Abstract
Background:
Knee injuries encountered in clinical practice can involve avulsions of the biceps femoris from the fibula and proximal tibia. Advances in tendon repair methods now allow for repairs with increased surface areas using modern suture anchor techniques. Despite descriptions of repair techniques, there are no biomechanical studies on the biceps femoris for comparison.
Purpose/Hypothesis:
The objective of this controlled laboratory study was to determine the failure load of the native biceps femoris distal insertion and to evaluate modern repair techniques. Our hypothesis was 2-fold: (1) Suture repairs to the tibia and fibula would perform better on tensile testing than repairs to the fibula alone, and (2) complex bridge repairs, similar to those frequently used in rotator cuff surgery, would perform better on tensile testing than simple repairs.
Study Design:
Controlled laboratory study.
Methods:
A total of 40 paired, fresh-frozen cadaveric specimens were dissected, identifying the biceps femoris and its insertion on the proximal tibia and fibula. The native biceps femoris footprint was left intact in 8 specimens and tested to failure on a uniaxial materials testing machine evaluating tensile properties, while in the other 32 specimens, the biceps femoris insertion was dissected using a No. 15 scalpel blade, underwent repair, and was then tested to failure on a uniaxial materials testing machine evaluating tensile properties. Four repair constructs were evaluated, with 8 specimens allocated for each: construct 1 involved a simple repair (ie, passing suture through tissue in a running Krackow fashion and tying at the anchor site) to the fibula with 2 suture anchors, construct 2 involved a simple repair to the fibula and tibia with 3 suture anchors, construct 3 was a fibular repair with a tibial suture bridge involving the fibula and tibia and 3 suture anchors, construct 4 involved a transosseous repair through the fibula and 1 suture anchor on the tibia. Analysis of variance was used to evaluate for significance of the mean failure load and stiffness between groups.
Results:
The mean (±95% CI) failure loads were the following: native biceps femoris, 1280 ± 247.0 N; simple fibular repair, 173 ± 84.6 N; simple fibular and tibial repair, 176 ± 48.1 N; fibular repair with tibial suture bridge, 191 ± 78.5 N; and transosseous repair, 327 ± 66.3 N. The mean stiffness values were the following: native, 46 ± 13.0 N/mm; simple fibular repair, 16 ± 5.1 N/mm; simple fibular and tibial repair, 14 ± 5.4 N/mm; fibular repair with tibial suture bridge, 13 ± 2.8 N/mm; and transosseous repair, 15 ± 2.5 N/mm. Interconstruct comparison of failure loads revealed no statistical difference between constructs utilizing anchors alone. The transosseous repair showed a significant difference for the failure load when compared with each anchor repair construct (P = .02, .02, and .04 for constructs 1, 2, and 3, respectively). Interconstruct comparison of stiffness revealed no statistical difference between all constructs (P > .86). None of the repair techniques re-created the failure load or stiffness of the native biceps femoris tendon (P = .02).
Conclusion:
In this biomechanical study, no difference was found between the mean failure loads of different biceps femoris repair constructs involving suture anchors alone and No. 2 braided polyester and ultra–high-molecular-weight polyethylene suture. A technique involving transosseous fibular tunnels and 2-mm suture tape illustrated a greater mean failure load than repairs relying on suture anchors for fixation.
Clinical Relevance:
Understanding the tensile performance of biceps femoris repair constructs aids clinicians with preoperative and intraoperative decisions. Current biceps femoris repair techniques do not approximate the native strength of the tendon. A transosseous style of repair offers the highest failure load.
Keywords: posterolateral corner, biceps femoris, repair, failure load
An avulsion injury of the distal biceps femoris can occur as an isolated injury or as part of a multiligament injury pattern, with or without concomitant injuries to additional lateral knee structures.‡ Because of the functional importance of the biceps femoris, surgical intervention is frequently considered after an avulsion injury.§ While this injury is rare, we have occasionally seen it in our clinical practice. Multiple surgical repair techniques have been described, including sutures alone, suture anchors, and transosseous fixation.4,5,8,11,15,19,28 As advances in tendon repair methods have been made, there are now methods of repair with increased surface areas using modern suture anchor techniques. However, a biomechanical study evaluating simple methods of repair as well as advanced complex methods is lacking. As we have encountered these injuries in clinical practice, we have questioned the superiority of modern techniques to historical techniques. Understanding the tensile performance of biceps femoris repair constructs will aid clinicians with preoperative and intraoperative decisions.
