Abstract
Objective
To evaluate the effects of the population-based, person-centred and integrated care service ‘Embrace’ at twelve months on three domains comprising health, wellbeing and self-management among community-living older people.
Methods
Embrace supports older adults to age in place. A multidisciplinary team provides care and support, with intensity depending on the older adults’ risk profile. A randomised controlled trial was conducted in fifteen general practices in the Netherlands. Older adults (≥75 years) were included and stratified into three risk profiles: Robust, Frail and Complex care needs, and randomised to Embrace or care as usual (CAU). Outcomes were recorded in three domains. The EuroQol-5D-3L and visual analogue scale, INTERMED for the Elderly Self-Assessment, Groningen Frailty Indicator and Katz-15 were used for the domain ‘Health.’ The Groningen Well-being Indicator and two quality of life questions measured ‘Wellbeing.’ The Self-Management Ability Scale and Partners in Health scale for older adults (PIH-OA) were used for ‘Self-management.’ Primary and secondary outcome measurements differed per risk profile. Data were analysed with multilevel mixed-model techniques using intention-to-treat and complete case analyses, for the whole sample and per risk profile.
Results
1456 eligible older adults participated (49%) and were randomized to Embrace (n(T0) = 747, n(T1) = 570, mean age 80.6 years (SD 4.5), 54.2% female) and CAU (n(T0) = 709, n(T1) = 561, mean age 80.8 years (SD 4.7), 55.6% female). Embrace participants showed a greater–but clinically irrelevant–improvement in self-management (PIH-OA Knowledge subscale effect size [ES] = 0.14), and a greater–but clinically relevant–deterioration in health (ADL ES = 0.10; physical ADL ES = 0.13) compared to CAU. No differences in change in wellbeing were observed. This picture was also found in the risk profiles. Complete case analyses showed comparable results.
Conclusions
This study found no clear benefits to receiving person-centred and integrated care for twelve months for the domains of health, wellbeing and self-management in community-living older adults.
Introduction
Older adults prefer to remain living at home for as long as possible–‘to age in place’–and to participate in society [1–3]. However, this preference is compromised by age-related health problems [4,5], leading to an increasing level of dependency and service-use, a growing sense of loss of control and insecurity, and the threat of ultimate relocation to an institution [6–9]. A major challenge is how to support older people to age in place in the face of increasing decline associated with ageing [6,7]. The current healthcare systems are insufficiently able to address these challenges for many ageing individuals and need to be reorganised in such a way that they promote ageing in place [10].
A model of increasing importance and popularity in healthcare reform is the Chronic Care Model (CCM) [11–13]. The CCM addresses the needs of chronically ill patients by offering comprehensive, person-centred, proactive and preventive care and support. It encourages patients to be informed and activated, meaning that they should formulate personal goals and a plan to improve their health, and should have the motivation, information, skills, and confidence necessary to deal with the consequences of their diseases [14]. Two randomised controlled trials on the CCM targeted older adults, but both have limitations regarding the study populations included as only frail older adults with a limited health status were included and not those who were still healthy [15,16]. In order to provide care and support to the total community-living population of older adults, the CCM can be combined with a Population Health Management (PHM) model. PHM models assess an entire population in a community and not just those in need of urgent care. PHM-based care and support can be targeted to individual needs by classifying population subgroups into risk profiles [17].
Embrace is an integrated care service based on the complete CCM and a PHM model (Kaiser Permanente [KP] Triangle) [13] targeting all community-living older adults [18]. Embrace’s goal is to support older adults to age in place by providing person-centred, integrated, proactive and preventive care and support. Embrace classifies older adults into three risk profiles based on the complexity of their care needs [19] and their level of frailty [20,21]. Care and support are tailored to the risk profile and the needs of the older adults. A qualitative study of Embrace has already shown that older adults felt safe, secure and more in control due to Embrace [6].
In this study, we intend to evaluate the effects of the population-based, person-centred and integrated care service Embrace on patient-reported outcomes at 12 months on three domains comprising health, wellbeing and self-management among community-dwelling older people.
Methods
Study design and setting
Between October 2011 and March 2013, we conducted a randomised controlled trial (RCT) with stratification into three risk profiles based on the level of frailty and complexity of care needs and balanced allocation within general practitioner (GP) practices to the intervention (Embrace) or care as usual (CAU) groups. The RCT was performed in three semi-rural municipalities in the province of Groningen (in the northern Netherlands). Participants were followed for twelve months between January 2012 and March 2013. The Medical Ethical Committee of the University Medical Center of Groningen has assessed the study proposal and concluded that approval was not required (Reference METc2011.108). The study was performed in accordance with the tenets of the Declaration of Helsinki [22]. All participants gave informed consent. The study protocol has been published previously [18]. The trial was registered at the Netherlands National Trial Register NTR3039 (http://www.trialregister.nl; see S1 and S2 Files). The CONSORT statement was followed to report the findings and the checklist is available as supporting information (S3 File).
Study population and procedure
First, we invited all GPs working in the three municipalities to participate in the study. Recruitment stopped after fifteen GPs–proportionally distributed according to the size of the municipalities–agreed to participate as they had enough eligible participants to obtain the sample size needed. Next, all older adults aged 75 and over who were registered with one of the participating GPs and were living at home or in a home for the elderly, were invited to participate. Exclusion criteria at baseline were long-term admission to a nursing home (not just for rehabilitation), receiving an alternative type of integrated care and participating in another research study. Eligible participants received a letter from their GP with general information about Embrace and the study. After having provided informed consent, participants completed self-report questionnaires at baseline (T0: October-December 2011) and twelve months after starting (T1: January-March 2013), with support by a family member, friend or volunteer if needed. We sent reminders to non-respondents, followed by telephone calls to all persistent non-respondents. Respondents who submitted questionnaires with missing values were called by help desk assistants or visited by volunteers to complete the missing items.
Stratified randomisation and blinding
We first stratified participants into one of three risk profiles, using results of the baseline assessment of complexity of care needs (measured using the INTERMED for the Elderly Self-Assessment [INTERMED-E-SA]) [19] and the level of frailty (measured using the Groningen Frailty Indicator [GFI]) [20]. These risk profiles are ‘Complex care needs’ for participants with complex care needs and at risk for assignment to a hospital or nursing home (INTERMED-E-SA ≥16), ‘Frail’ for participants at risk of complex care needs (INTERMED-E-SA <16 and a GFI ≥5) and ‘Robust’ for participants at risk for the consequences of ageing (INTERMED-E-SA <16 and GFI <5).
