shahmoradi M, Bertassoni LE, Elfallah HM, et al. Fundamental Structure and Properties of Enamel, Dentin and Cementum. Advances in Calcium Phosphate Biomaterials. 2014;2(17):511–47. [Google Scholar]
- 23.Nanci A. Ten Cate’s Oral Histology: Development, Structure, and function. 7th. Mosby-Year Book Inc; 2008. [Google Scholar]
- 24.Ivancik J, Naranjo M, Correa S, et al. Differences in the microstructure and fatigue properties of dentine between residents of North and South America. Arch Oral Biol. 2014;59(10):1001–12. doi: 10.1016/j.archoralbio.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garberoglio R, Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21(6):355–62. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- 26.Schilke R, Lisson JA, Bauss O, et al. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol. 2000;45(5):355–61. doi: 10.1016/s0003-9969(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho ET, Moraes d’Almeida JR, et al. Evaluation of microstructural parameters of human dentin by digital image analysis. Mater Res. 2007;10(2):153–9. [Google Scholar]
- 28.Carvalho RM, Fernandes CA, Villanueva R, Wang L, Pashley DH. Tensile strength of human dentin as a function of tubule orientation and density. J Adhes Dent. 2001;3(4):309–14. [PubMed] [Google Scholar]
- 29.Giannini M, Carvalho RM, Martins LR, et al. The influence of tubule density and area of solid dentin on bond strength of two adhesive systems to dentin. J Adhes Dent. 2001;3(4):315–24. [PubMed] [Google Scholar]
- 30.Mannocci F, Pilecki P, Bertelli E, et al. Density of dentinal tubules affects the tensile strength of root dentin. Dent Mater. 2004;20(3):293–6. doi: 10.1016/S0109-5641(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 31.Arola D, Ivancik J, Majd H, et al. On the microstructure and mechanical behavior of radicular and coronal dentin. Endodontic Topics. 2009;20:30–51. [Google Scholar]
- 32.Montoya C, Arango-Santander S, Peláez-Vargas A, et al. Effect of aging on the microstructure, hardness and chemical composition of dentin. Arch Oral Biol. 2015;60(12):1811–20. doi: 10.1016/j.archoralbio.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Arola D, Bajaj D, Ivancik J, et al. Fatigue of biomaterials: Hard tissues. Int J Fatigue. 2010;32(9):1400–12. doi: 10.1016/j.ijfatigue.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson C, Kirkham J, Shore R. Dental enamel: formation to destruction. Boca Raton, FL: CRC Press; 1995. pp. 151–52. [Google Scholar]
- 35.He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav of Biomed Mater. 2008;1(1):18–29. doi: 10.1016/j.jmbbm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 36.An B, Wang R, Zhang D. Role of crystal arrangement on the mechanical performance of enamel. Acta Biomater. 2012;8(10):3784–93. doi: 10.1016/j.actbio.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Macho GA, Jiang Y, Spears IR. Enamel microstructure–a truly three-dimensional structure. J Hum Evol. 2003;45(1):81–90. doi: 10.1016/s0047-2484(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 38.Lynch CD, O’Sullivan VR, Dockery P, McGillycuddy CT, Sloan AJ. Hunter-Schreger Band patterns in human tooth enamel. J Anat. 2010;217(2):106–15. doi: 10.1111/j.1469-7580.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj D, Nazari A, Eidelman N, et al. A comparison of fatigue crack growth in human enamel and hydroxyapatite. Biomater. 2008;29(36):4847–54. doi: 10.1016/j.biomaterials.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajaj D, Arola D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater. 2009;5(8):3045–56. doi: 10.1016/j.actbio.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechtle S, Habelitz S, Klocke A, et al. The fracture behaviour of dental enamel. Biomater. 2010;31(2):375–84. doi: 10.1016/j.biomaterials.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 42.Yahyazadehfar M, Bajaj D, Arola DD. Hidden contributions of the enamel rods on the fracture resistance of human teeth. Acta Biomater. 2013;9(1):4806–14. doi: 10.1016/j.actbio.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix–limitations of restorative dentistry hidden on the nanometer scale. Acta Biomaterialia. 2012;8(7):2419. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertassoni LE, Swain MV. The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater. 2014;38:91–104. doi: 10.1016/j.jmbbm.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Bertassoni LE, Kury M, Rathsam C, et al. The role of proteoglycans in the nanoindentation creep behavior of human dentin. J Mech Behav Biomed Mater. 2015;55:264–70. doi: 10.1016/j.jmbbm.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25(11):781–806. [PubMed] [Google Scholar]
