Abstract
Physical activity plays a crucial role in the prevention and treatment of type 2 diabetes. Therefore, it is important to understand why so few adults with type 2 diabetes regularly engage in physical activity. The role of self-regulation in the context of health-related behavior adherence, especially in terms of physical activity engagement and adherence, has largely been reviewed based on the strength energy model. Building on this line of research, the aim of this theoretical work was to highlight how self-regulation and ego depletion can influence the lower rate of physical activity participation among adults with type 2 diabetes, compared to adults from the general population.
Keywords: exercise, motivation, physical activity, self-control, self-regulation, type 2 diabetes
Introduction
According to the World Health Organization (WHO) (2006, 2016), type 2 diabetes (T2D) is a chronic disease that occurs when either the pancreas does not produce enough insulin or the body uses insulin ineffectively, which is commonly associated with elevated blood glucose. T2D differs from type 1 diabetes in that insulin intake is not required for survival. The increasing prevalence of T2D is a major public health problem, with approximately 13 percent of the population worldwide having a diabetes diagnosis, of which around 90 percent is a type 2 and an estimated total annual cost of US$348 billion in North America, which correspond to almost 14 percent of the total health budget of the region (International Diabetes Federation, 2015). Until recently, T2D manifested mainly in adults 40 years and older, but in the last 20 years, an increase in the prevalence of T2D among youth has been observed (Dabelea et al., 2014). Physical activity (PA) plays a crucial role in the treatment of T2D (Colberg et al., 2016; Sigal et al., 2006; Zanuso et al., 2010). By following the recommendations of practicing at least 150 minutes of moderate to vigorous PA (MVPA) per week (including aerobic and resistance exercises), regular PA practice promotes the management of core metabolic goals for T2D, glycemia, arterial pressure, and cholesterol (American Diabetes Association (ADA), 2014, 2017; Colberg et al., 2016). It is worth noting that only 13 percent of patients are achieving the composite goal of these three clinical indicators (Leiter et al., 2013). Despite the benefits of PA for T2D, between 60 and 70 percent of adults with diabetes in the United States and in Canada are not practicing enough PA or are not considered physically active (Health Canada, 2002; Morrato et al., 2007; Palakodeti et al., 2015), compared to between 40 and 50 percent of adults in the non-diabetic US and Canadian populations (Health Canada, 2002; Morrato et al., 2007; Statistics Canada, 2014; Ward et al., 2016)
Given these participation trends, it has become essential to understand why so few adults with T2D (hereafter T2D adults) regularly practice PA, despite the existing documentation on its beneficial effects for their health. To examine this question, some authors have published work focusing on PA self-reported barriers in T2D adults. For instance, in a systematic review including 13 studies totaling 3465 participants from six different countries (United Kingdom, United States, South Africa, Kuwait, Australia, and Canada), Korkiakangas et al. (2009) have summarized and organized the main barriers reported by T2D adults in seven categories: lack of motivation, health problems, negative emotions (e.g. shame), lack of social support, lack of facilities for exercises, cultural barriers, and weather.
A promising theoretical approach to understanding and solving this concern is the strength energy model (SEM, also known as the strength model of self-control, the self-regulatory strength model, or simply the strength model), developed by Baumeister and colleagues (Baumeister et al., 1998, 2007; Baumeister and Heatherton, 1996; Muraven and Baumeister, 2000; Vohs and Heatherton, 2000). According to the SEM, self-control is a finite resource that determines the capacity for effortful control over dominant responses and, once expended, it leads to impaired self-control in task performance, known as ego depletion. More precisely, “ego depletion refers to the state of diminished self-control resources, when one cannot or does not successfully implement further control” (Maranges and Baumeister, 2016). Building on the SEM’s assumptions, Hagger et al. (2009, 2010a, 2010b) recently conducted a literature review offering researchers and practitioners a better understanding of self-regulation and ego depletion in the context of health-related behavior adherence, especially in terms of PA engagement and adherence. Within their work, these authors describe extensively how the SEM can explain behavioral self-regulation in a PA participation context.
The SEM, its principal components, and its implications for PA practice are described below. Then, based on this model’s assumptions regarding self-regulation resources, the herein work aims to explain how self-regulation could play a major role in the difficulties experienced by T2D adults related to PA practice compared to adults from the normal population. More specifically, section “Components of the SEM” presents the two key concepts of the SEM, namely, self-control and self-regulation. Section “Underlying assumptions of the SEM” explains the underlying hypotheses of the SEM as self-control resource exertion, replenishment, and improvement. Section “The physiological energy resource of self-regulation” reports the latest theoretical advances in terms of the physiological counterpart of the SEM and its “energy reserve.” Section “Fatigue, ego depletion, and self-regulatory failure” presents key concepts related to self-regulatory exertion and its expected consequences, while section “Using the SEM to explain the low rate of PA participation among T2D adults” introduces how, according to the SEM, T2D adults could be vulnerable to chronic ego depletion, which can explain their lower PA participation. Several strategies to overcome ego depletion are then provided in section “Strategies for preventing self-regulatory failure in the context of PA participation among T2D adults,” followed by suggestions for future research in section “Implications and future directions.” Of note is that although few sections look at self-regulation physiological substrates (i.e. glucose, brain structures, and executive functions), the main focus of this work consists of examining the psychological (versus physiological) perspective of self-regulation and its impact on PA engagement.
Components of the SEM
Self-control
According to the SEM, self-control is a capacity that depends on a global limited resource that can become depleted or replenished following different behaviors. This capacity tends to conserve energy and can be improved with training (Vohs and Baumeister, 2016). Self-control resources are global and limited because every task that requires self-control uses the same resources. No matter what caused the depletion, if the resource is so low that self-control cannot be exerted efficiently, successful completion of the task will be impeded (Hagger et al., 2010b; Muraven and Baumeister, 2000). More details on these underlying assumptions of the SEM (conservation, recovery, and training) are presented further below.
Self-control has major positive impacts on a variety of life domains: work, school, relationships, health, health-related behaviors, and longevity (Maranges and Baumeister, 2016). It also seems that the benefits of increased self-control are linear, never reaching a point where more self-control would be detrimental (De Ridder et al., 2012; Tangney et al., 2004). Moreover, self-control as a trait (dispositional self-control) is strongly associated with the formation and maintenance of habits, as well as breaking habits and making life choices that reduce exposure to temptations (De Ridder et al., 2012; Vohs and Baumeister, 2016). Punctual, specific acts of self-control (Maranges and Baumeister, 2016), like refraining the urge to eat a doughnut before an aerobic class, are considered sub-processes of self-regulation, “those that aim to override unwanted, prepotent impulses or urges” (Hofmann et al., 2012).
Self-regulation
The term “self-regulation” is considered as “the ability to alter one’s responses based on rules, goals, ideals, norms, plans, and other standards” or as “a set of psychological and perceptual processes by which individuals work toward the achievement of goals and objectives by keeping them on track and minimizing distractions or impulses” (Baumeister and Vohs, 2016; Carver and Scheier, 2001). Generally speaking, self-regulation arises in response to a clash between two desires, one that is more focused on immediate interests and another that is more distant and that takes into consideration what would be best, all things considered (Baumeister and Vohs, 2007). Self-regulation is a process working as a feedback loop in which the actual state is compared with the desired state and in which adjustments (punctual acts of self-control) are made when deviations from the goal occur (Carver and Scheier, 2016). For example, when people engage in a new PA routine, they may face barriers, including fatigue. Using self-regulation would mean remembering that a goal has not yet been achieved and then choosing to push oneself to engage in the activity, despite feeling fatigued. In this example, pushing oneself is a form of exerting self-control to stay in line with a goal.