Repair strength is of significance and is often studied because of its implications for healing potential and rehabilitation protocols. Through qualitative, quantitative, and biomechanical study, the anatomy and strength of the fibular collateral ligament, popliteofibular ligament, and popliteus tendon have been thoroughly described.3,9,10,16,22 While the anatomy of the biceps femoris has also been quantitatively and qualitatively described2,9,10,13,20–24 (Figure 1), biomechanical testing of its tendinous insertion and repair technique is lacking. Furthermore, previously reported repair methods have been simplistic and have not attempted to re-create the normal anatomy; repair techniques have historically addressed only the insertion on the proximal fibula without consideration of the insertion on the tibia.2,4,5,8,11,15,19,24,28
Figure 1.
The insertional locations and footprint areas of the biceps femoris, fibular collateral ligament (FCL), and anterolateral ligament, with footprint surface area presented as the mean (range) in mm2. Reprinted with permission from Branch and Anz.2
The objective of this controlled laboratory study was to determine the failure load of the native biceps femoris distal insertion and to evaluate modern repair techniques of the distal biceps femoris. Our hypothesis was 2-fold: (1) Suture repairs to the tibia and fibula would perform better on tensile testing than repairs to the fibula alone, and (2) complex bridging repairs, similar to those frequently used in rotator cuff surgery, would perform better on tensile testing than simple repairs. Evaluation of the failure load and stiffness for different biceps femoris repair constructs will aid the clinician in decisions regarding the optimal repair technique.
Methods
Forty fresh-frozen cadaveric specimens were acquired from Science Care and used for the study. The mean specimen age was 59.3 ± 6.89 years (range, 28-66 years), the mean height was 66.9 ± 3.52 inches (range, 61-72 inches), and the mean weight was 200.0 ± 56.90 lb (range, 100-315 lb). Eighteen specimens were from male donors, and 22 were from female donors. The specimens were randomly assigned to 1 of 5 groups (n = 8 each) to test the native strength of the biceps femoris, simple repair to the fibula alone, simple repair to the fibula and tibia, fibular repair with a tibial suture bridge to the fibula and tibia, and transosseous repair to the fibula and tibia. Of the 40 specimens, 32 were matched-pair knees from 16 donors. To ensure a more even distribution of samples and avoid bias, we avoided allocating both specimens of a pair to a single group. The mean and SD of the groups were calculated for donor age, weight, and height as well as male versus female ratio (Table 1).
TABLE 1.
Donor Morphological Characteristicsa
| Native | Simple Fibular Repair | Simple Fibular and Tibial Repair | Fibular Repair With Tibial Suture Bridge | Transosseous Repair | |
|---|---|---|---|---|---|
| Age, y | 58.5 ± 6.74 (46-66) | 60.0 ± 4.03 (53-64) | 56.6 ± 12.30 (28-66) | 61.1 ± 4.61 (53-66) | 60.1 ± 3.68 (53-66) |
| Height, inches | 65.8 ± 2.58 (61-70) | 65.0 ± 2.67 (61-70) | 67.4 ± 4.06 (62-72) | 67.9 ± 3.68 (62-72) | 68.1 ± 4.08 (64-73) |
| Weight, lb | 210 ± 39.2 (160-260) | 191 ± 45.9 (120-250) | 203 ± 77.1 (100-315) | 202 ± 70.6 (100-315) | 192 ± 55.9 (100-260) |
| Male:female ratio | 3:5 | 3:5 | 4:4 | 4:4 | 4:4 |
aValues are expressed as mean ± SD (range) unless otherwise specified. There were no significant differences between donor group demographics (P > .05 for all).
Our medical education institution is licensed to receive cadaveric specimens for research and training purposes, and our institution does not require institutional review board approval for cadaveric biomechanical studies.
Specimens were dissected using a lateral approach, and all skin was removed. The iliotibial band and biceps femoris were identified and separated, and the biceps femoris was freed down to its insertion on the fibular head. The cadaveric specimens ended at the midtibia without distal ankle structures including the syndesmosis. The tibia and fibula were cleared of all remaining soft tissues, and the knee disarticulated. The proximal tibial fibular ligaments were kept intact, which represents the injury pattern encountered in clinical practice. Specimens were then potted using polyvinyl chloride (PVC) cylinders and automotive body filler (Bondo; 3M).
