Abstract
Chimeric antigen receptor (CAR) T-cells targeting CD19 mediate potent effects in relapsed/refractory pre-B cell acute lymphoblastic leukemia (B-ALL) but antigen loss is a frequent cause of resistance to CD19-targeted immunotherapy. CD22 is also expressed on most B-ALL and usually retained following CD19 loss. We report results from a phase I trial testing a novel CD22-CAR in twenty-one children and adults, including 17 previously treated with CD19-directed immunotherapy. Dose dependent anti-leukemic activity was observed with complete remission in 73% (11/15) of patients receiving ≥ 1 × 106 CD22-CART cells/kg, including 5/5 patients with CD19dim/neg B-ALL. Median remission duration was 6 months. Relapses were associated with diminished CD22 site density that likely permitted escape from killing by CD22-CART cells. These results are the first to eastablish the clinical activity of a CD22-CAR in pre-B cell ALL, including in leukemia resistant to anti-CD19 immunotherapy, demonstrating comparable potency to CD19-CART at biologically active doses in B-ALL. They also highlight the critical role played by antigen density in regulating CAR function. (Funded by NCI Intramural Research Program)
Graphical Abstract
INTRODUCTION
Cure rates for children with B-ALL approach 90%, but outcomes for those with relapsed and chemotherapy refractory disease remain poor.1,2 Adults with B-ALL experience survival rates <50%, even when treated with pediatric-inspired, risk-adapted, multi-agent regimens.3–5 Risk-adapting therapy can diminish the prevalence of severe late effects in survivors, but long-term morbidity remains substantial, especially in patients treated with intensive regimens for high risk-disease.6,7
Immunotherapies targeting CD19 have recently provided a new class of effective therapeutics for B-ALL. Blinatumomab, a CD19xCD3 bispecific antibody, mediates impressive effects in patients with overt8,9 and minimal residual disease (MRD) levels of B-ALL10. T cells expressing chimeric antigen receptors (CARs) targeting CD19 have also demonstrated impressive antileukemic effects in children and adults with relapsed/refractory B-ALL with remission rates ranging from 70–90%.11–13 However, the likelihood of durable remission following CD19 targeted immunotherapy remains unknown. Although CD19 is expressed on essentially all cases of B-ALL at clinical presentation14,15, relapses with loss or diminished surface expression of CD19 are increasingly recognized as a cause of treatment failure.12,16–18
Like CD19, CD22 is expressed on most cases of pre-B cell ALL14,19,20, and normal tissue expression is restricted to the B cell lineage. Substantial clinical experience and success has been reported with monoclonal antibody (mAb)-based therapeutics targeting CD22.21–29 We report the first clinical experience using a CD22-CAR in pre-B ALL19,30. Our data demonstrate that CD22-CAR expressing T cells have a similar safety profile to CD19-CARs and mediate similarly potent anti-leukemic effects, in both immunotherapy-naïve patients and patients with CD19 dim/negative relapse following CD19-directed immunotherapy. These results are the first to establish that CAR expressing T cells targeting antigens other than CD19 can mediate similarly potent antineoplastic effects and the first to demonstrate that resistance to immunotherapy via antigen loss can be overcome by treatment with CAR T cells targeting an alternative antigen, opening the way to dual targeted immunotherapeutics.
RESULTS
Patient Characteristics
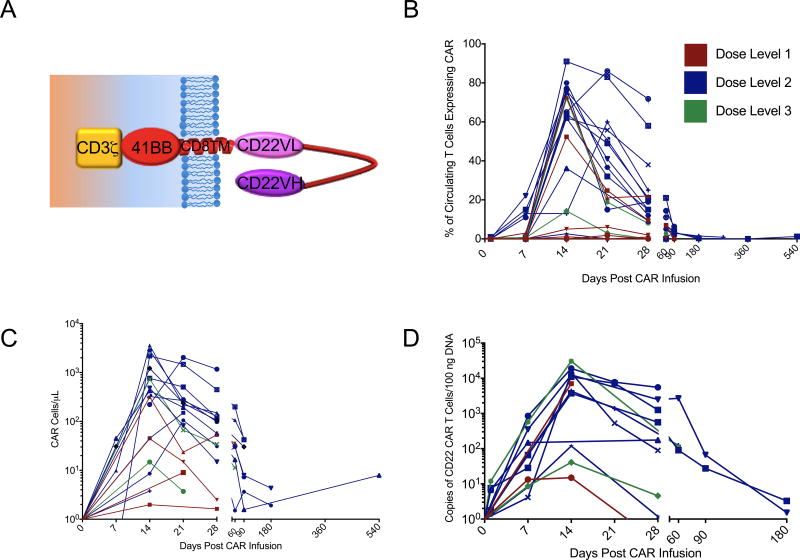
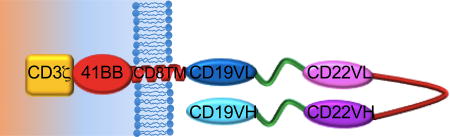
The first twenty-one consecutive patients with relapsed or refractory B-ALL treated with CD22 CAR T cells are included in this analysis. The median age was 19 years (range 7–30 years), and all patients had undergone at least 1 prior hematopoietic stem cell transplantation (HSCT) and 2 patients had received 2 prior HSCTs (Supplementary Table 1). Seventeen patients had received prior CD19 directed immunotherapy, including 15 who received prior CD19-CAR therapy. Lymphoblasts were CD19-negative or dim in 10 patients, including 9 following CD19-CAR therapy and 1 following blinatumomab. Median marrow blast percentage was 70.5% (range 1%-99%) and all were CNS1 (< 5 WBC/mcL and no blasts). Median CD22 site density was 2839 molecules per cell (range 613–13452). Fourteen patients manifested B-cell aplasia at enrollment (B cell counts < 50 cells/mcL), including 7 patients who had received prior therapy with a CD19-CAR suggesting ongoing effects of the previous CAR therapy please clarify why this is important. You need to provide more information for the general reader.Also, please describe in more detail the design of the CAR so that readers know from the outset that it is CD22.BB.z. All treated patients received the intended protocol-specified cell dose of T cells modified to express the anti-CD22 CAR construct which is based on a binding domain previously reported30 and modified to incorporate a 41BB endodomain which has been shown to improve persistence.31 A schematic of the anti-CD22 CAR construct is shown in Figure 1A. Product characteristics are shown in Supplemental Table 2.
Figure 1. Expansion of CD22 CAR T cells infused following lymphodepleting chemotherapy.
A. Percentage of circulating T-cells which express CD22 CAR as measured by flow cytometry. B. Absolute number of circulating CAR T-cells per mcL blood calculated by multiplying the percent CD22 CAR positive by the absolute CD3+ T cells/mcL. C. Copies of integrated CD22-CAR transgene per 100 mcg of DNA obtained from peripheral blood mononuclear cells.
Toxicity
The primary toxicity was cytokine release syndrome (CRS)11–13 occurring in 16/21 patients, coinciding with CAR T cell expansion, and with onset after day +5 in all patients. Protocol-defined (supplemental methods) grade 1 CRS occurred in 9 patients and grade 2 CRS occurred in 7 patients. Patient #2 experienced grade 3 self-limited, non-infectious, diarrhea during CRS which resolved with supportive care, but resulted in a dose limiting toxicity (DLT) at the first dose level requiring a protocol-specified expansion of the first dose level to 6 patients with no further DLTs observed. Three patients received dose level 2 (1×106/kg CD22-CAR T cells/kg) with no evidence of DLT. Two patients then proceeded to dose level 3 (3 × 106/kg CD22-CAR T cells/kg), and patient #10 developed dose limiting grade 4 hypoxia associated with rapid disease progression requiring brief intubation with complete resolution of the hypoxia within 24 hours after initiation of steroids. A second patient was safely treated at this dose level without DLT. Based on the single DLT at dose level 3 and clinical activity associated with significant CD22-CAR T cell expansion and persistence at dose level 2, 1×106/kg CD22-CAR T cells/kg was identified as the recommended phase II dose and the cohort was expanded to ten additional patients (n=13 total at dose level 2). One subject (patient #14) with a pre-enrollment history of multi-organ failure due to sepsis, died from gram negative rod sepsis developing after resolution of CRS and neutrophil count recovery to >1000 cells/mcL. Prospective neurotoxicity evaluations demonstrated no irreversible neurotoxicity or seizure. Amongst the first 16 patients with complete assessments, transient visual hallucinations (n=2), mild unresponsiveness (n=1), mild disorientation (n=1) and mild-moderate pain (n=2) were observed but returned to baseline by day 28 post-infusion. B-cell aplasia (< 50 cells/mcL) was noted in all patients achieving remission, including patients who were not previously B-cell aplastic. Grade 3–4 toxicities possibly, probably or definitely attributed to the CD22-CAR are listed in Supplemental Table 3.