When we feel the desire to act in a certain way, but we choose to act in a way that would be more appropriate or consistent with our goals, we show self-regulation, which is based on four variables: the standard, monitoring, energy, and motivation (Baumeister and Vohs, 2007). The standard is the criterion or the goal according to which we want to align our behavior. For example, when people attend fitness classes, they try to replicate the movements made by the instructor. They do not execute movements impulsively, as the standard in such a situation is to imitate the instructor. It could also be a more personal and broader goal, such as being physically active. Monitoring refers to focusing attention on a behavior and to the desire to undertake and compare different standards. In other words, it is the action of comparing the actual state with the desired state and ensuring the actual behavior stays in line with the goal. According to the SEM, controlling, changing, or retaining a behavior; making a decision; and showing willingness require energy. This energy, also known as self-regulatory strength, a self-control resource, or willpower, is considered a global limited internal resource (Baumeister et al., 1998; Baumeister and Vohs, 2016; Hagger et al., 2009; Vohs et al., 2008). Of note is that the capacity and effectiveness to regulate oneself depend on the available energy level. If this level is so low that behaviors requiring self-control are affected negatively or the person lacks willingness, the individual will enter a state of ego depletion (Baumeister and Vohs, 2007). For example, in the morning after a good night’s sleep, it is more likely that one will have enough energy to undertake a new resolution, such as starting a new workout program, compared to in the evening. However, after a day of particularly demanding work, it is possible that one will not have enough energy to regulate him or herself. In this situation, it will be more difficult to undertake the same resolution. The motivation level is the fourth variable on which the self-regulation capacity stands (Baumeister and Vohs, 2007). For someone to self-regulate voluntarily, one must be motivated to observe the standard, to use its resources, and, therefore, to self-regulate. Thus, even when the first three variables are favorable, if the person does not consider it important or is not motivated to self-regulate, he or she will not. Worth mentioning is that the four variables are all-important, but not all necessary. For example, to some extent, motivation can compensate for a lack of energy, allowing for successful self-regulation even within a state of ego depletion, but only temporarily (Baumeister and Vohs, 2007, 2016; Hagger et al., 2010b). Eventually, the fatigue will become insurmountable, and replenishing activities will become necessary.
Underlying assumptions of the SEM
As outlined by Hagger et al. (2010a, 2010b), the SEM is based on three main underlying hypotheses: the conservation, the recovery, and the training hypotheses. According to the conservation hypothesis, as self-control is drawn from a limited energy reserve, individuals must allocate such energy strategically while considering future events. Therefore, if individuals consider that they will have to engage in a demanding self-regulatory task in the future, they will be more likely to conserve their resources. By doing so, tasks requiring self-regulation could be affected negatively in the meantime. The recovery hypothesis states that following depletion, the self-regulation capacity can be restored. Just as with a muscle, the time for recovery depends on the intensity and duration of the depleting task. The more severe the exertion, the longer the recovery will be. A sufficient amount of breaks, relaxation sessions, and sleep are essential in the replenishment of self-control resources (Krizan and Garrett, 2016; Tyler and Burns, 2008). Finally, the training hypothesis supposes that the self-regulation capacity can be developed and increased through training (Hagger et al., 2010b; Muraven, 2010). Just as a muscle would develop through regular, specific exercises, appropriate exposure to self-control tasks is expected to lead to improvements in self-regulation capacity. Noteworthy is that such improvements are not limited to training conditions; they could also be generalized to other activities. For instance, as reported by Hagger et al. (2010b), if someone trains the self-regulation capacity by forcing him or herself to use a non-dominant hand for a certain amount of time, the increase in the self-regulation capacity could also benefit his or her adherence to a new PA. However, enough recovery time and experience with self-regulatory success are needed for the training to produce self-regulation performance improvements (Hagger et al., 2010b).
The physiological energy resource of self-regulation
Even though studies have reported a significant relationship between glucose levels and ego depletion (Baumeister and Vohs, 2016; Hagger and Chatzisarantis, 2013; Hagger et al., 2010a), others have not succeeded in replicating these results (Boyle et al., 2016; Dang, 2016; Vadillo et al., 2016; Zahn et al., 2016). In response to the increasing number of published arguments against the direct link between glucose and self-control, Baumeister and Vohs (2016) suggested that glucose alone is not sufficient to explain the SEM, because ego depletion (the state of self-control resource exertion) appears long before glucose exertion. Therefore, other research avenues have been suggested to replace the glucose hypothesis as a physiological equivalent to willpower, such as adenosine (Baumeister and Vohs, 2016). Even if glucose were the equivalent of willpower (the limited physiological energy resource used by self-regulation), self-regulation alone would not be a threat to the body’s global reserve of glucose (Baumeister, 2016; Baumeister and Vohs, 2016; Vohs and Baumeister, 2016). Self-regulation relies on several executive functions (e.g. inhibiting, monitoring, and shifting) (Hirt et al., 2016) and, by extension, brain structures (Magen et al., 2014; Vohs and Baumeister, 2016) that rely on glucose, but the energy consumption of self-regulation is too small to disrupt glucose levels in the brain (Segerstrom et al., 2016). According to Baumeister and Vohs (Baumeister and Vohs, 2016; Vohs and Baumeister, 2016), the main taxing effect of self-regulation would not be on the glucose reserve, but on brain receptiveness, attention, and mental effort.
Fatigue, ego depletion, and self-regulatory failure
Similar to the muscles, neurological processes associated with self-regulation are incapacitated by continuous solicitation (Wagner and Heatherton, 2016). Even though the body’s energy levels are still largely sufficient to sustain effort for a longer period, an increase in fatigue leads to decreased performance (Krizan and Garrett, 2016). Only appropriate replenishment conditions (e.g. taking breaks, doing enjoyable activities, and sleeping) can counteract the deleterious effects. It has been suggested that this feeling of fatigue could be attributed to a conservation tendency toward the executive functions required by self-regulation (Krizan and Garrett, 2016). This tendency would limit uses of executive functions and self-regulation to preserve brain receptiveness for other processes. When willpower runs low after self-control exertion, the individual enters a state of self-regulatory fatigue called ego depletion (Baumeister, 2014). It is important to note that even though general fatigue can affect self-control and therefore ego depletion, ego depletion is not fatigue (Baumeister et al., 2006; Hagger et al., 2016; Vohs et al., 2011). Within a state of ego depletion, there are not enough resources left to self-regulate, which increases the chance of a self-regulatory failure.
A self-regulatory failure is characterized by a lack of self-control or by its opposite, impulsivity, and it takes the form of either under regulations (“failure to exert control over oneself”) or misregulations (“exerting control in a way that fails to produce the desired result”) (Baumeister et al., 1994; Sayette and Creswell, 2016). In other words, a self-regulatory failure occurs when the self-regulation process fails. In a PA context, a self-regulatory failure would imply, for instance, the skipping of a training session after an exerting workday because one does not feel like training. A self-regulatory failure is expected to occur when (1) a desire becomes too strong, (2) self-regulatory fatigue becomes too strong, or (3) the importance of the goal (motivation) is not strong enough (Heatherton and Wagner, 2011; Krizan and Garrett, 2016). Physiologically, a self-regulatory failure has been proposed to be the result of a connectivity impairment among the brain structures responsible for the executive functions essential to self-regulation (Vohs and Baumeister, 2016).
Using the SEM to explain the low rate of PA participation among T2D adults
The limited resource model of self-control, as conceptualized by the SEM, could prove useful in explaining the low PA participation among T2D adults (Adriaanse et al., 2013; Cradock et al., 2017). As the integration of regular PA practice is one of the many complex tasks T2D adults must accomplish to manage their condition optimally (Powers et al., 2017), having a sufficient self-regulation capacity acts as a facilitator and is positively associated with goal achievement (Baumeister et al., 1998; Muraven and Baumeister, 2000). Conversely, the self-regulation capacity becomes an important barrier to PA engagement if it becomes exhausted by previous self-control demands, too many self-control tasks undertaken simultaneously, or the anticipation of a future activity requiring self-regulation (Dorris et al., 2012; Hagger et al., 2010b; Martin Ginis and Bray, 2010; Muraven et al., 2006). In such a situation, one is left in a state of exhaustion or ego depletion, and such consequences as self-regulatory failure are expected (Hagger et al., 2009). Because T2D adults must engage in several tasks to manage and cope with their condition, such as monitoring their diets (what to eat, when to eat, what portion size, etc.), glucose levels (keeping it within the acceptable range), and medications (when to take what) (Powers et al., 2017), they are left with few self-regulatory resources to integrate and sustain PA into their daily life. This in itself requires a high level of self-control (Hagger et al., 2009).
In line with the issue raised above regarding PA engagement and self-regulation, a highly interesting research avenue pertains to the SEM’s capacity to explain the low rate of PA participation among T2D adults compared to that among adults from the general population. Accordingly, the potential effects of (1) glucose, brain structures, and executive functions; (2) mood regulation; and (3) T2D-related symptoms on ego depletion and, as a consequence, on PA participation among T2D adults are presented below and resumed in Figure 1. Each topic is described as a potential predisposition for T2D adults to be ego depleted, rendering them more vulnerable to self-regulatory failures when attempting to include PA in their daily life.
Figure 1.
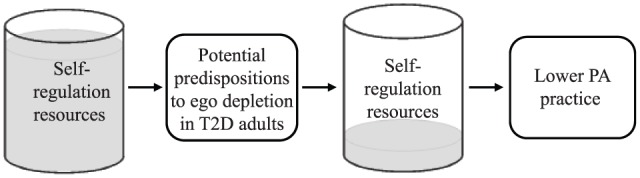
How diabetes management related tasks influence self-regulation resources and PA practice among T2D adults.