We selected 8 specimens per group, as this number has proven to be a sufficient sample size in previous mechanical testing of the native medial and lateral structures of the knee.9,29 Additionally, on power analysis, it was determined that a sample size of 8 specimens per group would yield at least 80% power to detect the minimal between-group difference in the maximum failure load of 75 N assuming a nonparametric comparison of 4 groups, a group SD of ≤50 N, and a type I error probability of .05.
The first group of 8 specimens served to evaluate native tensile properties without dissection of the biceps femoris from the fibula/tibia. The remaining 4 groups all underwent repair of the biceps femoris after its insertions were dissected free with a No. 15 scalpel blade. Four repair constructs were evaluated: simple repair involving 2 suture anchors and repair to the fibula alone (simple fibular repair), simple repair involving 3 suture anchors and repair to the fibula and tibia (simple fibular and tibial repair), complex repair involving 3 suture anchors and repair to the fibula and tibia in a transosseous-equivalent fashion (fibular repair with a tibial suture bridge), and transosseous repair involving tunnels across the fibula and 1 suture anchor on the tibia (transosseous repair) (Figures 2 -5). We had encountered the simple fibular repair in training and within the literature,8,11 the senior author (A.W.A.) had adopted the simple fibular and tibial repair after completion of a recent anatomic study on the biceps femoris,2 we designed the fibular repair with a tibial suture bridge for testing based on the senior author’s experience with double-row rotator cuff repair, and the transosseous repair was designed after a review of rotator cuff biomechanical studies.27 A transosseous repair to the proximal fibula was mentioned in the original case report of isolated avulsions of the biceps femoris insertion by Sebastianelli et al.19
Figure 2.
Biceps femoris repair construct: simple fibular repair.
Figure 3.

Biceps femoris repair construct: simple fibular and tibial repair.
Figure 4.

Biceps femoris repair construct: fibular repair with tibial suture bridge.
Figure 5.
Biceps femoris repair construct: transosseous repair.
The simple fibular repair construct (Figure 2) involved 1 double-loaded 4.5-mm biocomposite suture anchor (Corkscrew; Arthrex) inserted at the proximal fibular insertion of the biceps femoris and 1 single-loaded 3.0-mm biocomposite suture anchor (SutureTak; Arthrex) inserted at the distal fibular insertion of the biceps femoris. For each suture, 1 limb was passed through the tendon and then run proximally for 4 locking passes and returned distally for 4 locking passes in a Krackow fashion to create a leading limb.7 The remaining limb of each suture was passed once through the tissue, creating a post limb. The leading limb and post limb were tied using 2 same-sided hitches followed by 3 alternating half-hitches.
The simple fibular and tibial repair construct (Figure 3) involved 1 double-loaded 4.5-mm suture anchor (Corkscrew) at the proximal fibular insertion of the biceps femoris, one 3.0-mm suture anchor (SutureTak) at the distal fibular insertion of the biceps femoris, and one 3.0-mm suture anchor (SutureTak) at the tibial insertion of the biceps femoris. All sutures were passed and tied as described above for each suture anchor.
The fibular repair with a tibial suture bridge construct (Figure 4) involved one 3.0-mm suture anchor (SutureTak) at the proximal fibular insertion of the biceps femoris, one 4.5-mm suture anchor (Corkscrew) at the distal fibular insertion of the biceps femoris, and one 2.9-mm friction-securing suture anchor (PushLock; Arthrex) at the tibial insertion of the biceps femoris. The suture of the proximal fibular 3.0-mm suture anchor and 1 suture of the double-loaded 4.5-mm suture anchor were passed and tied in a running Krackow fashion as described above. The remaining suture of the 4.5-mm suture anchor was passed in a horizontal fashion through the distal component of the biceps femoris and then placed into a 2.9-mm friction-securing suture anchor (PushLock) on the tibia, producing a transosseous-equivalent construct.
