Abstract
This work challenges the popular notion that pharmaceutical salts are more soluble than cocrystals. There are cocrystals that are more soluble than salt forms of a drug and vice-versa. It all depends on the interplay between the chemistry of both the solid and solution phases. Aqueous solubility, pHmax, and supersaturation index (SA = SCC/SD or Ssalt/SD) of cocrystals and salts of a basic drug, Lamotrigine (LTG), were determined, and mathematical models that predict the influence of cocrystal/salt Ksp and Ka were derived. Ksp and SA followed the order LTG-Nicotinamide cocrystal (18) > LTG-HCl salt (12) > LTG-Saccharin salt (5) > LTG-Methylparaben cocrystal (1) > LTG-Phenobarbital cocrystal (0.2). The values in parenthesis represent SA under non-ionizing conditions. Cocrystal/salt solubility and thermodynamic stability are determined by pH and will drastically change with a single unit change in pH. pHmax values ranged from 5.0 (Saccharin salt) to 6.4 (Methylparaben cocrystal) to 9.0 (Phenobarbital cocrystal). Cocrystal/salt pHmax dependence on pKsp and pKa shows that cocrystals and salts exhibit different behavior. Solubility and pHmax are as important as supersaturation index in assessing the stability and risks associated with conversions of supersaturating forms.
Introduction
Cocrystals constitute an important class of pharmaceutical materials that have the ability to enhance and fine-tune solubility. This property enables cocrystals to solve absorption and bioavailability problems of poorly water-soluble drugs. Cocrystals are thus receiving significant attention and numerous cocrystals have been reported.1–8 In spite of their promise to solve solubility problems, there is a wide gap between the principles that explain cocrystal solution behavior and their application to cocrystal characterization and development.
Unlike other supersaturating delivery systems, cocrystal stoichiometric nature predisposes them to huge and predictable changes in solubility and thermodynamic stability as solution conditions change (pH, complexation, solubilization, among others). Cocrystals are therefore characterized not only by their solubility but also by the supersaturation that they generate. For cocrystals whose constituents may ionize in solution, understanding the influence of pH on cocrystal solubility and supersaturation is essential to correctly evaluate their stability and potential for conversion to less soluble forms. The purpose of the work presented here is to develop mathematical relationships and determine thermodynamic parameters that explain the stability and solubility-pH dependence of cocrystals and salts of weakly basic drugs. We also wish to address the commonly held notion that salts are more soluble than cocrystals. To this end, we have studied the solubility behavior of salts and cocrystals of lamotrigine (LTG), a weakly basic drug of equimolar composition. The LTG cocrystal and salt constituents studied are summarized in Table 1.
Table 1.
pKa values of cocrystal and salt constituents.
Materials and Methods
Materials
Cocrystal and Salt Constituents
LTG was purchased from Jai Radhe Sales, India with a purity of 99.6% w/w and was used as received. Nicotinamide (NCT) and saccharin (SAC) were purchased from Sigma-Aldrich and used as received. X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC) analyses were performed on all materials to confirm phase purity prior to use. Lamotrigine monohydrate (LTG·H2O) was prepared by suspending anhydrous LTG for 72 hours in water at 25±0.1°C. The slurry was vacuum filtered and the solid phase was confirmed by XRPD and DSC.
Solvents and Buffer Components
HPLC grade methanol was purchased from Fisher Scientific (Pittsburgh, PA). Trifluoroacetic acid spectrophometric grade 99% was purchased from Aldrich Company (Milwaukee, WI). All water used in this study was filtered through a double deionized purification system (Milli Q Plus Water System) from Millipore Company (Bedford, MA).
pH 2.0 phosphate buffer was prepared using sodium phosphate dibasic heptahydrate from Acros Organics, potassium dihydrogen phosphate from Sigma-Aldrich, and phosphoric acid from Acros Organics. pH 4.5 acetate buffer was prepared using acetic acid purchased from Sigma-Aldrich and sodium acetate purchased from Sigma Chemical Company. pH 6.8 phosphate buffer was prepared using monobasic potassium phosphate purchased from Sigma- Aldrich and sodium hydroxide pellets (NaOH) from J.T. Baker (Philipsburg, NJ).
Methods
Buffer Preparation
All buffers were prepared according to the pharmacopeial protocol.14,15 pH 2.0 phosphate buffer was prepared by dissolving 1.41 g sodium phosphate dibasic heptahydrate and 0.85 g potassium dihydrogen phosphate in 250 mL of water. pH was adjusted to 2.0 using phosphoric acid (1.8 mL total). pH 4.5 acetate buffer was prepared by dissolving 147.5 mg sodium acetate and 3.5 mL of 2 M acetic acid in 246.5 mL of water. 2 M acetic acid was prepared adding 28.8 mL of acetic acid to 250 mL of water. pH 6.8 phosphate buffer was prepared by adding 22.4 mL of 0.2 M NaOH solution to 50 mL of 0.2 M monobasic potassium phosphate solution. 0.2 M NaOH solution was prepared by dissolving 0.8 g NaOH pellets in 100 mL of water. 0.2 M monobasic potassium phosphate solution was prepared by dissolving 2.7 g monobasic potassium phosphate in 100 mL of water. All buffers were prepared by stirring at room temperature.
Cocrystal and Salt Synthesis
LTG-NCT·H2O was synthesized by reaction crystallization method (RCM)16. An aqueous solution of 2% w/w SLS and 3.5 M NCT was prepared, to which anhydrous LTG was added and stirred for 72 hours at ambient temperature. Solid phases were isolated, dried, and analyzed by XRPD, DSC, and HPLC to confirm crystal form and phase purity. LTG-SAC salt was synthesized by adding stoichiometric ratio of anhydrous LTG to a SAC solution. The solid phase was filtered after approximately 24 hours and confirmed to be LTG-SAC salt by XRPD and DSC.