Glucose, brain structures, and executive functions
Over the past years, glucose has been proposed as a physiological equivalent of willpower, the self-control resource that becomes depleted with usage (Gailliot and Baumeister, 2007; Gailliot et al., 2007). However, recent studies, as reported by the meta-analysis of Vadillo et al. (2016), have failed to establish a clear link between glucose and self-regulation, casting doubt on their hypothesized link. Nevertheless, some evidence shows that glucose still plays an important role in self-regulation (Baumeister and Vohs, 2016). This seems an important consideration for T2D adults, given that their condition is intrinsically related to blood glucose monitoring (Levin et al., 1999; WHO, 2016). For instance, the impact of glucose on self-regulation among T2D adults could be indirect by means of its effect on brain structures. This assumption is partially supported by a recent suggestion that a self-regulatory failure could be the result of reduced coupling between brain regions, mainly in the prefrontal cortex (PFC) (Wagner and Heatherton, 2016). In fact, three sub-regions of the PFC (ventromedial PFC, lateral PFC, and anterior cingulate cortex), each linked to executive functions and self-regulatory processes, are largely interconnected with one another and have connections with other self-regulation-related brain regions. For example, the ventromedial PFC is associated with maintaining self-control, the lateral PFC with recognizing when to switch tasks, and the anterior cingulate cortex with the capacity to keep a behavior in line with a goal. Additionally, many studies have linked ego depletion to reduced connectivity between and from those three regions (Wagner and Heatherton, 2016).
The connectivity explanation of a self-regulatory failure concurs with the SEM idea that self-regulation depends on a common resource that becomes depleted through exertion. It could explain why prior studies have found a significant relation between glucose and self-regulation. As reported by a recent meta-analysis, swishing a glucose solution around in the mouth is the only way glucose may significantly be implicated in self-control (Dang, 2016). This could be explained by the activation of self-regulation-related brain regions by glucose-sensitive receptors in the mouth (Wagner and Heatherton, 2016). Moreover, according to the results of a meta-analysis examining the impact of diabetes on cognitive functions (McCrimmon et al., 2012), as well as a meta-analysis surveying the impact of T2D on memory and executive functions (Sadanand et al., 2016), T2D significantly burdens cognitive functioning. Among the reported affected cognitive functions, executive functions and attention are essential to self-regulation (Vohs and Baumeister, 2016). T2D is also associated with the deterioration of many brain structures linked with those cognitive functions, such as the frontal lobe (Moulton et al., 2015), being highly responsible for self-regulation and normal functioning (Vohs and Baumeister, 2016). Thus, even though the exact dynamic between glucose and self-control remains to be explained, it is still accepted that glucose plays a significant role in self-regulation. Given the intrinsic relation between glucose and their condition, T2D adults could be physiologically more prone to experience self-regulatory failures.
Mood regulation
Depression, anxiety, stress, and diabetes-related distress
Compared to the non-diabetic population, T2D adults are more prone to suffer from depression (Mezuk et al., 2008; Nouwen et al., 2010; Roy and Lloyd, 2012), anxiety disorders, and distress (Fisher et al., 2008), which can seriously hinder diabetes self-management (Powers et al., 2017), including PA participation (Ciechanowski et al., 2000). Moreover, T2D adults tend to feel negative emotions about PA. They feel ashamed to train with people who do not have T2D, and they are afraid of getting hurt and of experiencing a hypoglycemic episode, which could impede their PA engagement (Korkiakangas et al., 2009). According to the SEM conceptual framework, regarding the assumption that the self-control capacity is global and limited, it is expected that negative emotional states, such as depressive and anxious symptoms related to PA or not, would have a negative impact on the self-control capacity (Baumeister et al., 1999; Muraven and Baumeister, 2000). Therefore, T2D adults who are particularly vulnerable to negative emotional states would also be more prone to experience ego depletion. In such a state, a self-regulatory failure is more likely to occur, and thus, PA practice and diabetes self-management are likely to be impeded.
It is also important to consider the impact of stressful life events on diabetes self-management and PA adherence among T2D adults. According to Baumeister et al. (1999), stressful life events tax the self-control capacity, and therefore, fewer resources remain to cope with the demands associated with other activities. Moreover, the act of coping in the face of stress depletes self-control resources and negatively affects self-control performance (Muraven and Baumeister, 2000). As the integration of PA into one’s life requires a great amount of self-control, it is proposed that stress can affect PA adherence (Hagger et al., 2009, 2010b). In addition, as stress directly draws from one’s self-regulation capacity (Baumeister et al., 1999), it is susceptible to affect negatively the executive functions and brain structures (McEwen, 2016) associated with self-regulation and to worsen diabetes management and glucose levels (ADA, 2017). Prior research supports the negative impact of depression (Katon et al., 2010), anxiety (Lipscombe et al., 2014), and perceived stress (Delahanty et al., 2006) on PA engagement among T2D adults.
In addition to daily stress, the multiple taxing tasks related to diabetes self-management are directly predictive of a specific form of stress called diabetes distress, which is highly common among people with diabetes (Young-Hyman et al., 2016). According to the ADA (2017), diabetes distress is associated with “significant negative psychological reactions related to emotional burdens and worries specific to an individual’s experience in having to manage a severe, complicated, and demanding chronic disease such as diabetes.” T2D adults are exposed to a daily burden associated with a high stress potential, which is the self-management of their condition. Indeed, the ADA, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics state, “Diabetes is a chronic disease that requires a person with diabetes to make a multitude of daily self-management decisions and to perform complex care activities” (Powers et al., 2017). This statement has two main implications regarding the self-regulation capacity and therefore ego depletion for T2D adults. First, it requires a person newly diagnosed with T2D to learn and incorporate into his or her life and daily routine complex new habits. Second, even after learning is achieved or years after diagnosis, T2D still requires daily complex self-management decisions. These two implications have an important impact on self-control resources, making T2D adults more prone to a state of chronic ego depletion, which has the potential to burden a wide variety of life aspects, including PA engagement (Wang et al., 2015).
T2D-related symptoms
According to Grootenhuis et al. (1994), the most common T2D-associated symptoms include frequent urination, significant thirst, a lack of energy, irritability, fatigue, a lack of concentration, palpitations, shortness of breath, pain, loss of sensation, sensations of tingling or prickling, and vision deterioration. All of these symptoms are potential discomforts with which T2D adults must cope, including during their PA session. In line with prior work by Solberg Nes et al. (2009, 2010, 2011) on chronic multi-symptom illnesses and on coping with chronic pain, the authors herein suggest that symptoms related to T2D may diminish self-control resources. Moreover, T2D adults are often afflicted with poor sleep quality (Luyster and Dunbar-Jacob, 2011) and sleep disorders (Davies et al., 2006; Shaw et al., 2008; West et al., 2006). Those who do not have enough sleep and who have a poor sleep quality also tend to present poorer glycemic control and therefore higher HbA1c levels (Trento et al., 2008), which in turn leads to more severe diabetes-related symptoms, including fatigue. As explained above, self-control resources become replenished, among others, through sleep. If sleep sessions are deficient or incomplete, T2D adults potentially cannot restore fully the energy level required to self-regulate their daily lives and attain their goals.
Conclusion on the SEM’s capacity to explain the low rate of PA participation among T2D adults
Compared to the general population, T2D adults engage less frequently in PA (Health Canada, 2002; Morrato et al., 2007; Nelson et al., 2002; Plotnikoff et al., 2011; Statistics Canada, 2014; Ward et al., 2016). In authors’ view, this could be explained by the fact that T2D adults present higher self-regulation vulnerabilities compared to others. Although the hypothesis according to which glucose is the equivalent of willpower could potentially explain this difference (given that glucose regulation is a core problem associated with T2D), recent developments in the SEM tend to confirm that glucose is not the equivalent of willpower, although it still plays an important role in self- regulation (Baumeister and Vohs, 2016; Vadillo et al., 2016). Therefore, besides the hypothetical direct link between glucose and self-regulation, other factors can explain why T2D adults, given their condition, would be more susceptible to chronic ego depletion. First, T2D has been shown to tax significantly cognitive functioning, including the executive functions essential to self-regulation, and to deteriorate brain regions underlying these functions. Second, T2D adults present higher depressive and anxious symptoms than the general population, directly impeding their self-control. Finally, symptoms associated with T2D can also drain self-regulation resources, if only because of pain and impaired sleep quality. Specifically, every T2D-related symptom comes with a potential self-control cost given that individuals need to cope with discomfort or pain through daily activities, including PA sessions.