After stratification, we performed an anonymised and computerised balanced randomisation process within each GP practice. Therefore, participants were equally distributed within each GP practice to Embrace and CAU, taking into account predetermined patient characteristics deemed capable of affecting intervention outcomes, for example age, gender, number of chronic conditions and living situation [23]. Elderly Care Team members did not know if someone was randomised to CAU or had declined participation, but knew who was randomised to Embrace. Participating older adults were informed in writing whether they were assigned to Embrace or CAU. Data collectors (volunteers available when necessary for helping filling in questionnaires, and help desk assistants) were blinded for randomisation and stratification, as were the data analysts (SS and RU) until the point of data analysis. For practical reasons, the data manager was not blinded.
Intervention: Embrace
Embrace (in Dutch: SamenOud [ageing together]) is a person-centred and integrated care service for community-living older adults. A multidisciplinary Elderly Care Team–consisting of the older adults’ GP, a nursing home physician [24] and two case managers (district nurse and social worker)–provides care and support to older adults. This care is in addition to care as usual. Before starting the intervention, team members followed an intensive training program (three days for the GPs and nursing home physicians, eight days for the case managers) that focused on working according to the Embrace principles and methods. Also, team members were coached during the intervention to support the cultural change in professionals’ deep-rooted working patterns [18].
The intensity, focus, and individual or group approach of the care and support depended on the participant’s risk profile. We invited all participants to follow a self-management support and prevention program focusing on staying healthy and independent for as long as possible. The program included regular Embrace community meetings, in which self-management abilities were encouraged and during which local healthcare and welfare organisations provided information on health maintenance, physical and social activities, and dietary recommendations. In addition, frail people and those with complex care needs received individual support from a case manager. They jointly developed an individual care and support plan targeting all health-related problems, which had to be agreed upon by the Elderly Care Team before implementation. The case managers monitored changes in the medical, psychosocial, or living situation, and navigated the plan’s delivery. The Elderly Care Team discussed and evaluated the participants’ health status and social situation in monthly meetings. If necessary, they took proactive steps in dialogue with participants to prevent deterioration. People with a ‘Robust’ profile were encouraged to contact the team in the event of changes in their health or living situation. Details of the implementation of Embrace have been published in the study protocol [18].
Care as usual
The control group received care as usual as provided by their GPs and local health and community organisations. Municipalities are in charge of social care, disease prevention and health promotion. Once a health problem is found, patients enter the health care system–in most cases with a visit to their GP. In the Netherlands, GPs are family physicians who usually have a long-term relationship with their patients. They act as gatekeepers for specialised services in the Dutch healthcare system: patients need a referral to enter specialised medical care. The mean number of GP visits increases with age from six visits per year at age 45–64 to fifteen visits per year for people aged 75 years and older [25], and a regular GP visit takes about ten minutes [26].
Patient-reported primary and secondary outcomes
We used eight different questionnaires to assess patient-reported outcomes in three domains: ‘Health,’ ‘Wellbeing’ and ‘Self-management,’ as these outcomes are important to ageing in place and to participation in society. Primary and secondary patient-reported outcomes differed per risk profile, as we expected problems to vary per profile (see Table 1) [18].
Table 1. Primary and secondary measurement instruments per risk profile.
| Complex care needs | Frail | Robust | ||||
|---|---|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | Primary | Secondary | |
| Health | ||||||
| EQ-5D-3L | X | X | X | |||
| INTERMED-E-SA | X | X | X | |||
| GFI | X | X | X | |||
| Katz-15 | X | X | X | |||
| Wellbeing | ||||||
| GWI | X | X | X | |||
| QoL | X | X | X | |||
| Self-management | ||||||
| SMAS-30 | X | X | X | |||
| PIH-OA | X | X | X | |||
EQ-5D-3L = EuroQol-5D-3L including the EuroQol visual analogue scale; GFI: Groningen Frailty Indicator; GWI = Groningen Well-being Indicator; INTERMED-E-SA = INTERMED for the Elderly Self-Assessment; PIH-OA = Partners in Health scale for older adults; QoL = Quality of life; SMAS-30 = Self-Management Ability Scale version 2.
Health
The ‘Health’ domain included the outcomes ‘Health status,’ ‘Complexity of care needs,’ ‘Level of frailty’ and ‘Limitations in Activities of Daily Living (ADL).’ We measured Health status using the EuroQol-5D three-level version (EQ-5D-3L), which is a short self-report questionnaire measuring health in five dimensions [27,28] in combination with a visual analogue scale (EQ-VAS) [29].
We measured Complexity of care needs using the INTERMED-E-SA, which includes twenty questions in the biological, psychological, social and healthcare domains [19].
We measured Level of frailty in the physical, social, cognitive and psychological domains with the GFI self-report version (fifteen items) [20].
We measured Limitations in ADL using the Katz-15, which measures independence in six physical ADLs (PADL), seven instrumental ADLs (IADL) and two additional ADL items. We calculated ADL performance as the total number of disabilities [30]. Subscale scores were calculated for PADL and IADL.
Wellbeing
The ‘Wellbeing’ domain included ‘Wellbeing’ and ‘Quality of Life’ (QoL). Wellbeing was measured using the Groningen Well-being Indicator (GWI), covering eight sources of wellbeing in daily experiences: enjoying eating and drinking, sleeping and resting well, having good relationships and contacts, being active, managing oneself, being oneself, feeling healthy in body and mind, and living pleasantly. Participants had to indicate whether each source of wellbeing was important to them and, if so, whether they were satisfied with that source. The Well-being Satisfaction Score is the number of important sources divided by the number of satisfactory sources [unpublished manuscript].
We assessed QoL using two items derived from the self-perceived health questions of the RAND-36 [31]. The first item measured self-rated QoL, while the second item compared the current self-rated QoL with QoL a year earlier. Both questions are rated on a 5-point scale ranging from 1 to 5.
