Abstract
IMPORTANCE
The ability to explore associations between reports of subjective cognitive decline (SCD) and biomarkers of early Alzheimer disease (AD) pathophysiologic processes (accumulation of neocortical β-amyloid [Aβ] and tau) provides an important opportunity to understand the basis of SCD and AD risk.
OBJECTIVE
To examine associations between SCD and global Aβ and tau burdens in regions of interest in clinically healthy older adults.
DESIGN, SETTING, AND PARTICIPANTS
This imaging substudy of the Harvard Aging Brain Study included 133 clinically healthy older participants (Clinical Dementia Rating Scale global scores of 0) participating in the Harvard Aging Brain Study who underwent cross-sectional flortaucipir F 18 (previously known as AV 1451, T807) positron emission tomography (FTP-PET) imaging for tau and Pittsburgh compound B carbon 11–labeled PET (PiB-PET) imaging for Aβ. The following 2 regions for tau burden were identified: the entorhinal cortex, which exhibits early signs of tauopathy, and the inferior temporal region, which is more closely associated with AD-related pathologic mechanisms. Data were collected from June 11, 2012, through April 7, 2016.
MAIN OUTCOMES AND MEASURES
Subjective cognitive decline was measured using a previously published method of z-transforming subscales from the Memory Functioning Questionnaire, the Everyday Cognition battery, and a 7-item questionnaire. The Aβ level was measured according to a summary distribution volume ratio of frontal, lateral temporal and parietal, and retrosplenial PiB-PET tracer uptake. The FTP-PET measures were computed as standardized uptake value ratios. Linear regression models focused on main and interactive effects of Aβ, entorhinal cortical, and inferior temporal tau on SCD, controlling for age, sex, educational attainment, and Geriatric Depression Scale score.
RESULTS
Of the 133 participants, 75 (56.3%) were women and 58 (43.6%) were men; mean (SD) age was 76 (6.9) years (range, 55–90 years). Thirty-nine participants (29.3%) exhibited a high Aβ burden. Greater SCD was associated with increasing entorhinal cortical tau burden (β = 0.35; 95% CI, 0.19-.52; P < .001) and Aβ burden (β = 0.24; 95% CI, 0.08-.40; P = .005), but not inferior temporal tau burden (β = 0.10; 95% CI, −0.08 to 0.28; P = .27). This association between entorhinal cortical tau burden and SCD was largely unchanged after accounting for Aβ burden (β = 0.36; 95% CI, 0.15-.58; P = .001), and no interaction influenced SCD (β = −0.36; 95% CI, −0.34 to 0.09; P = .25). An exploratory post hoc whole-brain analysis also indicated that SCD was predominantly associated with greater tau burden in the entorhinal cortex.
CONCLUSIONS AND RELEVANCE
Subjective cognitive decline is indicative of accumulation of early tauopathy in the medial temporal lobe, specifically in the entorhinal cortex, and to a lesser extent, elevated global levels of Aβ. Our findings suggest multiple underlying pathways that motivate SCD that do not necessarily interact to influence SCD endorsement. As such, multiple biological factors must be considered when assessing SCD in clinically healthy older adults.
Report of subjective cognitive decline (SCD) represents a known risk factor for future progression to Alzheimer disease (AD) dementia,1 particularly in individuals with high β-amyloid (Aβ) levels.2 Current preclinical staging criteria argue for the appearance of SCD in stage 3 of the preclinical trajectory after the presence of abnormal levels of Aβ and neurodegeneration are evident.3 In clinically healthy adults, SCD is associated with abnormal levels of AD biomarkers of Aβ and neurodegeneration, such as cerebrospinal fluid4 and neuroimaging markers of Aβ burden,5 smaller entorhinal cortical6 and hippocampal7 volumes, and reduced brain glucose metabolism in AD regions of interest.8 Supporting evidence from postmortem studies also indicates that increased neuritic plaques,9 diffuse plaques, and neurofibrillary tangles are present inclinically healthy older adults with endorsement of SCD symptoms proximate to death.10 Building on previous observations relating SCD to Aβ levels,5 we sought to clarify whether the anatomical distribution of tauopathy seen with positron emission tomography (PET) can lend explanatory power to the development of SCD in clinically healthy older adults. Early signs of tauopathy are apparent in the entorhinal cortices, regardless of the presence of Aβ according to in vivo neuroimaging11–14 and postmortem studies. 15–17 In AD, tau deposits expand into adjacent neocortical regions as Aβ burden increases, and an increasing Aβ level is associated with greater tau burden in the entorhinal and neocortical regions.11 We sought to identify the context of successive tau deposition that is associated with SCD.
Specifically, we related the development of SCD to the specific anatomy of tau deposition revealed with flortaucipir F 18 (previously known as AV 1451, T807) PET (FTP-PET) in clinically healthy older adults. Our hypothesis was that associations between SCD and tau-specific anatomy (ie, with one or both of the entorhinal or inferior temporal FTP-PET regions) could inform the earliest appearance of SCD. We also investigated how tau deposition in these regions might interact with Aβ, as measured with carbon 11–labeled Pittsburgh compound BPET (PiB-PET), to influence SCD and found that greater Aβ level was not associated with stronger associations between SCD and tau deposition.
