Abstract
BACKGROUND
Our aim was to create and establish a database called “Persian Registry Of cardioVascular diseasE (PROVE)” in order to be used for future research and in addition, as a tool to develop national guidelines for diagnosis, treatment, and prevention of cardiovascular disease (CVD). In this paper, the design and methodology of the PROVE pilot study will be discussed, launched in Isfahan, Iran, in 2015-2016.
METHODS
Through establishing PROVE, patients' data were collected from hospitals and outpatient clinics prospectively or retrospectively and followed up for a maximum of three years based on the type of CVDs. The inclusion criteria were as patients with acute coronary syndrome (ACS), ST elevation myocardial infarction (STEMI), stroke, atrial fibrillation (AF), heart failure (HF), congenital heart disease (CHD), percutaneous coronary intervention (PCI), and chronic ischemic cardiovascular disease (CICD). Specific protocols, questionnaires, and glossaries were developed for each registry. In order to ensure the validation of the protocols, questionnaires, data collection, management, and analysis, a well-established quality control (QC) protocol was developed and implemented. Data confidentiality was considered.
RESULTS
In order to register patients with ACS, STEMI, stroke, HF, PCI, and CICD, the hospital recorded data were used, whereas, in case of AF and CHD registries, the data were collected from hospitals and outpatient clinics. During the pilot phase of the study in Isfahan, from March 2015 to September 2016, 9427 patients were registered as ACS including 809 as STEMI, 1195 patients with HF, 363 with AF, 761 with stroke, 1136 with CHD, 1200 with PCI, and 9 with CICD. Data collection and management were performed under the supervision of the QC group.
CONCLUSION
PROVE was developed and implemented in Isfahan as a pilot study, in order to be implemented at national level in future. It provides a valuable source of valid data that could be used for future research, re-evaluation of current CVD management and more specifically, gap analysis and as a tool for assessment of the type of CVDs, prevention, treatment, and control by health care decision makers.
Keywords: Cardiovascular Disease, Registries, Disease Management, Data Collection
Introduction
The gradual reduction in birth and death rates, along with the increase in life expectancy has led to an unprecedented growing rate of the older adult population.1 There are 600 million people aged 60 years and over, across the world and the number expected to double by 2025.2 This is a global burden and Iran is not an exception. According to the Iranian Ministry of Health Statistics on the age pyramid, Iran is currently transitioning from a dominantly young to a dominantly older population.3
Aging can affect cardiovascular (CV) function, consequently causes diseases and results in mortality and morbidity.4 Several studies have reported a higher prevalence of cardiovascular diseases (CVD) and its associated risk factors in Iranian population.5-11
Globally, CVD is the leading cause of death, and CV death in Iran, constitutes 38% of all deaths, compared to the global rate of 31%.12
Since 2001, The Isfahan Cohort Study (ICS), Iran, conducted on more than 6000 healthy adult subjects, followed up for the occurrence of CV events, reported a high incidence and mortality rate due to CVD, and urged health policy-makers to take action to prevent and control CVD.13-15
A disease registry can enhance the quality of care, and encourages health care providers to lean towards a more patient self-care approach, and to adopt better health care services by identifying the gaps and challenges in treatment and care. Having a registry in place will be very helpful to generate research data, in order to raise public awareness and take more effective steps, towards disease prevention and control. It could also help experts developing therapeutic guidelines for more effective and evidence-based disease management options.16
Considering the benefits of having a disease registry database, Isfahan Cardiovascular Research Center (ICRC), a the World Health Organization (WHO) collaborating center, primarily had established a CVD registry for acute coronary syndromes (ACS) and stroke in 1999, based on the WHO Multinational Monitoring of trends and determinants in Cardiovascular disease (MONICA) method.17 Through that earlier registry, data from over 150,000 patients with ACS and 37000 patients with stroke were collected and patients were followed up for mortality or morbidity; details of this registry have been previously reported.18-25 The aforementioned experience led us to design and develop a more comprehensive and improved registry database for patients with different types of CVD, namely the Persian Registry Of cardioVascular diseasE (PROVE), in 2015. Data collection, protocols, and questionnaires as well as frequency, method, and duration of follow-ups differ, according to the type of CVDs.