Self-management
The ‘Self-management’ domain included ‘Self-management ability’ and ‘Self-management knowledge and behaviour’. We assessed Self-management ability using the Self-Management Ability Scale (SMAS-30) version 2, which contains thirty items and six subscales. The total SMAS score was calculated as the average of the subscale scores [32,33].
We measured Self-management knowledge and behaviour with the culturally adapted and validated version of the Partners in Health scale (PIH) [34]: the PIH scale for older adults (PIH-OA) [35]. The PIH-OA includes three subscales measuring eight items on an 8-point scale. Originally, we defined the PIH as a secondary outcome measurement for quality of care. However, the new, adapted version–PIH-OA–measures self-management and is therefore included in the present study.
Adaptations to the trial protocol
When effects are found on an outcome measurement, follow-up analyses will be performed using the subscales of that particular measurement instrument–if applicable.
Sample size
We used the primary outcome Health status (EQ-VAS) to calculate the sample size needed [18]. We considered a change in outcome of six points (SD 14 points) on the EQ-VAS of participants in the smallest sample, i.e. the risk profile ‘Frail,’ clinically relevant. With a power of 80% (α = 0.05, two-sided), a total number of 1062 older adults had to be included in the analysis. Taking into account an estimated non-response rate of 30% and a loss-to-follow-up rate of 30%, 2178 patients had to be invited to participate.
Statistical analyses
Differences between respondents and non-respondents were tested using Chi-square tests for categorical variables and t-tests for continuous variables. Differences in reasons for dropout in the intervention and control groups were tested using Chi-square tests.
We assessed differences in change between the intervention and control groups using multilevel mixed-effects analyses with regression coefficients (B) with 95% confidence intervals (CI) at α = 0.05 (two-sided), with adjustment for age and sex. Individual measurements (difference score per outcome, calculated as the difference between the T0 score and T1 score) were included as the first level and GP practices as the second level. We estimated the clinical relevance of the effects using Cohen’s effect sizes (ES) for statistically significant differences (p<0.05), with an ES of ≥0.20 reflecting a clinically relevant difference [36,37].
We performed intention-to-treat (ITT) analyses [38] for the whole sample and per profile. Missing data were imputed at item level by multiple imputation techniques, with the fully conditional specification approach–which uses the Bayesian framework [39]. Variables group, risk profile, GP, sex, age, marital status, living situation, educational level, income and receiving help with completing the questionnaire were used as covariates of the missing predictor models, generating twenty imputed data sets. Missing scale scores due to loss to follow-up were imputed using the mean change in deterioration of completed cases, as we assumed that older adults deteriorate over time [40]. This process was performed per risk profile for each scale. ITT outcomes were compared with those of complete case analyses including participants having both T0 and T1 measurements [41].
We performed all analyses using SPSS Statistics version 23.0 and used Mplus version 7.1 to impute the data.
Results
Participants
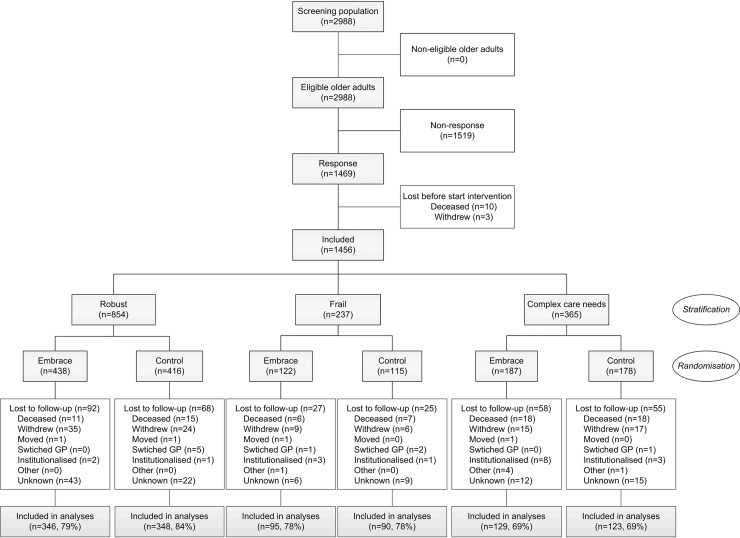
Fig 1 presents the flow of participants in the study. We included 1456 of the 2988 eligible older adults in the study and analyses (48.7%). The main reasons for non-participation included poor health or having a partner with poor health, good health, questionnaire length and lack of interest. Non-respondents differed from respondents (all p-values <0.01) regarding gender (more women declined to participate), age (oldest older adults consented less often) and degree of urbanisation (more older adults living in rural areas declined to participate) (S1 Table).
Fig 1. CONSORT flow diagram of the Embrace study.
Table 2 shows the baseline characteristics of participants. There were no statistically significant differences in the baseline characteristics between Embrace and CAU. After twelve months, 570 (76.3%) Embrace recipients and 561 (79.1%) CAU recipients completed the follow-up questionnaire. Dropouts (Embrace n = 177,23.7%; CAU n = 148, 20.9%) were significantly (all p-values <0.01) older, more frail, with more complex care needs and with poorer health. There were no significant differences in attrition rates between Embrace and CAU for the whole sample and per profile.
Table 2. Baseline characteristics of participants (n = 1456).
Values are numbers (percentages) unless stated otherwise.