Methods
Study Description
A total of 133 clinically healthy participants from the Harvard Aging Brain Study (HABS) underwent Aβ imaging with PiB-PET and FTP-PET from June 11, 2012, through April 7, 2016. To be enrolled in the HABS study, participants needed to score 0 on the Clinical Dementia Rating Scale (CDR) global score (range, 0–0.5, with higher scores indicating greater dementia),18 greater than 25 on the Mini-Mental State Examination (range, 25–30, with higher scores indicating poorer cognitive performance),19 and less than 11 on the Geriatric Depression Scale (GDS; range, 0–11 for our study, with higher scores indicating greater endorsement of depression)20 and perform within validated educational attainment–adjusted norms on Logical Memory II delayed recall.21 To be included in this study, participants were also required to have FTP-PET imaging within 18 months of neuropsychological testing. Median delay between PET and neuropsychological testing was 111 days (range, −5 to 536 days). Baseline demographic characteristics can be found in Table 1. Participants provided written informed consent and underwent investigation under protocols approved by the Partners Human Research Committee at Massachusetts General Hospital, Boston.
Table 1.
Baseline Demographic Characteristics According to Aβ Status
| Characteristic | Study Groupa | P Value | ||
|---|---|---|---|---|
| All (n = 133) | Aβ Negative (n = 94) | Aβ Positive (n = 39)b | ||
| Age, y | 75.9 (7.0) | 74.9 (7.2) | 78.4 (5.7) | .003 |
| Educational attainment, y | 15.8 (3.0) | 15.8 (3.1) | 15.7 (2.9) | .94 |
| Female, No. (%) | 75 (56.4) | 54 (57.4) | 21 (53.8) | .85 |
| SCD compositec | 0.01 (0.8) | −0.09 (0.7) | 0.11 (0.9) | .15 |
| GDSd | 3.33 (2.8) | 3.31 (2.8) | 3.38 (2.9) | .90 |
| Aβ FLR DVR (PVC) | 1.42 (0.4) | 1.18 (0.1) | 2.00 (0.4) | <.001 |
| EC FTP-PET SUVR (PVC) | 1.33 (0.3) | 1.22 (0.2) | 1.43 (0.4) | <.001 |
| IT FTP-PET SUVR (PVC) | 1.45 (0.2) | 1.41 (0.1) | 1.48 (0.2) | .02 |
| High Aβ burden, No. (%) | 39 (29.3) | NA | NA | NA |
| APOEε4 carrier, No. (%) | 35 (26.3) | 14 (14.9) | 23 (59.0) | <.001 |
Abbreviations: Aβ, β-amyloid; APOEε4, apolipoprotein E ε4 allele; DVR, distribution volume ratio; EC, entorhinal cortex; FLR, frontal, lateral, and retrosplenial tracer; FTP, flortaucipir F 18; GDS, Geriatric Depression Score; IT, inferior temporal cortex; NA, not applicable; PVC, partial volume correction; SCD, subjective cognitive decline; SUVR, standardized uptake value ratio.
Unless otherwise indicated, data are expressed as mean (SD).
Indicates cutoff of Pittsburgh compound B PVC DVR FLR of greater than 1.34 using gaussian mixture modeling Aβ.
Calculated as the mean z-transformed subscales from the Memory Functioning Questionnaire,22 the Everyday Cognition battery,23 and a 7-item questionnaire adapted from the Structured Telephone Interview for Dementia Assessment. Scores represent a z-score composite ranging from −1.15 to 2.81, with higher scores indicating greater subjective concern.
Scores range from 0 to 11, with higher scores indicating greater development of depression.
Development of the SCD composite has been published previously.24 In brief, the composite was created by calculating the mean z-transformed subscales from the Memory Functioning Questionnaire22 (the first 18 items of the General Frequency of Forgetting), the Everyday Cognition battery23 (the Memory section from the self-report version), and a 7-item questionnaire adapted from the Structured Telephone Interview for Dementia Assessment (using the present sample as the reference group).24 The Memory Functioning Questionnaire was reversed before calculating the mean with the other measures. To account for the potential confounding of poor mood related to SCD, we used the GDS score to measure depressive symptoms. The GDS was adjusted for the present analyses to remove 4 items that featured elements of cognitive concern (Do you feel you have more problems with memory than most [question 14]? Do you have trouble concentrating [question 26]? Is it easy for you to make decisions [question 29]? and Is your mind as clear as it used to be [question 30]?), which resulted in a total score of 26.
For Aβ PET imaging, acquisition of PiB-PET data in the HABS has previously been described in detail.25 In brief, PiB-PET images were acquired with an 8.5- to 15.0-mCi bolus injection, followed by a 60-minute dynamic acquisition in 69 volumes (12 × 15 seconds and 57 × 60 seconds). For tau PET imaging, FTP-PET (also known as 18F-labeled AV1451 or T807) was prepared at Massachusetts General Hospital as previously described.26 Images were acquired from 80 to 100 minutes in 4 × 5-minute frames after a mean (SD) bolus injection of 10.0 (1.0) mCi. All PET data were reconstructed, attenuation corrected, evaluated for head motion, and coregistered to the corresponding T1 image for each patient using 6 df rigid body registration. For PiB-PET and FTP-PET, cerebellar gray matter was used as the reference region from the FreeSurfer aseg atlas as previously described,11,27 with FTP-PET measures computed as standardized uptake value ratios (SUVRs), whereas for PiB-PET, a summary distribution volume ratio (DVR) was used. For PiB-PET and FTP-PET, we performed partial volume correction by using the geometric transform matrix method28,29 as implemented in FreeSurfer software (version 6.0; https://surfer.nmr.mgh.harvard.edu/) and described by Greve and colleagues.30 We used a slightly modified FreeSurfer atlas mapped to each participant’s native structural space that included regions of interest for cerebrospinal fluid, white matter, and extracerebral structures. The partial volume correction processing was performed assuming a uniform 6-mm point spread function.