To the best of our knowledge, PROVE is the first national comprehensive CVD registry database, established in Iran with the less similar registry in the Eastern Mediterranean region. This paper describes the design and implementation of PROVE in Isfahan, as a feasibility study.
Materials and Methods
PROVE is a registry, in which patients’ data from hospitals or outpatient clinics are collected. It was initiated in late 2014 and launched in March 2015. Ethics approval was obtained from the Ethics Committee of Isfahan University of Medical Sciences.
Patients with ACS, stroke, heart failure (HF), atrial fibrillation (AF), percutaneous coronary intervention (PCI), and congenital heart disease (CHD) were recruited and followed up, since 2015 and more recently, chronic ischemic cardiovascular disease (CICD) was incorporated, in 2016. All diagnoses were made based on the International Classification of Diseases (ICD 10).
Since we aimed to use the registered data in PROVE, as a potential tool for developing national diagnosis, treatment, and prevention guidelines for patients with CVD, we identified interested cardiologists as principle investigator (PI) of each type of CVDs, all members of the steering committee of PROVE. Furthermore, a data collection team was formed, consisting of trained nurses and general practitioners, worked under the supervision of cardiologists and a neurologist.
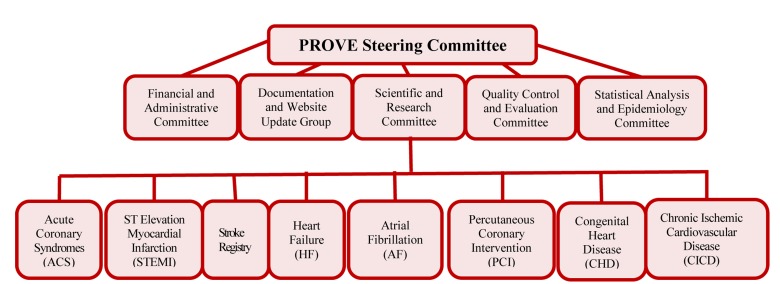
The quality control (QC) committee, consisting of epidemiologist, statisticians, specialized physicians, and information technology staff was developed, concomitantly with the steering committee and the head was selected from the steering committee members. Figure 1 shows the management flowchart of the PROVE program.
Figure 1.
The management flowchart of the Persian Registry Of cardioVascular diseasE (PROVE) program
Each type of CVD registry had a team who carried out their own search and analysis, in order to develop a methodology for collecting data, monitoring data entry, and following up the patients. Each team developed their relevant questionnaire protocol and a glossary specific to the type of CVD registry. Concomitantly, all staff responsible for the data collection, either through medical records or face to face interviews with patients, received a basic systematic training for the AF, ST elevation myocardial infarction (STEMI), HF, and CICD, joined the European Observational Research Program (EORP).26
Data were collected, according to the established protocol and glossary, using the questionnaires. Since the registry of patients with ACS and stroke had begun earlier in 1999, using WHO MONICA protocol and questionnaires,17 the data collection and registration were incorporated in PROVE; meanwhile, the registration of ACS was continued, using the same protocol, but the stroke registry method was derived from a WHO Stepwise approach27 and the European Registry.28
Two methods were used for data collection: cold pursuit method where the patients’ data were collected from the medical records, and hot pursuit method, whereby patients’ data were collected by face to face interviews with patients.29 Eventually, a website was created for PROVE, in order to present a brief introduction to the program.30
Data collection methods for different type of CVDs
PROVE/Stroke: Patients with stroke were originally registered, based on the WHO MONICA protocol, and were followed-up for 28 days, since the events occurred. Using the initial questionnaire, data regarding the patients’ demographic characteristic, symptoms of the disease, medical history and used prescribed medications, diagnostic tests, treatment course, physical examination, and presence of risk factors were collected. The follow-up form inquired brief data on the patients’ medical status and conditions. The MONICA questionnaire does not have any option to investigate and register the type of stroke, however, the newly developed protocol by WHO, named WHO Stepwise does.
By proceeding into the PROVE program, the WHO Stepwise protocol and the European registry were used to develop a new questionnaire to register patients with stroke using cold pursuit method.27 The stroke registration personnel received three initial trainings of two-hour long and monthly one-hour retraining session.
