Abstract
Alzheimer’s disease (AD) patients display hippocampal atrophy, memory impairment, and cognitive decline. New neurons are generated throughout adulthood in two regions of the brain implicated in AD, the dentate gyrus of the hippocampus and the sub-ventricular zone of the olfactory bulb. Disruption of this process contributes to neurodegenerative diseases including AD and many of the molecular players in AD are also modulators of adult neurogenesis. However, the genetic mechanisms underlying adult neurogenesis in AD have been underexplored. To address this gap, we performed a gene-based association analysis in cognitively normal and impaired participants using neurogenesis pathway-related candidate genes curated from existing databases, literature mining, and large-scale genome-wide association study findings. A gene-based association analysis identified ADORA2A as significantly associated with hippocampal volume and the association between rs9608282 within ADORA2A and hippocampal volume was replicated in the meta-analysis after multiple comparison adjustments (p=7.88×10−6). The minor allele of rs9608282 in ADORA2A is associated with larger hippocampal volumes and better memory.
Keywords: Neurogenesis, ADORA2A, hippocampal volume, memory, Alzheimer’s Disease, gene-based association analysis, NMDA-receptor antagonist
1. Introduction
Adult neurogenesis occurs throughout life in specific regions of the brain in humans. In rodents, neural stem cells differentiate to new neurons in several regions of the brain, but studies show that adult neurogenesis is limited to the dentate gyrus (DG) of the hippocampus and the sub-ventricular zone of the olfactory bulb in humans (Eriksson, Perfilieva et al. 1998, Spalding, Bergmann et al. 2013, Bond, Ming et al. 2015). The hippocampus is the most important region of the brain for new learning and episodic/spatial memory. The new neurons generated during adult neurogenesis are incorporated into hippocampal network circuitry during construction and maintenance of neural circuits and contribute to learning and memory (Aimone, Li et al. 2014, Winner and Winkler 2015). The progenitor cells in DG divide periodically, and DG experiences stability in neurogenesis throughout life (Scharfman and Bernstein 2015, Horgusluoglu, Nudelman et al. 2016). In rodents, neural stem cells (NSCs) in the DG make approximately 8,000 to 10,000 new neurons per day. However, the proportion of hippocampal neurogenesis decline in human is smaller than mice with aging (Amrein, Isler et al. 2011, Spalding, Bergmann et al. 2013). In 1998, the presence of adult-born neurons in the dentate gyrus of the human hippocampus had been identified by using cancer patients who had received the labelled 5-bromo-2'-deoxyuridine (BrdU) in hippocampal neurons (Eriksson, Perfilieva et al. 1998). In adults, the annual turnover of stem cells into neurons is 1.75% with a modest decline during aging (Spalding, Bergmann et al. 2013). By contrast, the estimated annualized hippocampal atrophy rate is 1.41% per year for cognitively normal older people and 4.66% for patients with AD pathology (Barnes, Bartlett et al. 2009). Disruption of adult neurogenesis process has been postulated to contribute to neurodegenerative diseases including AD. Alterations in hippocampal neurogenesis in AD could either provide protection by proliferation of neural progenitor cells or cause accelerated neural degeneration due to impairment of neuronal networks and synaptic plasticity. Several studies in mice have combined structural MRI and histological approaches to investigate newborn neurons and neural stem/progenitor cells in neurogenesis-related brain regions and found that neurogenesis was associated with increased gray matter volume (Biedermann, Fuss et al. 2016). The relationship between hippocampal volume and adult neurogenesis in the human brain has not been studied yet.
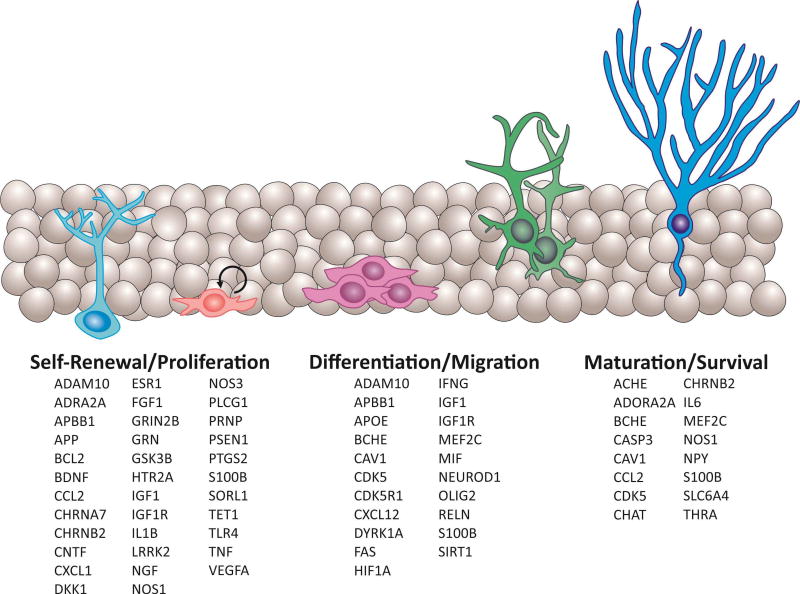
Many molecular mechanisms and pathways play a role in the hippocampal neurogenesis process, including the proliferation of neural progenitor cells, the differentiation, migration, and maturation of adult neurons (Urban and Guillemot 2014, Horgusluoglu, Nudelman et al. 2016). Known modulators of adult neurogenesis include signaling transduction, vascular and immune systems, metabolic factors, and epigenetic regulation (Urban and Guillemot 2014, Borsini, Zunszain et al. 2015, Horgusluoglu, Nudelman et al. 2016). In particular, multiple factors such as neurotrophic factors, transcription factors, and cell cycle regulators control NSC proliferation, maintenance in neurogenic niche, and differentiation into mature neurons; these factors play role in networks of signaling molecules that influence each other during construction of neural circuits, and contribute to learning and memory (Fig. 1).
Figure 1. Genes playing roles in stem cells proliferation, differentiation, migration, and survival to new neurons during adult neurogenesis process.
Glial-like radial stem cells (light blue); progenitors (pink); neuroblasts (purple); immature neurons (green); mature neurons (blue).
Disruption of the neurogenesis process has been postulated to contribute to neurodegenerative diseases including AD (Marlatt and Lucassen 2010). However, the mechanisms by which AD pathology affects neurogenesis are not completely understood. Alterations in the early stages of AD, such as amyloid-β deposition and inflammation, may impair the maturation of newborn neurons and inhibit hippocampal neurogenesis (Mu and Gage 2011). Genetic changes in neurogenesis-related pathways and genes may also play important roles in the alteration of NSCs maturation into newborn neurons (Horgusluoglu, Nudelman et al. 2016). Pathway- or gene-based association analysis has been used to study a number of complex neurodegenerative diseases, including AD, using a wide variety of phenotypes, including cerebrospinal fluid Aβ1–42 peptide level (Han, Schellenberg et al. 2010, Kim, Swaminathan et al. 2011), cerebral amyloid deposition (Swaminathan, Shen et al. 2012), brain glutamate levels (Baranzini, Srinivasan et al. 2010), and episodic memory (Ramanan, Kim et al. 2012). However, no study to date has evaluated the association between candidate neurogenesis-related genes and hippocampal volume. Thus, the goal of the present study was to perform a gene-based association analysis of neurogenesis pathway-related candidate genes in cognitively normal and impaired participants from ADNI cohort. Identification of genes that play a role in both hippocampal neurogenesis and AD may hold great promise for better understanding the role of neurogenesis in AD, as well as to aid in discovery of novel therapeutic targets for AD.
We used well-characterized participants from extensively studied cohort Alzheimer’s Diasease Neuroimaging Initiatives (ADNI), which uniquely have GWAS data sets on the same participants as well as multi-modal structural and functional neuroimaging (MRI, PET) data. A quantitative phenotype approach to genetic association studies provides the advantage of increased power sizes to detect significant genetic effects as compared to a traditional case-control design. We used hippocampal volume as a quantitative phenotype measured by MRI imaging, metabolic activity and amyloidosis in the hippocampus measured by PET imaging, and composite memory scores as quantitative traits to investigate that adult hippocampal neurogenesis-related genes and pathways are significantly associated with AD-related endophenotypes.