| Whole sample | Complex care needs | Frail | Robust | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1456) | (n = 365) | (n = 237) | (n = 854) | |||||||||||||
| Embrace | CAU | Embrace | CAU | Embrace | CAU | Embrace | CAU | |||||||||
| (n = 747) | (n = 709) | (n = 187) | (n = 178) | (n = 122) | (n = 115) | (n = 438) | (n = 416) | |||||||||
| Age in years, mean (SD) | 80.6 | (4.5) | 80.8 | (4.7) | 81.8 | (4.6) | 81.5 | (4.9) | 81.6 | (5.1) | 82.8 | (5.5) | 79.9 | (4.0) | 79.9 | (4.1) |
| Female | 405 | (54.2) | 394 | (55.6) | 121 | (64.7) | 115 | (64.6) | 82 | (67.2) | 80 | (69.6) | 202 | (46.1) | 199 | (47.8) |
| Widowed/divorced/single | 320 | (42.8) | 290 | (41.0) | 87 | (46.5) | 79 | (44.4) | 77 | (63.1) | 72 | (63.2) | 156 | (35.6) | 139 | (33.5) |
| In sheltered accommodation/home for the elderly | 93 | (12.5) | 99 | (14.0) | 37 | (19.9) | 40 | (22.6) | 20 | (16.4) | 26 | (22.8) | 36 | (8.3) | 33 | (8.0) |
| Low educational level1 | 370 | (49.9) | 374 | (53.4) | 106 | (57.0) | 116 | (66.3) | 66 | (54.1) | 69 | (60.0) | 198 | (45.7) | 189 | (46.0) |
| Low income2 | 261 | (44.1) | 231 | (42.4) | 80 | (54.1) | 77 | (54.2) | 53 | (55.8) | 51 | (54.8) | 128 | (36.7) | 103 | (33.2) |
| Number of chronic conditions, median (IQR) | 2 | (1–3) | 2 | (1–3) | 3 | (2–5) | 3 | (2–5) | 3 | (1–4) | 3 | (2–4) | 1 | (1–2) | 1 | (1–2) |
| Receiving home care | 89 | (12.1) | 69 | (9.8) | 47 | (26.4) | 42 | (23.9) | 24 | (20.0) | 14 | (12.4) | 18 | (4.1) | 13 | (3.2) |
| Receiving help with filling in the questionnaire | 243 | (32.8) | 245 | (35.0) | 99 | (53.8) | 106 | (60.2) | 48 | (39.3) | 43 | (37.7) | 96 | (22.1) | 96 | (23.4) |
CAU = Care as usual; IQR = Interquartile range; SD = Standard deviation.
1 Low: (Less than) primary school or low vocational training.
2 Low: <€1350 per month.
Values are based on complete data. There were no significant differences between CAU and Embrace–neither for the whole sample nor per risk profile. This was tested using independent t-tests for continuous variables, Chi-square tests for categorical variables, and Mann-Whitney U tests for non-normally distributed continuous variables and ordinal variables.
Differences in effects between Embrace and CAU
Whole sample
We found no clear beneficial effects of Embrace in the whole sample as compared to CAU. Regarding the Health domain, Embrace participants showed a significantly greater deterioration in ADL (p = 0.047, ES = 0.10) and PADL performance (p = 0.011, ES = 0.13) compared to CAU–although these effect sizes indicated not clinically relevant changes. We found no differences in the changes observed between Embrace and CAU regarding Wellbeing outcomes (p>0.05, ES≤0.05. Regarding Self-management, Embrace participants showed a significantly greater improvement in the ‘Knowledge domain of self-management knowledge and behaviour’ compared to CAU, but this difference did not reach clinical relevance (p = 0.009, ES = 0.14) Table 3 and S2 Table).
Table 3. Patient-reported outcomes at 12-month follow-up in the Embrace study: Overview of the results of the intention-to-treat multilevel analyses for the whole sample and per risk profile.
| Whole sample | Complex care needs | Frail | Robust | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1456) | (n = 365) | (n = 237) | (n = 854) | |||||||||||||||
| Embrace | CAU | Embrace | CAU | Embrace | CAU | Embrace | CAU | |||||||||||
| Scale scores (range) |
Higher score* | Mean change | Mean change | p-value† | ES | Mean change | Mean change | p-value† | ES | Mean change | Mean change | p-value† | ES | Mean change | Mean change | p-value† | ES | |
| Health | ||||||||||||||||||
| EQ-5D-3L | -0.33–1.00 | + | 0.00 | 0.00 | 0.670 | 0.02 | -0.02 | -0.01 | 0.521 | 0.07 | -0.02 | 0.0 | 0.223 | 0.16 | 0.01 | 0.01 | 0.630 | 0.03 |
| EQ-VAS | 0–100 | + | -0.5 | -0.6 | 0.878 | 0.01 | -0.1 | 1.6 | 0.323 | 0.10 | -1.7 | -3.0 | 0.387 | 0.11 | -0.4 | -0.9 | 0.511 | 0.05 |
| INTERMED-E-SA | 0–60 | - | -0.1 | -0.2 | 0.597 | 0.03 | -1.9 | -2.6 | 0.149 | 0.15 | 1.4 | 1.3 | 0.608 | 0.06 | 0.3 | 0.5 | 0.540 | 0.04 |
| GFI | 0–15 | - | 0.1 | 0.1 | 0.998 | 0.00 | 0.1 | 0.0 | 0.552 | 0.06 | -0.6 | -0.7 | 0.586 | 0.07 | 0.4 | 0.5 | 0.411 | 0.06 |
| Katz-151 | 0–15 | - | 0.35 | 0.19 | 0.047 | 0.10 | 0.58 | 0.33 | 0.204 | 0.13 | 0.28 | 0.39 | 0.660 | 0.06 | 0.26 | 0.08 | 0.035 | 0.14 |
| PADL | 0–6 | - | 0.14 | 0.06 | 0.011 | 0.13 | 0.32 | 0.14 | 0.058 | 0.20 | 0.14 | 0.10 | 0.561 | 0.08 | 0.07 | 0.01 | 0.089 | 0.12 |
| IADL2 | 0–7 | - | 0.19 | 0.13 | 0.185 | 0.07 | 0.27 | 0.16 | 0.363 | 0.10 | 0.11 | 0.25 | 0.355 | 0.12 | 0.18 | 0.08 | 0.063 | 0.13 |
| Wellbeing | ||||||||||||||||||
| GWI SF Score3 | 0–1 | + | -0.02 | -0.02 | 0.892 | 0.01 | -0.02 | -0.03 | 0.512 | 0.07 | -0.04 | 0.0 | 0.478 | 0.09 | -0.02 | -0.02 | 0.900 | 0.01 |
| QoL general | 0–5 | - | 0.08 | 0.10 | 0.636 | 0.02 | 0.17 | 0.14 | 0.587 | 0.06 | 0.12 | 0.09 | 0.818 | 0.03 | 0.03 | 0.08 | 0.289 | 0.07 |