Strategies for preventing self-regulatory failure in the context of PA participation among T2D adults
The main objective of the present theoretical review was to highlight the importance of the SEM when it comes to explaining the lower rate of PA participation among T2D adults compared to adults from the general population. As such, the authors herein presented the latest theoretical developments in the SEM, as well as the underlying rationale or reasons why T2D adults would be more susceptible to chronic ego depletion, which, in turn, favors self-regulatory failure in PA engagement. In accordance with the SEM, challenging new behaviors, such as PA practice, should be integrated carefully and progressively to avoid ego depletion and dropout. T2D adults are often asked to work on multiple, complex, and challenging tasks and to perform new behaviors, such as acquiring knowledge related to diabetes management, changing their diets, monitoring their glucose levels, and, if required, discontinuing drinking alcohol and smoking. This list cannot be undertaken all at once. Trying to do so could render a person more vulnerable to burnout (Schmidt et al., 2007) and could lead to a state of chronic ego depletion (Hagger et al., 2010a). Indeed, when managing their condition, T2D adults may experience an overload of demanding self-control tasks combined with a lack of breaks and self-regulatory success. As outlined by Hagger et al. (2010a), such a situation could be the equivalent of over-training, which in this case would lead to a state of chronic ego depletion.
In consideration of the above-mentioned points, T2D adults can use various strategies to cope with their condition, while being careful with their self-regulation capacity and other demanding activities. The next section aims to present strategies that can prevent self-regulatory failures in the context of PA participation and T2D adult-related vulnerabilities. Because few examples in the literature exist regarding the promotion of self-regulation in T2D adults in a PA participation context, strategies to prevent ego depletion or to restore the self-regulation capacity are suggested based on research conducted with non-T2D individuals.
Sequential integration of new behaviors
The first technique to prevent ego depletion and increase PA engagement in T2D adults pertains to the progressive integration of new behaviors. A new behavior integrated into T2D adults’ routines is more likely to be maintained over time if this additional demand on self-control is well planned to occur when self-control resources are expected to be available (Hagger et al., 2010b). If several new behaviors must be integrated into one’s routine, as is the case for adults newly diagnosed with T2D (e.g. acquiring knowledge associated with T2D management, managing a diet, and engaging in PA regularly), patients are advised to undertake one new behavior at the time. Even though other behaviors are important, individuals are encouraged to initiate each progressively and to select attainable goals (Hagger et al., 2010b). Trying to modify or to integrate too many novel behaviors at the same time can be too demanding on self-control resources, therefore increasing the risk of self-regulatory failure and PA dropout. Results obtained by Jenkins et al. (2016) corroborate this assumption among adults diagnosed with T2D trying to improve their dietary adherence. These researchers found that the more severe the overexertion of self-control, the sooner the dietary relapse.
Self-regulation training
Self-regulation training is another strategy to counter ego depletion and increase PA engagement among T2D adults. Self-regulation training can be undertaken in the same way as muscle training. Regular exposure to demanding situations in which one experiences self-regulatory success, followed by sufficient breaks and rest to allow for the replenishment of self-control resources, is expected to lead to self-regulation improvements (Hagger et al., 2010b). Every task requiring self-control, from weak urges to those that challenge self-regulatory capacity limits, is a potential self-regulation training opportunity, provided one remains within the limits of his or her current capacity. Exceeding one’s capacity, even for a training exercise, can lead to ego depletion and self-regulatory failure. However, just as the benefits of developing self-regulation are transferable to other situations, the costs of ego depletion are too. Thus, self-regulation improvements or ego depletion developed during a training task could affect primary goal-related self-control tasks. Training self-regulation to achieve a specific goal can be achieved in a goal-related context with time and practice or in a second, easier goal-related context that will bolster self-regulation in general, which will also benefit the main goal. It is expected that these additional self-control challenges will promote PA self-regulation. In other words, additional self-control challenges will improve the likelihood of self-regulatory success in PA engagement and adherence by improving individuals’ self-regulation capacity through prior integration of an easier behavior. For instance, T2D adults could train their self-regulation skill by increasing the amount of vegetables they eat daily. Once this behavior has been assimilated into their routine, eating more vegetables won’t require much self-regulatory resources anymore and PA practice will be easier to initiate. Stated otherwise, the self-regulation process associated with PA practice is expected to be facilitated by prior self-regulatory success related to vegetables consumption. Then, breaks within and between self-control tasks, including quality sleep and relaxation, are to be planned according to the intensity of the self-control demands.
The greater the depletion, the longer the resting period must be. As previously mentioned, sleep quality has multiple positive effects on the lives of T2D adults. Regarding brain function, sleep has been shown to play an important role in brain connectivity in the frontal brain regions (Verweij et al., 2014), which are considered to play a central role in self-regulation. Furthermore, reduced brain connectivity is actually considered a potential physiological equivalent of ego depletion (Vohs and Baumeister, 2016). Therefore, increasing sleep quality could improve the capacity to self-regulate among T2D adults.
Engaging in PA to diminish T2D-related self-regulatory vulnerabilities
A third way to forestall ego depletion and promote PA participation in T2D adults is actually to initiate and sustain PA engagement. In fact, engaging in PA regularly has been found to be associated with an increase in brain volume, as well as better cognitive functioning, particularly in terms of attention, executive functions, and the brain regions associated with self-regulation in non-clinical populations (Buckley et al., 2014; Colcombe et al., 2006; Colcombe and Kramer, 2003; Daly et al., 2014). Likewise, engaging in PA regularly has been found to increase executive functions in T2D adults (Indelicato, 2009; Vincent, 2014), which are important for self-regulation. Therefore, PA could help counteract the damaging effects of T2D on the brain and as such could diminish the self-regulatory vulnerability of T2D adults. In addition, PA has been shown to aid in blood glucose regulation, improve well-being, and diminish some T2D-related symptoms (Colberg et al., 2016; Sigal et al., 2013; Young-Hyman et al., 2016; Zanuso et al., 2010). Finally, as indicated further above, the integration of regular PA practice in one’s daily routine represents an opportunity for self-regulation training. When PA is performed appropriately, with enough rest and chances for success, it can increase the self-regulation capacity and render future self-regulation tasks easier (Hagger et al., 2010b).
Forming habits
Another strategy to hamper ego depletion and increase PA participation among T2D adults is converting PA into a habit and making life choices that reduce exposure to temptations (e.g. going to a gym close to one’s workplace or choosing a route between work and home that does not pass by one’s favorite fast-food restaurant). These are effective strategies to reduce self-control demands (De Ridder et al., 2012; Maranges and Baumeister, 2016). Unsurprisingly, it has been suggested that to preserve self-control resources, people benefit from working on automatizing their behaviors (Baumeister and Alquist, 2009). When a behavior becomes a habit, the action occurs almost automatically, without really paying attention to the pros and cons and requiring less decision-making, thus necessitating less self-control. T2D adults have many complex decisions to make daily to manage their condition. To lighten this self-regulation burden, they could attempt to convert as many decisions as possible into habits. For instance, if one decides to incorporate PA practice into his or her daily life, automating this action could involve finding a parking spot a 10-minute moderate walk from work and using this same parking spot every workday. Eventually, using this parking spot and taking this moderate walk will become a habit, and no self-control will be necessary.
Using planning techniques
Planning is another self-regulatory strategy that can facilitate PA participation and limit ego depletion, because the execution of such a strategy does not require self-control resources (Gollwitzer, 1999; Hagger et al., 2010b). Once a goal has been chosen and the intention of acting toward this goal has been set, planning concretizes the idea and helps in automating future decisions related to goal-associated behaviors (Hagger and Luszczynska, 2014). It allows for deliberation and decision-making in advance so that when the time comes to act, one will be able to follow the plan automatically, almost without thinking or making any decisions, which will lighten the self-regulation burden. Planning techniques facilitate the realization of one’s intention and favor its translation into action by defining a specific context in which the action will take place (when and where) and the details of said action (how) (Hagger and Luszczynska, 2014). Worth noting is that planning techniques can be learned, practiced, and improved to help people develop their self-regulatory capacity (Schwarzer, 2008). However, one must be cautious when making several plans because elaboration requires self-regulatory resources, which makes the elaboration of multiple plans particularly taxing (Hagger and Luszczynska, 2014). Having too many plans could hinder the adoption of each specific plan as a result of the increased risk of triggering opposing goals (Hagger and Luszczynska, 2014).