The transosseous repair construct (Figure 5) involved drilling two 2-mm tunnels from posterior to anterior through the fibula. The proximal tunnel entered the posterior aspect of the fibula approximately 8 mm from the posterior tip of the styloid and exited the anterior aspect of the fibula approximately 8 mm from the anterior/proximal aspect of the fibula. The distal tunnel entered the posterior aspect of the fibula approximately 14 mm from the posterior tip of the styloid and exited the anterior aspect of the fibula approximately 14 mm from the anterior/proximal aspect of the fibula. The tunnels were drilled in a fashion to leave an approximate 4-mm bone bridge between the two. A 2-mm suture tape (FiberTape; Arthrex) was passed proximally for 4 passes in a running Krackow fashion and returned distally for 4 passes in the same fashion. The suture tape was passed through the bone tunnels and tied over the bone bridge created between the tunnels. The limbs of the suture tape were passed in a horizontal fashion through the distal component of the biceps femoris and then placed into a 3.5-mm biocomposite screw-in suture anchor (SwiveLock; Arthrex) at the tibial insertion of the biceps femoris.
All suture anchors were loaded with No. 2 braided polyester and ultra–high-molecular-weight polyethylene suture (FiberWire; Arthrex), and sutures were passed utilizing a free curved needle. Insertion of the suture anchors followed the manufacturer’s recommended technique.
All specimens were tested to failure on a uniaxial materials testing machine (Model 5565; Instron) with a 1 degree of freedom holder and a screw side-action specimen mount with freeze augmentation (Figure 6). After the specimen was secured with the screw side-action clamp, dry ice was loaded into the clamp until specimen freeze was confirmed with complete ice crystal formation over the entirety of the clamp and adjacent tendon-muscle tissue. Specimens were kept moist with saline solution after dissection and repair. Tensile testing was performed within an hour of repair construct placement. The tensile testing protocol included a 5-N preload period for 10 seconds, a preconditioning period with cyclic loading, and a period of load to failure. Throughout testing, tensile load and displacement were recorded at 10 Hz. The preconditioning period involved 20 cycles from 5 to 30 N at 0.5 mm/s, and the load-to-failure period involved an increase in force at a rate of 0.5 mm/s until failure. The tensile load was applied parallel to the fibular shaft to simulate the knee in full extension. Throughout the entire testing process, actuator force and displacement were captured, and a displacement curve was generated by use of mechanical testing software (Bluehill 2; Instron). The failure load was defined as the first loss of structural integrity as illustrated by the initial peak on the displacement curve. Stiffness values were determined by calculating the slope of the load-displacement curve during initial loading of the construct from 5 to 30 N. The included region was linear and sufficiently below the failure load to ensure that nonlinear deformation was excluded. Specimens were monitored for slippage within the clamp visually during testing as well as on posttest analysis of the displacement curve. Data are presented as means with 95% CIs. All data were checked for normality of distribution, and analysis of variance was chosen to evaluate for statistical significance of the maximum failure load and stiffness between groups. Statistical significance was defined as P < .05.
Figure 6.
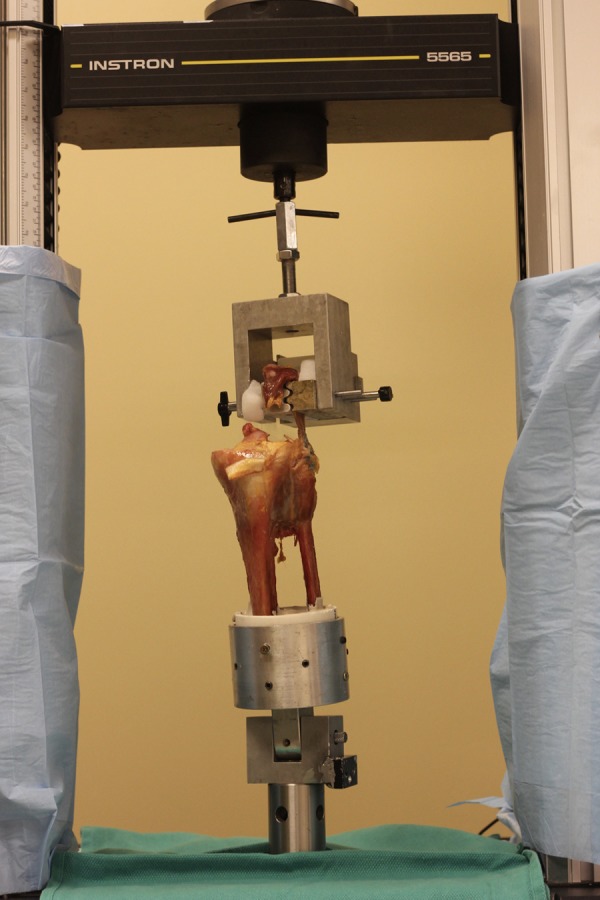
Uniaxial testing construct.