2. Materials and Methods
2.1. Participants
We used the participants of the Alzheimer's Disease Neuroimaging Initiative Phase 1 (ADNI-1) and its subsequent extensions (ADNI-GO/2) for this study. ADNI was launched in 2004 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration (FDA), private pharmaceutical companies, and nonprofit organizations as a public-private partnership. The aim of ADNI has been to identify whether serial MRI, positron emission tomography (PET), sensitive and specific other markers, and clinical and neuropsychological assessments could be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Participants aged 55–90 in ADNI cohort include approximately 400 cognitively normal older individuals (CN), 100 individuals with significant memory concerns (SMC), 800 individuals diagnosed with MCI, and 300 individuals diagnosed with AD. Clinical and neuroimaging procedures and the other information about the ADNI cohort can be found at http://www.adni-info.org/.
After the initial analysis, a meta-analysis was conducted with ADNI (Nho, Corneveaux et al. 2013, Weiner, Veitch et al. 2013) and two independent datasets, including the AddNeuroMed study (N=218; 66 CN, 77 MCI, 76 AD) (Lovestone, Francis et al. 2009, Nho, Corneveaux et al. 2013), and the Indiana Memory and Aging Study (IMAS) study (N=59; 29 CN, 24 MCI, 6 AD) (Nho, Corneveaux et al. 2013).
Written informed consent was obtained from each participant and all protocols were approved by each participating study and site’s Institutional Review Board.
2.1.1. Subject selection
Only non-Hispanic Caucasian participants were selected for this analysis by genetic clustering with CEU (Utah residents with Northern and Western European ancestry from the CEPH collection) and TSI (Tuscans in Italy) populations using HapMap 3 genotype data and multidimensional scaling (MDS) analysis after performing standard quality control (QC) procedures for genetic markers and participants (Ramanan, Risacher et al. 2014). Overall, 1,563 non-Hispanic Caucasian participants were included, as their genome-wide association study (GWAS) data passed the above population stratification and all other standard QC procedures (Kim, Swaminathan et al. 2011). Demographic information is shown in Table 1 for these participants.
Table 1.
Demographic and clinical characteristics of ADNI participants at the time of MRI scan
| CN | SMC | EMCI | LMCI | AD | |
|---|---|---|---|---|---|
| N | 367 | 94 | 280 | 512 | 310 |
| Age | 74.59 (5.57) | 71.77 (5.65) | 71.14 (7.26) | 73.52 (7.65) | 74.65 (7.79) |
| Gender (M/F) | 192/175 | 38/56 | 158/122 | 318/194 | 176/134 |
| Education | 16.32 (2.68) | 16.81 (2.57) | 16.08 (2.67) | 15.97 (2.91) | 15.23 (2.97) |
| APOE (ε4−/ε4+) | 267/99 | 62/32 | 160/119 | 232/280 | 104/206 |
2.2. Identification of candidate genes
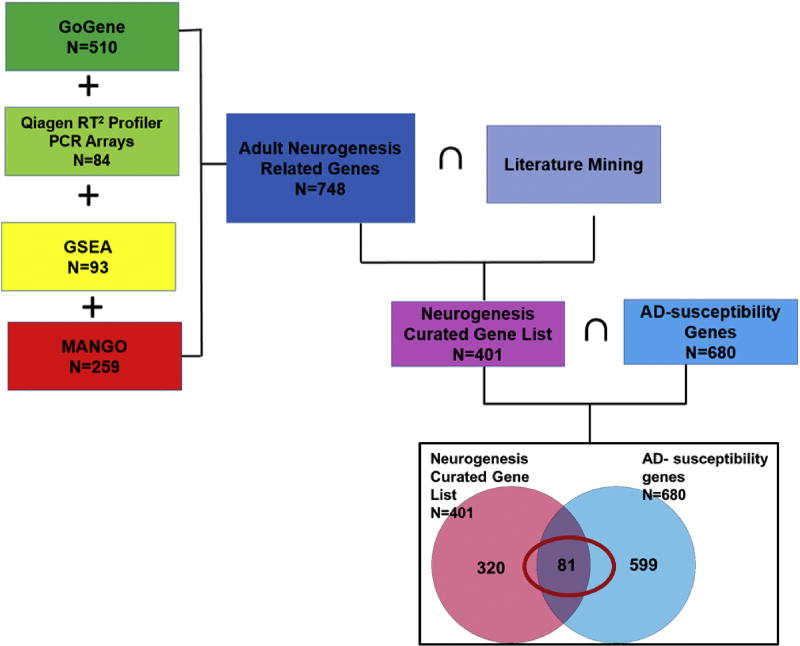
Candidate genes which control the turnover process of neural stem cells/precursors to new functional neurons during adult neurogenesis were manually curated using a pathway-based systems biology approach (Fig. 1). Genes from known modulators of adult neurogenesis include those involved in signaling transduction, vascular and immune system function, metabolic factors, and epigenetic regulation (Nho, Corneveaux et al. 2013, Funa and Sasahara 2014, Urban and Guillemot 2014, Yao and Jin 2014, Borsini, Zunszain et al. 2015). Pathway-based approaches were used to manually curate these hippocampal neurogenesis-related genes through a review of existing databases and literature mining, resulting in a final gene list (Fig. 2). Specifically, we identified hippocampal neurogenesis-related genes using four publicly available databases (GoGene, Qiagen RT2 Profiler PCR Arrays, Gene Ontology (GO) with Gene Set Enrichment Analysis (GSEA), and Mammalian Adult Neurogenesis Gene Ontology (MANGO)) and literature mining.
Figure 2. Venn diagram of Adult neurogenesis-related genes and AD-susceptibility genes.
Manually curated neurogenesis and AD related gene. {(GoGene) ∪ (Mango) ∪ (Qiagen) ∪ (GSEA)} ∩ {Pubmed Mining} = 401. AD-susceptibility genes (N=680) from AlzGene database and large-scale GWAS results. Eighty-one common genes were identified associated with both neurogenesis and AD. ∪: Union; ∩: Intersection.
GoGene contains high-quality manual annotation with high-throughput text mining from literature using ontology terms. This database includes associations between genes and gene-related terms for ten model organisms extracted from more than 18,000,000 PubMed entries that cover process, function, location of genes and their relationship with diseases and compounds (http://gopubmed.org/gogene) (Plake, Royer et al. 2009). We performed a search for GoGene term “adult neurogenesis” to identify neurogenesis-related genes.
Qiagen RT2 Profiler PCR Arrays are the one of the most reliable tools to analyze the expression of genes related to specific pathways. The human Neurogenesis RT2 Profiler™ PCR Array contains 84 genes, manually curated using literature mining, which are highly related to the process of neurogenesis, such as neural stem/progenitor cells proliferation, differentiation, migration, and maturation into newborn neurons (www.qiagen.com). Growth factors, inflammatory cytokines, cell adhesion molecules, and cell signaling genes involved in the neurogenesis process were also represented in this profiling array. We included all genes from this Neurogenesis array in our gene list.
DAVID (the Database for Annotation, Visualization, and Integrated Discovery) is a publicly available functional tool which includes annotations from Gene Ontology (GO). Gene Set Enrichment Analysis (GSEA) pathway annotations were downloaded from Molecular Signatures Database version 5.0 (http://software.broadinstitute.org/gsea/msigdb). This annotation data comprised a collection of GO. A GO annotation contains a GO term associated with a specific reference which expresses the target or analysis associated with a specific gene product. Each GO term belongs to molecular function (Naveh, Shahar et al.), cellular component (CC), or biological process (BP). “Neurogenesis” was used as the GO term in this analysis to identify neurogenesis related gene sets from the GSEA database.