Drug Solubility Measurement
Intrinsic LTG·H2O solubility (S0,LTG·H2O) was measured in water by adding excess solid to solution. Solutions were magnetically stirred and maintained at 25.0 ± 0.1°C using a water bath for 72 hours. At 24 hour intervals, 0.50 mL aliquots were collected and filtered through a 0.45 µm pore membrane. After dilution with mobile phase, solution concentrations were analyzed by HPLC. LTG·H2O was confirmed as the equilibrium solid phase by XRPD and DSC.
Cocrystal and Salt Solubility Measurements
Cocrystal equilibrium solubilities were measured in pH 2.0 phosphate buffer, pH 4.5 acetate buffer, pH 6.8 phosphate buffer, and water at the eutectic point, where LTG·H2O and LTG-NCT·H2O were in equilibrium with solution. The eutectic point between LTG-NCT·H2O and LTG·H2O was approached by equilibrating both solid phases in aqueous media. LTG-SAC solubilities were measured by suspending salt in aqueous media, adjusting pH with pH 2 phosphate buffer or 0.1 M NaOH and allowing suspensions to equilibrate. Suspensions were stirred for 72 hours under constant temperature of 25.0 ± 0.1°C using a water bath. At 24 hour intervals, 0.50 mL aliquots were collected and filtered through a 0.45 µm pore membrane. Solid phases were analyzed by XRPD and DSC to characterize the solid phases at equilibrium. The equilibrium pH of the solution media was measured prior to dilution with mobile phase for concentration measurement of LTG cocrystal and salt constituents by HPLC. Solutions were considered to have reached equilibrium with solid phases when less than 5% change in concentration was detected in either component of the cocrystal or salt.
Stoichiometric solubility of the 1:1 cocrystal was calculated according to
| (1) |
from measured drug and coformer concentrations at the eutectic point ([LTG]T,eu and [NCT]T,eu).17–19
Stoichiometric solubility of the 1:1 salt was calculated by a similar expression according to
| (2) |
Ksp for LTG-NCT·H2O and LTG-SAC was calculated from solubility determinations from the above equations, as described in the results section.
X-ray Powder Diffraction (XRPD)
XRPD patterns of solid phases were recorded with a Rigaku MiniFlex X-ray diffractometer (Danvers, MA) using Cu Ka radiation (λ = 1.5418 Å), a tube voltage of 30 kV, and a tube current of 15 mA. The intensities were measured at 2θ values from 2° to 35° with a continuous scan rate of 2.5°/min.
Thermal Analysis
Crystalline samples of 2–4 mg were analyzed by DSC using a TA instrument (Newark, DE) 2910 MDSC system equipped with a refrigerated cooling unit. DSC experiments were performed by heating the samples at a rate of 10°C/min under a dry nitrogen atmosphere. Temperature and enthalpy calibration of the instruments was achieved by using a high purity indium standard. Standard aluminum sample pans were used for all measurements.
High Performance Liquid Chromatography (HPLC)
Solution concentrations of drug and coformer were analyzed by Waters HPLC (Milford, MA) equipped with a UV/vis spectrometer detector. A reversed phase C18 Atlantis column (5 µm, 4.5 × 250 mm) at ambient temperature was used to separate the drug and the coformer or counterion. An isocratic method of 55% methanol, 45% water, and 0.1% trifluoroacetic acid mobile phase was used with a flow rate of 1 mL/min. Sample injection volume was 20µL. Absorbance of the drug and coformer or counterion analytes was monitored between 210–300nm. The peak areas were integrated using Empower™ software.
Results and Discussion
Solubility-pH dependence and supersaturation index
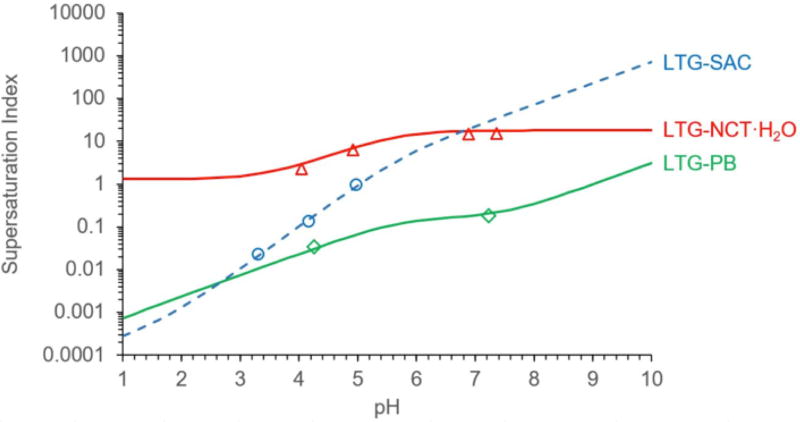
Figure 1 shows the solubility-pH dependence of the weakly basic drug LTG, a salt with a moderately strong acid, SAC, and two cocrystals, with a weakly basic coformer, NCT, or with a weakly acidic drug, phenobarbital (PB). We recently published the solubility-pH dependence of LTG-PB cocrystal and include it here for comparison.1 These results demonstrate that pH has a huge influence on solubility and that both cocrystal and salt can exhibit a pHmax where their solubility curves intersect with the drug solubility curve. Furthermore, the cocrystal and salt solubility behaviors differ as a result of the ionization properties of their constituents.
Figure 1.