CD22 CARs Demonstrate Robust Expansion and Anti-leukemic Activity
CAR expansion, persistence and response for individual patients are summarized in Supplemental Table 4. As shown in Figure 1, CD22-CAR T cells were detected in the peripheral blood of 19/21 treated patients, peaking on Day 14. Median peak expansion was 62% of circulating T cells expressing the CD22-CAR (Figure 1B, range 1–91%), median circulating CAR T cell number was 316/mcl (Figure 1C, range 1–3593/mcl), and median peak expansion in the first 16 patients, measured by PCR for CAR DNA sequence was 7007 copies/100ng DNA (Figure 1D, range 15–30,500 copies/100ng DNA). On Day 28, CAR T cells remained detectable in the peripheral blood of 15/21 patients, in the bone marrow in 15/19 patients for whom bone marrow flow was performed (median 25%, range 0–78.2%) and in the cerebrospinal fluid in 12/17 patients for whom CSF analysis was performed (range 21–71.6%). CAR-T cells remained detectable in the blood of 7 of 9 patients evaluated 3 months post-infusion, in 2 of 3 patients evaluated at 6 months post-infusion, and in one patient each evaluated 9 and 18 months post-infusion who were in ongoing remission at these timepoints.
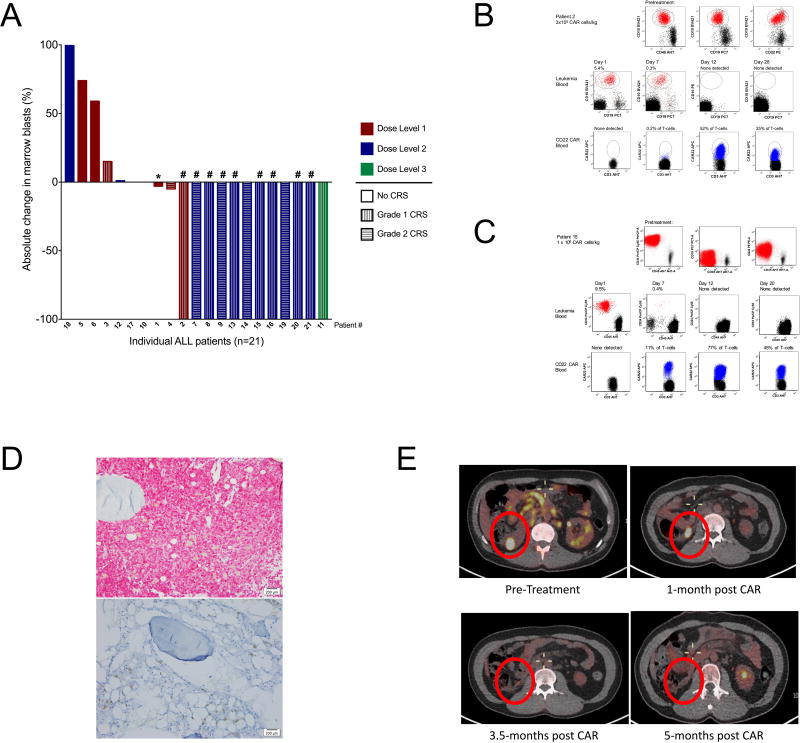
Twelve patients (57%) achieved a complete remission (CR) (Figure 2A); 9 were MRD negative (Supplemental Table 4). Response varied with administered cell dose. At the first dose level (3×105 CD22-CAR/kg) 1 of 6 patients attained a CR compared to 11 of 15 patients (73%) at doses ≥ 1 × 106 CD22-CAR/kg (p<0.001, Fisher’s exact test). Among patients receiving ≥ 1 × 106 CD22-CAR/kg, complete remissions occurred in 9/10 patients who had received prior CD19 directed immunotherapy, including 5/5 who enrolled with CD19dim/neg B-ALL and 1 patient who was refractory to both CD19-CAR and blinatumomab (patient #11). Thus, we saw no evidence that previous CD19-directed immunotherapy or diminished expression of CD19 impacted response to CD22-CAR T cells. Figure 2B illustrates response in patient #2 whose leukemia had dim CD19 expression with substantial disease burden and experienced an MRD negative complete remission following infusion of 3×105 CAR T cells/kg. Similarly, patient #15 had complete absence of surface CD19 expression and high disease burden, but attained MRD negative remission with grade 1 CRS and considerable lymphocyte expansion (Figure 2C and D). Reponses were also seen in patients with extramedullary disease, including patient #13 who attained MRD negative marrow remission at day 28, with steady decrease in FDG-glucose uptake in lymphomatous disease, which fully resolved by 5 months post CAR infusion (Figure 2E).
Figure 2. CD22 CAR T cells induce remission in patients with relapsed and refractory pre-B ALL including CD19 CAR-resistant ALL.
A. Waterfall plot demonstrating percent change in bone marrow aspirate blast frequency from baseline to day 28 (+/− 4 days) and cytokine release syndrome (CRS) grading in the first 21consecutive patients treated, color coded by dose level. *Asterisks designates a patient with progressive disease (PD) defined as greater than 50% increase in circulating blasts. #Denotes minimal residual disease (MRD) negative complete remission. B. Eradication of CD19dim ALL relapsing after CD19-CAR therapy following infusion of 3×105/kg CD22 CAR T cells in patient #2. Top 3 dot plots show leukemia cell surface antigen expression in bone marrow. Bottom 2 rows of dot plots demonstrate clearance of circulating blasts (top) and CAR expansion (bottom) in blood. C. CAR expansion and clearance of CD19neg CD19-CAR resistant ALL following infusion of 1×106/kg CD22 CAR T cells in patient #15. C. Immunohistochemistry (staining CD79a, a pan-B cell marker) of a bone marrow biopsy of patient #15 demonstrating MRD negative complete remission and B-cell aplasia one month following CD22 CAR T cell infusion. Magnification 200x. D. Serial PET scans showing pre-treatment disease and evolution to full resolution in post-treatment evaluations in patient #13.
Among the 4 non-responders at dose levels 2 and 3, two (patients #10 and #18) demonstrated very high disease burden with rapid disease progression, which may have contributed to lack of response. Two additional non-responders (patients #12 and #17) expressed dim or partial CD22 on leukemic blasts at the time of enrollment, which emerged following inotuzumab ozogamicin, an anti-CD22/calicheamicin conjugate,29 administered immediately prior to enrollment on this study.
Serum cytokines were measured serially during the first month in all patients (Supplemental Figures 1 and 2, representative images). Similar to previous reports following CD19-CAR therapy, we saw a general correlation between high leukemia burden and high peak cytokine levels, as illustrated by patient #4 and patient #9.