We also used MANGO, which consists of 259 genes designed and curated by Overall et al. (Overall, Paszkowski-Rogacz et al. 2012). In MANGO, all genes are classified by their positive, negative, or neutral effect on hippocampal neurogenesis and thusly annotated. We used recently updated MANGO version 3.1 to annotate genes.
We identified 510 genes from GoGene, 84 genes from Neurogenesis RT2 Profiler™ PCR Array, 259 genes from MANGO database, and 93 genes from GSEA/Molecular Signatures Database. We combined all genes related to neurogenesis from the four databases (N=748). Then, each gene related to hippocampal neurogenesis was annotated from relevant literature. Initial Pubmed search using the keywords “adult hippocampal neurogenesis” for papers published until 9/2016 included 2,717 articles. We used Pubmed to identify if all 748 genes are related to hippocampal neurogenesis from 2,717 articles. After literature mining, among 748 genes, only 401 genes were related to adult hippocampal neurogenesis.
Finally, since a key goal was to identify candidate genes playing a role in both adult hippocampal neurogenesis and AD pathology, we focused on hippocampal neurogenesis-related genes which are also implicated in AD. For this purpose, we identified AD-associated susceptibility genes using the AlzGene database (http://www.alzgene.org/), which provides a comprehensive meta-analysis of genes previously identified in various AD association studies, and large-scale AD GWAS results (N=680) (Lambert, Ibrahim-Verbaas et al. 2013). The AlzGene database consists of a comprehensive, unbiased, publicly available catalog of all genetic association studies in the field of AD, which was identified from published papers by pubMed search using keywords “alzheimer* AND (genet* OR associat*)”. The gene list in AlzGene represents a summary of promising AD candidate genes. We then compared this gene list to the 401 genes previously identified as involved in adult hippocampal neurogenesis to filter the lists to 81 common genes related to both hippocampal neurogenesis and AD. These 81 genes were used in the association analysis.
2.3. Endophenotypes
Pre-processed baseline 1.5T and 3T MRI scans from 1,563 participants were downloaded from ADNI public website (http://adni.loni.usc.edu). FreeSurfer version 5.1 was used to extract total hippocampal and hippocampal subfield volumes, as well as total intracranial volume (ICV) (Dale, Fischl et al. 1999, Fischl, Sereno et al. 1999, Risacher, Kim et al. 2013, Risacher, Kim et al. 2015). Total hippocampal volume, as well as selected adult neurogenesis-related subfield volumes (CA1, CA23, CA4, and DG) (N=1,563), were used as endophenotypes for the association analysis. In addition, we used a composite score of episodic memory (N=1,563) (Crane, Carle et al. 2012) and CSF total tau levels (N=1,112) as endophenotypes to further characterize neurodegeneration (Shaw, Vanderstichele et al. 2009, Shaw, Vanderstichele et al. 2011).
2.4. Genotyping and Quality Control
ADNI samples were genotyped using Human 610-Quad, HumanOmni Express, and HumanOmni 2.5M BeadChips. Sample and SNP quality control procedures of GWAS data such as SNP call rate < 95%, Hardy-Weinberg equilibrium test p < 1 × 10−6, and frequency filtering (MAF ≥ 5%) were performed (Purcell, Neale et al. 2007, Saykin, Shen et al. 2010, Hohman, Koran et al. 2014, Ramanan, Risacher et al. 2014). Imputation of un-genotyped SNPs was performed using MaCH (Markov Chain Haplotyping) software based on the 1000 Genomes Project as a reference panel (Howie, Fuchsberger et al. 2012).
2.5. Association Analysis and Meta-Analysis
SNPs from the 81 candidate genes were located in untranslated regions (Laussu, Khuong et al. 2014), 3′ UTR, 5′ UTR, coding regions, intronic regions, and regulatory regions (±20 kb of upstream and downstream regions). A gene-based association analysis of hippocampal neurogenesis pathway-related candidate genes was performed in an additive genetic model using a set-based test in Plink v1.07 (http://pngu.mgh.harvard.edu/~purcell/plink/) (Purcell, Neale et al. 2007). After frequency and genotyping pruning, 18407 SNPs remained from 81 genes. (Purcell, Neale et al. 2007). After frequency and genotyping pruning, 18407 SNPs remained from 81 genes. A mean test statistic for each SNP within a gene was computed to determine other SNPs in linkage disequilibrium (LD; i.e., r2>0.5). A quantitative trait analysis (QT) was then performed with each SNP. For each gene (set), the top independent SNPs (i.e., not in LD; maximum of 5) were selected if their p-values were less than 0.05 in the QT analysis. The SNP with the smallest p-value was selected first; subsequent independent SNPs were selected in order of decreasing statistical significance. From these subsets of SNPs, the statistic for each set was calculated as the mean of these single SNP statistics. The analysis was performed to estimate the additive effect of the selected SNP minor allele on the phenotypic mean (Purcell, Neale et al. 2007, Swaminathan, Shen et al. 2012). Covariates included gender, age, years of education, ICV, MRI field strength (1.5T vs 3T) and diagnosis status. An empirical p-value (20,000 permutations) was reported for each gene. In the discovery sample (ADNI-1/GO/2), a conservative significance threshold (p < 0.00061) was used based on Bonferroni correction for 81 genes. We subsequently performed a meta-analysis for genes and SNPs associated with hippocampal volume using data from the ADNI-1/GO/2, AddNeuroMed, and IMAS cohorts. The gene-based meta-analysis was performed using the weighted z statistic test (Stouffer’s weighted z statistic) as implemented in R, with weight accounting for the sample size of each cohort. For SNP-based meta-analysis, METAL was used with a fixed-effect inverse variance model (Willer, Li et al. 2010). For meta-analysis, effect sizes were provided by standardized β coefficients from linear regression.
We also evaluated the effect of the minor allele rs9608282-T on hippocampal volume and composite memory score after the participants, which included only those diagnosed with MCI or AD, were classified as either a Memantine user (NMDA (+)) or a Memantine non-user (NMDA (−)) using two-way analysis of covariance (ANCOVA) for continuous variables and a chi-square for categorical variables implemented in SPSS 23.0. In addition, two-way analysis of covariance (ANCOVA) was used to examine the effect of the minor allele (rs9608282-T) on hippocampal volume in both amyloid-negative and amyloid-positive participants (classified as positive or negative by either baseline [18F]Florbetapir PET scans and/or CSF Aβ1–42 level).
3. Results
3.1. Gene-based and SNP-based analysis of mean volumes of hippocampus and hippocampal sub-regions
The manual gene/pathway curation for hippocampal neurogenesis yielded 18407 SNPs in 81 genes (Fig. 2). In the discovery sample, the gene-based association analysis showed that APOE and ADORA2A were significantly associated with hippocampal volume after Bonferroni correction (p-value=5×10−5; Table 2).
Table 2.