Solubility values for cocrystals and salt were determined under equilibrium conditions and calculated according to equations 1 and 2. Some of the solubility values in Figure 1 represent supersaturated conditions with respect to drug free base and are useful as a measure of the supersaturation index (SA=SCC/SD) that a cocrystal or a salt may generate, as presented in Figure 2.
Figure 2.
SA is the driving force for conversions from cocrystal to the less soluble drug. The change in Gibbs free energy for this conversion is
| (3) |
where S is solubility and subscripts CC and D refer to cocrystal and drug phases. In this equation SCC is expressed in terms of moles of drug. A phase conversion from cocrystal to drug is favorable when ΔG is negative, or when SCC>SD, SA>1. A similar analysis applies to salts and SCC can be substituted for Ssalt in equation 3. When SCC=SD, SA=1, and ΔG = 0, the system is at equilibrium and the pH corresponding to this equilibria is pHmax.
Supersaturation can be sustained for some time until nucleation occurs, which is the reason for the importance of supersaturating drug delivery systems. The higher supersaturation is, the more negative is the free energy for conversion, the shorter is the time for nucleation to occur, and the higher is the nucleation rate. This means, that cocrystals with higher SA values may experience lower supersaturations and shorter induction times before conversion. In the context of the work presented here, SA is used to quantify the propensity of cocrystal conversions as their SA changes with pH.
The utility of SA vs pH plot (Figure 2) in assessing the risk of cocrystal and salt conversions to drug is shown by considering reported dissolution results for LTG-NCT·H2O cocrystal and LTG-SAC salt.3,20 The cocrystal was reported to transform to the drug whereas the salt did not transform during dissolution in aqueous unbuffered media at 25°C. The dissolution media pH was reported to be 6.6 for the cocrystal and 5 for the salt. Considering the SA for cocrystal (18 at pH 6.6) and salt (1 at pH 5), one would expect the salt to be stable (as the dissolution pH is at pHmax) whereas the cocrystal will have a risk of conversion. In fact, the cocrystal was reported to transform to the less soluble drug as an SA of 18 was not sustainable and the salt did not transform and was reported to be stable.
A common mistake in cocrystal solubility and stability studies is that solution conditions are not considered. Even when initial pH conditions are known, in aqueous or buffered solutions, the pH during cocrystal dissolution can change considerably, causing huge errors in data interpretation.
A word of caution about kinetically obtained Cmax values during cocrystal/salt dissolution studies is in order. Cmax is a result of two opposing kinetic processes, cocrystal/salt dissolution and drug precipitation, and is not proportional to solubility. Cmax is a kinetic parameter and its values are variable due to its kinetic nature and to the differences in changing experimental conditions. Integrating dissolution and thermodynamic data provides a useful conceptual context to assess the risks associated with changing factors and conditions.
Relationships between solubility, pKa, and pH
LTG-NCT·H2O is composed of two weakly basic components, which means the change in slope of the solubility-pH profile (Figure 1) is determined by the ionization behavior of both the drug (pKa = 5.7) and the coformer (pKa = 3.4). Cocrystal solubility remains constant above the drug pKa as both components are predominantly nonionized. However, as the components become increasingly ionized, cocrystal solubility increases quite substantially as pH approaches the drug pKa and even more so as pH approaches the coformer pKa.
A multidrug cocrystal of basic LTG and acidic PB (pKa,1 = 7.5, pKa,2 = 11.8), exhibits a U-shaped solubility curve due the different ionization ranges of LTG and PB. Cocrystal solubility increases with LTG ionization and PB ionization. A pH of minimum solubility occurs between the pKa of the basic and acidic constituents. The cocrystal and drug solubility curves intersect at a pHmax, which for this system is pH 9.
LTG-SAC salt with the acidic counterion SAC (pKa = 1.6) also exhibits a U-shaped curve, including a plateau region between pH values of 2.5 and 5.0. Salt solubility increases below pH 2.5 and above pH 5.0 as ionization of the basic and acidic constituents change. A pHmax is observed at pH 5.
Solubilities of these four forms of LTG as a function of pH were predicted according to the following equations using pKa values shown in Table 1. The drug solubility (SLTG·H2O) was predicted from
| (4) |
where S0,LTG·H2O represents the solubility of LTG monohydrate under nonionizing conditions, also referred to as intrinsic drug solubility. S0,LTG·H2O was evaluated to be 6.6 × 10−4 M by fitting the above equation to the data.
For LTG-NCT·H2O cocrystal the solubility expression is given by
| (5) |
where Ksp is the cocrystal solubility product. This equation was derived by considering the mass balance of all cocrystal species in solution and expressing them in terms of the equilibrium constants that correspond to cocrystal dissociation (Ksp) and ionization (Ka)
Cocrystal Ksp was evaluated from Equation 5 and cocrystal solubility (SCC) values obtained from equation 1 by measurement of cocrystal component concentrations in equilibrium with cocrystal and drug phases at specific pH equilibrium values and pKa values in Table 1.
The salt solubility-pH relationship is
| (6) |
This well recognized equation is analogous to that of the cocrystal (Equation 5) as it considers dissociation and ionization equilibrium reactions.21–23 In this case, however, the salt Ksp is the product of activities of ionized constituents, whereas for a cocrystal, it is the non-ionized constituents. As a result of the different ionization states in the dissociation and ionization reactions, the ionization terms in the solubility equations for cocrystal and salt also have different signs.