The new questionnaire included patients’ demographic characteristics, personal medical and drug history, risk factors, heart and brain imaging, blood test results, treatments received in the hospital and complications (if any), discharge prescriptions, and patient’s status, as well as the definite diagnosis of the type of stroke, according to computed tomography (CT) scan or magnetic resonance imaging (MRI).
The patients were followed up by the phone or in person at the 1st, 3rd, and 12th months, using the Barthel Index and the Modified Rankin Scale (MRS). The reliability and validity of these two questionnaires had previously been investigated, in Iranian population.18 Moreover, secondary prevention measures, rehabilitation status, and the incidence of new CV events were examined in the follow-up.
PROVE/AF: Data regarding patients with AF were jointly registered with EORP, using population-based protocol. We gathered patients’ data from two Isfahan University of Medical Sciences (IUMS)-affiliated hospitals, and the main electrophysiology (EPS) clinics in Isfahan.
Case report forms (CRF) were completed by on-site trained nurses, and then reviewed by an EPS expert, before submitting to EORP database. All nurses received six initial trainings of two-hour long and a retraining session every two months. The registered patients’ were followed up on annual basis, up to three years.
PROVE/HF: A questionnaire was developed, based on the Swedish Heart Failure Registry (S-HFR)31 and the Acute Decompensated Heart Failure Registry (ADHERE).32 The validity of the questionnaire was investigated and approved by the QC committee. This registry was performed on patients with HF who were hospitalized, due to decompensated or acute HF. The questionnaire collected data on demographic characteristics, medical history, sign and symptoms at admission, treatment regimen, and diagnostic tests. The HF registry personnel received three initial trainings of two-hour long and a monthly retraining session.
The patients’ follow-up protocol was either by phone or, if necessary, a visit by a specialist physician on the 1st, 6th, and 12th months after discharge. The follow-up form inquired the patients’ status, used medications, new diagnostic tests, and in case of death, place and the reason of death.
After one year of the implementation of a pilot registry for HF, as planned to join EORP/HF, the initial PROVE/HF protocol switched to EORP/HF protocol, for alignment on a joint effort.
PROVE/PCI: The PCI registry was started in the largest referral hospital of cardiac and cardiosugery in the province. Data collection initiated by interventional cardiology fellows and residents, alongside a research nurse, and under supervision of PIs.
PCI questionnaire included information on demographic characteristics, medical history and records on previous diagnostic and treatment measures, results of coronary artery angiography, details on PCI intervention, acute and sub-acute complications of the PCI (if any), and in-hospital and long-term outcomes. Several training and re-training sessions were held and are still ongoing. Patients who underwent PCI for any reason were recruited in this registry. The follow-up protocol is similar to the PROVE/HF registry.
PROVE/CHD: A population-based protocol was used to register CHD. The questionnaire was developed by cardiac pediatricians and validated by the QC experts. It was used to collect the following data on different types of CHDs: demographic characteristics, medical history, pregnancy history, maternal diseases, family history of CHDs, clinical presentations, diagnostics findings, used medications and recommended follow-up, and disease management plans. Data were collected by five pediatric cardiologists and their fellows, at three university-affiliated referral hospitals and private clinics.
Patients were followed up by phone calls or a visit at the clinic, depending on the type and severity of the disease and interventions or treatments they received, the time of admission, performance and type of cardiac surgery, and the probability of medication side effects or new medical condition occurring during the course of treatment.
PROVE/STEMI: In order to register patients with STEMI, and since our plan was to join the EORP/STEMI registry, their questionnaire and protocol were used, when all the alignments and feasibility studies were completed. The registration of patients with STEMI was begun in three IUMS-affiliated hospitals, and then the number of hospitals was gradually increased. The inclusion criteria comprised of all patients with STEMI diagnosis based on electrocardiography (ECG), new left bundle branch block (LBBB), and those who died upon admission with at least one STEMI diagnosis on ECG. All completed CRFs were carefully reviewed by two cardiologists, before submitting to EORP. Data were collected based on past medical history, significant events in the course of hospitalization [from the onset of chest pain until seeking medical help, referrals, diagnoses, hospitalizations, and dispatches (if any)], examination upon arrival, and the type and time of treatment. Furthermore, angiography and PCI results (if any), laboratory data, left ventricular function, disease course and complications during hospitalization, type and dosage of medications before and during hospitalization, and at the time of discharge, and ultimately the patients’ status at the time of discharge, and future rehabilitation plans were studied. The nurses appointed to collect the data, received ten initial trainings of 2-hour long and a monthly one session of retraining.