A composite PiB-PET DVR measure of cortical Aβ burden consisting of frontal, lateral, and retrosplenial tracer (FLR) uptake31 was determined for each participant by calculating the median PiB uptake value across voxels in the precuneus, rostral anterior cingulate, medial orbitofrontal, superior frontal, rostral middle frontal, inferior parietal, inferior temporal, and middle temporal regions of interest from both hemispheres divided by the median PiB-PET DVR from cerebellar gray matter. The regions constituting the FLR are known to show elevated PiB binding in patients with AD dementia.32
For FTP-PET, we focused our analyses on 2 structurally defined regions of interest, the entorhinal and inferior temporal cortices. The entorhinal cortex was chosen because it is among the first regions to develop tau pathologic changes, even in the absence of Aβ. The inferior temporal cortex was used as the current best choice of a surrogate marker of early AD-related tauopathy in the neocortex; inferior temporal cortex FTP-PET shows the largest effect size between impaired and non-impaired individuals as reported by Johnson and colleagues.11
Whole-brain maps were also used for post hoc analyses to determine SCD-associated tau and Aβ maps; these maps were created by taking the participants’ image-mapping native PET images to fs average surface in FreeSurfer and smoothing with the equivalent of an 8-mm Gaussian kernel. Uncorrected thresholds (P < .025, 1-tailed test) were used for the purposes of exploratory analysis for the general linear model of FTP-PET SUVR approximated as SCD + age. Analyses were conducted using MATLAB (generalized linear model scripts can be accessed at http://mrtools.mgh.harvard.edu/). For the whole-brain PiB analysis, the generalized linear model was conducted with Aβ FLR DVR approximated as SCD + age.
Statistical Analysis
We used statistical package R (version 3.2.2; https://cran.r-project.org/bin/windows/base/old/3.2.2/), along with the QuantPsyc, car, MASS, relaimpo, sjPlot, lm.ridge, npreg, and sjmisc extension packages. Demographic comparisons were made using independent-sample and 2-tailed t tests and χ2 tests of independence. A series of linear hierarchical regressions were conducted to determine the influence of tau and Aβ on SCD after accounting for covariates (age, educational attainment, sex, depressive symptoms [adjusted GDS score], and delay from neuropsychological testing to FTP-PET scanning) and subsequently within the context of an interaction between tau and Aβ. Multiple comparisons for these 8 linear models were accounted for using a Šidak-corrected α of 1 − (1 − 0.05)1/8 = 0.006. Because Aβ FLR data are positively skewed, comparison nonparametric analyses for robustness were run using rigid local linear kernel-based estimation analyses33 and bootstrapped with 399 independently identically distributed draws (with bootstrapped P values included in Table 2). Model 1 approximated SCD with covariates; model 2a, SCD with entorhinal cortex FTP-PET + covariates; model2b, SCD with inferior temporal cortex FTP-PET + covariates; model 3, SCD with Aβ FLR + covariates; model 4a, SCD with entorhinal cortex FTP-PET × Aβ FLR + covariates; and model4b, SCD with inferior temporal cortex FTP-PET × Aβ FLR + covariates, where SCD is defined as described above and covariates included age, educational attainment, sex, depressive symptoms, and delay time from neuropsychological testing to FTP-PET scan.
Table 2.
Regression Coefficients Estimating SCD From EC and IT Tau and Covariatesa
| Covariate by Model (R2 Value) | β (95% CI) | P Value | Bootstrapped P Valueb | Relative Importance (% Explained of R2 Value)c |
|---|---|---|---|---|
| Model 2a (0.24) | ||||
| EC tau | 0.35 (0.19 to 0.52) | <.001 | <.001 | 47 |
| Age | 0.06 (−0.11 to 0.23) | .49 | .36 | 11 |
| Male | 0.11 (−0.04 to 0.27) | .15 | .69 | 4 |
| Educational attainment | −0.06 (−0.21 to 0.10) | .48 | .59 | 0 |
| Adjusted GDS score | 0.30 (0.14 to 0.45) | <.002 | <.001 | 36 |
| Time from NP to FTP-PET | 0.05 (−0.10 to 0.20) | .53 | .53 | 0 |
| Model 2b (0.14) | ||||
| IT tau | 0.10 (−0.08 to 0.28) | .27 | .32 | 10 |
| Age | 0.14 (−0.04 to 0.32) | .13 | .10 | 25 |