| QoL vs 1 year ago | 0–5 | - | 0.08 | 0.04 | 0.320 | 0.05 | -0.04 | 0.01 | 0.471 | 0.08 | 0.11 | 0.17 | 0.425 | 0.10 | 0.13 | 0.02 | 0.018 | 0.16 |
| Self-management | ||||||||||||||||||
| SMAS-30 | 0–100 | + | -1.1 | -0.8 | 0.411 | 0.04 | -2.0 | 0.2 | 0.015 | 0.26 | -0.4 | -0.7 | 0.705 | 0.05 | -0.9 | -1.2 | 0.664 | 0.03 |
| INIT | 0–100 | + | -2.3 | -2.5 | 0.709 | 0.02 | -2.8 | -2.1 | 0.530 | 0.07 | -1.7 | -2.3 | 0.658 | 0.06 | -2.2 | -2.8 | 0.485 | 0.05 |
| SE | 0–100 | + | -0.8 | -0.9 | 0.455 | 0.04 | -2.1 | 1.7 | 0.020 | 0.24 | 0.0 | -1.3 | 0.619 | 0.07 | -0.4 | -2.0 | 0.585 | 0.04 |
| INVEST | 0–100 | + | -1.1 | 0.0 | 0.802 | 0.01 | -1.3 | 0.8 | 0.005 | 0.30 | -0.3 | 0.5 | 0.412 | 0.11 | -1.2 | -0.4 | 0.068 | 0.13 |
| POSITIVE | 0–100 | + | -0.2 | 0.2 | 0.542 | 0.03 | -0.2 | 1.2 | 0.217 | 0.13 | -0.3 | 0.5 | 0.680 | 0.05 | -0.1 | -0.3 | 0.835 | 0.01 |
| MULT | 0–100 | + | -0.8 | -0.4 | 0.124 | 0.08 | -1.9 | 1.1 | 0.126 | 0.16 | -1.1 | -1.7 | 0.609 | 0.07 | -0.2 | -0.6 | 0.383 | 0.06 |
| VAR | 0–100 | + | -1.3 | -0.8 | 0.461 | 0.04 | -3.2 | -1.3 | 0.177 | 0.14 | 1.2 | 0.3 | 0.450 | 0.10 | -1.2 | -0.8 | 0.649 | 0.03 |
| PIH-OA4 | 8–64 | + | 0.8 | 0.4 | 0.285 | 0.06 | 1.1 | 1.1 | 0.976 | 0.00 | 1.7 | -0.8 | 0.020 | 0.31 | 0.4 | 0.4 | 0.936 | 0.01 |
| Knowledge | 2–16 | + | 0.8 | 0.3 | 0.009 | 0.14 | 0.8 | 0.3 | 0.113 | 0.17 | 1.0 | -0.2 | 0.015 | 0.32 | 0.7 | 0.4 | 0.245 | 0.08 |
| Management | 2–16 | + | 0.1 | 0.0 | 0.691 | 0.02 | 0.2 | 0.2 | 0.969 | 0.00 | 0.2 | -0.2 | 0.398 | 0.11 | -0.1 | -0.1 | 0.965 | 0.00 |
| Coping | 4–32 | + | 0.0 | 0.1 | 0.659 | 0.02 | 0.1 | 0.6 | 0.336 | 0.10 | 0.6 | -0.4 | 0.119 | 0.21 | -0.2 | 0.0 | 0.355 | 0.06 |
CAU = Care as usual; EQ-5D-3L = EuroQol-5D-3L; EQ-VAS = EuroQoL-5D visual analogue scale; ES = Effect size d, thresholds <0.2 trivial, ≥ 0.2–0.5 small, ≥0.5–0.8 medium, ≥ 0.8 large; GFI = Groningen Frailty Indicator; GWI SF Score = Groningen Well-being Indicator Satisfaction Score; IADL = Instrumental Activities of Daily Living; INIT = Taking initiatives subscale; INTERMED-E-SA = INTERMED for the Elderly Self-Assessment; INVEST = Investment behaviour subscale; MULT = Multi-functionality of resources subscale; PADL = Physical Activities of Daily Living; PIH-OA = Partners in Health scale for older adults; POSITIVE = Positive frame of mind subscale; QoL = Quality of life; SE = Self-efficacy beliefs subscale; SMAS-30 = Self-Management Ability Scale version 2; VAR = Variety in resources subscale.
* + Higher score means improvement;—higher score means deterioration.
† Values are corrected for age and sex; bold values indicate p<0.05.
1 Percentage of missing items at baseline before imputation 7.4%
2 Percentage of missing items at baseline before imputation 5.4% and 6.1% at follow-up.
3 Percentage of missing items at baseline before imputation 12.7%
4 Percentage of missing items at baseline before imputation 5.7%
Bold text and orange filling: Significant (p<0.05) or clinically relevant (ES ≥0.20) deterioration
Bold text and green filling: Significant (p<0.05) or clinically relevant (ES ≥0.20) improvement
Complex care needs
We found no significant differences in the changes observed in the domains of Health and Wellbeing after twelve months between Embrace and CAU. However, there was a significant and clinically relevant difference in change in the Self-management outcomes ‘Self-management abilities,’ ‘Self-efficacy beliefs’ and ‘Investment behaviour’, as Embrace participants performed worse after twelve months, whereas those in CAU showed a small improvement (Table 3 and S3 Table).
Frail
We found no significant differences in the change observed between Embrace and CAU regarding Health and Wellbeing, but Embrace participants did show a significantly greater improvement in the ‘Self-management knowledge and behaviour’ Self-management outcome, as well as in its ‘Knowledge’ domain, compared to a deterioration for those in CAU (Table 3 and S4 Table).
Robust
We found no significant differences in the Health domain, except for significantly worse ADL performance compared to CAU–although this difference was not clinically relevant. Furthermore, Embrace participants showed a significantly larger deterioration in the Wellbeing outcome ‘QoL comparison item’ compared to CAU, but this difference was not clinically relevant either. We found no differences in the changes observed between groups regarding Self-management (Table 3 and S5 Table).
Missing data and sensitivity analyses
Missing scale scores ranged from 0.0% to 12.7%, with 37 of the 42 scales and subscales having less than 5.0% missing values (Table 3). Sensitivity analyses with complete cases showed the same pattern of results, except for 1) a significant deterioration in PADL performance of the complex Embrace participants, and 2) a no longer significant–but still clinically relevant–improvement on the total PIH-OA score for the frail Embrace participants (S6–S10 Tables).