The long-term follow-up of the patients was performed, over the phone and in person in a one-year period. The follow-up form includes data on vital status, physical activity, history of CV events, history of hospitalization for any cardiac or non-cardiac reasons, and history of surgical or interventional CV procedures performed within one year of the STEMI diagnosis, a complete medication history, left ventricular function, and some biochemical factors. All the patients with STEMI were also registered in the older ongoing ACS registry based on WHO MONICA protocole.17
PROVE/CICD: The CICD registry was initiated based on the hot pursuit method, in the same hospital that the PCI registry had been implemented and is ongoing. This registry started as part of EORP26 and based on their protocol. Cardiologists familiar with EORP/CICD began to register and follow up all registered patients. Eligible patients were all aged above 18 years with stable angina and a history of myocardial infarction (MI) or heart revascularization. Patients’ demographic data, medical history, risk factors, diagnostic tests, and treatment measures were registered. The patients’ were followed up for a year, in person or by phone.
The follow-up after discharge data were the same as the enrollment one.
Table 1 presents the registered diseases and their details in PROVE.
Table 1.
Characteristics of cardiovascular disease registry based on different types of disease
| Type of diseases | Questionnaire based on | Follow-ups | Methods | Settings |
|---|---|---|---|---|
| Stroke | Researcher-made | 1, 3, and 12 months | Cold pursuit: Refer to archive patient records | Hospital based |
| AF | EORP | Once/year for 3 years | Hot pursuit: Interviews with patients | Population based (hospital and clinics) |
| HF | Researcher-made | 1, 6, and 12 months | Cold pursuit: Refer to archive patient records and switch to Hot pursuit later | Hospital based |
| PCI | Researcher-made | 1, 6, and 12 months | Hot pursuit | Hospital based |
| CHD | Researcher-made | Depending on the patient’s disease and conditions | 1) Hot pursuit: Interviews with patients 2) Cold pursuit (in one of the centers): Refer to archive patient records | Population based (hospital and clinics) |
| STEMI | EORP | 12 months | Hot pursuit: Interviews with patients | Hospital based |
| CICD | EORP | 12 months | Hot pursuit: Interviews with patients | Hospital based |
| ACS and stroke | WHO MONICA8 | 28 days | Cold pursuit: Refer to archive patient records | Hospital based |
AF: Atrial Fibrillation; HF: Heart Failure; PCI: Percutaneous Coronary Intervention; CHD: Congenital Heart Disease; STEMI: ST Elevation Myocardial Infarction; CICD: Chronic Ischemic Cardiovascular Disease; ACS: Acute Coronary Syndrome; EORP: European Observational Research Program; MONICA: Multinational Monitoring of trends and determinants in Cardiovascular disease
Consent and Confidentiality
All the patients were ensured of the confidentiality of their data and signed an informed consent form, for having their data collected. In order to maintain a full confidentiality of the patients’ information, a registration code (additional to a national code) was assigned, to be used for research purposes, instead of the first and last name. The registration code is unique to each patient and formed by a non-identifiable combination of patient information, such as the first and last name, and date of birth, as inspired by Huffman coding.33,34 With this coding system, the software used to create PROVE has the capability to capture each patient’s data and categorize it in a right place even if the patient has been hospitalized for a different type of CVD, on any other occasion. We created a second code to ensure the confidentiality and to have an alternative, in case the first code is lost.