Fig. 1 shows excellent agreement between predicted and experimental solubility behavior. LTG-NCT cocrystal is more soluble than drug at pH > 3 and reaches solubilities 18 times the drug solubility at pH ≥ 6. The cocrystal is also more soluble than the SAC salt at pH < 7, and is orders of magnitude more soluble than the salt at pH < 3. The SAC salt has a pHmax around pH 5, below which it is less soluble than the drug. Experimental measurement of salt solubility above pHmax was not possible as the equilibrium pH decreased to pH 5 due to salt conversion to the less soluble drug at initial pH values of about 6.
The above results are compared with those recently reported for LTG-PB cocrystal.1 The cocrystal solubility dependence on pH is given by
| (7) |
The cocrystal solubility curve generated by this equation24 (Figure 1) predicts a pHmax of 9 and a solubility minimum between the pKa of LTG and the first pKa of PB.19,24,25 Another pHmax with PB occurs at pH 2.6 (not shown in this plot). The PB cocrystal is the least soluble of the four forms considered between pH 3 and 9. PB is also the least soluble constituent among the cocrystals. In fact, its intrinsic solubility is only about 10 times higher than the drug. The ratio of coformer to drug solubilites (SCF/SD) ≤ 10 has been generally observed to result in cocrystals that are less soluble than drug.17,26 Solubility is however determined by pH and these trends will change with the ionization of cocrystal and salt constituents as demonstrated in Figure 1.
Solubility product, Ksp
Whereas solubility is a conditional constant and dependent on pH, the solubility product, Ksp, is not. Ksp is the constant associated with the equilibrium between cocrystal or salt and a solution phase. It involves dissociation of cocrystal and salt into its constituents according to
| (8) |
for a cocrystal, and
| (9) |
for a salt. A and B represent cocrystal constituents (or ionized constituents A− and BH+ for a salt), y and z are the stoichiometric coefficients. The forward reaction is dissociation and represents dissolution, while the reverse reaction is association and represents precipitation. Ksp for a cocrystal is
| (10) |
and for a salt
| (11) |
where a represents activities, and subscripts represent cocrystal or salt constituents. Since the activity of the solid phase is assumed to be constant and equal to 1, Ksp is an activity product. Activities are approximated by concentrations under the assumption of ideality, and Ksp becomes
| (12) |
for a cocrystal, and
| (13) |
for a salt.
Ksp values for several salts and cocrystals of LTG are presented in Table 2. The Ksp expression for LTG-NCT cocrystal is
| (14) |
For the saccharin salt, Ksp is
| (15) |
Similar equations with the corresponding coformers or counterions were used to evaluate Ksp of other LTG forms as they were all of equimolar stoichiometry.
Table 2.
Solubility product (Ksp), intrinsic solubility (S0,CC), and solubility advantage ( ) of LTG cocrystals and salts.
| Solid Phase | Ksp (M2) | S0,CC or S0,Salt (M) | SA |
|---|---|---|---|
| LTG-NCT Cocrystal | (1.4±0.4) ×10−4 | 1.2 × 10−2 | 18 |
| LTG-HCl Salt | (6.7±0.53) × 10−5 a | 8.2 × 10−3 | 12 |
| LTG-SAC Salt | (1.1±0.2) × 10−5 | 3.3 × 10−3 | 5 |
| LTG-MP Cocrystal | (5.3±0.47) × 10−7 b | 7.3 × 10−4 | 1.1 |
| LTG-PB Cocrystal | (1.2±0.5) × 10−8 | 1.1 × 10−4 | 0.2 |
Ksp of LTG-HCl was calculated from reported solubility of 0.46 mg/mL at 37°C (pH 1.2) from reference27. Ksp of the salt was estimated at 25°C from [LTGH+] and [Cl−] concentrations calculated from the dissolved salt and HCl concentrations, using a heat of solution value of 30 kJ/mol.
Steady state concentration from dissolution data in reference (3) was used to calculate the Ksp of lamotrigine-methylparaben (LTG-MP) cocrystal, where cocrystal was reported to be stable.
Results in Table 2 show that NCT cocrystal Ksp is higher than that of SAC and HCl salts. The PB cocrystal has the lowest Ksp among these forms. These findings challenge the popular notion that salts are more soluble than cocrystals. In fact, NCT cocrystal intrinsic solubility is 18 times higher than drug and even higher than the HCl and SAC salts.
It is important to note that Ksp is the product of only the cocrystal or salt constituents in the non-ionized or ionized state, respectively. Ksp ≠ [A]T [B]T when there are molecular species in solution different from those in the corresponding solid phase. The reader should be cautious of incorrect Ksp values calculated from total analytical concentrations of cocrystal or salt constituents that do not correspond to the Ksp definition according to the equilibrium in Equations 12 and 13.
Ksp values for cocrystals of several drugs in aqueous media are presented in Table 3 in terms of pKsp. The low values of Ksp make use of pKsp = −log Ksp more reasonable. Higher pKsp values refer to lower Ksp values. The range of values is similar to those reported for pharmaceutical salts.28–30 pKsp values for CBZ cocrystals are in the range of 2 to 9, indicating increases in Ksp by orders of magnitude. When solubility is determined by solvation and not by solid-state lattice energy,17,31 cocrystal solubility is dependent on the solubility of its components. Nicotinamide and glutaric acid are among the most soluble coformers and generally correspond to cocrystals with higher Ksp values for a given drug. The highest 1:1 cocrystal Ksp corresponds to theophylline-nicotinamide with a pKsp of 0.7, among the most soluble combination of cocrystal constituents.
Table 3.