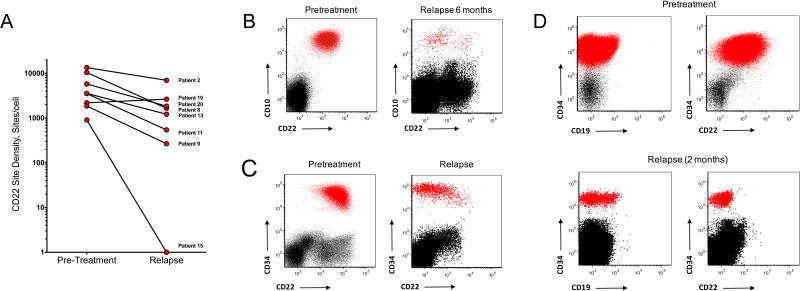
Relapse is Associated with Diminished CD22 Site Density Without Detectable CD22 Mutations or Changes in CD22 mRNA Level
Among the 12 patients who attained complete remission, 3 remain in ongoing complete remission at 21 mos, 9 mos, and 6 mos (Supplemental Table 4). Eight patients relapsed 1.5–12 mos (median 6 months) post CD22-CAR infusion and relapse was associated with diminished CD22 expression in 7 patients (Figure 3A). Illustrative examples include patient #9, who enrolled with CD19 negative leukemia and experienced relapse 6 months following CD22-CAR therapy with blasts that were negative for both CD19 and CD22 (Figure 3B). Two patients who enrolled after receiving inotuzumab ozogamicin experienced early relapse including Patient #11, who relapsed at 1.5 mos following anti-CD22-CAR infusion with low but variable CD22 expression on leukemic blasts (Figure 3C) and patient #15 with CD19 negative leukemia following CD19-CAR therapy who relapsed with variable dim to negative CD22 expression 2 months following CD22-CAR infusion (Figure 3D), despite high-levels of circulating CD22-CAR cells (Figure 2C).
Figure 3. Changes in CD22 expression level in a subset of patients following CD22 CAR T cells.
A. Change in CD22 expression levels in patients achieving remission following CD22 CAR T cell therapy who subsequently developed relapse. Site density measured by flow cytometry as described in methods.. B. Patient #9 who enrolled with CD19 negative B-ALL, experienced an MRD negative remission following CD22-CAR, with subsequent relapse at 6 months demonstrating emergence of 2 leukemic populations with altered CD22 expression compared to pretreatment. C. Relapse with CD22 negative ALL following MRD negative remission in Patient #15 with pretreatment CD19 negative ALL. Last therapy prior to anti-CD22 CAR was with inotuzumab ozogamicin. At the time of relapse, 23% of T-cells in the marrow and 14.4% of T-cells in the peripheral blood were CD22 CAR positive. D. Patient #11 relapsed 1.5 mos following CD22 CAR infusion with CD22lo B-ALL. Therapy administered immediately prior to anti-CD22 CAR was inotuzumab ozogamicin
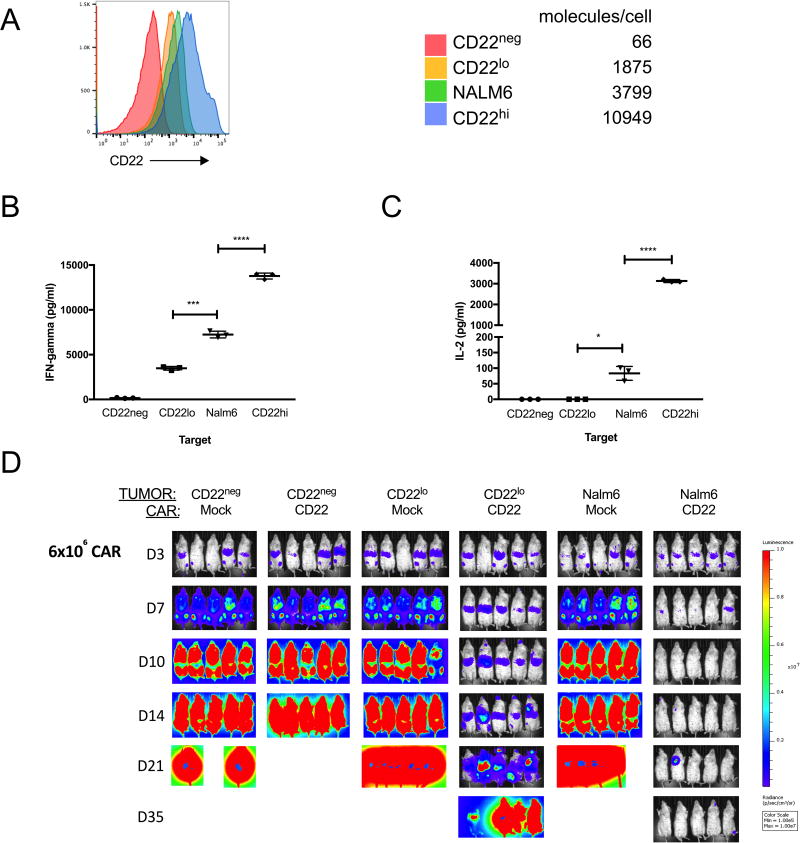
Rather than the persistent and complete absence of target that has been reported following CD19 directed immunotherapy due to preferential expression of CD19 splice variants in resistant leukemia lacking the targeted epitope16, we observed a pattern of acquired resistance to CD22-CAR associated with diminished (Figure 3) and variable (Supplemental Figure 3) CD22 site density assessed by flow cytometry as described in Methods. To determine whether diminished CD22 site density may have had a causal role in relapse in this setting by enabling escape from CD22-CAR T cells, we assessed functionality of the CD22-CAR against NALM6 derived cell lines engineered to express varying CD22 site densities reflecting the range observed in this study (Figure 4A). IFN-gamma (Figure 4B) and IL-2 (Figure 4C) production by CD22-CAR T cells was reduced following exposure to NALM6 expressing low CD22 density corresponding to levels observed at the time of relapse in patients following CD22-CAR therapy. Finally, although CD22 CAR T cells delay in vivo progression of CD22lo ALL, leukemia progression eventually ensues (Figure 4D). These results implicate diminished CD22 expression on B-ALL as a mechanism for relapse following CD22-CAR therapy.
Figure 4. CD22 site density limits CD22 CAR functionality.
A. Histogram of CD22 expression on CRISPR/Cas9 edited (as described in methods) CD22 negative NALM6 B-ALL lines transduced to express varying levels of CD22. Table shows site density using the same flow cytometry-based assay used to measure CD22 on patient samples and described in supplemental methods. NALM6 refers to the parental cell line. Interferon gamma (B) and IL-2 (C) production by CD22 CAR transduced T cells upon co-culture with NALM6 cell lines expressing varying CD22 site densities. *=p<0.05, ***=p <0.005, ****=p<0.001 by one way analysis of variance (ANOVA). Data shown in B and C is representative of 3 independent experiments. Lines represent means +/− standard error of measurent of triplicate wells. D. Xenograft model (as described in Methods) demonstrating clearance of parental NALM6 by CD22 CAR T cells at a dose of 6×106 per mouse (NOD SCID gamma (NSG)) administered at day 3 following leukemia injection but failure of the same CAR T cells to eradicate NALM6 expressing low CD22 site density despite initial delay in leukemia progression. Representative of 3 independent experiments.