Results
The mean (95% CI, low-high) failure loads were the following: native, 1280 (1033-1527) N; simple fibular repair, 173 (88.4-257.6) N; simple fibular and tibial repair, 176 (127.9-224.1) N; fibular repair with tibial suture bridge, 191 (112.5-269.5) N; and transosseous repair, 327 (260.7-393.3) N. The mean (95% CI, low-high) stiffness values were the following: native, 46 (33-59) N/mm; simple fibular repair, 16 (10.9-21.1) N/mm; simple fibular and tibial repair, 14 (8.6-19.4) N/mm; fibular repair with tibial suture bridge, 13 (10.2-15.8) N/mm; and transosseous repair, 15 (12.5-17.5) N/mm. Interconstruct comparison of the mean failure load between the 3 repair groups involving suture anchors alone revealed no statistical difference. The transosseous repair had a significantly higher failure load when compared with simple fibular repair (P = .02), simple fibular and tibial repair (P = .02), and fibular repair with tibial suture bridge (P = .04). Interconstruct comparison of stiffness revealed no statistical difference between all constructs (P > .86). None of the repair techniques re-created the failure load or stiffness of the native biceps femoris tendon (P = .02). The native biceps femoris tendon exhibited the highest mean failure load and stiffness values (Table 2).
TABLE 2.
Maximum Failure Load and Stiffness of Repair Constructsa
| Maximum Failure Load, N | Stiffness, N/mm | |
|---|---|---|
| Native | 1280 (1033-1527) | 46 (33-59) |
| Simple fibular repair | 173 (88.4-257.6) | 16 (10.9-21.1) |
| Simple fibular and tibial repair | 176 (127.9-224.1) | 14 (8.6-19.4) |
| Fibular repair with tibial suture bridge | 191 (112.5-269.5) | 13 (10.2-15.8) |
| Transosseous repair | 327 (260.7-393.3) | 15 (12.5-17.5) |
aValues are expressed as mean (95% CI, low-high).
The mode of failure for all suture anchor repair constructs was sequential anchor pullout, with the exception of 1 specimen in the simple fibular and tibial repair group failing because of knot failure. The primary mode of failure for the transosseous repair construct was suture cut-through of the transosseous tunnel, with the exception of 1 specimen failing because of knot failure and 1 specimen failing because of suture tape failure.
Discussion
This cadaveric study evaluated the tensile strength of 4 distal biceps femoris repair constructs: simple repair to the fibula, simple repair to the fibula and tibia, fibular repair with a tibial suture bridge to the fibula and tibia, and transosseous repair to the fibula and tibia. While there was not a superior construct regarding stiffness, transosseous repair to the fibula and tibia had a higher mean failure load than all other constructs, with a mean of 327 N. While we hypothesized that a fibular repair with a tibial suture bridge would prove stronger than a simple repair and that repair to the tibia and fibula would prove stronger than repair to the fibula alone, this was not observed in our testing. In all tested repair constructs, it should be noted that the tested technique is a function of the entire construct, including tendinous quality, suture type, suture quantity, anchor type, anchor number, and anchor position.
The majority of described biceps femoris repair techniques have involved either direct suture repair at the myotendinous junction, suture anchor repair onto the fibula, or transosseous repair.4,5,8,11,15,19,28 Despite descriptions of repair techniques, there are no biceps femoris biomechanical studies for comparison. The fibular repair with a tibial suture bridge construct in this study was designed with previous transosseous-equivalent rotator cuff studies in mind to achieve fixation to a larger area of the footprint. In our study, all repair constructs utilizing suture anchors performed similarly on tensile testing, while a transosseous-style repair performed superiorly to those involving suture anchors alone. The differences observed in this study may be attributable to the porous nature of the proximal fibular head, as reflected in the predominant mode of failure being anchor cutout and suture cut-through of the fibula. This anatomic limitation should be considered clinically when surgeons evaluate repair options.
Isolated tension in the biceps femoris during both activities of daily living and rehabilitation is difficult to define. Past studies have focused on the hamstring complex as opposed to isolated intratendinous tension of single muscle units during activity. Furthermore, a biomechanical study has shown variability in both absolute maximum stress and corresponding knee flexion angle between flexible and inflexible persons.12 In the absence of formal biomechanical data regarding the forces generated by the biceps femoris, a conservative postoperative protocol is warranted. Sebastianelli et al19 utilized immobilization for 4 weeks at 60° of flexion, between 4 to 6 weeks active motion is allowed within 30° to 60°, progressing to free range of motion after 6 weeks, and progressive resistive exercises after 8 weeks. With modern repair techniques, we have not limited range of motion after biceps femoris repair but only limited patients to nonweightbearing for 6 weeks.