- 47.Ji B, Gao H. Mechanical properties of nanostructure of biological materials. J Mech Phys Solids. 2004;52(9):1963–1990. [Google Scholar]
- 48.Elfallah HM, Bertassoni LE, Charadram N, et al. Effect of tooth bleaching agents on protein content and mechanical properties of dental enamel. Acta Biomater. 2015;20:120–8. doi: 10.1016/j.actbio.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 49.Elfallah HM, Swain MV. A review of the effect of vital teeth bleaching on the mechanical properties of tooth enamel. N Z Dent J. 2013;109(3):87–96. [PubMed] [Google Scholar]
- 50.Yahyazadehfar M, Arola D. The role of organic proteins on the crack growth resistance of human enamel. Acta Biomater. 2015;19:33–45. doi: 10.1016/j.actbio.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang T, Ma X, Wang Y, et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008;13(014019):1–9. doi: 10.1117/1.2870114. [DOI] [PubMed] [Google Scholar]
- 52.Zimmerman B, Datko L, Cupelli M, Alapati S, Dean D, Kennedy M. Alteration of dentin-enamel mechanical properties due to dental whitening treatments. J Mech Behav Biomed Mater. 2010;3(4):339–46. doi: 10.1016/j.jmbbm.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubarsky GV, Lemoine P, Meenan BJ, et al. Enamel proteins mitigate mechanical and structural degradation in mature human enamel during acid attack. Mater Res Expr. 2014;1(2):1–20. [Google Scholar]
- 54.Reed R, Xu C, Liu Y, et al. Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentin. Arch Oral Biol. 2015;60(5):690–7. doi: 10.1016/j.archoralbio.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arola D. Fracture and Aging in Dentin. In: Curtis R, Watson T, editors. Dental Biomaterials: Imaging, Testing and Modeling. Woodhead Publishing; Cambridge, UK: 2007. [Google Scholar]
- 56.Kruzic JJ, Ritchie RO. Fatigue of mineralized tissues: cortical bone and dentin. J Mech Behav Biomed Mater. 2008;1:3–17. doi: 10.1016/j.jmbbm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Ivancik J, Majd H, Bajaj D, et al. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomater. 2012;8(7):2737–46. doi: 10.1016/j.actbio.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivancik J, Neerchal NK, Romberg E, et al. The reduction in fatigue crack growth resistance of dentin with depth. J Dent Res. 2011;90(8):1031–6. doi: 10.1177/0022034511408429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivancik J, Arola DD. The importance of microstructural variations on the fracture toughness of human dentin. Biomaterials. 2013;34(4):864–74. doi: 10.1016/j.biomaterials.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montoya C, Arola D, Ossa EA. Importance of tubule density to the fracture toughness of dentin. Arch Oral Biol. 2016;67:9–14. doi: 10.1016/j.archoralbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Yahyazadehfar M, Mutluay MM, Majd H, et al. Fatigue of the resin-enamel bonded interface and the mechanisms of failure. J Mech Behav Biomed Mater. 2013;21:121–32. doi: 10.1016/j.jmbbm.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arola D, Reprogel RK. Effects of aging on the mechanical behavior of human dentin. Biomaterials. 2005;26(18):4051–61. doi: 10.1016/j.biomaterials.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Rivera C, Arola D, Ossa A. Indentation damage and crack repair in human enamel. J Mech Behav Biomed Mater. 2013;21:178–84. doi: 10.1016/j.jmbbm.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imbeni V, Kruzic JJ, Marshall GW, et al. The dentin-enamel junction and the fracture of human teeth. Nat Mater. 2005;4(3):229–32. doi: 10.1038/nmat1323. [DOI] [PubMed] [Google Scholar]
- 65.Bechtle S, Fett T, Rizzi G, et al. Crack arrest within teeth at the dentinoenamel junction caused by elastic modulus mismatch. Biomaterials. 2010;31(14):4238–47. doi: 10.1016/j.biomaterials.2010.01.127. [DOI] [PubMed] [Google Scholar]
- 66.Yilmaz ED, Schneider GA, Swain MV. Influence of structural hierarchy on the fracture behaviour of tooth enamel. Philos Trans A Math Phys Eng Sci. 2015;373(2038) doi: 10.1098/rsta.2014.0130. [DOI] [PubMed] [Google Scholar]