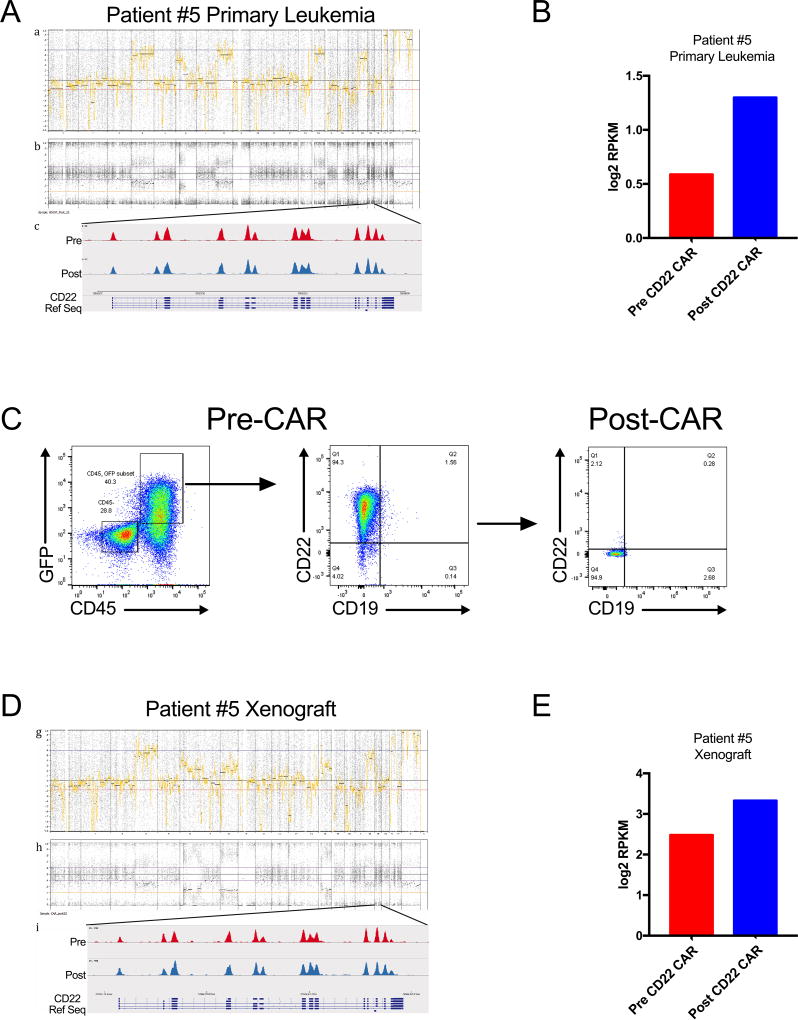
To identify possible genetic or transcriptomic mechanisms underlying the observed alteration in CD22 site density, longitudinal assessment of the CD22 genomic locus and corresponding mRNA was evaluated in two patients who relapsed following CD22-CAR with diminished CD22 site density. Genome-wide copy number profiles of the leukemias remained stable despite substantial changes in CD22 site density. Heterozygosity of the CD22 locus was maintained and no focal copy number changes were observed. Mutation analysis comparing the pre-treatment and post-treatment samples demonstrated no aquired mutations within the CD22 locus (Figure 5A, Supplemental Figure 4A). Corresponding analysis of CD22 mRNA levels pre- and post-CD22 CAR treatment demonstrated no qualitative change in the observed CD22 transcripts, and no evidence for diminished transcription of CD22, since in each case, total CD22 mRNA levels were slightly increased at the time that CD22 site density was diminished (Figure 5B, Supplemental Figure 4B). We saw no evidence for alternative CD22 isoforms as a cause of the downmodulation of CD22 expression observed although a novel isoform cannot be ruled out. Furthermore, we studied a patient derived xenograft model in mice subjected to pressure with CD22-CAR T cells which induced complete loss of CD22 surface expression. Remarkably, such leukemias again showed no evidence for genetic mutation, changes in gene copy number or isoform expression and no evidence for diminished mRNA expression (Figure 5D and E). Together, the data implicate post-transcriptional effects in modulating CD22 protein levels rather genomic mutation, modulation of gene expression or altered isoform expression.
Figure 5. Whole Exome and RNAseq profiling of CD22 in Primary Patient Samples and Patient Derived Xenograft (PDX) recurring in the presence of CD22 CAR Immunotherapeutic Immune pressure.
(A) Genome-wide copy number profiling of the primary and relapsed leukemia sample from Patient #5 demonstrate no net ploidy changes across the genome. Interrogation of the CD22 locus demonstrated no exon level alteration in the pre or post treatment sample. (B) Analysis of RNAseq for Patient #5 showed maintenance of the CD22 mRNA. (C) A patient-derived xenograft (PDX) developed from patient #5 was treated in vivo with CD22 CAR which showed tumor response followed by CD22 flow cytometry-negative relapse. (D) Genome-wide copy number and CD22 exon analysis demonstrated genomic stability from the primary tumor with no gains, losses and maintenance of the ploidy status before and after therapy. (E) CD22 transcript levels in the pre and post treatment sample again showed maintenance of consistent mRNA expression.
A bispecific CAR targeting both CD19 and CD22 can recognize and kill CD19+CD22+, CD19−CD22+ and CD22+CD19− B-ALL
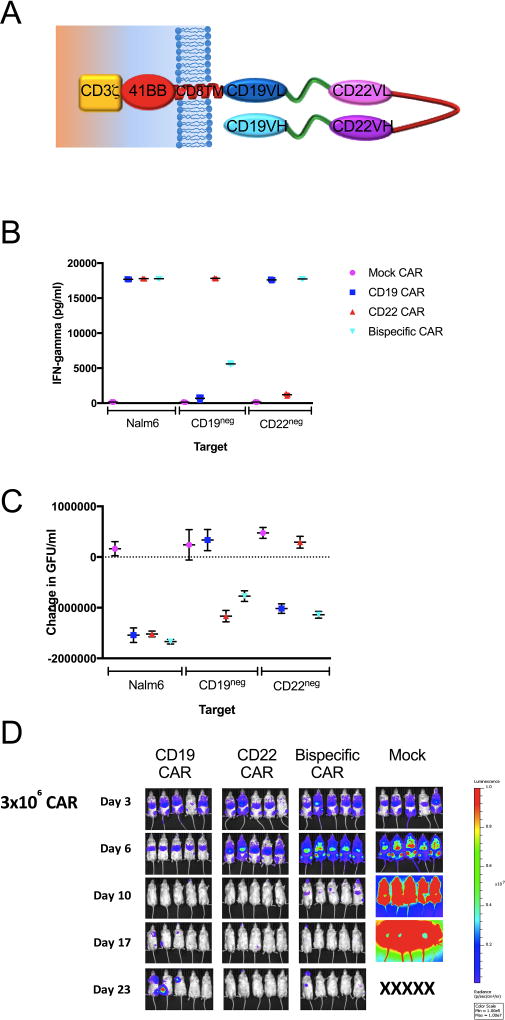
Multi-agent combination chemotherapy is a central tenet of ALL therapy. The results presented here credential a novel CAR-based therapeutic for the treatment of B-ALL, but also illustrate the challenges associated with sequential administration of CD19 CAR followed by CD22 CAR therapy. We therefore sought to develop a multi-specific CAR T cell which could simultaneously recognize CD19 or CD22 expressing targets. Using a single CAR construct that incorporates both CD19 and CD22 scFv sequences into one bivalent receptor (Figure 6A), we demonstrate in vitro cytokine production against and killing of CD19+/CD22+, CD19+/CD22−, and CD22−CD19+ cell B-ALL cell lines (Figures 6B and C, Supplemental Figure 5). T cells transduced with this mutispecific construct administered intravenously at a dose 3 × 106 into NSG mice 3 days after injection of luciferase-expressing B-ALL demonstrate the ability to clear B-ALL (Figure 6D). Thus, the binding domain in the CD22 CAR validated in the clinical trial reported here can be combined with a CD19 binding domain validated as a CAR construct in multiple clinical trials to generate a single multitarget CAR with the potential to overcome leukemic resitance to either CAR administered alone.
Figure 6. CD22xCD19 bispecific CAR demonstrates in vitro and in vivo activity against CD19- and CD22- ALL.
A. Schematic representation of the CD19xCD22 CAR construct. Interferon gamma production measured by enzyme-linked immun in response to (B) and killing in an in vitro assay measuring loss of GFP-labeled targets of (C) parental NALM6 and CRISPR/Cas9 edited CD19neg and CD22neg (as described in Methods) NALM6 cell lines. In vitro killing of GFP-expressing NALM6 ALL measured by loss of GFP+ cells. Data presented in panels B and C is representative of 2 independent experiments. D. Eradication of NALM6 in NOD SCID gamma (NSG) mice (as described in Methods) by CAR expressing T cells as indicated and is representative of 4 independent experiments.