Discussion
This RCT examined the effects of ‘Embrace,’ a person-centred and integrated care service for older adults, for the total sample and by respective risk profiles. We found no clear clinically relevant changes after receiving twelve months of care and support by Embrace on health, wellbeing and self-management in the total sample of community-living older adults and neither in the risk profiles. Overall, Embrace participants showed a greater–but clinically irrelevant–improvement in self-management knowledge and a greater–but clinically irrelevant–deterioration in ADL compared to CAU. This heterogeneous picture was also found in the risk profiles.
Interpretation of findings
The care and support offered by Embrace had fewer beneficial effects–and sometimes even unbeneficial effects–on the domains of Health, Wellbeing and Self-management than we anticipated, which confirms the heterogeneous outcomes previously reported in RCTs on integrated care programs for community-living older adults [15, 16, 42–56].
Our finding of no clear benefits for Embrace on the outcomes measured could be due to the duration of the intervention, the nature of the intervention, the selection of outcomes or methodological limitations.
Firstly, the intervention may not have worked or may not yet have worked. We may have been dealing with an investment effect [57], as this multifaceted and complex intervention requires a cultural change in professionals’ deep-rooted working patterns, which could take more time than only twelve months despite an intensive training and coaching program before and during the intervention. Assessment among participating professionals of whether the care and support provided was in accordance with the Chronic Care Model underlined this assumption. We found a clinically relevant increase in the perceived level of implementation of integrated care from a ‘basic level’ at the start to a ‘reasonably good level’ after twelve months–indicating clinically relevant improvements with room for further improvement [58]. Evaluation of effects in the longer term is therefore needed, as well as follow-up coaching for further support of the cultural change in professionals’ working behaviour and evaluation of protocol adherence.
Secondly, the contrast between our intervention and CAU may have been too small to detect differences over the first twelve-month period. The Dutch healthcare system is already of a quite high standard, as all inhabitants have health insurance and healthcare is easily accessible, leaving little room for improvement [59]. This was confirmed by our finding that only the frail Embrace participants showed a significant increase in self-management knowledge and behaviour. These participants had received little or no care before the start of the intervention, in contrast with the complex participants, the majority of whom already received home care.
Thirdly, we had to deal with the heterogeneity and instability of the older population, which increased measurement error and thus reduced the likelihood of observing effects [60].
Fourthly, the measurement instruments for health and wellbeing may not have been specific enough for this type of intervention and may not have been sensitive enough to detect changes in clinical practice [61]. This could explain why we did find effects on two specifically developed measurement instruments: the PIH-OA, which is a version of the PIH for the evaluation of self-management knowledge and behaviour in older adults [35], and the PAIEC [62], which is used in another Embrace study for evaluation of perceived quality of integrated care and support [58].
Strengths and limitations
The strengths of this study are its design–a RCT targeting all community-living older adults–and its stratification of participants into risk profiles, thereby enabling professionals to provide person-centred care and support. Moreover, we were able to perform predefined subgroup analyses to examine the effect of integrated care in subgroups at a higher risk of deterioration [63].
We must also acknowledge some potential limitations. We randomised within GP practices, which increased the risk of contamination. Although we instructed GPs to provide care as usual to patients who were not assigned to the intervention, we may have underestimated the effect on CAU participants. However, regular GP visits are brief and only take about ten minutes [26], with little time to discuss the topic of concern–let alone other health-related topics [64]. Moreover, CAU participants did not receive any additional support that was part of the intervention. Furthermore, a potential limitation is the non-response rate at baseline of about 50%. The differences between respondents and non-respondents concerning gender, age and degree of urbanisation may limit the generalisability of our findings to some extent.
Implications for practice, policy and research
The present study showed that receiving twelve months of integrated care has no clear beneficial effect on patient-reported outcomes. Based on these results, the implementation of integrated care services for older adults cannot be recommended. However, a parallel study on Embrace showed that perceived quality of care improved [58]. Moreover, in a qualitative study of Embrace, older adults indicated that they felt safe and secure due to Embrace care and support [6]. These results could contribute to decision-making and show the need for mixed method evaluations [65]. Mixed method evaluation could also offer an explanation for the absence of clear effects in the present study [65]. Furthermore, future research should focus on the long-term effects of Embrace and should use outcomes–for example on dependency, age-related fears and coping–and specifically developed measurement instruments appropriate for this older population and type of intervention. A future cost-effectiveness study could help policy makers and professionals decide whether to implement Embrace. The effects of Embrace should also be evaluated in other geographical areas and in other cultures with different healthcare systems. Finally, stratification into risk profiles was the starting point for delivering care and support at a suitable care intensity level. Future studies could also target different risk profiles.
Conclusion
The present study showed that receiving twelve months of person-centred and integrated care and support from Embrace has no clear beneficial effect on patient-reported health status and neither on wellbeing and self-management outcomes. Future research should provide insight into the long-term effects of Embrace. Moreover, specifically developed measurement instruments suitable for the target population and intervention should be used in future studies. As this is the first CCM-based RCT to include a population-based sample of community-living older adults, it contributes to the design of future research on population-based integrated care.