A limitation of this study is the advanced age of the specimens and the lack of bone density study on the specimens, which could be used to more effectively allocate the groups. Biceps femoris tears are more likely in younger patients with active lifestyles. The mean age of the specimens in this study was 59.3 years, which has an effect on suture anchor fixation. Cadaveric repair analysis is also limited, as it tests time-zero repair strength, which does not account for strength that occurs as tissue heals. Additionally, this study involved unidirectional tensile force, whereas a repair in vivo will be subject to a more complex combination of tensile, shear, and torsional forces during physiological knee motion. Using a nonanatomic loading profile in the study could have led to sequential anchor/suture failure and variability in loading angle, and consequently to inaccurate strength values. An additional weakness is that the specimens in the transosseous repair group were not paired to another group. A better design may have been to pair the transosseous repair group to the fibular repair with a tibial suture bridge group, leaving the native group unpaired.
A possible confounder between the transosseous-equivalent and transosseous repair groups is the difference in the tibial knotless suture anchor used: 2.9-mm push-in versus 3.5-mm screw-in. Variation in the suture anchor number and suture material is also a weakness of this study. Ideally, more variables regarding design would have been held constant; however, we designed the study to test the tensile properties of 4 constructs that we have used clinically, instead of what was biomechanically ideal for the study design. For example, in addition to the design of the transosseous repair contributing to its superior testing result, one also must consider that 2-mm suture tape was used in this group as opposed to No. 2 braided polyester and ultra–high-molecular-weight polyethylene suture.
The uniaxial materials testing machine applied force in a vector that represented near–full extension of the knee. Clinical case reports have indicated that this is the position of injury.5,8,19,28 We felt that a design mimicking the injury scenario would be the best tensile setup; however, it could be argued that after surgery, maximal hamstring contraction could be a possible mode of failure of repair, which occurs in knee flexion.
In conclusion, this cadaveric study demonstrated that transosseous biceps femoris repair is stronger than suture anchor repair. Repair strength and stiffness did not approach the native values of the biceps femoris.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Arthrex provided financial support for the acquisition of cadaveric specimens and material support with suture anchors. A.W.A. is a paid speaker for and receives research funding from Arthrex and Smith & Nephew.
Ethical approval was not sought for the present study.
References
- 1. Alioto RJ, Browne JE, Barnthouse CD, Scott AR. Complete rupture of the distal semimembranosus complex in a professional athlete. Clin Orthop Relat Res. 1997;336:162–165. [DOI] [PubMed] [Google Scholar]
- 2. Branch EA, Anz AW. Distal insertions of the biceps femoris: a quantitative analysis. Orthop J Sports Med. 2015;3(9):23259 67115602255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinkman J-M, Schwering P, Blankevoort L, Koolos J, Luites J, Wymenga A. The insertion geometry of the posterolateral corner of the knee. J Bone Joint Surg Br. 2005;87(10):1364–1368. [DOI] [PubMed] [Google Scholar]
- 4. David A, Buchholz J, Muhr G. Tear of the biceps femoris tendon. Arch Orthop Trauma Surg. 1994;113(6):351–352. [DOI] [PubMed] [Google Scholar]
- 5. Fortems Y, Victor J, Dauwe D, Fabry G. Isolated complete rupture of biceps femoris tendon. Injury. 1995;26(4):275–276. [DOI] [PubMed] [Google Scholar]
- 6. Jensen I, Kramhøft M. Distal rupture of the biceps femoris muscle. Scand J Med Sci Sports. 1994;4(4):259–260. [Google Scholar]