| Male | 0.08 (−0.08 to 0.25) | .34 | .42 | 5 |
| Educational attainment | −0.02 (−0.19 to 0.14) | .79 | .91 | 0 |
| Adjusted GDS | 0.28 (0.11 to 0.44) | .001 | <.001 | 58 |
| Time from NP to FTP-PET | 0.05 (−0.11 to 0.22) | .53 | .53 | 1 |
| Model 3 (0.18) | ||||
| Aβ FLR | 0.24 (0.08 to 0.40) | .005 | .005 | 29 |
| Age | 0.13 (−0.04 to 0.30) | .13 | .11 | 19 |
| Male | 0.08 (0.14 to 0.46) | .31 | .39 | 4 |
| Educational attainment | −0.03 (−0.20 to 0.13) | .67 | .77 | 0 |
| Adjusted GDS | 0.30 (0.14 to 0.46) | <.001 | <.001 | 47 |
| Time from NP to FTP-PET | 0.05 (−0.11 to 0.21) | .56 | .56 | 0 |
| Post hoc EC tau and IT tau model (0.25) | ||||
| EC tau | 0.45 (0.24 to 0.65) | <.001 | <.001 | 45 |
| IT tau | −0.16 (−0.37 to 0.04) | .12 | .03 | 6 |
| Age | 0.09 (−0.08 to 0.27) | .29 | .18 | 10 |
| Male | 0.10 (−0.05 to 0.26) | .20 | .40 | 4 |
| Educational attainment | −0.04 (−0.20 to 0.12) | .61 | .87 | 0 |
| Adjusted GDS | 0.29 (0.14 to 0.45) | <.001 | <.001 | 33 |
| Time from NP to FTP-PET | 0.04 (−0.11 to 0.20) | .59 | .59 | 0 |
| Model 4a (0.25) | ||||
| EC tau | 0.36 (0.15 to 0.58) | .001 | <.001 | 35 |
| Age | 0.04 (−0.13 to 0.21) | .66 | .58 | 8 |
| Male | 0.10 (−0.05 to 0.26) | .20 | .09 | 4 |
| Educational attainment | −0.06 (−0.21 to 0.10) | .48 | .54 | 0 |
| Adjusted GDS | 0.30 (0.14 to 0.46) | <.01 | <.001 | 35 |
| Aβ FLR | 0.13 (−0.06 to 0.33) | .18 | 0.05 | 11 |
| EC tau × Aβ FLR | −0.36 (−0.34 to 0.09) | .25 | .18 | 4 |
| Time from NP to FTP-PET | 0.03 (−0.12 to 0.19) | .67 | .67 | 0 |
| Model 4b (0.19) | ||||
| IT tau | −0.14 (−0.76 to 0.47) | .65 | .79 | 5 |
| Age | 0.14 (−0.05 to 0.32) | .15 | .11 | 16 |
| Male | 0.08 (−0.08 to 0.25) | .31 | .53 | 4 |
| Educational attainment | −0.04 (−0.20 to 0.13) | .66 | .78 | 0 |
| Adjusted GDS | 0.30 (0.14 to 0.46) | <.001 | <.001 | 48 |
| Aβ FLR | 0.22 (0.03 to 0.41) | .03 | .02 | 25 |
| IT tau × Aβ FLR | 0.16 (−0.47 to 0.79) | .61 | .64 | 0 |
| Time from NP to FTP-PET | 0.05 (−0.11 to 0.21) | .53 | .53 | 0 |
Abbreviations: Aβ, β-amyloid; APOEε4, apolipoprotein E ε4 allele; DVR, distribution volume ratio; EC, entorhinal cortex; FLR, frontal, lateral, and retrosplenial; FTP-PET, flortaucipir F 18 positron emission tomography; GDS, Geriatric Depression Score; IT, inferior temporal cortex; NA, not applicable; NP, neuropsychological testing; PVC, partial volume correction; SCD, subjective cognitive decline; SUVR, standardized uptake value ratios.
All variables are centered. Models are described in the Statistical Analysis subsection of the Methods section.
Nonparametric local linear kernel-based estimation using independently identically distributed draw bootstrapping (time from NP not included in model).
Relative importance using the metric of Lindemann et al.34
The fit of each increasingly complex model was tested against the fit of the prior model by using analysis of variance. A more complex model was rejected in favor of a simpler model if the analysis of variance revealed P > .05. Pearson correlations between all covariates and the variables of interest were included to denote the associations between covariates and the dependent variable.
Two post hoc exploratory, whole-brain, voxel-wise general linear models were also conducted to identify evidence of positive associations between (1) voxel-wise FTP-PET SUVR and SCD after covarying age and (2) voxel-wise Aβ FLR DVR and SCD after covarying age. Owing to the exploratory nature of these analyses, parsimonious models were run that accounted only for age. The rationale behind these analyses was to determine the spatial specificity of positive associations between SCD and tau and between SCD and Aβ.
Results
We included 133 patients, of whom 75 (56.3%) were women and 58 (43.6%) were men, with a mean (SD) age of 76 (6.9) years (range, 55–90 years). Increasing SCD was associated with greater age (r131 = 0.23; P = .007) and greater adjusted GDS score (ρ131 = 0.28; P = .001). No effect of sex (t123.4 = −1.01; P = .31) or years of education (ρ131 = 0.01; P = .89) was found on SCD. The mean score on the SCD composite was 0.015 (range, −1.15 to2.81, with a slight positive skew), with a score above the mean indicating greater cognitive concern.