DISCUSSION
CD22, a sialic acid-binding immunoglobulin-like lectin (SIGLEC), expressed exclusively within the B cell lineage, is expressed on the vast majority of B cell malignancies, including B-ALL, indolent and high-grade non-Hodgkin lymphoma, chronic lymphocytic leukemia, and hairy cell leukemia.14,19,20,32,33 Numerous CD22 directed therapeutics have been studied in clinical trials. Epratuzumab, an unconjugated anti-CD22 mAb, mediated modest clinical activity in adult and pediatric ALL.21,26,27 CD22-immunotoxins mediated clinical activity in hairy cell leukemia23 and B-ALL22, but benefits were transient due to the agent’s short half-life, and substantial immunogenicity. A recent phase 3 trial of inotuzumab ozogamicin, an anti-CD22 mAb conjugated to the toxin calicheamicin, demonstrated complete responses in 80.7% of patients with relapsed/refractory B-ALL.29 However, liver toxicity limited the dose intensity that could be safely delivered and the agent is associated with higher rates of veno-occlusive disease following subsequent HSCT.24,34
We generated a novel CAR targeting CD22, which we optimized by comparing potency across multiple scFvs and multiple costimulatory domains.19,30,35 Therapeutics incorporating the m971 binding domain30 have not been tested previously in clinical trials. The data presented here demonstrate no evidence for off-target toxicity, modest cytokine release syndrome and potent anti-leukemic activity following CD22-CAR therapy. Doses ≥ 1 × 106 CD22-CAR cells/kg mediated substantial anti-leukemic activity, comparable to results with CD19-CAR, with 11/15 patients rendered into complete remission, including 8/10 patients previously treated with CD19 based immunotherapies, and 5/5 patients who enrolled with CD19 neg/dim relapse. Notably, all patients enrolled had undergone previous HSCT and patient #7 who had undergone two previous HSCTs prior to receiving CD22 CAR T-cells, remains in remission 21 months following CD22-CAR therapy with persistent CAR T cells and has received no additional anti-leukemic therapy since treatment with CD22-CAR T cells. Together, the data provides no evidence to suggest that previous chemotherapy or CD19-based immunotherapy diminishes the likelihood of remission induction following bioactive doses of CD22-CAR T cells. The CD22-CAR studied here incorporates a 4-1BB costimulatory endodomain, and the cells demonstrate a pattern of expansion and persistence that is similar to that observed following CD19-CAR T cells incorporating a 4-1BB endodomain, consistent with previous evidence that CAR costimulatory domains play a dominant role in modulating the rate of expansion and the likelihood of persistence following CAR therapy.35 CD22-CAR cells also migrated efficiently to the CSF, but we observed no evidence for severe neurologic toxicity or seizures, which have been observed in studies of CD19-CAR therapy.11–13
CARs targeting CD19 have demonstrated remarkable activity in B-ALL and diffuse large B cell lymphoma (DLBCL), and we demonstrate similar complete response rates in B-ALL with the CD22-CAR tested here. This is notable since numerous non-CD19 targeted CARs have entered clinical trials for B cell malignancies, (CD20-CAR36, κ-CAR37, CD138-CAR38, BCMA-CAR39), AML40 and solid tumors, yet none of these have demonstrated complete responses rates of >50%. This work therefore provides the first evidence that CD19 is not a uniquely effective CAR T cell target and raise the prospect that similarly effective CARs could ultimately be developed for an array of antigenic targets. Future studies are needed to determine whether the high rates observed with CD22-CARs in B-ALL will translate into similarly significant response rates in DLBCL, where CD22 expression is common.
CD19 is universally expressed at high levels on B-ALL at the time of diagnosis and is retained following cytotoxic therapy.41–43 However, since the introduction of CD19-based immunotherapies, relapse with diminished or absent surface CD19 has been increasingly observed and has emerged as the dominant mechanism of resistance to this class of therapeutics. CD19 immune escape was first reported following blinatumumab therapy10, but has now been observed by several groups following CD19-CAR therapy.12,44 In a recent report of 50 patients rendered into remission with CD19-CAR therapy, with a median follow-up of 10.6 months, 40% of patients had relapsed, and loss of the CD19 target accounted for 65% of the total relapses.17 Thus, while the true incidence is unknown, CD19 immune escape is emerging as the most common cause of relapse following CD19-CAR therapy for B-ALL. Recent investigation into the biology of “CD19 negative B-ALL” has revealed that the majority of cases retain mRNA specific for CD19, but are enriched for CD19 isoforms that preferentially remain intracellular and/or lack the epitopes targeted by all CD19-CARs currently under study as well as blinatumumab.16 An alternative pathway for loss or diminished CD19 expression involves lineage switch of blasts with acquisition of myeloid markers and characteristics.45 None of the patients enrolled on this study appeared to manifest CD19 loss associated with lineage switch.
The data presented here demonstrate that diminished CD22 site density, rather than total loss of expression, is sufficient to permit escape of leukemia from CD22-directed CAR therapy. This is not unique to the CD22-CAR, but rather reflects a pattern observed across chimeric antigen receptors, which demonstrate a higher requirement for antigen induced activation than is seen for native T cell receptors46–49. Decrease in CD22 site density or complete CD22 loss were observed in 7/8 patients who relapsed following CD22-CAR induced remission. Given that CD22 expression is often variable at presentation, it appears likely that this phenomena represents selection of pre-existing CD22lo cells within the heterogeneous leukemia population. Notably, we identified no genetic basis for the change in expression level and no evidence for diminished mRNA levels in leukemic cells demonstrating low CD22 site density, thus implicating post-transcriptional mechanisms in this biology. Regardless of the mechanism, decades of experience with cytotoxic chemotherapy for B-ALL have convincingly established that multimodal chemotherapy is required to achieve long-term remission. Based on clear evidence for CD19 antigen loss or downregulation following CD19-directed immunotherapy, and evidence for diminished CD22 expression contributing to relapse following sequential CAR therapy, we propose that simultaneous immunotherapeutic targeting of multiple antigens may diminish the likelihood of antigen loss escape. Proof-of-principle for bioactivity of a multispecific CD19/CD22-CAR in mice is demonstrated here.
The majority of patients who develop CD19 immune escape following CD19 directed immunotherapy retain CD22 expression and this report demonstrates that such leukemias retain susceptible to immune-based targeting. Furthermore, while we demonstrate proof-of principle that sequential immunotherapeutic targeting of a second antigen can mediate clinical benefit, we also observe a high rate of relapse associated with diminution in CD22 expression using this approach, raising the prospect that simultaneous multispecific targeting may be a more effective approach to enhance the durability of immunotherapy-induced remissions in B-ALL. Toward this end, we have developed a chimeric antigen receptor that simultaneously targets both CD19 and CD22, which could prove more effective at inducing remissions and be less susceptible to relapse associated with antigen escape50. Clinical trials testing this CD19/CD22 mutispecific target CAR are underway.
METHODS
Trial Design and Toxicity Monitoring
This Phase I, first-in-human, dose escalation trial conducted at the Pediatric Oncology Branch of the National Cancer Institute was designed to test the safety and feasibility of CD22-CAR T cell therapy in children and young adults, aged 1–30 years, with relapsed or refractory CD22 expressing hematopoietic malignancies which had not responded to or had recurred following standard regimens. The protocol was approved by the NCI Institutional Review Board and the NIH Recombinant DNA Advisory Committee (NCT02315612). All authors reviewed, discussed and interpreted the study results and vouch for the data and analyses. Written informed consent for participation was obtained from patients or the parents according to the Declaration of Helsinki and the protocol was approved by the National Cancer Institute Institutional Review Board.. This report represents an interim analysis from the first, consecutively treated 21 patients with pre-B ALL who received CD22-CAR T cell infusions between December 2014 and August 2016 with a data cut-off of June 1, 2017. A total of 23 subjects were enrolled, but in one case T lymphocyte numbers were inadequate for CAR cell production and thus this patient was not treated on study; in a second case the diagnosis was diffuse large B cell lymphoma, which is not included in this analysis of efficacy in B-ALL.
A standard 3 + 3 Phase I dose escalation design was used. If 2/6 patients experienced a DLT at dose level 1, safety would have been evaluated in a de-escalated dose of 1 × 105 transduced T cells/kg. Once the maximum tolerated dose (or highest level evaluated) was reached, enrollment into an expansion cohort of a total of 12 patients in two strata proceeded to provide additional information regarding the feasibility, safety and efficacy of this treatment. In the expansion cohort, patients who have previously received CD19-CAR T cells will be evaluated as a separate stratum from CAR-naïve patients. All doses allowed a range of ± 20% of the prescribed dose to allow for potential variations in the cell products.