Using mental contrasting
Another self-regulatory technique used to increase PA participation while minimizing self-control demands is mental contrasting. This technique consists of contrasting the achievement of a goal with the obstacles of the current reality (Oettingen, 2000, 2012). Mental contrasting has been shown to support T2D adults in the self-management of their condition, including PA participation (Adriaanse et al., 2013). To illustrate this technique, if one has the goal to engage in PA for 30 minutes per day, he or she could think about four desirable characteristics related to the achievement of this goal and then think about four current obstacles to achieving this goal. This technique brings the present and the future together, allowing for current obstacles to be perceived as being in the way, which forces individuals to take position, according to the perceived feasibility of the task (Oettingen and Gollwitzer, 2010). If the goal is deemed achievable, one will feel the need to surmount any obstacles to realize the goal. If expectations of success are low, the goal is likely to be abandoned. Therefore, mental contrasting helps distinguish achievable from non-achievable goals, thus preventing the waste of personal resources and focusing on goals having higher chances of success (Oettingen et al., 2010; Oettingen and Gollwitzer, 2010). Moreover, mental contrasting favors the informed selection of a reasonable goal, evaluates its feasibility, and provides engagement and energy for its achievement (Oettingen and Cachia, 2016; Oettingen et al., 2010; Sheeran et al., 2005). Simply put by Oettingen et al. (2010), “Mental contrasting is a self-regulation strategy to select effective means to an end.” Interestingly, the positive impact of mental contrasting does not require a precise end goal. One can simply focus on the benefits of making progress toward the goal and contrast the obstacles hindering this progress (Oettingen et al., 2010).
Worth mentioning is that recent research has demonstrated that the combination of mental contrasting and planning was associated with greater outcomes in terms of goal attainment than each one alone, although it requires self-control during the elaboration stage (Hagger and Luszczynska, 2014; Oettingen et al., 2015). It is thus recommended that those techniques be used together with careful consideration of self-regulatory resource availability. More specifically, using mental contrasting in combination with planning techniques can lead individuals to (1) choose a goal considered important and achievable (e.g. 30 minutes of PA/day); (2) realize the positive outcomes associated with goal completion (e.g. better diabetes-related symptom management); (3) become aware of the obstacles separating the present state from the goal achievement state (e.g. poor weather and tiredness); (4) plan where, when, and how they will engage in their activity (e.g. after work, I will go to the 30 minute aerobics class on my way home); and (5) elaborate on strategies to overcome potential barriers (e.g. I took a class inside so weather will not be an issue and if I feel tired, I will adapt the intensity of my exercise).
Distracting and reframing
Distracting and reframing are also self-regulatory strategies that can be used to increase PA participation while minimizing ego depletion. When exercising self-control, focusing directly on the demanding task has been shown to decrease performance, while focusing on something else increases it (Alberts et al., 2008). Focusing on the “cost” of the activity may add more weight to the impulse one is trying to control by rendering it more tempting (Alberts et al., 2008). For instance, if one only concentrates on how difficult and demanding his or her aerobics class is, engagement toward achieving the goal could diminish and the temptation of leaving class early could become more appealing. Yet, focusing on anything other than the current activity, such as completing an intense mental calculation, should improve self-control and engagement toward achieving the goal (Alberts et al., 2008).
By contrast, instead of thinking about anything but the actual task requiring self-control (which does not affect the “weight” of the temptation or goal), one could remember that his or her future exercise-related goal (e.g. to run a marathon, to lose 10 pounds, and to be healthy) has more value than the temporary pleasure of leaving the aerobics class early. This strategy is called reframing and it refers to considering the importance of a present desire as secondary to a future goal (Magen et al., 2014). Thus, while mental contrasting compares a future goal with current obstacles, reframing allows for the comparison of a future goal with a more current, unanticipated one, usually associated with temptation. Reframing could even be integrated as part of the strategies used to overcome potential barriers. For instance, one could tell himself or herself, “If my friends or my colleagues invite me to have a cocktail after work, I will reframe the importance of going to have a cocktail versus going to my aerobics class.” In this example, the person would diminish the importance of the immediate goal, “having fun,” compared to that of the future goal associated with the choice of going to the aerobics class (e.g. controlling blood glucose level to feel healthier). In sum, reframing helps one maintain perspective of the reasons for his or her actions.
Examining one’s motivation
The reasons or motivations behind a behavior also play an important role in terms of the self-regulation resource exertion associated with PA practice (Vohs and Baumeister, 2016). According to the latest version of the SEM, motivation is considered one of the fundamental pillars of self-regulation (Baumeister and Vohs, 2007). As some authors working with self-determination theory (SDT) have suggested (Muraven, 2008; Ryan and Deci, 2008), motivation quality is important in terms of self-regulation. These authors proposed that when motivated by controlled reasons (engaging in an activity because of internal or external pressures, such as avoiding guilt or gaining social approval), PA engagement requires more self-regulation resources, given that the behavior follows internal or external pressures. By contrast, when PA is sustained by autonomous motives (engaging in an activity for the inherent fun associated with it or for personally relevant outcomes, such as being healthy), the behavior does not require self-control and could even replenish self-regulation resources. Thus, individuals with T2D would benefit in terms of self-regulation from engaging in PA for autonomous reasons, such as enjoying it or personally valuing its impact on their health.
While examining the effects of each motivational regulation on a specific outcome (a variable-centered approach to motivation) is acceptable, it is limited (Chemolli and Gagné, 2014), as it does not consider that for a given activity, different motivational configurations may be present in an individual (Deci and Ryan, 2002; Patrick, 2014; Vallerand, 1997). Using a person-centered approach with motivational profiles allows for a better overview of individual motivational configurations (Pintrich, 2003; Vansteenkiste et al., 2009). In line with this proposition, some researchers have examined SDT-based motivational profiles in the PA domain. While most have investigated motivational profiles within the general adult population (Friederichs et al., 2015; Guerin and Fortier, 2012; Matsumoto and Takenaka, 2004; Stephan et al., 2010; Ullrich-French and Cox, 2009), very few (Castonguay and Miquelon, 2017; Gourlan et al., 2016) have explored these issues among T2D adults. In brief, Gourlan et al. (2016) demonstrated that compared to other T2D adults, participants presenting a self-determined profile (i.e. individuals simultaneously showing higher levels of autonomous motivation and lower levels of controlled motivation and amotivation) reported more time spent practicing PA over a 12-month period. Similarly, Castonguay and Miquelon (2017) found that T2D adults presenting a self-determined profile reported engaging in PA more frequently, and they were more likely to adhere to the PA guidelines recommended for T2D adults. These results highlight that beyond having a high level of autonomous motivation toward PA, T2D adults must also possess low levels of controlled motivation to perform and maintain PA practice.
Implications and future directions
The aim of this review was to highlight the importance of considering the SEM when it comes to explaining the rate of lower PA participation among T2D adults compared to adults from the general population. Based on this theoretical review, significant research avenues can be proposed. The first research agenda pertains to the verification of the hypothesis that states T2D adults are effectively more susceptible to chronic ego depletion than are adults from the normal population, favoring self-regulatory failure in PA engagement. To examine this assumption, a questionnaire specifically assessing day-to-day ego depletion could be developed. Such a measure should be adapted to T2D and operationalized in line with prior works according to which self-regulatory success is mainly characterized by engagement in a behavior despite obstacles, and it is highly dependent on sufficient rest (Baumeister and Vohs, 2016; Carver and Scheier, 2001; Young, 2016). Based on prior work, to gain a complete perspective of ego depletion (and its link to PA participation) among T2D adults and from the general population, such a questionnaire should measure (1) participants’ current self-regulation capacity level (Ciarocco et al., 2012) (e.g. “If I had to engage in PA right now, I would give up easily”), (2) self-regulation efficiency (Neal and Carey, 2005) (e.g. “Today, to what extent did you keep track of your progress toward your PA goals?”), and (3) overall fatigue (Arbuckle et al., 2009) (e.g. “Today, to what extent did you abandoned or give up your PA goals because you were too tired or lack energy?”).
Using this questionnaire, it would be possible to measure ego depletion in adults with and without T2D (presenting no other chronic health problems either) and to compare the results obtained within both populations, controlling for such key variables as sex, age, health issues, and body mass index. As for PA frequency, it should be measured with both subjective (self-reported) and objective (accelerometer) measures on a daily basis among participants from both populations. The main obstacles participants encounter in their day-to-day PA participation (e.g. lack of time or facilities and weather conditions) should also be measured. To assess these constructs daily over a period of 1 or 2 weeks, researchers could use the ecological momentary assessment (also known as the daily diary method or experience sampling method). Such a research design would allow for the verification of whether T2D adults tend to be more chronically ego depleted, as well as how often they gave up on their PA participation because of self-regulatory failure. Based on the herein work’s assumptions, the number of times T2D adults (who should also be more susceptible ego depletion) abandoned their PA goals because of self-regulatory failure should be higher than the number of adults from the normal population.