Cocrystal and Salt pKsp
| Cocrystal (drug-coformer) or Salt (drug-counterion) |
Solid Form | Stoichiometry (drug:coformer or drug:counterion) |
pKsp |
|---|---|---|---|
| Caffeine-Salicylic acid | Cocrystal | 1:1 | 3.117 |
| Carbamazepine-Nicotinamide | Cocrystal | 1:1 | 2.317 |
| Carbamazepine-Glutaric acid | Cocrystal | 1:1 | 2.517 |
| Carbamazepine-Salicylic acid | Cocrystal | 1:1 | 5.924 |
| Carbamazepine-Saccharin | Cocrystal | 1:1 | 6.032 |
| Carbamazepine-Malonic acida | Cocrystal | 2:1b | 6.117 |
| Carbamazepine-Oxalic acid | Cocrystal | 2:1b | 8.017 |
| Carbamazepine-Succinic acid | Cocrystal | 2:1 | 8.233 |
| Carbamazepine-4-aminobenzoic acid hydrate | Cocrystal | 2:1 | 8.924 |
| Danazol-Hydroxybenzoic acid | Cocrystal | 1:1 | 8.034 |
| Danazol-Vanillin | Cocrystal | 1:1 | 8.534 |
| Gabapentin Lactam-4-aminobenzoic acid | Cocrystal | 1:1 | 3.119 |
| Gabapentin Lactam-Fumaric acid | Cocrystal | 2:1 | 3.419 |
| Gabapentin Lactam-Benzoic acid | Cocrystal | 1:1 | 3.519 |
| Gabapentin Lactam-4-hydroxybenzoic acid | Cocrystal | 1:1 | 3.719 |
| Gabapentin Lactam-Gentisic acid | Cocrystal | 1:1 | 3.919 |
| Indomethacin-Saccharin | Cocrystal | 1:1 | 8.932 |
| Ketoconazole-Oxalic acid | Salt | 1:1 | 5.9c |
| Ketoconazole-Adipic acid | Cocrystal | 1:1 | 7.535 |
| Ketoconazole-Succinic acid | Cocrystal | 1:1 | 7.635 |
| Ketoconazole-Fumaric acid | Cocrystal | 1:1 | 8.835 |
| Lamotrigine-Nicotinamide | Cocrystal | 1:1 | 3.9 |
| Lamotrigine-Hydrochloride | Salt | 1:1 | 4.2d |
| Lamotrigine-Saccharin | Salt | 1:1 | 5.0 |
| Lamotrigine-Methylparaben | Cocrystal | 1:1 | 6.3e |
| Lamotrigine-Phenobarbital | Cocrystal | 1:1 | 7.91 |
| Nevirapine-Maleic acid | Cocrystal | 1:1 | 4.725 |
| Nevirapine-Saccharin | Cocrystal | 2:1 | 10.025 |
| Nevirapine-Salicylic acid | Cocrystal | 2:1 | 10.425 |
| Pterostilbene-Caffeine | Cocrystal | 1:1 | 5.336 |
| Pterostilbene-Piperazine | Cocrystal | 2:1 | 6.337 |
| Piroxicam-Saccharin | Cocrystal | 1:1 | 7.134 |
| Theophylline-Nicotinamide | Cocrystal | 1:1 | 0.717 |
| Theophylline-Salicylic acid | Cocrystal | 1:1 | 3.817 |
Form B (hydrated cocrystal).38
Disordered crystal structure that does not provide definitive stoichiometry.26
Calculated from reported solubility of 0.90 mg/mL at 25°C and pH 3.4.39
Calculation described in Table 2 footnote a.
Calculated from steady state concentration during reported cocrystal dissolution as reported in reference (3).
Comparing the pKsp values of salts and cocrystals of the same drug (Table 3) shows that some cocrystals are more soluble than salts, or less soluble depending on the coformers and counterions. Cocrystals with higher ratio of the less soluble drugs also have higher pKsp values, as observed for 2:1 vs 1:1 cocrystals.
pHmax of cocrystals and salts
Knowledge of pHmax is important to determine phase stability regions. In this article, we are concerned with the LTG and cocrystal or salt pHmax, where LTG is the least soluble constituent under the conditions studied.
The pHmax of cocrystals is expected to vary with coformers over a wider pH range than that of salts due to the range of ionization properties of cocrystal constituents. Cocrystals can be composed of all possible combinations of acidic, basic, and nonionized constituents, unlike salts. For instance, binary cocrystals can be composed of two basic, two acidic, one basic and one acidic, or one or both nonionizable constituents.
We derived equations that predict cocrystal/salt pHmax values from Ka, Ksp, and S0,D. Since at the pHmax the solution is doubly saturated with two solid phases, cocrystal or salt and drug, pHmax equations were derived by setting the cocrystal solubility equal to drug solubility and solving for pHmax. This approach is similar to that described for salts.40–42
pHmax values for cocrystals and salts with the ionization properties of the LTG solid forms studied here are shown in Table 4. These were calculated from the below relationships. Equations are presented in terms of [H+]max and K as they are simpler than in terms of pH or pK.
Table 4.
pHmaxa for LTG cocrystals and salts
| Solid Phase | pHmax | SpHmax (M) |
|---|---|---|
| LTG-NCT·H2O Cocrystal | Noneb | − |
| LTG-MP Cocrystal | 6.4 | 8.0 × 10−4 |
| LTG-PB Cocrystal | 9.0 | 6.5 × 10−4 |
| LTG-SAC Salt | 5.0 | 3.7 × 10−3 |
For cocrystals with a basic drug and a basic coformer, such as LTG-NCT·H2O, [H+]max is
| (16) |
where S0,D is the drug intrinsic solubility and Ksp is the cocrystal or salt solubility product.