Dose limiting toxicity was defined as any ≥ Grade 3 toxicity attributed as possibly, probably, or likely related to either the lymphodepletion regimen or the CD22-CAR T cells with exception of low electrolyte levels responding to supplementation, tumor lysis syndrome, hypoalbuminemia, liver dysfunction resolved to < grade 2 within 14 days, transient (< 72 hours) grade 4 hepatic enzyme abnormality, pre-existing coagulopathy, grade 3 or 4 fever lasting 7 days or less, grade 3 diarrhea that resolves to grade 2 within 4 days, grade 3 nausea and/or anorexia and any infusion related toxicity occurring within 24 hours that would resolve with minimal intervention. and grade 3 cytokine release syndrome. Cytokine release syndrome was graded and managed based on Lee et al.51 Additionally, subjects with abnormal blood counts at baseline due to marrow involvement were not evaluable for hematologic toxicity.
To evaluate possible neurotoxic effects, psychologists administered a brief (<1 hour) neurocognitive battery to patients prior to (baseline) and 1-month after (day 21–28) CD22 CAR T cell infusion. The test battery consisted of the NIH Toolbox computerized test1 evaluating attention, working memory, cognitive flexibility, and a paper/pencil test assessing processing speed2. At baseline and then at approximately 14 and 21 days post-infusion, the caregiver of the patient or the adult patient completed a neuro-symptom checklist that assessed fever, auditory or visual hallucinations, responsiveness to commands, disorientation, depressed mood, and pain.52
Eligibility
Patients were eligible if their disease had recurred after standard upfront therapy and at least one salvage therapy. There was no limit on the number of previous salvage therapies the patient may have received. Prior allogeneic stem cell transplant was allowed if at least 100 days had elapsed since transplant, there was no evidence of GVHD and the patient was off systemic immunosuppression for at least 30 days prior to enrollment. Patients who had received prior CD19-CAR therapy were eligible if at least 30 days had elapsed since CD19-CAR infusion and circulating levels of genetically modified cells were <5% by flow cytometry.
Eligibility required a performance status of ≥ 50% by Karnofksy for patients > 16 years of age, or ≥ 50% using the Lansky scale for patients < 16 years of age. Minimal weight for eligibility was 15 kg. Patients with asymptomatic CNS1 or CNS2 leukemia were eligible, whereas patients with symptomatic or CNS3 leukemia were ineligible, as previously described.12 Patients with isolated CNS or testicular leukemia were not eligible.
Patients with uncontrolled intercurrent infection, other malignancies, illnesses or conditions that would limit their ability to tolerate or comply with the study requirements were ineligible. Patients were also ineligible if they demonstrated seropositivity for HIV or HCV or positive testing for HBV surface antigen or if they had a history of hypersensitivity to agents required for the treatment regimen. Other exclusion criteria included inadequate liver function defined as total bilirubin > 2× upper limit of normal (ULN) (except for patients with documented Gilbert’s) or transaminase levels > 3× ULN; renal function < 60 mL/min/1.73 m2 or hyperleukocytosis (≥50,000 circulating blasts/uL).
Systemic chemotherapy must have been completed > 2 weeks prior to enrollment (> 6 weeks for clofarabine or nitrosureas), radiation therapy must have been completed > 3 weeks prior to enrollment, monoclonal antibody (mAb) therapy must have been completed at least 30 days or 5 half-lives prior to enrollment and investigational anti-neoplastic therapy must have been completed at least 30 days prior to enrollment. No washout period was required for intrathecal chemotherapy, hydroxyurea (provided no increase in dose within 2 weeks prior to enrollment), standard maintenance ALL therapy or physiologic steroid replacement.
CD22 CAR Construct and Manufacturing of CD22-CAR T cells
The CD22-CAR contains a fully human single chain fragment variable region generated from a human B cell phage library30, a CD8 transmembrane domain and CD3 zeta plus 4-1BB signaling chains (CD22.BB.z) as previously described and as illustrated in Figure 1A.19,35 All patients received the identical preparative regimen consisting of fludarabine 25 mg/m2/d on Days −4, −3, −2 and cyclophosphamide 900 mg/m2 on day −2, with CD22-CAR T cell infusion on Day 0. Additional details can be found in the Supplementary Methods.
CD22-CAR T cells were produced in the Cell Processing Section of the Department of Transfusion Medicine, NIH Clinical Center. This laboratory operates under principles of Good Manufacturing Practices and Good Clinical Laboratory Practice with established Standard Operating Procedures (SOPs) and/or protocols for sample receipt, processing, freezing, and analysis. All patients underwent leukapheresis within 5 days following enrollment. Leukapheresed peripheral blood mononuclear cells were either placed directly into culture or cryopreserved prior to culture initiation on a subsequent day. In cases where substantial numbers of myeloid cells were present in the apheresis products, elutriation and/or plastic adherence53 was performed based on evidence that such cells inhibited expansion54. The lentiviral vector containing CD22.BB.Z-CAR, was produced by Lentigen Inc. Cells were expanded in sterile bags in a 37oC incubator for 9 days, with an additional 3 days of culture permitted to allow resolution of intercurrent clinical events. If > 3 days was required, cells were cryopreserved then thawed immediately prior to infusion. Final product release criteria required the following: cell viability ≥ 70%, cell number within 20% of planned dose, % CAR T cells ≥ 15% as measured using CD22-Fc fusion protein, endotoxin ≤ 5 EU/mL, Mycoplasma negative, gram stain and culture negative, VSV-G DNA (as a surrogate marker for replication competent lentivirus (RCL)) negative by qPCR. In process culture-based testing was also performed on an aliquot of the product at the National Gene Vector Laboratory (Indiana University) using assays for p24 antigen and product-enhanced reverse transcriptase assay (PERT) to complete testing for RCL.
Response Monitoring
Baseline bone marrow aspirate and biopsy and lumbar puncture were performed within 14 days prior to beginning the lymphodepletion preparative regimen, and then response was monitored via bone marrow aspirate and biopsy and lumbar puncture performed on Day 28 ± 4 days from cell infusion, and at months 2, 3, 6, 9 and 12 months. Complete remission (CR) was defined by morphologic assessment of the bone marrow as M1 (<5% leukemic blasts) with no evidence of extramedullary disease. Minimal residual disease (MRD) was assessed by multiparametric flow cytometry conducted at the NCI Laboratory of Pathology using standard techniques.
Cytokine Assays, PCR and Flow cytometry
Plasma was cryopreserved before measurement of cytokines in a multiplex format according to manufacturer's instructions (MesoScaleDiscovery, Gaithersburg, MD, USA). CD22-CAR T cell expansion was measured by quantitative PCR (qPCR) (Life Technologies, Grand Island, NY, USA), and adapted from published methods55. Briefly, measured CAR copies per 100 ng DNA were normalised to the input quantity of amplifiable DNA by measurement of the single-copy gene, CDKN1a.
Specimens for flow cytometry were processed within 12 h of collection and stained with a panel of antibodies to quantitate leukemic burden and measure CAR T cells numbers. Briefly whole blood lysis was performed using ammonium chloride prior to staining for 30 minutes at room temperature with the following two cocktails (antibody concentration according to manufacturer’s recommendations): Cocktail A- CD16FITC (clone DJ130c, Dako), CD19PE (clone SJ25C1), CD3PerCP (clone SK7, BD), CD13PECy7 (L138, BD), CD34APC (clone 8G12, BD), CD14 APC H7 (MP9, BD), CD56v450 (clone B159, BD), CD45 v500 (clone HI30, BD); and Cocktail B-CD66bFITC (clone G10F5, BD), CD22PE (clone S-HCL-1, BD), CD34PerCP5.5 (clone8G12, BD), CD19PECy7 (clone SJ25C1, BD), CD24APC (clone SN3 A5-2H10, eBioscience), CD45 APC H7 (clone 2D1, BD), CD10BV421 (cloneHI10a, BD) and CD38BV510 (clone HB-7, Biolegend). At least 1 million cells were acquired per tube using an 8-color multiparametric approach on a 3-laser FACS Canto II (BD Biosciences, San Jose, CA) with DiVa 6.1.1 software and analyzed by FCS Express 4 software (DeNovo Software, Los Angeles, CA). The validated limit of detection of leukemic blasts with this assay is 0.002% of cells.