81 genes of gene-based association results in the discovery sample for hippocampal volume using common variants (MAF ≥ 0.05) where empirical p-values were calculated using 20,000 permutations in PLINK
| Gene | Number of SNPs in gene |
Number of significant SNPs (p<0.05, r2<0.5) |
Empirical gene-based p-value |
List of significant SNP |
|---|---|---|---|---|
| ADORA2A | 55 | 1 | 5×10−5 | rs9608282 |
| APOE | 85 | 5 | 5×10−5 | rs429358|rs7259620|rs34095326|rs4803770|rs157580 |
| TLR4 | 58 | 2 | 0.01499 | rs11789302|rs10759930 |
| BCHE | 201 | 4 | 0.02797 | rs2686409|rs1355538|rs12107166|rs6807910 |
| CXCL10 | 42 | 1 | 0.04795 | rs4256246 |
| S100B | 220 | 1 | 0.05994 | rs118078026 |
| CXCL12 | 306 | 4 | 0.06194 | rs11238990|rs11238991|rs1144472|rs17659345 |
| TET1 | 387 | 3 | 0.06593 | rs113716271|rs12776586|rs12221107 |
| BCL2 | 368 | 5 | 0.07493 | rs9957149|rs28564323|rs7236090|rs6567334|rs11872403 |
| PTGS2 | 52 | 2 | 0.08492 | rs7547677|rs2206593 |
| ACHE | 51 | 2 | 0.08791 | rs13245899|rs73714210 |
| SORL1 | 233 | 2 | 0.09091 | rs9665907|rs643010 |
| SLC6A4 | 63 | 1 | 0.09191 | rs16965628 |
| GRIN2B | 1054 | 5 | 0.0999 | rs34870448|rs11612709|rs12582848|rs11611667|rs2300256 |
| NGFR | 86 | 5 | 0.0999 | rs584589|rs11466150|rs2072444|rs535717|rs2537710 |
| MEF2C | 232 | 1 | 0.1019 | rs1065861 |
| CXCL1 | 39 | 1 | 0.1039 | rs2968710 |
| NR3C1 | 238 | 5 | 0.1209 | rs4912912|rs17209237|rs10050756|rs7719514|rs12653301 |
| DKK1 | 40 | 2 | 0.1249 | rs11001581|rs7100461 |
| GRN | 37 | 1 | 0.1319 | rs114641762 |
| CHAT | 189 | 5 | 0.1379 | rs885834|rs11101179|rs1720367|rs4615945|rs74981858 |
| CHRNB2 | 39 | 5 | 0.1499 | rs9616|rs9427094|rs12072348|rs67860750|rs4845653 |
| Syn3 | 1388 | 5 | 0.1718 | rs2157188|rs9609643|rs2710348|rs180958069|rs5749521 |
| CCL2 | 43 | 1 | 0.1758 | rs111843487 |
| IGF1 | 89 | 4 | 0.1888 | rs1549593|rs10860862|rs12821878|rs80280982 |
| VEGFA | 59 | 3 | 0.1908 | rs9381248|rs3025006|rs699946 |
| NGF | 135 | 2 | 0.1978 | rs6537860|rs4320778 |
| MIF | 77 | 1 | 0.2008 | rs738807 |
| IL6 | 77 | 2 | 0.2338 | rs2069840|rs62449498 |
| CASP3 | 94 | 2 | 0.2378 | rs4647634|rs2696059 |
| MMP9 | 57 | 2 | 0.2537 | rs73112805|rs3918253 |
| PRNP | 94 | 2 | 0.2747 | rs6052766|rs67017873 |
| SNCA | 444 | 1 | 0.2927 | rs187644542 |
| CDK5 | 53 | 2 | 0.3147 | rs4148853|rs34403003 |
| IL1B | 42 | 1 | 0.3147 | rs3917381 |
| ADAM10 | 232 | 1 | 0.3197 | rs544282 |
| NOS3 | 71 | 1 | 0.3197 | rs12666075 |
| NRG1 | 2784 | 5 | 0.3227 | rs147179882|rs2466068|rs7829383|rs2347071|rs11998153 |
| APBB1 | 119 | 1 | 0.3487 | rs11040880 |
| LRRK2 | 360 | 3 | 0.3526 | rs189800607|rs10878411|rs11564173 |
| OLIG2 | 123 | 2 | 0.3626 | rs17632819|rs76708155 |
| NOS1 | 559 | 5 | 0.3826 | rs67313272|rs4767542|rs816284|rs12228022|rs10850829 |
| HIF1A | 82 | 1 | 0.4186 | rs12891737 |
| FGF1 | 221 | 5 | 0.4326 | rs1808258|rs2070715|rs249925|rs10041541|rs13179022 |
| TGFB1 | 131 | 1 | 0.5135 | rs4803459 |
| IGF1R | 520 | 5 | 0.5325 | rs11631965|rs4966039|rs3743254|rs7166348|rs2272037 |
| FAS | 175 | 1 | 0.5774 | rs12767306 |
| APP | 554 | 5 | 0.6214 | rs2829960|rs6516705|rs13046930|rs6516715|rs117104544 |
| NTRK2 | 605 | 4 | 0.6264 | rs17087710|rs28580203|rs1047896|rs1006446 |
| DYRK1A | 380 | 1 | 0.6593 | rs28550863 |
| ESR2 | 412 | 4 | 0.6983 | rs1152576|rs4986938|rs10146107|rs12587140 |
| RELN | 1552 | 5 | 0.7932 | rs2299373|rs3819491|rs39377|rs1476446|rs694894 |
| ESR1 | 810 | 1 | 0.7972 | rs55650062 |
| ABCA2 | 61 | 0 | 1 | NA |
| ADRA2A | 27 | 0 | 1 | NA |
| BDNF | 122 | 0 | 1 | NA |
| CAV1 | 129 | 0 | 1 | NA |
| CDK5R1 | 24 | 0 | 1 | NA |
| CHRNA7 | 2 | 0 | 1 | NA |
| CNTF | 29 | 0 | 1 | NA |
| FOS | 48 | 0 | 1 | NA |
| GSK3B | 329 | 0 | 1 | NA |
| HTR2A | 203 | 0 | 1 | NA |
| NEUROD1 | 54 | 0 | 1 | NA |
| NPY | 131 | 0 | 1 | NA |
| PLCG1 | 60 | 0 | 1 | NA |
| PSEN1 | 154 | 0 | 1 | NA |
| SIRT1 | 98 | 0 | 1 | NA |
| THRA | 82 | 0 | 1 | NA |
| TNF | 40 | 0 | 1 | NA |
| TNFR1A | 4 | 0 | 1 | NA |
| TNFR1B | 54 | 0 | 1 | NA |
| DLD | 24 | 0 | 1 | NA |
| GLP1R | 98 | 0 | 1 | NA |
| IFNG | 19 | 0 | 1 | NA |
| Plau | 30 | 0 | 1 | NA |
| PSEN2 | 19 | 0 | 1 | NA |
| SOD2 | 5 | 0 | 1 | NA |
| tp53 | 5 | 0 | 1 | NA |
| CST3 | 92 | 0 | 1 | NA |
| CHRM1 | 47 | 0 | 1 | NA |
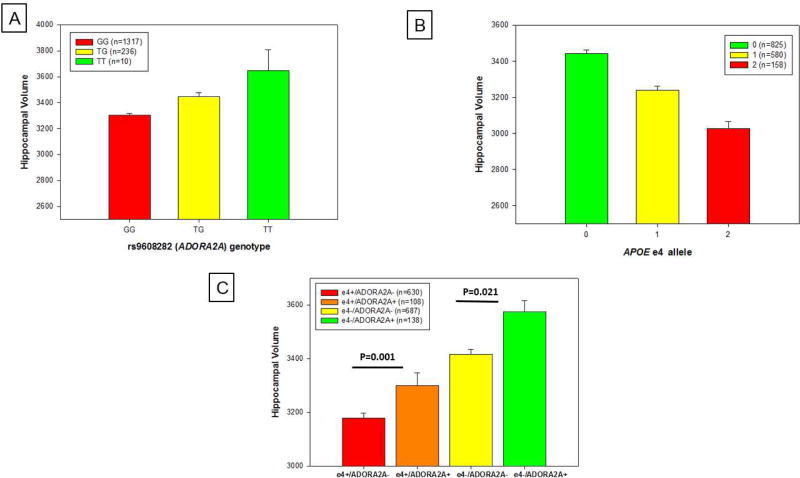
One SNP (rs9608282) upstream of ADORA2A was most significantly associated with total hippocampal volume and sum of the hippocampal sub-region volumes (p = 1.14×10−5 and 2.5×10−4, respectively; Table 3). Specifically, participants with no copies of the minor allele (N=1,317; GG genotype) had a smaller mean hippocampal volume compared to participants with one copy of the minor allele (N=236; TG genotype) or participants with two copies of the minor allele (N=10; TT genotype).
Table 3.