For cocrystals with a basic drug and a nonionic coformer, such as LTG-MP, [H+]max is
| (17) |
For cocrystals with a basic drug and an acidic coformer, such as LTG-PB [H+]max is
| (18) |
Equation 18 is for a monoprotic acid. While PB is a diprotic acid, the second pKa is 11.8 and the assumption of only using the lower pKa is justified at pH values below 10.
For salts with a basic drug and an acidic counterion, such as LTG-SAC, [H+]max is
| (19) |
Results in Table 4 show the range in pHmax and solubility at pHmax, SpHmax, for the cocrystals and salts studied. Of forms that exhibit a pHmax, the salt has the lowest pHmax and highest SpHmax. This is consistent with SAC having the lowest pKa and higher solubility than PB and MP. The NCT cocrystal approaches the drug solubility as pH approaches the NCT pKa (3.4) at a solubility of about 0.6 M, although a pHmax is not achieved (Figure 1).
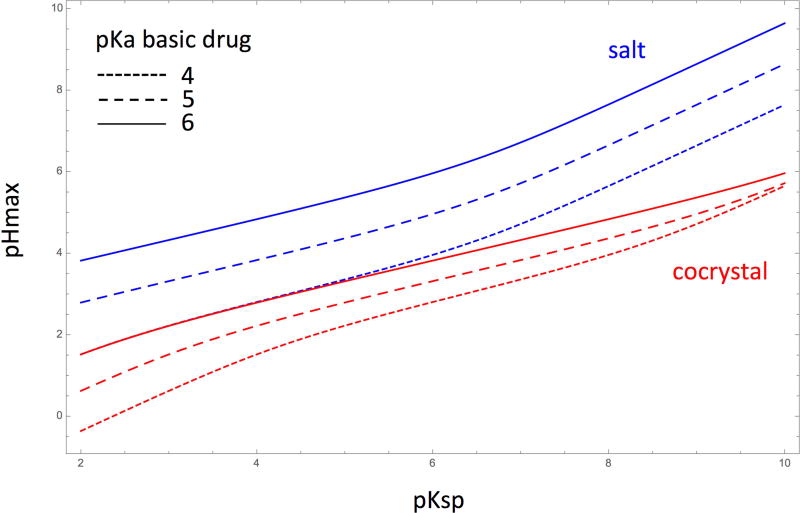
How pHmax changes with pKsp for basic drugs and acidic coformers/counterions is shown in Figures 3 and 4 by using the above equations. The pKa values for the simulations were selected for the purpose of understanding the influence of pKsp and pKa on cocrystal/salt pHmax and SpHmax, even though it is generally accepted that salts may form when ΔpKa (ΔpKa = pKa(base) - pKa(acid)) is greater than 2 or 3.43–45
Figure 3.
Figure 4.
Figure 3 illustrates the influence of coformer/counterion pKa on cocrystal/salt pHmax dependence on pKsp. Both cocrystal and salt pHmax values increase with pKsp. Cocrystals have lower pHmax values than salts. Cocrystal pHmax values also show higher sensitivity to changes in counterion pKa values.
Figure 4 shows how the pHmax dependence on pKsp varies with pKa of a basic drug when the acidic coformer/counterion pKa = 2.0. At the same drug pKa, salts have higher pHmax values. Salt pHmax changes by about one unit when drug pKa is varied by one unit, and this trend appears to be almost constant throughout the studied pKsp range. However, changes in cocrystal pHmax are smaller than those for salts, and they are not constant with changes in drug pKa for all pKsp values. While one unit change in drug pKa results in about one unit change in pHmax at pKsp 2, pHmax varies by 0.5 units at pKsp 6 and by less than 0.2 units at pKsp 10.
These findings are consistent with the fact that salt pHmax values typically occur in the salt solubility plateau region where the counterion is almost completely ionized.21,41,46,47 Changes in counterion pKa (Figure 3) have a small effect on pHmax dependence on pKsp when ΔpKa is large enough for the counterion to be fully ionized at pHmax. However, changes in drug pKa will result in greater variability in pHmax vs pKsp dependence as the solubility-pH dependencies of both the drug and salt shift. Unlike salts, the pHmax of cocrystals of basic drugs and acidic coformers can occur on either side of the solubility minimum depending on pKsp and S0,D. pHmax occurs above minimum solubility for LTG-PB (Figure 1),1 but has been shown to occur below minimum solubility for some ketoconazole and nevirapine cocrystals.25,31 For cocrystals with the same S0,D, high pKsp values show greater pHmax variability with changes in coformer pKa (Figure 3), but low pKsp show greater pHmax variability with changes in drug pKa (Figure 4).
The analysis presented shows that cocrystals and salts exhibit different dependencies of pHmax on pKsp. These cocrystal/salt properties are critical for selection, stability, and solubility assessments.
Conclusions
This work shows that cocrystals can be more soluble than salts or vice-versa. The stoichiometric nature of cocrystals and salts predisposes them to huge, yet predictable changes in solubility, supersaturation index, and thermodynamic stability as solution pH changes. Supersaturation index is as important as solubility and pHmax in anticipating the potential for phase conversions. Cocrystal and salt solubility/supersaturation dependence on pH as well as pHmax, can be predicted from knowledge of Ksp and pKa.