- 67.Yahyazadehfar M, Ivancik J, Majd H, et al. On the mechanics of fatigue and fracture in teeth. Appl Mech Rev. 2014;66(3):0308031–3080319. doi: 10.1115/1.4027431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chai H, Lee JJ, Constantino PJ, et al. Remarkable resilience of teeth. Proc Natl Acad Sci. 2009;106(18):7289–93. doi: 10.1073/pnas.0902466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myoung S, Lee J, Constantino P, et al. Morphology and fracture of enamel. J Biomech. 2009;42(12):1947–51. doi: 10.1016/j.jbiomech.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Porter AE, Nalla RK, Minor A, et al. A transmission electron microscopy study of mineralization in age-induced transparent dentin. Biomaterials. 2005;26(36):7650–60. doi: 10.1016/j.biomaterials.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 71.Kinney JH, Nalla RK, Pople JA, et al. Age-related transparent root dentin: mineral concentration, crystallite size, and mechanical properties. Biomaterials. 2005;26(16):3363–76. doi: 10.1016/j.biomaterials.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj D, Sundaram N, Nazari A, et al. Age, dehydration and fatigue crack growth in dentin. Biomaterials. 2006;27(11):2507–17. doi: 10.1016/j.biomaterials.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 73.Koester KJ, Ager JW, 3rd, Ritchie RO. The effect of aging on crack-growth resistance and toughening mechanisms in human dentin. Biomaterials. 2008;29(10):1318–28. doi: 10.1016/j.biomaterials.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Nazari A, Bajaj D, Zhang D, et al. Aging and the reduction in fracture toughness of human dentin. J Mech Behav Biomed Mater. 2009;2(5):550–9. doi: 10.1016/j.jmbbm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shinno Y, Ishimoto T, Saito M, et al. Comprehensive analyses of how tubule occlusion and advanced glycation end-products diminish strength of aged dentin. Sci Rep. 2016;6:19849. doi: 10.1038/srep19849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–55. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 77.Miura J, Nishikawa K, Kubo M, et al. Accumulation of advanced glycation end-products in human dentine. Arch Oral Biol. 2014;59(2):119–24. doi: 10.1016/j.archoralbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 78.Park S, Wang DH, Dongsheng Z, et al. Mechanical properties of human enamel as a function of age and location in the tooth. J Mat Sci: Mat Med. 2008a;19(6):2317–24. doi: 10.1007/s10856-007-3340-y. [DOI] [PubMed] [Google Scholar]
- 79.Zheng Q, Xu H, Song F, et al. Spatial distribution of the human enamel fracture toughness with aging. J Mech Behav Biomed Mater. 2013;26:148–54. doi: 10.1016/j.jmbbm.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 80.Park S, Quinn JB, Romberg E, et al. On the brittleness of enamel and selected dental materials. Dent Mater. 2008b;24(11):1477–85. doi: 10.1016/j.dental.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yahyazadehfar M, Zhang D, Arola D. On the importance of aging to the crack growth resistance of human enamel. Acta Biomater. 2016;32:264–74. doi: 10.1016/j.actbio.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bertacci A, Chersoni S, Davidson CL, et al. In vivo enamel fluid movement. Eur J Oral Sci. 2007;115(3):169–73. doi: 10.1111/j.1600-0722.2007.00445.x. [DOI] [PubMed] [Google Scholar]
- 83.He B, Huang S, Zhang C, et al. Mineral densities and elemental content in different layers of healthy human enamel with varying teeth age. Arch Oral Biol. 2011;56(10):997–1004. doi: 10.1016/j.archoralbio.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 84.Efeoglu N, Wood D, Efeoglu C. Microcomputerised tomography evaluation of 10% carbamide peroxide applied to enamel. J Dent. 2005;33(7):561–7. doi: 10.1016/j.jdent.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Mihailova B, Klocke A, et al. Side effects of a non-peroxide-based home bleaching agent on dental enamel. J Biomed Mater Res A. 2009;88(1):195–204. doi: 10.1002/jbm.a.31843. [DOI] [PubMed] [Google Scholar]
- 86.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21(1):3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Featherstone JD. The continuum of dental caries e evidence for a dynamic disease process. J Dent Res. 2004;83(Spec Iss C):C39e42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 88.Do D, Orrego S, Majd H, et al. Accelerated fatigue of dentin with exposure to lactic acid. Biomaterials. 2013;34(34):8650–9. doi: 10.1016/j.biomaterials.2013.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orrego S, Xu HHK, Arola DD. Degradation in the fatigue crack growth resistance of human dentin by lactic acid. Mater Sci Engr: C. 2017 doi: 10.1016/j.msec.2016.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]