Association of rs9608282 in ADORA2A with neuroimaging phenotypes and memory composite scores with and without diagnosis (DX) adjustment. SNP-based association results (p-values) in the discovery sample for hippocampal volume, neurogenesis-related hippocampal sub-regions, memory performance, and CSF total tau level.
| rs9608282 |
p-value after adjusting for DX |
p-value without adjusting for DX |
|---|---|---|
| Hippocampal Volume | 3.29×10−4 | 1.14×10−5 |
| Neurogenesis-Related Hippocampal Sub-regions | 4.55×10−3 | 2.50×10−4 |
| Memory Composite Score | 2.18×10−1 | 7.45×10−3 |
| CSF Total Tau | 2×10−1 | 2.3×10−2 |
For replication of our major significant SNP finding, we analyzed independent samples from the ADNI, AddNeuroMed IMAS cohorts. SNP-based meta-analysis of ADORA2A in three independent cohorts (ADNI1/GO/2, AddNeuroMed, and IMAS) identified that rs9608282-T in ADORA2A are significantly associated with hippocampal volume (p = 0.000043, N=1,840, Table 4; p = 7.88×10−6, N= 1,840, Table 5, respectively). In ADNI1/GO/2, AddNeuroMed cohorts except IMAS, rs9608282-T exhibited a positive direction of effect on hippocampal volume.
Table 4.
Meta-analysis of ADORA2A with hippocampal volume in three independent cohorts: ADNI, AddNeuroMed and IMAS.
| ADNI p-value |
AddNeuroMed p-value |
IMAS p-value |
Meta-analysis p-value |
|
|---|---|---|---|---|
| ADORA2A | 5×10−5 | 2.1×10−1 | 2.95×10−1 | 4.3×10−5 |
Table 5.
Meta-analysis of rs9608282 with hippocampal volume in three independent cohorts: ADNI, AddNeuroMed and IMAS.
| rs9608282 | N | Effect of rs9608282 (T) (β value) |
p-value |
|---|---|---|---|
| ADNI | 1563 | 146.9 | 1.14×10−5 |
| AddNeuroMed | 218 | 362.4 | 2.34×10−2 |
| IMAS | 59 | −263.2 | 4.59×10−2 |
| Meta-analysis | 1840 | 7.88×10−6 |
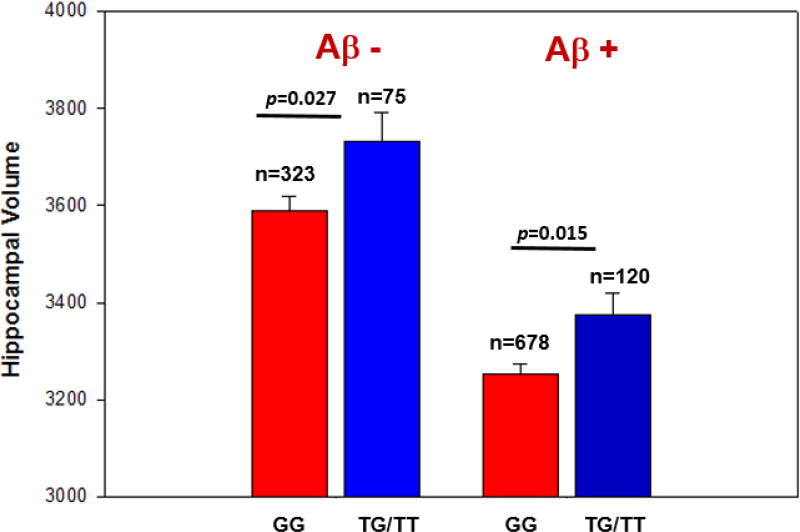
Following the SNP-based association analysis, we performed a post-hoc analysis to measure the interaction effect of APOE and the ADORA2A SNP on hippocampal volume. There was no evidence of epistasis, modeled as interaction between APOE ε4 status and the minor allele of rs9608282 (p = 0.54). However, ADORA2A rs9608282-T and APOE ε4 exhibited independent but opposite effects on hippocampal volume (Fig. 3A, 3B), with a comparable effect sizes between the APOE ε4 allele and the presence of at least one copy of the minor allele at rs9608282. Participants carrying at least one copy of the minor allele of the ADORA2A SNP have larger hippocampal volumes than those without the minor allele, even in participants with APOE ε4 (p=0.001; Fig. 3C). The positive effect of the ADORA2A rs9608282-T allele on hippocampal volume was seen in both amyloid-negative and amyloid-positive participants (classified as positive or negative by either baseline [18F]Florbetapir PET scans and/or CSF Aβ1–42 level). Specifically, rs9608282-T was significantly associated with larger hippocampal volumes in Aβ negative (p-value = 0.027) and Aβ positive participants (p-value = 0.015, Fig. 4). In addition, the association of the rs9608282-T allele with hippocampal volume and neurogenesis-related to sub-regions independent of diagnosis suggests that this effect might be a global phenomenon.
Figure 3. APOE ε4 and rs9608282 (ADORA2A) appear to exhibit independent, but opposite effect on hippocampal volume.
Baseline hippocampal volume (adjusted for age, gender, ICV, MRI field strength) ± standard errors are shown based on (a) rs9608282 in ADORA2A (A2aR) across genotype groups. Presence of at least one copy of the minor allele (T) of rs9608282 was significantly associated with increased hippocampal volume (p = 0.002). Baseline hippocampal volume is also shown by (b) the number of APOE ε4 allele copies. Presence of at least one copy of the ε4 allele was significantly associated with decreased hippocampal volume (p < 0.0001). (c) For participants having APOE ε4 allele copies, participants carrying minor allele of rs9608282 had larger hippocampal volume than those who did not carry minor allele of the ADORA2A rs9608282 (p = 0.001).
Figure 4. ADORA2A rs9608282 is associated with larger hippocampal volume in amyloid-positive participants (classified by PET scan and/or CSF-amyloid beta level).
For statistical analysis, each participant was classified by their amyloid status (positive versus negative) at the baseline visit (determined by standard cutoffs on [18F]Florbetapir PET scan and/or CSF amyloid level). The effect of the T allele on hippocampal volume was present in both amyloid-negative (left column) and amyloid-positive (right column) participants. Upon statistical analysis, rs9608282 is significantly associated with hippocampal volume in amyloid-negative and even amyloid-positive participants (p = 0.027, p =0.015, respectively).
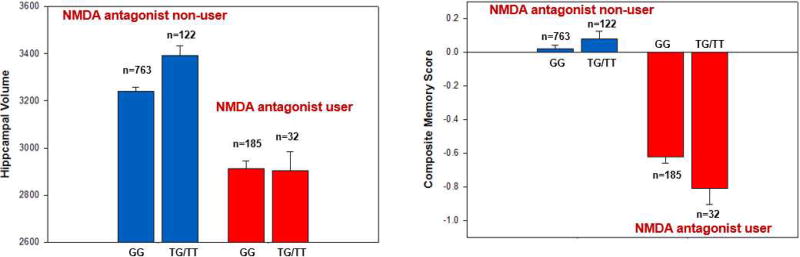
Since previous studies have suggested that ADORA2A plays an important role controlling NMDA-dependent synaptic toxicity and memory impairment (Tebano, Martire et al. 2005, Yee, Singer et al. 2007, Sarantis, Tsiamaki et al. 2015), we examined the interaction of taking Memantine, a NMDA-receptor antagonist, and ADORA2A rs9608282 on hippocampal volume and memory performance. Participants diagnosed with MCI or AD were classified as either a Memantine user (NMDA+) or a Memantine non-user (NMDA−). We found that NMDA-participants carrying at least one copy of the minor allele (T) of the ADORA2A rs9608282 had a larger mean hippocampal volume (p<0.001; Fig. 5A). There was a significant interaction effect of NMDA-receptor antagonist use and ADORA2A rs9608282 on memory performance (p = 0.009). NMDA+ participants carrying two copies of the major allele (G) of the ADORA2A rs9608282 had better memory performance (Fig. 5B).
Figure 5. ADORA2A rs9608282 is associated with larger hippocampal volume in Memantine non-users (NMDA (−)) and poorer memory performance in Memantine users (NMDA (+)).