- 7. Krackow KA, Thomas SC, Jones LC. A new stitch for ligament-tendon fixation: brief note. J Bone Joint Surg Am. 1986;68(5):764–766. [PubMed] [Google Scholar]
- 8. Kusma M, Seil R, Kohn D. Isolated avulsion of the biceps femoris insertion-injury patterns and treatment options: a case report and literature review. Arch Orthop Trauma Surg. 2007;127(9):777–780. [DOI] [PubMed] [Google Scholar]
- 9. LaPrade RF, Bollom TS, Wentorf FA, Wills NJ, Meister K. Mechanical properties of the posterolateral structures of the knee. Am J Sports Med. 2005;33(9):1386–1391. [DOI] [PubMed] [Google Scholar]
- 10. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854–860. [DOI] [PubMed] [Google Scholar]
- 11. Lempainen L, Sarimo J, Mattila K, Heikkilä J, Orava S. Distal tears of the hamstring muscles: review of the literature and our results of surgical treatment. Br J Sports Med. 2007;41(2):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnusson SP, Aagaard P, Simonsen EB, Bojsen-Møller F. Passive tensile stress and energy of the human hamstring muscles in vivo. Scand J Med Sci Sports. 2000;10(6):351–359. [DOI] [PubMed] [Google Scholar]
- 13. Marshall JL, Girgis FG, Zelko RR. The biceps femoris tendon and its functional significance. J Bone Joint Surg Am. 1972;54(7):1444–1450. [PubMed] [Google Scholar]
- 14. McGoldrick F, Colville J. Spontaneous rupture of the biceps femoris. Arch Orthop Trauma Surg. 1990;109(4):234–234. [DOI] [PubMed] [Google Scholar]
- 15. Meier SW, Meier JD. The effect of double-row fixation on initial repair strength in rotator cuff repair: a biomechanical study. Arthroscopy. 2006;22(11):1168–1173. [DOI] [PubMed] [Google Scholar]
- 16. Moorman CR, LaPrade RF. Anatomy and biomechanics of the posterolateral corner of the knee. J Knee Surg. 2005;18(2):137. [DOI] [PubMed] [Google Scholar]
- 17. Pan K, Ting F. Delayed repair of rupture of the biceps femoris tendon: a case report. Med J Malaysia. 2000;55(3):368–370. [PubMed] [Google Scholar]
- 18. Schilders E, Bismil Q, Sidhom S, Robinson P, Barwick T, Talbot C. Partial rupture of the distal semitendinosus tendon treated by tenotomy: a previously undescribed entity. Knee. 2006;13(1):45–47. [DOI] [PubMed] [Google Scholar]
- 19. Sebastianelli WJ, Hanks GA, Kalenak A. Isolated avulsion of the biceps femoris insertion: a case report. Clin Orthop Relat Res. 1990;259:200–203. [PubMed] [Google Scholar]
- 20. Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14(1):39–45. [DOI] [PubMed] [Google Scholar]
- 21. Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee: its anatomy and injury patterns associated with acute anterolateral-anteromedial rotatory instability. Am J Sports Med. 1996;24(1):2–8. [DOI] [PubMed] [Google Scholar]
- 22. Terry GC, LaPrade RF. The posterolateral aspect of the knee anatomy and surgical approach. Am J Sports Med. 1996;24(6):732–739. [DOI] [PubMed] [Google Scholar]
- 23. Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21(1):55–60. [DOI] [PubMed] [Google Scholar]
- 24. Tubbs RS, Caycedo FJ, Oakes WJ, Salter EG. Descriptive anatomy of the insertion of the biceps femoris muscle. Clin Anat. 2006;19(6):517–521. [DOI] [PubMed] [Google Scholar]
- 25. Valente M, Mancuso F, Alecci V. Isolated rupture of biceps femoris tendon. Musculoskelet Surg. 2013;97(3):263–266. [DOI] [PubMed] [Google Scholar]
- 26. Verburgh H, Keeman J. Complete ruptuur van de M biceps femoris-pees. Ned Tijdschr Geneeskd. 1991;135:1970–1971. [PubMed] [Google Scholar]
- 27. Waltrip RL, Zheng N, Dugas JR, Andrews JR. Rotator cuff repair: a biomechanical comparison of three techniques. Am J Sports Med. 2003;31(4):493–497. [DOI] [PubMed] [Google Scholar]
- 28. Werlich T. [Isolated rupture of the biceps tendon of the knee joint]. Unfallchirurg. 2001;104(2):187–190. [DOI] [PubMed] [Google Scholar]
- 29. Wijdicks CA, Ewart DT, Nuckley DJ, Johansen S, Engebretsen L, Laprade RF. Structural properties of the primary medial knee ligaments. Am J Sports Med. 2010;38(8):1638–1646. [DOI] [PubMed] [Google Scholar]