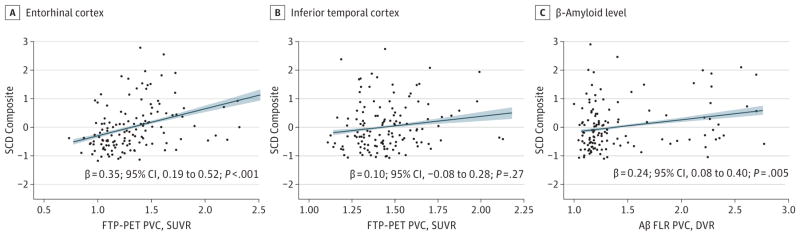
Greater entorhinal cortical tau deposition was found to be associated with significantly greater SCD beyond the covariates (β = 0.35; 95% CI, 0.19-.52; P < .001) (Figure 1A). The adjusted GDS score was also found to be associated with significantly greater SCD. Including entorhinal cortical tau in model 2a elicited a significantly better-fitting model than that including only covariates (model 1 vs model 2a: change in F = 17.6; P < .001). No association was found between SCD and inferior temporal cortex tau (β = 0.10; 95% CI, −0.08 to 0.28; P = .27) (model 2b) (Figure 1B), and model fit was not better after the inclusion of inferior temporal cortex tau as a factor (model 1 vs 2b: change in F = 1.2; P = .27). Table 2 depicts model estimates for all regression models. Greater Aβ FLR was found to be associated with significantly greater SCD (β = 0.24; 95% CI, 0.80–.40; P = .005; model 3).
Figure 1. Partial Regression Plots.
Plots depict the associations between a composite measure of subjective cognitive decline (SCD; described by Amariglio et al24; greater numbers indicate greater development of SCD) and flortaucipir F 18 positron emission tomography (FTP-PET; previously known as AV 1451, T807) standardized uptake value ratio (SUVR) in the entorhinal and inferior temporal cortices and between SCD and β-amyloid (Aβ) frontal, lateral, and retrosplenial tracer (FLR) uptake summary distribution ratio (DVR). Diagonal lines indicate lines of best fit for the correlational analysis; shading, 95% CI; and data points, study participants. PVC indicates partial volume correction.
A post hoc calculation of relative importance that used the metric of Lindeman et al34 was run for each factor in the linear regression entorhinal cortex tau model (model 2a in Table 2). The entorhinal cortical tau was found to account for most of overall explained variance in the model (47%), followed by the adjusted GDS score (36%) and other covariates (16%). A posthoc linear regression was run to determine whether entorhinal cortical tau maintained an association with SCD after including the effects of inferior temporal cortical tau and covariates because entorhinal cortical and inferior temporal cortical tau are associated (r131 = 0.65; P < .001). In this model, entorhinal cortical tau was found to be significantly associated with SCD (β = 0.45; 95% CI, 0.24-.65; P < .001) (Table 2).
In model 4a, no interactive effect between Aβ FLR and entorhinal cortical tau was found on SCD (Table 2). Furthermore, including Aβ FLR in the model did not create a significantly better-fitting model (model 2 vs 4a: change in F = 1.4; P = .24). Simply assessing the main effects of Aβ FLR and entorhinal cortical tau (without any interactive term) resulted in a significant effect of entorhinal cortical tau on SCD (β = 0.30; 95% CI, 0.11-.49; P = .002) and no significant effect of Aβ FLR (β = 0.09; 95% CI, −0.09 to 0.28; P = .32). In the inferior temporal cortical tau model (model 4b), no interactive effect of inferior temporal cortical tau and Aβ FLR was found on SCD (β = 0.05; 95% CI, −0.15 to 0.26; P = .61).
To examine any effects of multicolline arity, we present correlations between covariates and SCD in the eTable in the Supplement; we found moderate significant correlations between age and inferior temporal cortical tau (r131 = 0.37; P < .001), age and entorhinal cortical tau (r131 = 0.31; P < .001), and age and Aβ (r131 = 0.18; P = .03); however, these correlations did not explain more than 13% of the variance in the model and thus were not considered to impact collinearity assumptions.35
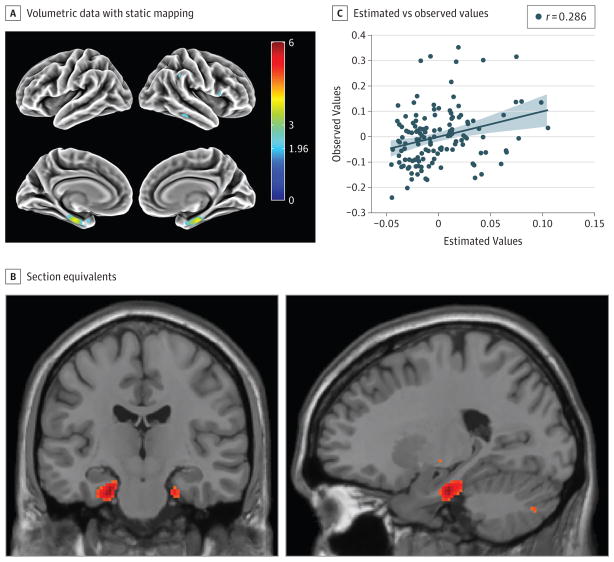
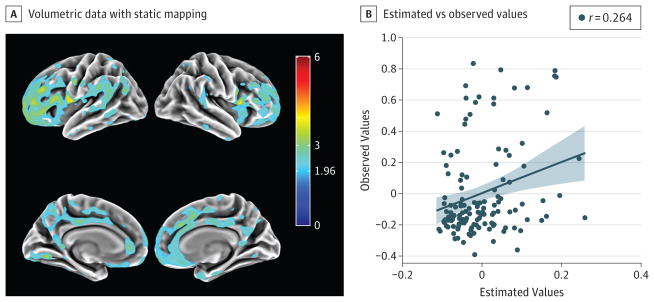
A post hoc exploratory whole-brain voxel-wise general linear model was conducted to identify evidence of positive associations between voxel-wise FTP-PET SUVR and SCD after taking into account the main effects of age. Whole-brain analysis supported the notion that greater SCD was moderately associated with greater FTP-PET SUVR in the entorhinal cortical region (peak effect: r = 0.30) after accounting for age (Figure 2). The association was most apparent in the left hemisphere and was not found convincingly in any other regions. By contrast, SCD was found to be associated with PiB-PET DVR in a widespread neocortical distribution prominently in the medial and lateral frontal regions and the cingulate after accounting for age (Figure 3); no association was found in medial temporal regions. After accounting for entorhinal cortex FTP-PET, most associations became attenuated, with only a few very-low-magnitude effect clusters remaining in lateral frontal regions.