Acknowledgments
We gratefully acknowledge the partial support from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number R01GM107146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaur R, Cavanagh KL, Rodríguez-Hornedo N, Matzger AJ. Multidrug Cocrystal of Anticonvulsants: Influence of Strong Intermolecular Interactions on Physiochemical Properties. Cryst Growth Des. 2017;17(10):5012–5016. doi: 10.1021/acs.cgd.7b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caira MR, Bourne SA, Samsodien H, Engel E, Liebenberg W, Stieger N, Aucamp M. Cocrystals of the antiretroviral nevirapine: crystal structures, thermal analysis and dissolution behaviour. Crystengcomm. 2012;14(7):2541–2551. [Google Scholar]
- 3.Cheney ML, Shan N, Healey ER, Hanna M, Wojtas L, Zaworotko MJ, Sava V, Song SJ, Sanchez-Ramos JR. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst Growth Des. 2010;10(1):394–405. [Google Scholar]
- 4.Childs SL, Hardcastle KI. Cocrystals of piroxicam with carboxylic acids. Cryst Growth Des. 2007;7(7):1291–1304. [Google Scholar]
- 5.Trask AV, Motherwell WDS, Jones W. Physical stability enhancement of theophylline via cocrystallization. International journal of pharmaceutics. 2006;320(1–2):114–123. doi: 10.1016/j.ijpharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 6.McMahon JA, Bis JA, Vishweshwar P, Shattock TR, McLaughlin OL, Zaworotko MJ. Crystal engineering of the composition of pharmaceutical phases. 3. Primary amide supramolecular heterosynthons and their role in the design of pharmaceutical co-crystals. Z Kristallogr. 2005;220(4):340–350. [Google Scholar]
- 7.Childs SL, Chyall LJ, Dunlap JT, Smolenskaya VN, Stahly BC, Stahly GP. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. Molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J Am Chem Soc. 2004;126(41):13335–13342. doi: 10.1021/ja048114o. [DOI] [PubMed] [Google Scholar]
- 8.Fleischman SG, Kuduva SS, McMahon JA, Moulton B, Walsh RDB, Rodríguez-Hornedo N, Zaworotko MJ. Crystal engineering of the composition of pharmaceutical phases: Multiple-component crystalline solids involving carbamazepine. Cryst Growth Des. 2003;3(6):909–919. [Google Scholar]
- 9.O'Neil MJ, Smith A, Heckelman PE. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck Research Laboratories; 2001. [Google Scholar]
- 10.GlaxoSmithKline, editor. Lamotrigine [package insert] Research Triangle Park, NC: 2009. [Google Scholar]
- 11.Bell RP, Higginson WCE. The Catalyzed Dehydration of Acetaldehyde Hydrate, and the Effect of Structure on the Velocity of Protolytic Reactions. Proceedings of the Royal Society of London Series A, Mathematical and Physical Sciences. 1949;197(1049):141–159. [Google Scholar]
- 12.Jellinek HHG, Wayne MG. Nicotinamide - Ultraviolet Absorption Spectra and Dissociation Constants. J Phys Colloid Chem. 1951;55(2):173–180. doi: 10.1021/j150485a002. [DOI] [PubMed] [Google Scholar]
- 13.Geiser L, Henchoz Y, Galland A, Carrupt PA, Veuthey JL. Determination of pK(a) values by capillary zone electrophoresis with a dynamic coating procedure. J Sep Sci. 2005;28(17):2374–2380. doi: 10.1002/jssc.200500213. [DOI] [PubMed] [Google Scholar]
- 14.European Pharmacopoeia (EP), editor. Strasbourg: Council of Europe. 2005. [Google Scholar]
- 15.United States Pharmacopeia National Formulary (USP40-NF35), editor. Rockville, Maryland: The United States Pharmacopeial Convention, Inc; 2017. [Google Scholar]
- 16.Rodríguez-Hornedo N, Nehm SJ, Seefeldt KF, Pagán-Torres Y, Falkiewicz CJ. Reaction Crystallization of Pharmaceutical Molecular Complexes. Mol Pharmaceut. 2006;3(3):362–367. doi: 10.1021/mp050099m. [DOI] [PubMed] [Google Scholar]
- 17.Good DJ, Rodríguez-Hornedo N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst Growth Des. 2009;9(5):2252–2264. [Google Scholar]
- 18.Nehm SJ, Rodríguez-Spong B, Rodríguez-Hornedo N. Phase solubility diagrams of cocrystals are explained by solubility product and solution complexation. Cryst Growth Des. 2006;6(2):592–600. [Google Scholar]
- 19.Maheshwari C, Andre V, Reddy S, Roy L, Duarte T, Rodríguez-Hornedo N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: effect of coformer ionization, pH(max) and solute-solvent interactions. Crystengcomm. 2012;14(14):4801–4811. [Google Scholar]
- 20.Chadha R, Saini A, Arora P, Jain DS, Dasgupta A, Row TNG. Multicomponent solids of lamotrigine with some selected coformers and their characterization by thermoanalytical, spectroscopic and X-ray diffraction methods. Crystengcomm. 2011;13(20):6271–6284. [Google Scholar]
- 21.Stahl PH, Wermuth CG. Handbook of pharmaceutical salts : properties, selection, and use. 2. Zürich: VHCA ; Weinheim: Wiley-VCH; 2011. p. xvi.p. 446. [Google Scholar]
- 22.Serajuddin ATM, Rosoff M. pH-Solubility Profile of Papaverine Hydrochloride and Its Relationship to the Dissolution Rate of Sustained-Release Pellets. J Pharm Sci. 1984;73(9):1203–1208. doi: 10.1002/jps.2600730905. [DOI] [PubMed] [Google Scholar]
- 23.Yalkowsky SH. Solubility and solubilization in aqueous media. Washington, D.C. New York ; Oxford: American Chemical Society ; Oxford University Press; 1999. p. xvi.p. 464. [Google Scholar]