Future studies should also examine the effects of the vulnerability factors associated with ego depletion and PA participation among T2D adults proposed in the herein work. For instance, using measures of sleep, psychological distress, and stress, researchers could verify the effects of sleep quality, depression, anxiety, and stress on ego depletion, as well as the effect of ego depletion on PA frequency. This investigation could also be realized by means of the ecological momentary assessment over a period of 1 to 2 weeks. Again, PA frequency should be assessed with both subjective (self-reported) and objective (e.g. accelerometer) measures on a daily basis. It would also be important to examine the presence of a direct or indirect link among the intensity of symptoms associated with T2D, ego depletion, and PA frequency.
As to the application of the knowledge presented in the herein work into practice, we believe that some propositions could be very useful for initiatives such as “Exercise is Medicine” (EIM). EIM is a global health initiative managed by the American College of Sports Medicine (ACSM) and focuses on encouraging primary care physicians and other health care providers to include PA when designing treatment plans for patients and referring their patients to EIM Credentialed Exercise Programs and Exercise Professionals. Indeed, a first step toward the application of the herein suggestions to practitioners or professionals involved in EIM could consists of advising T2D adults to carefully plan their PA practice, at moments when they will have enough “energy” to exert self-control (e.g. not just before, during, or right after another demanding task). Practitioners or professionals should also support their patients’ autonomy, a factor that has been found to positively influence autonomous motivation as well as glucose control in T2D adults (Julien et al., 2009; Williams et al., 1998), by, for instance, asking them “Which PA would you feel the more confident and comfortable to practice?” It could also be effective to show their patients how to recognize signs of self-regulatory failures or ego depletion, so they can learn to monitor their self-control capacity punctually, which could help them find the best time to engage in PA.
As outlined by this manuscript, knowledge on self-regulation capacity, combined with knowledge on T2D management, could positively influence PA practice among adults with T2D. As previously mentioned, diabetes education programs, which require T2D adults to learn the extensive details of diabetes management, pose a certain demand on self-regulatory resources, although they cover content expected to prevent ego depletion (e.g. action planning). It is only once the new information has been mastered that demands on self-regulation diminish and that knowledge then contributes to self-regulation. Therefore, education programs can foster a more appropriate use of self-regulatory resources and thus greater self-regulatory success. Consequently, we believe diabetes education programs should incorporate information regarding self-regulation into their curriculum and that their direct (i.e. learning techniques that promote self-regulation) and indirect (pointing out which behaviors are worth modifying) effects on self-regulatory skills should be evaluated. A simple way to evaluate the impact of these education programs on self-regulatory resources and skills could consist of a questionnaire assessing self-regulation efficiency (Neal and Carey, 2005). Participants could complete such a questionnaire before and at the end of the diabetes education program. Their scores could be compared to those of individuals who did not participated in this program.
In addition to these suggestions for future research, examining several strategies for preventing self-regulatory failure among T2D adults in a PA participation context, such as those presented in this work, would be worthwhile, given there are few examples in the literature of self-regulation promotion in T2D adults.
Conclusion
PA engagement requires self-regulation. This is especially true at the beginning when one first engages in a new PA, given the energy required to plan and weigh the pros and cons associated with the performance of a new behavior (Hagger and Chatzisarantis, 2009; Hagger et al., 2002, 2010a). T2D adults therefore need to use self-regulation to integrate PA into their lives, which impedes their self-regulation capacity, at least in the short term (Audiffren and André, 2015). Cautiousness is therefore recommended as to the amount of PA incorporated into the everyday lives of individuals with T2D. A gradual integration, starting with PA for as little as 10 minutes per day, can have real positive outcomes (Colberg et al., 2016; Sigal et al., 2013). Of note is that regular aerobic PA is a core recommendation for T2D adults given its crucial role in the management of their condition (Colberg et al., 2016; Powers et al., 2017). Cautiously integrating PA sessions into one’s routine has the potential to render future PA practice easier. In that sense, initiating PA increases the possibility of entering a loop promoting additional regular PA engagement, self-regulation, T2D management, and overall health.
The herein work presents avenues to increase PA participation according to the primary predisposition to self-regulatory failure in T2D adults. It is recommended that T2D adults, as well as their health care providers, consider their self-regulation capacity, chronic ego depletion and its implications, and daily self-regulatory demands, including those associated with PA participation. T2D and its management are complex and demanding. Because failure and dropout are not what individuals are looking for in terms of PA participation and diabetes management, ensuring one has the resources to integrate successfully the required new knowledge, skills, and behaviors is crucial to their success.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Adriaanse MA, De Ridder DT, Voorneman I. (2013) Improving diabetes self-management by mental contrasting. Psychology & Health 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Alberts HJEM, Martijn C, Nievelstein F, et al. (2008) Distracting the self: Shifting attention prevents ego depletion. Self and Identity 7: 322–334. [Google Scholar]
- American Diabetes Association (ADA) (2014) Standards of medical care in diabetes—2014. Diabetes Care 37(1): S14–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) (2017) Standards of medical care in diabetes—2017. Diabetes Care 40: S1-S135.27979885 [Google Scholar]
- Arbuckle RA, Humphrey L, Vardeva K, et al. (2009) Psychometric evaluation of the diabetes symptom checklist-revised (DSC-R)—A measure of symptom distress. Value in Health 12: 1168–1175. [DOI] [PubMed] [Google Scholar]
- Audiffren M, André N. (2015) The strength model of self-control revisited: Linking acute and chronic effects of exercise on executive functions. Journal of Sport and Health Science 4: 30–46. [Google Scholar]
- Baumeister RF. (2014) Self-regulation, ego depletion, and inhibition. Neuropsychologia 65: 313–319. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. (2016) Limited resources for self-regulation: A current overview of the strength model. In: Hirt ER, Clarkson JJ, Jia L. (eds) Self-Regulation and Ego Control. San Diego, CA: Academic Press, pp. 1–17. [Google Scholar]
- Baumeister RF, Alquist JL. (2009) Is there a downside to good self-control? Self and Identity 8: 115–130. [Google Scholar]
- Baumeister RF, Heatherton TF. (1996) Self-regulation failure: An overview Psychological Inquiry 7: 1–15. [Google Scholar]
- Baumeister RF, Vohs KD. (2007) Self-regulation, ego depletion, and motivation. Social and Personality Psychology Compass 1: 115–128. [Google Scholar]
- Baumeister RF, Vohs KD. (2016) Strength Model of self-regulation as limited resource: Assessment, controversies, update. In: Zanna JM, Oa MP. (eds) Advances in Experimental Social Psychology. Cambridge, MA: Academic Press, pp. 67–127. [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, et al. (1998) Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology 74: 1252–1265. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Faber JE, Wallace HM. (1999) Coping and ego depletion: Recovery after the coping process. In: Snyder CR. (ed.) Coping: The Psychology of What Works (1st edn). New York: Oxford University Press, pp. 50–69. [Google Scholar]
- Baumeister RF, Gailliot M, DeWall CN, et al. (2006) Self-regulation and personality: How interventions increase regulatory success, and how depletion moderates the effects of traits on behavior. Journal of Personality 74: 1773–1801. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF, Tice DM. (1994) Losing Control: How and Why People Fail at Self-Regulation. San Diego, CA: Academic Press. [Google Scholar]
- Baumeister RF, Vohs KD, Tice DM. (2007) The strength model of self-control. Current Directions in Psychological Science 16: 351–355. [Google Scholar]
- Boyle NB, Lawton CL, Allen R, et al. (2016) No effects of ingesting or rinsing sucrose on depleted self-control performance. Physiology & Behavior 154: 151–160. [DOI] [PubMed] [Google Scholar]
- Buckley J, Cohen JD, Kramer AF, et al. (2014) Cognitive control in the self-regulation of physical activity and sedentary behavior. Frontiers in Human Neuroscience 8: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. (2001) On the Self-Regulation of Behavior. New York: Cambridge University Press. [Google Scholar]
- Carver CS, Scheier MF. (2016) Self-regulation of action and affect. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 42–61. [Google Scholar]
- Castonguay A, Miquelon P. (2017) Motivational profiles for physical activity among adults with type 2 diabetes and their relationships with physical activity behavior. Health Psychology and Behavioral Medicine 5: 110–128. [Google Scholar]
- Chemolli E, Gagné M. (2014) Evidence against the continuum structure underlying motivation measures derived from self-determination theory. Psychological Assessment 26: 575–585. [DOI] [PubMed] [Google Scholar]