Supporting information
(PDF)
(PDF)
(PDF)
Values are numbers (percentages) unless stated otherwise.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the participating older adults and healthcare professionals from the fifteen GP practices, health care organisation Zorggroep Meander, welfare organisation Tinten welzijnsgroep and Coen Ronde BSc, without whom this study could not have been performed. We would also like to thank Nienke Verheij MSc, all research assistants and volunteers in welfare for their contribution to the data collection. In addition, we would like to thank Roy Stewart PhD and Josue Almansa Ortiz PhD, statisticians, for their statistical support and for their help and advice in imputing our data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Embrace study was funded by the Netherlands Organisation for Health Research and Development (ZonMw: grant number 314010201; http://www.zonmw.nl). The health care professionals involved are funded by the Dutch Healthcare Authority (NZa: file number 300-1021; http://www.nza.nl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sixsmith J, Sixsmith A, Fange AM, Naumann D, Kucsera C, Tomsone S, et al. Healthy ageing and home: the perspectives of very old people in five European countries. Soc Sci Med. 2014;106: 1–9. doi: 10.1016/j.socscimed.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Lofqvist C, Granbom M, Himmelsbach I, Iwarsson S, Oswald F, Haak M. Voices on relocation and aging in place in very old age—a complex and ambivalent matter. Gerontologist. 2013;53: 919–927. doi: 10.1093/geront/gnt034 [DOI] [PubMed] [Google Scholar]
- 3.Wiles JL, Leibing A, Guberman N, Reeve J, Allen RE. The meaning of "aging in place" to older people. Gerontologist. 2012;52: 357–366. doi: 10.1093/geront/gnr098 [DOI] [PubMed] [Google Scholar]
- 4.Spoorenberg SL, Reijneveld SA, Middel B, Uittenbroek RJ, Kremer HP, Wynia K. The Geriatric ICF Core Set reflecting health-related problems in community-living older adults aged 75 years and older without dementia: development and validation. Disabil Rehabil. 2015;37: 2337–2343. doi: 10.3109/09638288.2015.1024337 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Feeley TH. Social support, social strain, loneliness, and well-being among older adults: an analysis of the Health and Retirement Study. JSPR. 2014;31: 141. [Google Scholar]
- 6.Spoorenberg SL, Wynia K, Fokkens AS, Slotman K, Kremer HP, Reijneveld SA. Experiences of community-living older adults receiving integrated care based on the Chronic Care Model: a qualitative study. PLoS One. 2015;10: e0137803 doi: 10.1371/journal.pone.0137803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claassens L, Widdershoven GA, Van Rhijn SC, Van Nes F, Broese van Groenou MI, Deeg DJ, et al. Perceived control in health care: a conceptual model based on experiences of frail older adults. J Aging Stud. 2014;31: 159–170. doi: 10.1016/j.jaging.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 8.Lachman ME. Perceived control over aging-related declines: adaptive beliefs and behaviors. CDPS. 2006;15: 282. [Google Scholar]
- 9.Wolinsky FD, Wyrwich KW, Babu AN, Kroenke K, Tierney WM. Age, aging, and the sense of control among older adults: a longitudinal reconsideration. J Gerontol B Psychol Sci Soc Sci. 2003;58: S212–220. [DOI] [PubMed] [Google Scholar]
- 10.Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet. 2015;385: 658–661. doi: 10.1016/S0140-6736(14)61461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolte E, McKee M. Making it happen In: Nolte E, McKee M, editors. Caring for people with chronic conditions. A health system perspective. Maidenhead: Open University Press; 2008. pp. 222–244. [Google Scholar]
- 13.Singh D, Ham C. Improving care for people with long-term conditions: A review of UK and international frameworks. Birmingham: NHS Institute for Innovation and Improvement; 2006. [Google Scholar]
- 14.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20: 64–78. [DOI] [PubMed] [Google Scholar]
- 15.Hoogendijk EO, van der Horst HE, van de Ven PM, Twisk JW, Deeg DJ, Frijters DH, et al. Effectiveness of a Geriatric Care Model for frail older adults in primary care: results from a stepped wedge cluster randomized trial. Eur J Intern Med. 2016;28: 43–51. doi: 10.1016/j.ejim.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 16.Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. J Gen Intern Med. 2013;28: 612–621. doi: 10.1007/s11606-012-2287-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolte E, McKee M. Integration and chronic care: a review In: Nolte E, McKee M, editors. Caring for people with chronic conditions. A health system perspective. Maidenhead: Open University Press; 2008. pp. 64–91. [Google Scholar]
- 18.Spoorenberg SL, Uittenbroek RJ, Middel B, Kremer BP, Reijneveld SA, Wynia K. Embrace, a model for integrated elderly care: study protocol of a randomized controlled trial on the effectiveness regarding patient outcomes, service use, costs, and quality of care. BMC Geriatr. 2013;13: 62 doi: 10.1186/1471-2318-13-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters LL, Boter H, Slaets JP, Buskens E. Development and measurement properties of the self assessment version of the INTERMED for the elderly to assess case complexity. J Psychosom Res. 2013;74: 518–522. doi: 10.1016/j.jpsychores.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Peters LL, Boter H, Buskens E, Slaets JP. Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. 2012;13: 546–551. doi: 10.1016/j.jamda.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Steverink N, Slaets JPJ, Schuurmans H, Van Lis M. Measuring frailty: development and testing of the Groningen Frailty Indicator (GFI). Gerontologist. 2001;41: 236. [Google Scholar]
- 22.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310: 2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43: 215–221. doi: 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schols JM, Crebolder HF, van Weel C. Nursing home and nursing home physician: the Dutch experience. J Am Med Dir Assoc. 2004;5: 207–212. doi: 10.1097/01.JAM.0000123031.43619.60 [DOI] [PubMed] [Google Scholar]
- 25.Schäfer W, Kroneman M, Boerma W, Van den Berg M, Westert G, Deville W, et al. The Netherlands: health system review. Health Syst Transit. 2010;12: v–xxvii, 1–228. [PubMed] [Google Scholar]
- 26.Deveugele M, Derese A, Van den Brink-Muinen A, Bensing J, De Maeseneer J. Consultation length in general practice: cross sectional study in six European countries. BMJ. 2002;325: 472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. 2nd ed. Dordrecht, The Netherlands: Springer; 2007. [Google Scholar]
- 28.Lamers LM, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Ned Tijdschr Geneeskd. 2005;149: 1574–1578. [PubMed] [Google Scholar]
- 29.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37: 53–72. [DOI] [PubMed] [Google Scholar]
- 30.Laan W, Zuithoff NP, Drubbel I, Bleijenberg N, Numans ME, de Wit NJ, et al. Validity and reliability of the Katz-15 scale to measure unfavorable health outcomes in community-dwelling older people. J Nutr Health Aging. 2014;18: 848–854. doi: 10.1007/s12603-014-0479-3 [DOI] [PubMed] [Google Scholar]
- 31.Van der Zee KI, Sanderman R, Heyink JW, De Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996;3: 104–122. doi: 10.1207/s15327558ijbm0302_2 [DOI] [PubMed] [Google Scholar]