Figure 2. Association of Subjective Cognitive Decline (SCD) With Flortaucipir F 18 Positron Emission Tomography (FTP-PET) Findings.
Whole-brain voxel wise analysis with FTP-PET (previously known as AV 1451, T807) standardized uptake value ratio (SUVR) was performed after accounting for age. A, Surface rendering of volumetric data with static mapping from Montreal Neurological Institute (MNI) space to fs average from FreeSurfer software. B, Brain section equivalents are shown to better visualize the medial temporal lobe (chosen from apex t value in the region of interest; ie, MNI coordinates −22 − 18 − 26). Color bar indicates t values. C, Plot of the estimated and observed values from the apex of the entorhinal cortex region of interest from the general linear model of FTP-PET SUVR approximated as SCD + age. Diagonal lines indicate lines of best fit for the correlational analysis; shading, 95% CI; and data points, study participants.
Figure 3. Association of Subjective Cognitive Decline (SCD) With Pittsburgh compound B Carbon 11–Labeled Positron Emission Tomography (PiB-PET) Findings.
Whole-brain voxel wise analysis with summary distribution volume ratio (DVR) was performed after accounting for age. A, Surface rendering of volumetric data with static mapping from Montreal Neurological Institute space to fs average from FreeSurfer software. Color bar indicates t values. B, Plot of the estimated and observed values from the apex of the inferior lateral frontal region from the general linear model of PiB-PET DVR approximated as SCD + age. Diagonal lines indicate lines of best fit for the correlational analysis; shading, 95% CI; and data points, study participants.
Discussion
Subjective cognitive decline in clinically healthy older adults manifests in the context of increasing entorhinal cortical tauopathy. These findings existed regardless of whether we adjusted for subclinical levels of depressive symptoms, which have been found in previous literature to be associated with SCD.36 An exploratory whole-brain analysis demonstrated an entorhinal-specific FTP-PET association with SCD, supporting this notion of regional specificity. Although SCD was also found to be elevated with a higher Aβ burden, supporting previous study findings,15,37 this effect was weaker; an interactive effect between tau and Aβ on SCD was not apparent. Whole-brain analyses of Aβ with SCD revealed more diffuse associations across frontal and parietal regions, consistent with previous work,5 but no evidence of associations in medial temporal regions. Only individuals in the more developed stages of tau deposition (ie, late in the entorhinal cortex and earlier in the inferior temporal cortex)may drive the link between Aβ and SCD; however, this question will remain unclear until tau-stage–based measures can be quantified. Considering that FTP-PET and 11C-PiB uptake are associated in the HABS,11 this lack of interaction may also be interpreted as indicative of multiple pathologic pathways that motivate the endorsement of SCD symptoms. A similar mechanism has been reported in previous work,38 in which endorsement of SCD symptoms was elevated in the context of additive, rather than interactive, evidence of Aβ and neurodegeneration. Taken together, we posit that our findings support SCD as an early behavioral manifestation of entorhinal cortical tauopathy.
Our findings support a potential temporal sequence of events, with SCD marking one of the earliest behavioral changes among increasing pathologic change in clinically healthy older adults.39 Entorhinal tauopathy in the presence of minimal Aβ load is argued to be the earliest component of the AD pathologic continuum,40 and as such, endorsement of SCD symptoms may represent the earliest signs of preclinical AD in some cases. With consideration of evidence that inferior temporal cortical tau is representative of later stages in the tau topographic cascade,11,12,16,41,42 a lack of an association with SCD provides additional evidence for its appearance in the earliest stages of the preclinical trajectory. Inferior temporal cortical FTP-PET findings have been associated with increasing clinical impairment,42 as measured by clinical screening tools such as the Clinical Dementia Rating Scale sum of boxes, the Mini-Mental State Examination, and Logical Memory test,11 and are associated with episodic memory dysfunction.12,42 Therefore, a lack of an association between SCD and inferior temporal cortical tau may represent a subtle change of insight with the progression of AD-related tauopathy. We did not examine measures to interrogate this hypothesis; thus, future studies should examine associations between SCD and inferior temporal cortical tau in light of an informant’s report.