- Ciarocco NJ, Twenge JM, Muraven M, et al. (2012) The state self-control capacity scale: Reliability, validity, and correlations with physical and psychological stress. In: Society for Personality and Social Psychology annual convention, San Diego, CA, 26–28 January. [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE. (2000) Depression and diabetes: Impact of depression symptoms on adherence, function, costs. Archives of Internal Medicine 160: 3278–3285. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Yardley JE, et al. (2016) Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. (2003) Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science 14: 125–130. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, et al. (2006) Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 61: 1166–1170. [DOI] [PubMed] [Google Scholar]
- Cradock KA, ÓLaighin G, Finucane FM, et al. (2017) Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, et al. (2014) Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M, McMinn D, Allan JL. (2014) A bidirectional relationship between physical activity and executive function in older adults. Frontiers in Human Neuroscience 8: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J. (2016) Testing the role of glucose in self-control: A meta-analysis. Appetite 107: 222–230. [DOI] [PubMed] [Google Scholar]
- Davies M, Brophy S, Williams R, et al. (2006) The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 29: 1518–1522. [DOI] [PubMed] [Google Scholar]
- De Ridder DT, Lensvelt-Mulders G, Finkenauer C, et al. (2012) Taking stock of self-control: A meta-analysis of how trait self-control relates to a wide range of behaviors. Personality and Social Psychology Review 16: 76–99. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. (2002) Handbook of Self-Determination Research. Rochester, NY: University of Rochester Press. [Google Scholar]
- Delahanty LM, Conroy MB, Nathan DM, et al. (2006) Psychological predictors of physical activity in the diabetes prevention program. Journal of the American Dietetic Association 106: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris DC, Power DA, Kenefick E. (2012) Investigating the effects of ego depletion on physical exercise routines of athletes. Psychology of Sport and Exercise 13: 118–125. [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, et al. (2008) A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Medicine 25: 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederichs SA, Bolman C, Oenema A, et al. (2015) Profiling physical activity motivation based on self-determination theory: A cluster analysis approach. BMC Psychology 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF. (2007) The physiology of willpower: Linking blood glucose to self-control. Personality and Social Psychology Review 11: 303–327. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, et al. (2007) Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. Journal of Personality and Social Psychology 92: 325–336. [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM. (1999) Implementation intentions: Strong effects of simple plans. American Psychologist 54: 493–503. [Google Scholar]
- Gourlan M, Trouilloud D, Boiché J. (2016) Motivational profiles for physical activity practice in adults with Type 2 diabetes: A self-determination theory perspective. Behavioral Medicine 42: 227–237. [DOI] [PubMed] [Google Scholar]
- Grootenhuis PA, Snoek FJ, Heine RJ, et al. (1994) Development of a type 2 diabetes symptom checklist: A measure of symptom severity. Diabetic Medicine 11: 253–261. [DOI] [PubMed] [Google Scholar]
- Guerin E, Fortier M. (2012) Motivational profiles for physical activity: Cluster analysis and links with enjoyment. Revue PhénEPS/PHEnex Journal 4: 1–21. [Google Scholar]
- Hagger MS, Chatzisarantis NL. (2009) Integrating the theory of planned behaviour and self-determination theory in health behaviour: A meta-analysis. British Journal of Health Psychology 14: 275–302. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis NL. (2013) The sweet taste of success: The presence of glucose in the oral cavity moderates the depletion of self-control resources. Personality and Social Psychology Bulletin 39: 28–42. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Luszczynska A. (2014) Implementation intention and action planning interventions in health contexts: State of the research and proposals for the way forward. Applied Psychology: Health and Well-Being 6: 1–47. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis NL, Alberts HA, et al. (2016) A multilab preregistered replication of the ego-depletion effect. Perspectives on Psychological Science 11: 546–573. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis NLD, Biddle SJH. (2002) A meta-analytic review of the theories of reasoned action and planned behavior in physical activity: Predictive validity and the contribution of additional variables. Journal of Sport & Exercise Psychology 24: 3–32. [Google Scholar]
- Hagger MS, Wood C, Stiff C, et al. (2009) The strength model of self-regulation failure and health-related behaviour. Health Psychology Review 3: 208–238. [Google Scholar]
- Hagger MS, Wood CW, Stiff C, et al. (2010. a) Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin 136: 495–525. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood CW, Stiff C, et al. (2010. b) Self-regulation and self-control in exercise: The strength-energy model. International Review of Sport and Exercise Psychology 3: 62–86. [Google Scholar]
- Health Canada (2002) Diabetes in Canada (2nd edn). Available at: http://publications.gc.ca/Collection/H49-121-2002E.pdf
- Heatherton TF, Wagner DD. (2011) Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences 15: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt ER, Clarkson JJ, Jia L. (2016) Self-Regulation and Ego Control. Cambridge, MA: Academic Press. [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. (2012) Executive functions and self-regulation. Trends in Cognitive Sciences 16: 174–180. [DOI] [PubMed] [Google Scholar]
- Indelicato JA. (2009) Association between executive function, physical activity, and physical fitness in people with Type 2 diabetes. Master’s Thesis, Northeastern University, Boston, MA. [Google Scholar]
- International Diabetes Federation (2015) IDF Diabetes Atlas. Brussels: International Diabetes Federation. [Google Scholar]
- Jenkins BN, Rook KS, Borges-Garcia R, et al. (2016) Too much of a good thing? Overexertion of self-control and dietary adherence in individuals with Type 2 diabetes. British Journal of Health Psychology 21: 648–659. [DOI] [PubMed] [Google Scholar]
- Julien E, Senécal C, Guay F. (2009) Longitudinal relations among perceived autonomy support from health care practitioners, motivation, coping strategies and dietary compliance in a sample of adults with type 2 diabetes. Journal of Health Psychology 14: 457–470. [DOI] [PubMed] [Google Scholar]
- Katon WJ, Russo JE, Heckbert SR, et al. (2010) The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. International Journal of Geriatric Psychiatry 25: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkiakangas EE, Alahuhta MA, Laitinen JH. (2009) Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: A systematic review. Health Promotion International 24: 416–427. [DOI] [PubMed] [Google Scholar]
- Krizan Z, Garrett H. (2016) The essential role of sleep in self-regulation. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 182–202. [Google Scholar]
- Leiter LA, Berard L, Bowering CK, et al. (2013) Type 2 diabetes mellitus management in Canada: is it improving? Canadian Journal of Diabetes 37: 82–89. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. (1999) Brain glucose sensing and body energy homeostasis: Role in obesity and diabetes. American Journal of Physiology—Regulatory Integrative and Comparative Physiology 276: R1223–R1231. [DOI] [PubMed] [Google Scholar]
- Lipscombe C, Smith KJ, Gariepy G, et al. (2014) Gender differences in the relationship between anxiety symptoms and physical inactivity in a community-based sample of adults with type 2 diabetes. Canadian Journal of Diabetes 38: 444–450. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Dunbar-Jacob J. (2011) Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educator 37: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. (2012) Diabetes and cognitive dysfunction. The Lancet 379: 2291–2299. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2016) Central role of the brain in stress and adaptation: Allostasis, biological embedding, and cumulative change. In: Fink G. (ed.) Stress: Concepts, Cognition, Emotion, and Behavior. San Diego, CA: Academic Press, pp. 39–56. [Google Scholar]
- Magen E, Kim B, Dweck CS, et al. (2014) Behavioral and neural correlates of increased self-control in the absence of increased willpower. Proceedings of the National Academy of Sciences of the United States of America 111: 9786–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranges HM, Baumeister RF. (2016) Self-control and ego depletion. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 42–61. [Google Scholar]
- Martin Ginis KA, Bray SR. (2010) Application of the limited strength model of self-regulation to understanding exercise effort, planning and adherence. Psychology & Health 25: 1147–1160. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Takenaka K. (2004) Motivational profiles and stages of exercise behavior change. International Journal of Sport and Health Science 2: 89–96. [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, et al. (2008) Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 31: 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrato EH, Hill JO, Wyatt HR, et al. (2007) Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 30: 203–209. [DOI] [PubMed] [Google Scholar]
- Moulton CD, Costafreda SG, Horton P, et al. (2015) Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging and Behavior 9: 651–662. [DOI] [PubMed] [Google Scholar]
- Muraven M. (2008) Autonomous self-control is less depleting. Journal of Research in Personality 42: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M. (2010) Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology 46: 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. (2000) Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin 126: 247–259. [DOI] [PubMed] [Google Scholar]