CD22-CAR T cells were measured using a CD22-Fc (R&D Systems, Minneapolis, MN). Circulating CAR T-cell numbers were calculated on the basis of estimated blood volume and measured absolute lymphocyte counts. CAR T-cells were detected using the following cocktail: CD20FITC (B-Ly1, Dako), CD10PE (cloneHI10a, BD), CD34PerCP5.5 (clone8G12, BD), CD19PECy7 (clone SJ25C1, BD), CD22-Fc-APC (R&D Systems), CD3APC-H7 (clone SK7, BD), CD14 v450 (Mtems), CD3APC-H7 (clone SK7, BD), CD19PECy7 (clone SJ25C1, BD), , CD10PE s instructions (QuantiBRITE Beads, BD Biosciences, San Jose, CA, USA).E content of the Quanti-BRITE beads was constructed using QuantiCALC software. Circulating CAR T-cell numbers were calculated on the basis of estimated blood volume and measured absolute lymphocyte counts.
CD19 and CD22 Flow Cytometric Site Density Determination
CD22 and CD19 site density on blasts was enumerated by flow cytometry according to manufacturer's instructions (QuantiBRITE Beads, BD Biosciences, San Jose, CA, USA). The antibody bound per cell (ABC) was determined as previously described56,57 for anti-CD19PE (clone SJ25C1) and anti-CD22PE (clone S-HCL-1) (BD Biosciences, San Jose, CA) on leukemic blasts using saturating concentrations of antibody and the BD Biosciences QuantiBRITE system (QuantiBRITE standard beads and QuantiCALC software) for fluorescence quantitation. The ABC value represents the mean value of the maximum capacity of each cell to bind the antibody. QuantiBRITE PE beads are pre-calibrated standard beads containing known numbers of PE molecules bound per bead. QuantiBRITE beads were acquired on a FACSCanto™II (BD Biosciences, San Jose, CA) on the same day at the same instrument settings as the individual patient specimens. A standard curve comparing the geometric mean of fluorescence to known PE content of the Quanti-BRITE beads was constructed using QuantiCALC software. The regression analysis, slope, intercept, and correlation coefficient were determined. By gating based upon immunophenotype, blasts were distinguished from normal cells and the geometric mean fluorescence of CD19 and CD22 staining was reported for each population. The ABC values were generated from the measured geometric mean fluorescence of the gated cells using the QuantiBRITE standard curve. ABC values were only determined for populations containing 100 or greater events to achieve adequate precision. The geometric mean fluorescence of T- and NK-cells stained with the B-cell antibodies (negative control) has been previously determined and the negative ABC range is used to confirm positivity versus negativity. In addition, blasts with anti-CD19 or anti-CD22 staining less than or equal to T-cells (internal negative control) are considered negative.
CRISPR/Cas9 Editing of cell lines
Guide-RNAs were designed from the GeCKO human sgRNAs library, cloned into LentiCRISPR v2 plasmid (Addgene Plasmid 52961), and transformed into Stbl3 bacteria as previously published (hCD19F: 5’ CACCGTGGAATGTTTCGGACCTAGG 3’, hCD19R: 5’ AAACCCTAGGTCCGAAACATTCCAC 3’, hCD22F: 5’ CACCGTCTCCTTCTCGAATCGGCAT 3’, and hCD22R: 5’ AAACATGCCGATTCGAGAAGGAGAC 3’). Plasmids were co-transfected with packaging plasmids RRE, pMD-G, and REV into LentiX HEK293T cells (Clontech, Mountain View, CA, USA). After two days, CRISPR supernatants were harvested and filtered through a 0.45µm low protein binding membrane (Millipore, Billerica, Massachusetts, USA), concentrated using Lenti-X concentrator (Clontech, Mountain View, CA, USA), resuspended in PBS, and used immediately or stored at −80°C. For viral transduction, 1×105 leukemia cells were incubated with 10µl of concentrated viral supernatant for 2 days, followed by expansion in RPMI with 10%FBS, Pen/Strep, and Glutamax. Cell phenotype was assessed by flow cytometry, followed by sorting of cells with phenotypic alterations and single cell cloning. Sequencing was performed on single cell clones to confirm genotypic alterations by Plantinum PCR Supermix High Fidelity Kit (Invitrogen) (hCD19F Seq: 5’ TCTCCCTCTCCTGGGTG 3’, hCD19R Seq: 5’ CTCTCCCTCCCAGATCTCAG 3’, hCD22F Seq: 5’ AGGAGGGAAGGGGTACTG 3’, and hCD22R Seq: 5’ AGCCAACGTTTTGGATCTTCAG 3’). To obtain cell lines with various CD22 site densities, a complete sequence of cDNA human CD22 plasmid (Origene) was re-transduced into the CD22-negative cell line at different concentrations. Cell lines were single-cell cloned with resultant cell lines including CD22-negative (CD22neg), CD19-negative (CD19neg), CD2-low site density (CD22lo), and CD22-high site density (CD22hi) cell lines. QuantiBRITE Beads (BD Biosciences, San Jose, CA, USA) were used, as per above description to identify site density of CRISPR/Cas9-modified cell lines.
CD19xCD22 Bispecific CAR
A Bispecific CAR construct was developed using a CD19 binding domain derived from a clinically active CD19 CAR12 and a CD22 binding domain used for the CD22 CAR reported herein. A schematic of the CD19xCD22 bispecific CAR is shown in Figure 4A and the full sequence is provided Supplemental Figure 6.
The bispecific CAR was transduced onto human T cells using lentiviral vectors. The potency of the bispecific CAR was tested against the CRISPR/Cas9 knock-out cell lines as well as parental Nalm6 ALL cell line. Cytokine production was analyzed in cell co-culture supernatants using R&D ELISA Kit for Interferon-gamma and IL-2, following the product protocol (R&D Systems Inc, Minneapolis, MN, USA). In brief, CD22 CAR T cells were washed three times and co-incubated with varying tumor target cells at 1:1 effector-to-target ratio for 16–20 hours. Supernatant was collected from co-incubation and used for evaluation of cytokine production. Of note, supernatant was diluted 1:10 in media for Interferon-gamma analysis. Supernatant was not diluted for IL-2 evaluation. Optical density was determined within 30 minutes, using a microplate reader set to 450nm with wavelength correction of 540nm. In vitro killing was analyzed using an Incucyte assay (Essen bioscience). CAR cells were co-incubated with GFP-positive tumor target cells at a range of effector-to-target ratio. At the 24-hour time-point, Green Fluorescent Units per well (GFU/well) was calculated using Incucyte software, standardized to baseline GFU/well and normalized to tumor only wells. Assays were performed in triplicate and data is representative of multiple repeat experiments.
Xenograft Models
In vivo analysis of CAR activity was conducted using a xenograft model with NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG, Jackson Laboratories) mice. Mice were injected intravenously with 1×106 GFP-positive Nalm6 tumor cells on Day 0. On Day CAR-transduced T cells or Mock-transduced T cells were injected as indicated. Mice were imaged using IVIS technology and luciferin-D IP injections. All animal studies were approved by National Cancer Institute Animal Care and Use Committee.
Genomic Profiling
All primary patient samples were collected on IRB approved protocols for biological specimen research. Leukemia samples from bone marrow specimens containing greater than 90% leukemia were isolated by density gradient separation using Lymphocyte Separation Medium (Lonzo). The cells were lysed and nucleic acid extraction was performed using Qiagen Allprep Kits (Qiagen) per the manufactures protocol. DNA and RNA were quantified and assessed for quality using an Agilent 2100 BioAnalyzer. Poly-adenylated RNA libraries were generated and sequenced using TruSeq 4.0 chemistry on a Hiseq2500 (Illumina). Whole exome data was generated using Agilent SureSelectXT Human All Exon V5 and TruSeq V4 chemistry and sequenced to a median of 300× coverage using on a HiSeq2500 (Illumina).