- 24.Bethune SJ, Huang N, Jayasankar A, Rodríguez-Hornedo N. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Cryst Growth Des. 2009;9(9):3976–3988. [Google Scholar]
- 25.Kuminek G, Rodríguez-Hornedo N, Siedler S, Rocha HV, Cuffini SL, Cardoso SG. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem Commun (Camb) 2016;52(34):5832–5835. doi: 10.1039/c6cc00898d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Childs SL, Wood PA, Rodríguez-Hornedo N, Reddy LS, Hardcastle KI. Analysis of 50 Crystal Structures Containing Carbamazepine Using the Materials Module of Mercury CSD. Cryst Growth Des. 2009;9(4):1869–1888. [Google Scholar]
- 27.Floyd AG, Jain S. Pharmaceutical composition containing lamotrigine, editor. US 5942510A: Google Patents 1997
- 28.Thakral NK, Behme RJ, Aburub A, Peterson JA, Woods TA, Diseroad BA, Suryanarayanan R, Stephenson GA. Salt Disproportionation in the Solid State: Role of Solubility and Counterion Volatility. Mol Pharmaceut. 2016;13(12):4141–4151. doi: 10.1021/acs.molpharmaceut.6b00745. [DOI] [PubMed] [Google Scholar]
- 29.Guo J, Elzinga PA, Hageman MJ, Herron JN. Rapid Throughput Screening of Apparent KSP values for Weakly Basic Drugs Using 96-Well Format. J Pharm Sci. 2008;97(6):2080–2090. doi: 10.1002/jps.21149. [DOI] [PubMed] [Google Scholar]
- 30.Serajuddin AT, Sheen PC, Augustine MA. Common ion effect on solubility and dissolution rate of the sodium salt of an organic acid. J Pharm Pharmacol. 1987;39(8):587–591. doi: 10.1111/j.2042-7158.1987.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuminek G, Cao F, Bahia de Oliveira da Rocha A, Goncalves Cardoso S, Rodríguez-Hornedo N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Advanced drug delivery reviews. 2016;101:143–166. doi: 10.1016/j.addr.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhalaweh A, Roy L, Rodríguez-Hornedo N, Velaga SP. pH-Dependent Solubility of Indomethacin-Saccharin and Carbamazepine-Saccharin Cocrystals in Aqueous Media. Mol Pharmaceut. 2012;9(9):2605–2612. doi: 10.1021/mp300189b. [DOI] [PubMed] [Google Scholar]
- 33.Huang N, Rodríguez-Hornedo N. Engineering cocrystal solubility, stability, and pH(max) by micellar solubilization. J Pharm Sci. 2011;100(12):5219–5234. doi: 10.1002/jps.22725. [DOI] [PubMed] [Google Scholar]
- 34.Lipert MP . University of Michigan. Library. In: Predicting the Influence of Drug Solubilizing Agents on Cocrystal Solubility, Stability, and Transition Points, editor. Deep Blue. 2015. [Google Scholar]
- 35.Chen Y, Rodríguez-Hornedo N. Cocrystals Mitigate Negative Effects of High pH on Solubility and Dissolution of a Basic Drug. Submitted to Cryst Growth Des. 2017 doi: 10.1021/acs.cgd.7b01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultheiss N, Bethune S, Henck JO. Nutraceutical cocrystals: utilizing pterostilbene as a cocrystal former. Crystengcomm. 2010;12(8):2436–2442. [Google Scholar]
- 37.Bethune SJ, Schultheiss N, Henck JO. Improving the Poor Aqueous Solubility of Nutraceutical Compound Pterostilbene through Cocrystal Formation. Cryst Growth Des. 2011;11(7):2817–2823. [Google Scholar]
- 38.Childs SL, Rodríguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, Shipplett R, Stahly BC. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Crystengcomm. 2008;10(7):856–864. [Google Scholar]
- 39.Martin FA, Pop MM, Borodi G, Filip X, Kacso I. Ketoconazole Salt and Co-crystals with Enhanced Aqueous Solubility. Cryst Growth Des. 2013;13(10):4295–4304. [Google Scholar]
- 40.Bogardus JB, Blackwood RK. Solubility of Doxycycline in Aqueous-Solution. J Pharm Sci. 1979;68(2):188–194. doi: 10.1002/jps.2600680218. [DOI] [PubMed] [Google Scholar]
- 41.Serajuddin ATM. Salt formation to improve drug solubility. Advanced drug delivery reviews. 2007;59(7):603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Avdeef A. Solubility of sparingly-soluble ionizable drugs. Advanced drug delivery reviews. 2007;59(7):568–590. doi: 10.1016/j.addr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Huang KS, Britton D, Etter MC, Byrn SR. A novel class of phenol-pyridine co-crystals for second harmonic generation. J Mater Chem. 1997;7(5):713–720. [Google Scholar]
- 44.Bhogala BR, Basavoju S, Nangia A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4 '-bipyridines and isonicotinamide. From binary to ternary cocrystals. Crystengcomm. 2005;7:551–562. [Google Scholar]
- 45.Childs SL, Stahly GP, Park A. The salt-cocrystal continuum: The influence of crystal structure on ionization state. Mol Pharmaceut. 2007;4(3):323–338. doi: 10.1021/mp0601345. [DOI] [PubMed] [Google Scholar]
- 46.Bhattachar SN, Deschenes LA, Wesley JA. Solubility: it's not just for physical chemists. Drug Discov Today. 2006;11(21–22):1012–1018. doi: 10.1016/j.drudis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Elder DP, Holm R, de Diego HL. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm. 2013;453(1):88–100. doi: 10.1016/j.ijpharm.2012.11.028. [DOI] [PubMed] [Google Scholar]