- Muraven M, Shmueli D, Burkley E. (2006) Conserving self-control strength. Journal of Personality and Social Psychology 91: 524–537. [DOI] [PubMed] [Google Scholar]
- Neal DJ, Carey KB. (2005) A follow-up psychometric analysis of the self-regulation questionnaire. Psychology of Addictive Behaviors 19: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KM, Reiber G, Boyko EJ. (2002) Diet and exercise among adults with type 2 diabetes: Findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 25: 1722–1728. [DOI] [PubMed] [Google Scholar]
- Nouwen A, Winkley K, Twisk J, et al. (2010) Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia 53: 2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettingen G. (2000) Expectancy effects on behavior depend on self-regulatory thought. Social Cognition 18: 101–129. [Google Scholar]
- Oettingen G. (2012) Future thought and behaviour change. European Review of Social Psychology 23: 1–63. [Google Scholar]
- Oettingen G, Cachia JYA. (2016) Problems with positive thinking and how to overcome them. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 547–570. [Google Scholar]
- Oettingen G, Gollwitzer P. (2010) Strategies of setting and implementing goals: Mental contrasting and implementation intentions. In: Maddux JE, Tangney JP. (eds) Social Psychological Foundations of Clinical Psychology. New York: Guilford Press, pp. 114–135. [Google Scholar]
- Oettingen G, Kappes HB, Guttenberg KB, et al. (2015) Self-regulation of time management: Mental contrasting with implementation intentions. European Journal of Social Psychology 45: 218–229. [Google Scholar]
- Oettingen G, Stephens EJ, Mayer D, et al. (2010) Mental contrasting and the self-regulation of helping relations. Social Cognition 28: 490–508. [Google Scholar]
- Palakodeti S, Uratsu CS, Schmittdiel JA, et al. (2015) Changes in physical activity among adults with diabetes: A longitudinal cohort study of inactive patients with Type 2 diabetes who become physically active. Diabetic Medicine 32: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick H. (2014) Ascending Mount Maslow with oxygen to spare: A self-determination theory perspective. Psychological Inquiry 25: 101–107. [Google Scholar]
- Pintrich PR. (2003) A motivational science perspective on the role of student motivation in learning and teaching contexts. Journal of Educational Psychology 95: 667–686. [Google Scholar]
- Plotnikoff RC, Johnson ST, Loucaides CA, et al. (2011) Population-based estimates of physical activity for adults with type 2 diabetes: A cautionary tale of potential confounding by weight status. Journal of Obesity 2010: 561432 (5 pp.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MA, Bardsley J, Cypress M, et al. (2017) Diabetes self-management education and support in Type 2 diabetes; a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Educator 43: 40–53. [DOI] [PubMed] [Google Scholar]
- Roy T, Lloyd CE. (2012) Epidemiology of depression and diabetes: A systematic review. Journal of Affective Disorders 142: S8–S21. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. (2008) From ego depletion to vitality: Theory and findings concerning the facilitation of energy available to the self. Social and Personality Psychology Compass 2: 702–717. [Google Scholar]
- Sadanand S, Balachandar R, Bharath S. (2016) Memory and executive functions in persons with type 2 diabetes: A meta-analysis. Diabetes/Metabolism Research and Reviews 32: 132–142. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG. (2016) Self-regulatory failure and addiction. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 571–590. [Google Scholar]
- Schmidt K-H, Neubach B, Heuer H. (2007) Self-control demands, cognitive control deficits, and burnout. Work and Stress 21: 142–154. [Google Scholar]
- Schwarzer R. (2008) Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology 57: 1–29. [Google Scholar]
- Segerstrom SC, Boggero IA, Evans DR. (2016) Pause and plan; the physiology of self-regulation. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 131–145. [Google Scholar]
- Shaw JE, Punjabi NM, Wilding JP, et al. (2008) Sleep-disordered breathing and type 2 diabetes: A report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Research and Clinical Practice 81: 2–12. [DOI] [PubMed] [Google Scholar]
- Sheeran P, Webb TL, Gollwitzer PM. (2005) The interplay between goal intentions and implementation intentions. Personality and Social Psychology Bulletin 31: 87–98. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Armstrong MJ, Colby P, et al. (2013) Physical activity and diabetes. Canadian Journal of Diabetes 37(1): S40–S44. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Wasserman DH, et al. (2006) Physical activity/exercise and type 2 diabetes. Diabetes Care 29: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Solberg Nes L, Carlson CR, Crofford LJ, et al. (2010) Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain 151: 37–44. [DOI] [PubMed] [Google Scholar]
- Solberg Nes L, Carlson CR, Crofford LJ, et al. (2011) Individual differences and self-regulatory fatigue: Optimism, conscientiousness, and self-consciousness. Personality and Individual Differences. DOI: 10.1016/j.paid.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Nes L, Roach AR, Segerstrom SC. (2009) Executive functions, self-regulation, and chronic pain: A review. Annals of Behavioral Medicine 37: 173–183. [DOI] [PubMed] [Google Scholar]
- Statistics Canada (2014) Table 105-0501—Health Indicator Profile, Annual Estimates, by Age Group and Sex, Canada, Provinces, Territories, Health Regions (2013 Boundaries) and Peer Groups, Occasional. CANSIM (Database). Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1050501&&pattern=&stByVal=1&p1=1&p2=31&tabMode=dataTable&csid=
- Stephan Y, Boiché J, Le Scanff C. (2010) Motivation and physical activity behaviors among older women: A self-determination perspective. Psychology of Women Quarterly 34: 339–348. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. (2004) High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality 72: 271–324. [DOI] [PubMed] [Google Scholar]
- Trento M, Broglio F, Riganti F, et al. (2008) Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetologica 45: 225–229. [DOI] [PubMed] [Google Scholar]
- Tyler JM, Burns KC. (2008) After depletion: The replenishment of the self’s regulatory resources. Self and Identity 7: 305–321. [Google Scholar]
- Ullrich-French S, Cox A. (2009) Using cluster analysis to examine the combinations of motivation regulations of physical education students. Journal of Sport & Exercise Psychology 31: 358–379. [DOI] [PubMed] [Google Scholar]
- Vadillo MA, Gold N, Osman M. (2016) The bitter truth about sugar and willpower: The limited evidential value of the glucose model of ego depletion. Psychological Science 27: 1207–1214. [DOI] [PubMed] [Google Scholar]
- Vallerand RJ. (1997) Toward a hierarchical model of intrinsic and extrinsic motivation. In: Zanna MP. (ed.) Advances in Experimental Social Psychology, vol. 29 San Diego, CA: Academic Press, pp. 271–360. [Google Scholar]
- Vansteenkiste M, Sierens E, Soenens B, et al. (2009) Motivational profiles from a self-determination perspective: The quality of motivation matters. Journal of Educational Psychology 101: 671–688. [Google Scholar]
- Verweij IM, Romeijn N, Smit DJ, et al. (2014) Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neuroscience 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C. (2014) The effects of acute aerobic exercise on executive function in individuals with type 2 diabetes. Available at: https://uwspace.uwaterloo.ca/bitstream/handle/10012/8556/Vincent_Corita.pdf;sequence=3
- Vohs KD, Baumeister RF. (2016) Handbook of Self-Regulation: Research, Theory, and Applications. New York: Guilford Press. [Google Scholar]
- Vohs KD, Heatherton TF. (2000) Self-regulatory failure: A resource-depletion approach. Psychological Science 11: 249–254. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, Schmeichel BJ, et al. (2008) Making choices impairs subsequent self-control: A limited-resource account of decision making, self-regulation, and active initiative. Journal of Personality and Social Psychology 94: 883–898. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Glass BD, Maddox WT, et al. (2011) Ego depletion is not just fatigue. Social Psychological and Personality Science 2: 166–173. [Google Scholar]
- Wagner DD, Heatherton TF. (2016) The cognitive neuroscience of self-regulatory failure. In: Vohs KD, Baumeister RF. (eds) Handbook of Self-Regulation: Research, Theory, and Applications (3rd edn). New York: Guilford Press, pp. 111–130. [Google Scholar]
- Wang L, Tao T, Fan C, et al. (2015) The influence of chronic ego depletion on goal adherence: An experience sampling study. PLoS ONE 10: e0142220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Clarke TC, Nugent CN, et al. (2016) Early release of selected estimates based on data from the 2015 National Health Interview Survey. National Center for Health Statistics. Available at: https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201605.pdf
- West SD, Nicoll DJ, Stradling JR. (2006) Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 61: 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Freedman ZR, Deci EL. (1998) Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care 21: 1644–1651. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. Available at: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf
- World Health Organization (WHO) (2016) Global report on diabetes: WHO library cataloguing-in-publication data. Available at: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf
- Young G. (2016) Unifying Causality and Psychology: Being, Brain, and Behavior. Cham, Switzerland: SpringerInternational Publishing. [Google Scholar]
- Young-Hyman D, de Groot M, Hill-Briggs F, et al. (2016) Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care 39: 2126–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn D, Tug S, Wenzel M, et al. (2016) Glucose metabolism and self-regulation—Is insulin resistance a valid proxy of self-control? Personality and Individual Differences 99: 38–45. [Google Scholar]
- Zanuso S, Jimenez A, Pugliese G, et al. (2010) Exercise for the management of type 2 diabetes: A review of the evidence. Acta Diabetologica 47: 15–22. [DOI] [PubMed] [Google Scholar]