For statistical analysis, cognitively impaired participants were classified as either a Memantine user (NMDA (+)) or a Memantine non-user (NMDA (−)). (A) The rs9608282 T allele was associated with a larger mean hippocampal volume in Memantine non-user participants (p < 0.001). (B) Participants carrying at least one copy of minor allele (T) of the ADORA2A rs9608282 variant and using a NMDA-receptor antagonist had poorer memory performance.
3.2. Association of rs9608282 with episodic memory and CSF level of total tau
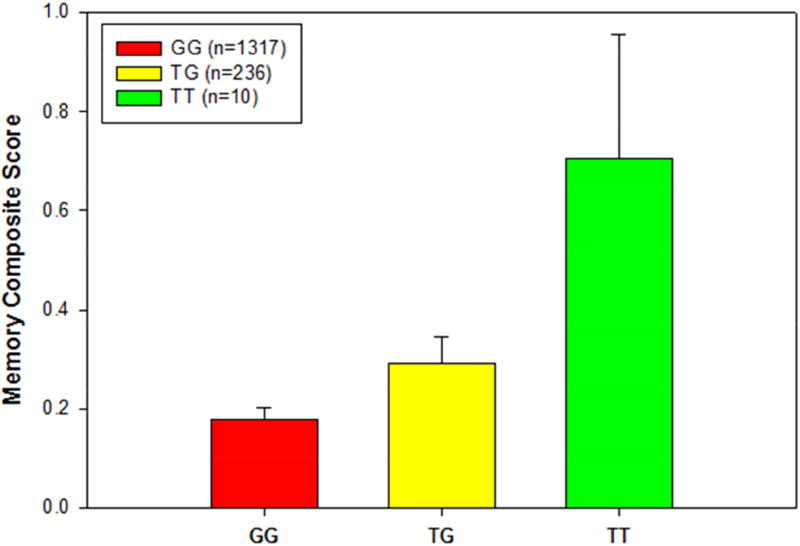
Given the association of ADORA2A rs9608282-T with larger hippocampal volume, we hypothesized that ADORA2A would also associated with episodic memory scores as they are highly related to hippocampal structure. As might be hypothesized, rs9608282-T was significantly associated with a better composite memory score (β=0.065; p=0.015) after controlling for age, gender, and years of education (Fig. 6).
Figure 6. Association of memory composite score with rs9608282 in ADORA2A across genotype.
Baseline memory composite score (adjusted for age, gender and education) ± standard errors are displayed based on rs9608282 genotype. Individuals with a TT genotype at the rs9608282 variant showed a 5% increase in memory performance relative to those with a GG genotype.
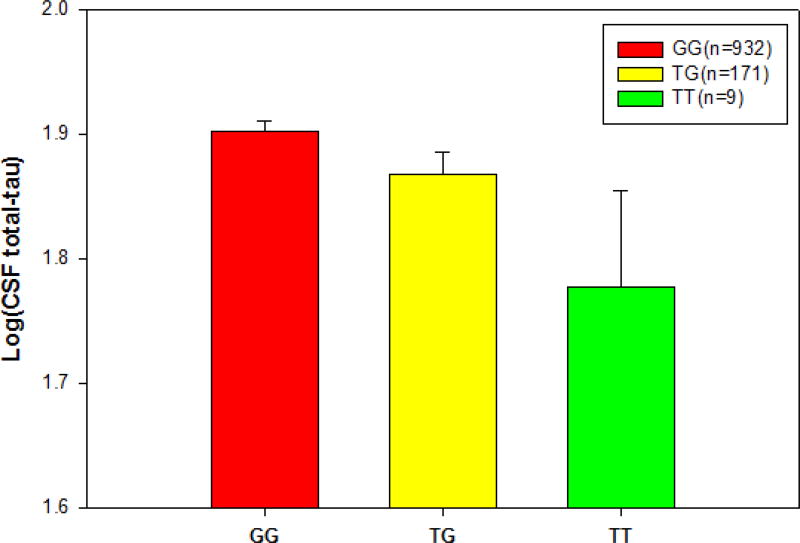
A previous study suggested that hyperphosphorylated tau decreases adult neurogenesis in mouse model (Komuro, Xu et al. 2015). Therefore, we also assessed the effect of the rs9608282-T minor allele on CSF total tau level. As we hypothesized, rs9608282-T carriers showed decreased CSF total tau levels relative to non-carriers (β=−0.061; p=0.039), after controlling for age and gender (Fig. 7). However, there is no correlations of rs9608282 with CSF Aβ and phospho-tau levels.
Figure 7.
Association of CSF tau level with rs9608282 in ADORA2A across genotype. CSF total tau level (adjusted for age and gender)) ± standard errors are displayed based on rs9608282 genotype. Individuals with a TT genotype at the rs9608282 variant showed significant decrease in CSF tau level relative to those with a GG genotype.
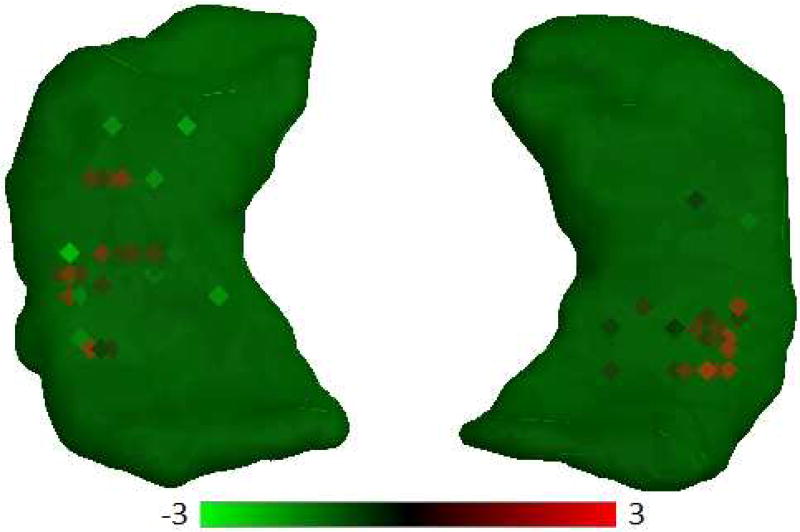
Finally, we used gene expression data from the Allen Human Brain Atlas to evaluate if ADORA2A was expressed in neurogenesis-related regions in normal brains. ADORA2A was in fact highly expressed across the major adult neurogenesis related regions of the brain (Fig. 8) and was especially highly expressed in CA1 and CA2.
Figure 8. ADORA2A expression profiles across the hippocampus region.
The square dot indicates the tissue sample location for human brain. In each square, heat map color represents the z-score over a probe ranging from green (z-score of −3 and below) through black to red (z-score of +3 and above). Red squares represents overexpression of the ADORA2A in specific locations, most especially in CA1 and CA2.
4. Discussion
We used well-characterized participants from the Alzheimer’s Disease Neuroimaging Initiatives (ADNI), an extensively studied cohort that includes cognitively normal older individuals (CN), as well as participants with significant memory concerns (SMC), amnestic mild cognitive impairment (MCI), and Alzheimer’s Disease (AD). Using targeted neurogenesis pathway-based gene analysis, we discovered a significant association of ADORA2A rs9608282-T with larger mean hippocampal volumes and volumes of neurogenesis-related hippocampal sub-regions, better episodic memory performance, and reduced CSF total tau. These findings suggested a protective effect of this SNP on brain structure and function in neurogenesis-related brain regions.
ADORA2A (Adenosine Receptor Subtype A2a) is a G-protein-coupled adenosine receptor that is involved with controlling synaptic plasticity in glutamatergic synapses (Cunha, Ferre et al. 2008, Krugel 2015). Previous work had indicated a physical and functional interaction of ADORA2A with dopamine D2 receptors (Chen, Moratalla et al. 2001). However, A1R–A2AR heteromer controls the affinity of agonist binding to A2a receptors in the striatum and localizes in glutamatergic nerve terminals to control glutamate release (Ciruela, Casado et al. 2006). In addition to its abundance in the striatum, ADORA2A also plays an important role in hippocampus, particularly in neurogenesis in the CA3 region. A previous study demonstrated that inhibition of the A2a receptor induced synaptic damage in rat hippocampal nerve terminals (Cunha, Canas et al. 2006).