Entorhinal cortical tau deposition is common with advancing age15,16,41,43 and is the earliest cortical involvement in the succession of tauopathy stages that, along with Aβ pathologic changes, define AD.15,40,41 Our present findings do not enable us to estimate whether an individual with an elevated entorhinal cortical tau PET signal will inevitably develop AD. Thus, the association that we found between the entorhinal cortex and SCD does not permit definitive conclusions about whether those individuals with a strong association will progress to AD. We are currently conducting serial evaluations to clarify this point. Our study does, however, raise the possibility that SCD signifies age-related neural insult and subsequent cognitive decline that is independent of fully expressed AD pathologic changes.44,45 In the present study, Aβ did not provide more than 1% added variance (R2 value) in models 4a and 4b, and did not affect entorhinal cortex–FTP-PET regression coefficients, findings that may support this possibility. Our findings were unable to fully characterize the Aβ contribution, however, possibly owing to PET limitations with measuring lower ranges of Aβ burden.46 Thus, we cannot definitively determine whether entorhinal cortical tauopathy and its SCD phenotypic feature are biologically distinct from early AD pathologic changes.
Another alternative explanation of our findings is that SCD is, in part, a manifestation of heterogenic strains of tau; some studies suggest a prion like propagation of tau, with neurodegenerative disease associated with different sets of strains.47 In addition, the appearance of neurofibrillary tangles is a byproduct of a range of causes48,49 that are maladaptive for optimal synaptic function.48,50 Regardless of whether SCD is AD specific, endorsement of SCD symtpoms reveals some level of underlying neuropathologic change.
Limitations
This study has a few limitations. The FTP-PET tracers were introduced into the HABS midway through the progression of the study; we chose the closest SCD measure from the time of the FTP-PET scan and covaried the delay between SCD and tau PET collections to account for cross-sectional idiosyncrasies. Unintentional biases may have related to the cross-sectional investigation of SCD after baseline; that is, individuals may have recalibrated their SCD according to their experience and judgment of their performance on cognitive testing that did not exist at the baseline of the HABS. Future studies will require longitudinal analyses that relate change in tau deposition to change in SCD. In addition, we used a composite of SCD that is honed on items related primarily to subjective concerns of memory decline; SCD from other cognitive domains may also be sensitive to PET markers of tauopathy in other regions. Finally, participants in the HABS cohort generally exhibited lower levels of SCD on our composite measure, which restricted our ability to measure very severe SCD and may have contributed to our null findings with inferior temporal cortical FTP-PET.
Conclusions
Our findings support the notion that SCD is an early indicator of tauopathy in clinically healthy older adults. This study is the first, to our knowledge, to show associations between SCD and region-specific tauopathy, with the implication that SCD robustly manifests with increasing tau deposition specific to the entorhinal cortical region regardless of Aβ burden. Future work will need to isolate the temporal pattern of associations between AD biomarkers and the appearance of SCD; however, we argue that our findings support the notion of SCD as an early behavioral marker of pathologic change. Further work should examine longitudinal change-on-change associations between these biologic and subjective markers. In sum, we argue that these data, and the work of others,51,52 support an association between SCD and AD biomarkers; however, an alternative argument that SCD is motivated by AD-unrelated tauopathy cannot be discounted and will also need to be examined. Our findings suggest multiple and potentially nonoverlapping pathologic drivers of endorsement of SCD symptoms. This complexity must be taken into consideration when assessing the genesis of SCD in clinically healthy older adults.
Supplementary Material
Key Points.
Question
How do concerns about subjective cognitive decline in clinically healthy older adults relate to tau burden in brain regions of interest and global β-amyloid burden?
Findings
In this imaging study of 133 clinically healthy adults, subjective cognitive decline was associated with greater tau burden only in the entorhinal cortical region and, to a lesser extent, greater global β-amyloid levels; however, neither pathologic factor exerted an interactive influence on subjective cognitive decline.
Meaning
Subjective cognitive decline is an important early indicator of abnormal tau and β-amyloid burden in clinically healthy adults with multiple underlying pathways; as such, multiple Alzheimer disease pathologic factors must be examined when considering the appearance of subjective cognitive decline in clinically healthy older adults.
Acknowledgments
Funding/Support: This study was supported by the Dementia Research Fellowship APP1105576 from the Australian National Health and Medical Research Council (Dr Buckley); grants P01 AG036694 (Drs Sperling and Johnson), P50 AG005134 (Drs Sperling and Johnson), R01 AG046396 (Dr Johnson), and K24 AG035007 (Dr Sperling) from the National Institutes of Health (NIH); grant SPD28094292 from the Belgian National Science Foundation; grant P16008 from the Belgian Foundation for Alzheimer Research (Dr Hanseeuw); and grant K23 AG044431 from the NIH (Dr Amariglio). This research was performed in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using Biotechnology Resource Grant P41EB015896 from the Center for Functional Neuroimaging Technologies, the National Institute of Biomedical Imaging and Bioengineering, NIH. This work also involved the use of instrumentation supported by grants S10RR021110, S10RR023401, and S10RR023043 from the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program.
Footnotes
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Drs Buckley and Amariglio had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Buckley, Hanseeuw, Rentz, Sperling, Johnson, Amariglio.
Acquisition, analysis, or interpretation of data: Buckley, Hanseeuw, Schultz, Vannini, Aghjayan, Properzi, Jackson, Mormino, Sperling, Johnson, Amariglio.
Drafting of the manuscript: Buckley, Aghjayan, Jackson, Johnson, Amariglio.
Critical revision of the manuscript for important intellectual content: Hanseeuw, Schultz, Vannini, Properzi, Jackson, Mormino, Rentz, Sperling, Johnson, Amariglio.