- 32.Steverink N. Self-management Ability Scale: SMAS-30; (Version 2/2008). Available: http://www.nardisteverink.nl/index.php?content=materials. [Google Scholar]
- 33.Schuurmans H, Steverink N, Frieswijk N, Buunk BP, Slaets JP, Lindenberg S. How to measure self-management abilities in older people by self-report. The development of the SMAS-30. Qual Life Res. 2005;14: 2215–2228. doi: 10.1007/s11136-005-8166-9 [DOI] [PubMed] [Google Scholar]
- 34.Battersby MW, Ask A, Reece MM, Markwick MJ, Collins JP. The Partners in Health scale: the development and psychometric properties of a generic assessment scale for chronic condition self-management. Aust J Prim Health. 2003;9: 41. [Google Scholar]
- 35.Veldman K, Reijneveld S, Lahr M, Uittenbroek R, Wynia K. The Partners in Health scale for older adults (PIH-OA): an examination of its construct validity and internal consistency in a Dutch population of older adults. Health Expect. doi: 10.1111/hex.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middel B, Stewart R, Bouma J, van Sonderen E, van den Heuvel WJ. How to validate clinically important change in health-related functional status. Is the magnitude of the effect size consistently related to magnitude of change as indicated by a global question rating? J Eval Clin Pract. 2001;7: 399–410. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. Hillsdale, New Jersey: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 38.Polit DF, Gillespie BM. Intention-to-treat in randomized controlled trials: recommendations for a total trial strategy. Res Nurs Health. 2010;33: 355–368. doi: 10.1002/nur.20386 [DOI] [PubMed] [Google Scholar]
- 39.van Buuren S, Brand J, Groothuis-Oudshoorn C, Rubin D. Fully conditional specification in multivariate imputation. JSCS. 2006;76: 1049–1064. [Google Scholar]
- 40.Gustafsson M, Kristensson J, Holst G, Willman A, Bohman D. Case managers for older persons with multi-morbidity and their everyday work—a focused ethnography. BMC Health Serv Res. 2013;13: 496 doi: 10.1186/1472-6963-13-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367: 1355–1360. doi: 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleijenberg N, Drubbel I, Schuurmans MJ, Dam HT, Zuithoff NP, Numans ME, et al. Effectiveness of a proactive primary care program on preserving daily functioning of older people: a cluster randomized controlled trial. J Am Geriatr Soc. 2016; 64: 1779–1788. doi: 10.1111/jgs.14325 [DOI] [PubMed] [Google Scholar]
- 43.Eklund K, Wilhelmson K, Gustafsson H, Landahl S, Dahlin-Ivanoff S. One-year outcome of frailty indicators and activities of daily living following the randomised controlled trial: "Continuum of care for frail older people". BMC Geriatr. 2013;13: 76 doi: 10.1186/1471-2318-13-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ. 1998;316: 1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo M, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. J Am Geriatr Soc. 1998;46: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 46.Metzelthin SF, van Rossum E, de Witte LP, Ambergen AW, Hobma SO, Sipers W, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ. 2013;347: f5264 doi: 10.1136/bmj.f5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298: 2623–2633. doi: 10.1001/jama.298.22.2623 [DOI] [PubMed] [Google Scholar]
- 48.Hammar T, Perala ML, Rissanen P. The effects of integrated home care and discharge practice on functional ability and health-related quality of life: a cluster-randomised trial among home care patients. Int J Integr Care. 2007;7: e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beland F, Bergman H, Lebel P, Clarfield AM, Tousignant P, Contandriopoulos AP, et al. A system of integrated care for older persons with disabilities in Canada: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61: 367–373. [DOI] [PubMed] [Google Scholar]
- 50.Tappenden P, Campbell F, Rawdin A, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess. 2012;16: 1–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huss A, Stuck AE, Rubenstein LZ, Egger M, Clough-Gorr KM. Multidimensional preventive home visit programs for community-dwelling older adults: a systematic review and meta-analysis of randomized controlled trials. J Gerontol A Biol Sci Med Sci. 2008;63: 298–307. [DOI] [PubMed] [Google Scholar]
- 52.Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371: 725–735. doi: 10.1016/S0140-6736(08)60342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Haastregt JC, Diederiks JP, van Rossum E, de Witte LP, Crebolder HF. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ. 2000;320: 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouman A, van Rossum E, Nelemans P, Kempen GI, Knipschild P. Effects of intensive home visiting programs for older people with poor health status: a systematic review. BMC Health Serv Res. 2008;8: 74 doi: 10.1186/1472-6963-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287: 1022–1028. [DOI] [PubMed] [Google Scholar]
- 56.You EC, Dunt D, Doyle C, Hsueh A. Effects of case management in community aged care on client and carer outcomes: a systematic review of randomized trials and comparative observational studies. BMC Health Serv Res. 2012;12: 395 doi: 10.1186/1472-6963-12-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toseland RW, O'Donnell JC, Engelhardt JB, Richie J, Jue D, Banks SM. Outpatient geriatric evaluation and management: is there an investment effect? Gerontologist. 1997;37: 324–332. [DOI] [PubMed] [Google Scholar]
- 58.Uittenbroek RJ, Kremer HP, Spoorenberg SL, Reijneveld SA, Wynia K. Integrated care for older adults improves perceived quality of care: results of a randomized controlled trial of Embrace. J Gen Intern Med. 2016. doi: 10.1007/s11606-016-3742-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Björnberg A. Euro Health Consumer Index 2014 Report. Brussels, Belgium: Health Consumer Powerhouse; 2015. [Google Scholar]
- 60.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11: 85 doi: 10.1186/1745-6215-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haywood KL, Garratt AM, Fitzpatrick R. Older people specific health status and quality of life: a structured review of self-assessed instruments. J Eval Clin Pract. 2005;11: 315–327. doi: 10.1111/j.1365-2753.2005.00538.x [DOI] [PubMed] [Google Scholar]
- 62.Uittenbroek RJ, Reijneveld SA, Stewart RE, Spoorenberg SL, Kremer HP, Wynia K. Development and psychometric evaluation of a measure to evaluate the quality of integrated care: the Patient Assessment of Integrated Elderly Care. Health Expect. 2016;4: 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin JS, Whitlock EP, Eckstrom E, Fu R, Perdue LA, Beil TL, et al. Challenges in synthesizing and interpreting the evidence from a systematic review of multifactorial interventions to prevent functional decline in older adults. J Am Geriatr Soc. 2012;60: 2157–2166. doi: 10.1111/j.1532-5415.2012.04214.x [DOI] [PubMed] [Google Scholar]
- 64.Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42: 1871–1894. doi: 10.1111/j.1475-6773.2006.00689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337: a1655 doi: 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Values are numbers (percentages) unless stated otherwise.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.