A reduction of A2a receptors in mice with traumatic brain injury has also been shown to decrease cognitive impairment (Ning, Yang et al. 2013). In fact, the adenosine 2a receptor localizes in microglial cells and may be a regulation of microglial function in response to brain damage (Cunha, Ferre et al. 2008). Since neuroinflammatory blockade is thought to enhance neural stem/progenitor cells activity and promote adult neurogenesis, A2a receptor-mediated control of neuroinflammation might be a vital mechanism in neurodegenerative diseases (Monje, Toda et al. 2003). Inhibition of the A2a receptor also prevents early Aβ-induced synaptotoxicity and memory dysfunction through a p38 MAPK-dependent pathway (Canas, Porciuncula et al. 2009), potentially suggesting additional roles for this receptor in AD. In fact, ADORA2A blockade prevented memory decline secondary to amyloid-beta accumulation, which is a major pathological hallmark in AD (Cunha, Canas et al. 2008). Another important role of ADORA2A is to modulate brain-derived neurotrophic factor (BDNF). Administration of an ADORA2A antagonist inhibits the actions of BDNF on GABA and glutamate release from the hippocampal nerve terminals (Vaz, Lerias et al. 2015). In addition, the A2a receptor is involved with control of N-methyl-D-aspartate (NMDA) receptor function by co-localizing with metabotropic glutamate 5 receptors (Glu5R) in hippocampal synapses (Tebano, Martire et al. 2005). The synaptic localization of A2a receptors plays a key role controlling NMDA-dependent synaptic transmission in the hippocampus (Rebola, Sachidhanandam et al. 2007). In fact, glutamate release is dependent on the activation of adenosine A2AR by endogenous adenosine (Canas, Porciuncula et al. 2009, Vaz, Lerias et al. 2015). Previous studies showed the relationship between NMDA receptor and Adenosine Receptor Subtype A2a, which supports our finding of a significant interaction effect of NMDA-receptor antagonist use and the ADORA2A rs9608282-T on memory performance.
rs9608282 is located upstream of ADORA2A (UCSC Genome Browser (GRCh37/hg19)) and is characterized by occurring during read-through transcription of two neighbor genes, SPECC1L (sperm antigen with calponin homology and coiled-coil domains 1-like) and ADORA2A (adenosine A2a receptor) on chromosome 22. This read-through transcription is a candidate for nonsense-mediated mRNA decay (NMD), which leads to no protein production. The inhibition of ADORA2A has been shown to enhance spatial memory and hippocampal plasticity through adult neurogenesis (Laurent, Burnouf et al. 2016). In the present study, rs9608282-T was associated with better memory and a larger hippocampal volume, suggesting that this variation may inhibit protein production of ADORA2A. Animal models or cell culture studies are needed to more completely characterize the function of this variation on brain structure and adult neurogenesis.
Based on gene expression data from postmortem human brains, the A2A receptor is highly expressed in neurogenesis-related regions (CA1, CA2, CA3 and dentate gyrus) of the hippocampus in the adult human brain. Since the dentate gyrus and CA3 regions are important for memory formation and pattern separation processes, as well as for learning new information, we believe the observed effect of the rs9608282-T variation may be protective for memory performance by altering neurogenesis in these regions. In addition, the association of the rs9608282-T allele with hippocampal volume and neurogenesis-related sub-regions independent of diagnosis suggests that this effect might be a global rather than AD-specific phenomenon. Consistent with protective effect of this variant, decreased CSF total-tau protein levels were also observed in participants with at least one minor allele (T) of rs9608282.
Interestingly, ADORA2A rs9608282-T and APOE ε4 exhibit an independent but opposite effect on hippocampal volume. In sum, we observed a significant protective effect of a variant (rs9608282) in the neurogenesis-related ADORA2A gene on brain structure and function, including increased hippocampal volume, better memory performance, and reduced CSF tau. This finding suggests that the adenosine A2a receptor warrants further investigation as a potential target for future therapeutics to treat neurodegenerative disease and cognitive decline.
The eQTL analysis using the BRAINEAC brain tissue microarray-based gene expression database (http://www.braineac.org/) revealed that rs9608282 in ADORA2A is marginally associated with ADORA2A gene expression levels in the hippocampus (p-value = 0.172). Individuals carrying minor allele rs9608282-T have decreased expression levels in the hippocampus, showing a potential protective effect consistent with our SNP-based association results with hippocampal volume and memory. Increased ADORA2A levels lead to synaptic toxicity and memory impairment (Tebano, Martire et al. 2005, Yee, Singer et al. 2007, Sarantis, Tsiamaki et al. 2015).
The limitation of the present report is that even though we used three independent publicly available databases to identify a curated gene list related to adult neurogenesis, it is possible that we may have missed other neurogenesis related genes not represented in these databases. The other limitation is that even though a few studies combined MRI-based hippocampal volume with immunochemistry to reveal that there is a significant hippocampal atrophy and the reduction of hippocampal neurogenesis in animal models, it is still not clear if hippocampal atrophy is related to adult neurogenesis in humans due to lack of data sources. Another limitation for this study is the lack of replication in the gene-based analysis. In the AddNeuroMed and IMAS, ADORA2A did not show a significant association with hippocampal volume but showed a trend. After combining three independent cohorts, the meta-analysis result was significant due to the increased detection power. In addition, future studies are needed to identify functional evidence to validate this SNP in ADORA2A. However, the present findings support that the ADORA2A gene plays a role in adult neurogenesis. AD is associated with hippocampal volume loss the observed effects indicates the potential importance of further investigation of this gene in independent cohorts.
Highlights.
Candidate pathways and genes which play a role in neurogenesis in the adult brain are manually-curated.
ADORA2A is significantly associated with hippocampal volume
A SNP (rs9608282) upstream of ADORA2A is associated with larger hippocampal volume and better memory performance.
rs9608282 may have a protective effect on brain structure and function in neurogenesis-related brain regions.
There is a significant interaction effect of NMDA-receptor antagonist use and the ADORA2A rs9608282-T on memory performance.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Samples from the National Cell Repository for AD (NCRAD), which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the National Institute on Aging (AIG), were used in this study. Funding for the WGS was provided by the Alzheimer’s Association and the Brin Wojcicki Foundation.