Whole exome and RNA-sequencing data was analyzed and mapped using the CCR Collaborative Bioinformatics Resource (CCBR) pipeline (https://bioinformatics.cancer.gov/). Reads were aligned to reference genome Hg19. Somatic variant calling was performed using MuTect58 and copy number alterations were analyzed using Nexus Copy Number Discovery Edition #9. (BioDiscovery). The integrity of the CD22 gene was interrogated by manual inspection using Integrative Genome Viewer (IGV). RNAsequencing reads for each sample were trimmed of their adapters and low quality bases using Trimmomatic software and aligned with reference human Hg38 and Gencode V24 transcripts using STAR software. Expression of CD22 transcript was evaluated using a log2 of the RPKM of RNAseq data.
Statistical Analyses
Doses of anti-CD22-CAR transduced T-cells were administered in a standard 3 + 3 dose escalation design until MTD was determined. After treatment of the first patient in the first cohort there was a four-week (28 day) safety assessment prior to treatment of the second patient. Subsequent patients in a cohort were treated after a one-week safety assessment period following cell infusion. Patients were enrolled sequentially; therefore, enrollment did not proceed to a higher dose level until all patients had been treated in the prior cohort and the last patient treated on the completed cohort had been observed for at least 4 weeks. If a minimum of 1 ×105 anti-CD22-CAR-transduced T cells per kg cannot be obtained for infusion, the patient may be treated but would not be evaluable for toxicity or response, but would be considered a feasibility failure. Up to 6 evaluable patients may be enrolled in cohorts 1 to 4 (24 total in order to determine MTD. In addition, the study will allow for up to 3 patients to be replaced in each of the dose cohorts 1 through 3 (9 additional patients) due to inability to achieve target doses. In addition, the study will allow for 6 total in-evaluable patients (patients enrolled but who cannot receive cells, either due to physical deterioration or withdrawn consent during cell growth).
Data Availability
Patient-related data not included in the manuscript may be restricted as it was generated in the context of an ongoing clinical trial and may be subject to patient confidentiality. All other data that support the findings of this study are provided in the manuscript (or accompanying Supplementary Material) or is available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
We gratefully acknowledge the study participants and their families, referring medical care teams, the faculty and staff of the NIH, and the data managers involved with this work.
This work was supported in part by the Intramural Research Program, National Cancer Institute, and NIH Clinical Center, National Institutes of Health, by a Stand Up to Cancer-St. Baldrick’s Pediatric Dream Team translational research grant (SU2C-AACR-DT113), and a St. Baldrick’s Foundation Scholar Award (DWL). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. CLM is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. C.L.M and R.J.O. are inventors on a patent for the CD22 CAR.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author roles
TJFr, RJO, DSD, BD, DWL and CLM designed the study. PW and SM designed and performed the neurotoxicity evaluation. The following authors conducted the study: TJFr, NNS, MS-S, CMY, CD, BY, HS, DFS, MS, YF, PW, SM, DWL. TJFr, MSS, CMY, CD, BY, TJFo, JFS, DFS, MS, LZ, SN, HQ, PW, SM, SR, RGM and CLM generated and analyzed the data. TJFr, NNS and CLM vouch for the data and the analysis, wrote the paper, and decided to publish the paper. No non-author wrote the first draft or any part of the paper.
References
- 1.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120:2497–2506. doi: 10.1002/cncr.28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rytting ME, et al. Augmented Berlin-Frankfurt-Munster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL) Cancer. 2014;120:3660–3668. doi: 10.1002/cncr.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram R, et al. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87:472–478. doi: 10.1002/ajh.23149. [DOI] [PubMed] [Google Scholar]
- 5.Faderl S, et al. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010;116:1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kero AE, et al. Health conditions associated with metabolic syndrome after cancer at a young age: A nationwide register-based study. Cancer Epidemiol. 2016;41:42–49. doi: 10.1016/j.canep.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Essig S, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. The Lancet. Oncology. 2014;15:841–851. doi: 10.1016/S1470-2045(14)70265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topp MS, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 9.Topp MS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. The Lancet. Oncology. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 10.Topp MS, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 11.Davila ML, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6 doi: 10.1126/scitranslmed.3008226. 224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raponi S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52:1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 15.Lucio P, et al. BIOMED-I concerted action report: flow cytometric immunophenotyping of precursor B-ALL with standardized triple-stainings. BIOMED-1 Concerted Action Investigation of Minimal Residual Disease in Acute Leukemia: International Standardization and Clinical Evaluation. Leukemia. 2001;15:1185–1192. doi: 10.1038/sj.leu.2402150. [DOI] [PubMed] [Google Scholar]
- 16.Sotillo E, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupp SA, et al. American Society of Hematology. Orlando, FL: 2015. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019) [Google Scholar]
- 18.Gardner R, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haso W, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NN, et al. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62:964–969. doi: 10.1002/pbc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen K, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wayne AS, et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreitman RJ, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarjian H, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119:2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevallier P, et al. (90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: a phase 1 dose-escalation study. Lancet Haematol. 2015;2:e108–117. doi: 10.1016/S2352-3026(15)00020-4. [DOI] [PubMed] [Google Scholar]
- 26.Chevallier P, et al. Vincristine, dexamethasone and epratuzumab for older relapsed/refractory CD22+ B-acute lymphoblastic leukemia patients: a phase II study. Haematologica. 2015;100:e128–131. doi: 10.3324/haematol.2014.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raetz EA, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children's Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer. 2015;62:1171–1175. doi: 10.1002/pbc.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant BW, et al. A phase 2 trial of extended induction epratuzumab and rituximab for previously untreated follicular lymphoma: CALGB 50701. Cancer. 2013;119:3797–3804. doi: 10.1002/cncr.28299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarjian HM, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao X, Ho M, Zhu Z, Pastan I, Dimitrov DS. Identification and characterization of fully human anti-CD22 monoclonal antibodies. MAbs. 2009;1:297–303. doi: 10.4161/mabs.1.3.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawalekar OU, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:712. doi: 10.1016/j.immuni.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Chevallier P, et al. Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases. Leukemia. 2009;23:806–807. doi: 10.1038/leu.2008.303. [DOI] [PubMed] [Google Scholar]
- 33.Olejniczak SH, Stewart CC, Donohue K, Czuczman MS. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest. 2006;35:93–114. doi: 10.1080/08820130500496878. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian H, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13:403–411. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 35.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Till BG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos CA, et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J Clin Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo B, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. Journal of Cellular Immunotherapy. 2016;2:28–35. [Google Scholar]
- 39.Ali SA, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritchie DS, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21:2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borowitz MJ, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borowitz MJ, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126:964–971. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pui CH, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. The Lancet. Oncology. 2015;16:465–474. doi: 10.1016/S1470-2045(15)70082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacoby E, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker AJ, et al. Tumor Antigen and Receptor Densities Regulate Efficacy of a Chimeric Antigen Receptor Targeting Anaplastic Lymphoma Kinase. Mol Ther. 2017 doi: 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe K, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 48.Caruso HG, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turatti F, et al. Redirected activity of human antitumor chimeric immune receptors is governed by antigen and receptor expression levels and affinity of interaction. J Immunother. 2007;30:684–693. doi: 10.1097/CJI.0b013e3180de5d90. [DOI] [PubMed] [Google Scholar]
- 50.Qin H, Haso W, Nguyen SM, Fry TJ. American Society of Hematology. Orlando, FL: 2015. Preclinical Development of Bispecific Chimeric Antigen Receptor Targeting Both CD19 and CD22. [Google Scholar]
- 51.Lee DW, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weintraub S, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stroncek DF, et al. Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells. J Transl Med. 2017;15:59. doi: 10.1186/s12967-017-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroncek DF, et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. doi: 10.1016/j.jcyt.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3 doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tembhare PR, et al. Quantification of expression of antigens targeted by antibody-based therapy in chronic lymphocytic leukemia. Am J Clin Pathol. 2013;140:813–818. doi: 10.1309/AJCPYFQ4XMGJD6TI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jasper GA, et al. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry B Clin Cytom. 2011;80:83–90. doi: 10.1002/cyto.b.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cibulskis K, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient-related data not included in the manuscript may be restricted as it was generated in the context of an ongoing clinical trial and may be subject to patient confidentiality. All other data that support the findings of this study are provided in the manuscript (or accompanying Supplementary Material) or is available from the corresponding author upon reasonable request.