Statistical analysis: Buckley, Hanseeuw, Schultz, Vannini, Properzi, Mormino, Johnson.
Obtained funding: Sperling, Johnson.
Administrative, technical, or material support: Properzi, Jackson, Johnson.
Study supervision: Hanseeuw, Rentz, Sperling, Johnson, Amariglio.
Conflict of Interest Disclosures: Dr Schultz reports sitting on an advisory board for Biogen in April 2016. Dr Sperling reports serving as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi; serving as a coinvestigator for Avid, Eli Lilly and Co, and Janssen Alzheimer Immunotherapy clinical trials; speaking at symposia sponsored by Eli Lilly and Co, Biogen, and Janssen; and receiving research support from Janssen Pharmaceuticals, and Eli Lilly and Co. Dr Johnson reports serving as a paid consultant for Bayer, GE Healthcare, Janssen Alzheimer’s Immunotherapy, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, ISIS Pharma, AZ Therapy, GEHC, Lundberg, and Abbvie; serving as a site coinvestigator for Lilly/Avid, Pfizer, Janssen Immunotherapy, and Navidea; and speaking at symposia sponsored by Janssen Alzheimer’s Immunotherapy and Pfizer. These relationships are not related to the content in the manuscript. No other disclosures were reported.
References
- 1.Luck T, Luppa M, Matschinger H, Jessen F, Angermeyer MC, Riedel-Heller SG. Incident subjective memory complaints and the risk of subsequent dementia. Acta Psychiatr Scand. 2015;131(4):290–296. doi: 10.1111/acps.12328. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RF, Maruff P, Ames D, et al. AIBL Study. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimers Dement. 2016;12(7):796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfsgruber S, Jessen F, Koppara A, et al. Subjective cognitive decline is related to CSF biomarkers of AD in patients with MCI. Neurology. 2015;84(12):1261–1268. doi: 10.1212/WNL.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 5.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22(5):747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 8.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 9.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanseeuw BJ, Betensky RA, Schultz AN, et al. FDG metabolism associated with tau-amyloid interaction predicts memory decline. Ann Neurol. 2017;81(4):583–596. doi: 10.1002/ana.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 17.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 20.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 22.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 23.Farias ST, Mungas D, Reed BR, et al. The measurement of Everyday Cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoup TM, Yokell DL, Rice PA, et al. A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J Labelled Comp Radiopharm. 2013;56(14):736–740. doi: 10.1002/jlcr.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker JA, Hedden T, Carmasin J, et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904–911. [PubMed] [Google Scholar]
- 29.Labbé C, Koepp M, Ashburner J, et al. Quantitative FBIWPET, editor. In: Absolute PET quantification with correction for partial volume effects within cerebral structures. Carson RE, Daube Witherspoon ME, Herscovitch P, editors. Bethesda, MD: National Institutes of Health; 1998. pp. 59–66. [Google Scholar]
- 30.Greve DN, Salat DH, Bowen SL, et al. Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–343. doi: 10.1016/j.neuroimage.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 32.Mormino EC, Betensky RA, Hedden T, et al. Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Racine J. Cross-validated local linear nonparametric regression. Stat Sin. 2004;14:485–512. [Google Scholar]
- 34.Lindeman RH, Merenda PF, Gold RZ. Introduction to Bivariate and Multivariate Analysis. London, England: Scott Foresman; 1980. [Google Scholar]
- 35.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 36.Hill NL, Mogle J, Wion R, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist. 2016;56(6):e109–e127. doi: 10.1093/geront/gnw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beach TG, Sue LI, Walker DG, et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer’s disease: implications for amyloid imaging. J Alzheimers Dis. 2012;28(4):869–876. doi: 10.3233/JAD-2011-111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amariglio RE, Mormino E, Vannini P, et al. Greater subjective cognitive concerns correspond with advancing stages of preclinical AD. Alzheimers Dement. 2014;10(4):566. [Google Scholar]
- 39.Colijn MA, Grossberg GT. Amyloid and tau biomarkers in subjective cognitive impairment. J Alzheimers Dis. 2015;47(1):1–8. doi: 10.3233/JAD-150180. [DOI] [PubMed] [Google Scholar]
- 40.Duyckaerts C, Braak H, Brion J-P, et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015;129(5):749–756. doi: 10.1007/s00401-015-1390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52(6):1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 42.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H, Thal DRMD, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 44.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontecorvo M, Devous M, Joshi A, et al. Relationships between 18F-AV-1451 (aka 18F-T807) PET tau binding and amyloid burden in cognitively normal subjects and patients with cognitive impairments suspected of Alzheimer’s disease. J Nucl Med. 2015;56(suppl 3):245. [Google Scholar]
- 46.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(pt 7):2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82(6):1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villemagne VL, Okamura N. Tau imaging in the study of ageing, Alzheimer’s disease, and other neurodegenerative conditions. Curr Opin Neurobiol. 2016;36:43–51. doi: 10.1016/j.conb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Beharry C, Cohen LS, Di J, Ibrahim K, Briffa-Mirabella S, Alonso AdC. Tau-induced neurodegeneration: mechanisms and targets. Neurosci Bull. 2014;30(2):346–358. doi: 10.1007/s12264-013-1414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jessen F, Amariglio RE, van Boxtel M, et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molinuevo JL, Rabin LA, Amariglio R, et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13(3):296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.