Additional support for data analysis was provided by NLM R01 LM012535, NIA R03 AG054936, NIA R01 AG19771, NIA P30 AG10133, NLM R01 LM011360, NSF IIS-1117335, DOD W81XWH-14-2-0151, NCAA 14132004, NIGMS P50GM115318, NCATS UL1 TR001108, NIA K01 AG049050, the Alzheimer’s Association, the Indiana Clinical and Translational Science Institute, and the IU Health-IU School of Medicine Strategic Neuroscience Research Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Isler K, Lipp HP. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci. 2011;34(6):978–987. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Srinivasan R, Khankhanian P, Okuda DT, Nelson SJ, Matthews PM, Hauser SL, Oksenberg JR, Pelletier D. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain. 2010;133(9):2603–2611. doi: 10.1093/brain/awq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, Thompson P, Fox NC. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30(11):1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann SV, Fuss J, Steinle J, Auer MK, Dormann C, Falfan-Melgoza C, Ende G, Gass P, Weber-Fahr W. The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct Funct. 2016;221(3):1353–1363. doi: 10.1007/s00429-014-0976-5. [DOI] [PubMed] [Google Scholar]
- Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17(4):385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38(3):145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29(47):14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98(4):1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, I. Alzheimer's Disease Neuroimaging Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6(4):502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Melo CS, Hockemeyer J, Muller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210(2):776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Oliveira CR, Cunha RA. Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience. 2006;141(4):1775–1781. doi: 10.1016/j.neuroscience.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14(15):1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168–181. doi: 10.1007/s11481-013-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MR, Schellenberg GD, Wang LS, I. Alzheimer's Disease Neuroimaging Genome-wide association reveals genetic effects on human Abeta42 and tau protein levels in cerebrospinal fluids: a case control study. BMC Neurol. 2010;10:90. doi: 10.1186/1471-2377-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Koran ME, Thornton-Wells TA, I. Alzheimer's Neuroimaging Genetic variation modifies risk for neurodegeneration based on biomarker status. Front Aging Neurosci. 2014;6:183. doi: 10.3389/fnagi.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2016 doi: 10.1002/ajmg.b.32429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44(8):955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, Craig DW, DeChairo BM, Aisen PS, Petersen RC, Weiner MW, Saykin AJ, I. Alzheimer's Disease Neuroimaging Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76(1):69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro Y, Xu G, Bhaskar K, Lamb BT. Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol Aging. 2015;36(6):2034–2042. doi: 10.1016/j.neurobiolaging.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel U. Purinergic receptors in psychiatric disorders. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, Malik E, Mariciniak E, Parrot S, Van der Jeugd A, Faivre E, Flaten V, Ledent C, D'Hooge R, Sergeant N, Hamdane M, Humez S, Muller CE, Lopes LV, Buee L, Blum D. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry. 2016;21(1):149. doi: 10.1038/mp.2014.151. [DOI] [PubMed] [Google Scholar]
- Laussu J, Khuong A, Gautrais J, Davy A. Beyond boundaries--Eph:ephrin signaling in neurogenesis. Cell Adh Migr. 2014;8(4):349–359. doi: 10.4161/19336918.2014.969990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Francis P, Kloszewska I, Mecocci P, Simmons A, Soininen H, Spenger C, Tsolaki M, Vellas B, Wahlund LO, Ward M, C. AddNeuroMed AddNeuroMed--the European collaboration for the discovery of novel biomarkers for Alzheimer's disease. Ann N Y Acad Sci. 2009;1180:36–46. doi: 10.1111/j.1749-6632.2009.05064.x. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ. Neurogenesis and Alzheimer's disease: Biology and pathophysiology in mice and men. Curr Alzheimer Res. 2010;7(2):113–125. doi: 10.2174/156720510790691362. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh GR, Shahar R, Brumfeld V, Weiner S. Tooth movements are guided by specific contact areas between the tooth root and the jaw bone: A dynamic 3D microCT study of the rat molar. J Struct Biol. 2012;177(2):477–483. doi: 10.1016/j.jsb.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Nho K, Corneveaux JJ, Kim S, Lin H, Risacher SL, Shen L, Swaminathan S, Ramanan VK, Liu Y, Foroud T, Inlow MH, Siniard AL, Reiman RA, Aisen PS, Petersen RC, Green RC, Jack CR, Weiner MW, Baldwin CT, Lunetta K, Farrer LA, S. Multi-Institutional Research on Alzheimer Genetic Epidemiology. Furney SJ, Lovestone S, Simmons A, Mecocci P, Vellas B, Tsolaki M, Kloszewska I, Soininen H, C. AddNeuroMed. McDonald BC, Farlow MR, Ghetti B, Indiana M, Aging S, Huentelman MJ, Saykin AJ, I. Alzheimer's Disease Neuroimaging Whole-exome sequencing and imaging genetics identify functional variants for rate of change in hippocampal volume in mild cognitive impairment. Mol Psychiatry. 2013;18(7):781–787. doi: 10.1038/mp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning YL, Yang N, Chen X, Xiong RP, Zhang XZ, Li P, Zhao Y, Chen XY, Liu P, Peng Y, Wang ZG, Chen JF, Zhou YG. Adenosine A2A receptor deficiency alleviates blast-induced cognitive dysfunction. J Cereb Blood Flow Metab. 2013;33(11):1789–1798. doi: 10.1038/jcbfm.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall RW, Paszkowski-Rogacz M, Kempermann G. The mammalian adult neurogenesis gene ontology (MANGO) provides a structural framework for published information on genes regulating adult hippocampal neurogenesis. PLoS One. 2012;7(11):e48527. doi: 10.1371/journal.pone.0048527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plake C, Royer L, Winnenburg R, Hakenberg J, Schroeder M. GoGene: gene annotation in the fast lane. Nucleic Acids Res. 2009;37(Web Server issue):W300–304. doi: 10.1093/nar/gkp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Kim S, Holohan K, Shen L, Nho K, Risacher SL, Foroud TM, Mukherjee S, Crane PK, Aisen PS, Petersen RC, Weiner MW, Saykin AJ, I. Alzheimer's Disease Neuroimaging Genome-wide pathway analysis of memory impairment in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging Behav. 2012;6(4):634–648. doi: 10.1007/s11682-012-9196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Swaminathan S, Shen L, Foroud TM, Hakonarson H, Huentelman MJ, Aisen PS, Petersen RC, Green RC, Jack CR, Koeppe RA, Jagust WJ, Weiner MW, Saykin AJ, I. Alzheimer's Disease Neuroimaging APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study. Mol Psychiatry. 2014;19(3):351–357. doi: 10.1038/mp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Sachidhanandam S, Perrais D, Cunha RA, Mulle C. Short-term plasticity of kainate receptor-mediated EPSCs induced by NMDA receptors at hippocampal mossy fiber synapses. J Neurosci. 2007;27(15):3987–3993. doi: 10.1523/JNEUROSCI.5182-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Kim S, Nho K, Foroud T, Shen L, Petersen RC, Jack CR, Jr, Beckett LA, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ, I. Alzheimer's Disease Neuroimaging APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11(12):1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, Petersen RC, Jack CR, Jr, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ, d. Alzheimer's Disease Neuroimaging Initiative The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis K, Tsiamaki E, Kouvaros S, Papatheodoropoulos C, Angelatou F. Adenosine A(2)A receptors permit mGluR5-evoked tyrosine phosphorylation of NR2B (Tyr1472) in rat hippocampus: a possible key mechanism in NMDA receptor modulation. J Neurochem. 2015;135(4):714–726. doi: 10.1111/jnc.13291. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, Thompson PM, Stein JL, Moore JH, Farrer LA, Green RC, Bertram L, Jack CR, Jr, Weiner MW, I. Alzheimer's Disease Neuroimaging Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimers Dement. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Bernstein HL. Potential implications of a monosynaptic pathway from mossy cells to adult-born granule cells of the dentate gyrus. Front Syst Neurosci. 2015;9:112. doi: 10.3389/fnsys.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, I. Alzheimer's Disease Neuroimaging Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, Siemers E, Potter W, Lee VM, Trojanowski JQ, I. Alzheimer's Disease Neuroimaging Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121(5):597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Shen L, Risacher SL, Yoder KK, West JD, Kim S, Nho K, Foroud T, Inlow M, Potkin SG, Huentelman MJ, Craig DW, Jagust WJ, Koeppe RA, Mathis CA, Jack CR, Jr, Weiner MW, Saykin AJ, I. Alzheimer's Disease Neuroimaging Amyloid pathway-based candidate gene analysis of [(11)C]PiB-PET in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. Brain Imaging Behav. 2012;6(1):1–15. doi: 10.1007/s11682-011-9136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem. 2005;95(4):1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- Urban N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. doi: 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz SH, Lerias SR, Parreira S, Diogenes MJ, Sebastiao AM. Adenosine A2A receptor activation is determinant for BDNF actions upon GABA and glutamate release from rat hippocampal synaptosomes. Purinergic Signal. 2015;11(4):607–612. doi: 10.1007/s11302-015-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Shen L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ, I. Alzheimer's Disease Neuroimaging The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9(5):e111–194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7(4):a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Jin P. Unlocking epigenetic codes in neurogenesis. Genes Dev. 2014;28(12):1253–1271. doi: 10.1101/gad.241547.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26(11):3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]