Quality Control
To ensure the quality of PROVE, and as an internal quality control measure, all PIs were responsible to collaborate with QC experts to evaluate their specific disease registry. Quality control measures taken, were as follows: setting a list of minimum required information to enter into the registry, ensuring the forms used to enter the data are user-friendly, providing a complete and easy-to-use protocol with a related glossary to be used in case of ambiguity, reviewing the data entry to ensure the quality, training all personnel responsible for data collection, ensuring the data are collected with the highest quality and least missing info, visual inspection of the forms, and regular interim analysis. The QC experts, with the collaboration of PIs, were responsible to incorporate into the registry protocols or questionnaires, all the recommendations based on interim analysis, evaluation, and monitoring of the results.35
To ensure a high quality data collection, in addition to the internal QC, an external audit was conducted by experts, not members of the PROVE team. They studied the protocols, questionnaires, glossaries, and inclusion criteria for each disease, before starting the audits. This would make the data more comparable and less biased, when compared with data from other countries (comparability). Moreover, the QC committee ensured 1) the timeliness of the data by registering the PROVE data in a preset time period, i.e. within two weeks of generating the medical record of a new patient at the target hospital and up to nine months, depending on the type of the disease; 2) the accuracy and validity of the data, by accurately registering the data, with no errors; and 3) lastly the completeness of data, by achieving the ultimate goal and by registering 90% of the admitted patients in hospitals, with any type of diseases .
A second expert also reviewed the diagnostic accuracy. To validate the clinical diagnosis, an independent cardiologist not affiliated with PROVE team, examined 10% of the records, using the diagnosis checklist in each protocol. The independent cardiologist then would report any error to the PIs. This was performed by examining 10% of the hospital records and comparing the registered and collected data. In case of any errors, the assessment team would report the error to the PIs.
Data were analyzed using SPSS software (version 22.0, IBM Corporation, Armonk, NY, USA,). Descriptive statistics were calculated, based on clinical characteristics of the registered patients. Quantitative variables were expressed as mean ± SD or median and interquartile range (if required). Qualitative variables were summarized as counts (percent). To assess content validity of questionnaires, we used a content validity ratio (CVR) and content validity index (CVI) scores.
Results
The number of patients whose data were entered into PROVE database by September 2016, for each type of disease was as follow:
A total of 1195 patients with HF were recruited, 928 based on clod pursuit method (61.96% men, and 38.4% women) and 267 based on hot pursuit method. There were 9427 registered patients with ACS (54.5% men and 45.5% women) of whom 809 registered with STEMI (79.97% men and 20.03% women). Patients registered with AF were 363 (50.68% men and 49.32% women), with stroke 761 cases (50.72% men and 49.28% women), with CHD 1136 subjects (51.4% men and 48.6% women); those who underwent PCI were 1200 (74% men and 26% women), and there were 9 cases of CICD. CRFs for cases of AF, STEMI, HF, and CICD cases were sent to the EORP site.
After assessing the data by QC committee on HF registry, and challenges faced, the PIs decided to change the HF registry to hot (prospective) pursuit.
Table 2 presents the number of recruits and sites, according to the type of CVDs.
Table 2.
Registered patients with cardiovascular disease in Isfahan, Iran, by September 2016
| Registered Diseases | Number of registered participant | Time of onset | Number of target centers and hospitals |
|---|---|---|---|
| ACS/WHO/MONICA | 9427* | March 2015 | All of the Isfahan hospitals |
| STEMI/EORP | 809 | September 2015 | 3 government hospitals affiliated to Isfahan University of Medical Sciences |
| Stroke/WHO/Stepwise and European registry | 761 | March 2015 | All of the Isfahan hospitals |
| HF | COLD: 928 | March 2015 | All of the Isfahan hospitals |
| HOT:267 | April 2016 | 2 government hospitals affiliated to Isfahan University of Medical Sciences | |
| AF | 363 | March 2015 | 2 government hospitals affiliated to Isfahan University of Medical Sciences and 1 referral clinic |
| CHD | 1136 | September 2015 | 3 government hospitals affiliated to Isfahan University of Medical Sciences, 2 referral clinics and 2 referral intensive clinics |
| PCI | 1200 | September 2015 | 1 government hospital affiliated to Isfahan University of Medical Sciences |
| CICD | 9 | June 2016 | 1 government hospital affiliated to Isfahan University of Medical Sciences |
ACS: Acute Coronary Syndrome; MONICA: Multinational Monitoring of trends and determinants in Cardiovascular disease; STEMI: ST Elevation Myocardial Infarction; EORP: European Observational Research Program; HF: Heart Failure; AF: Atrial Fibrillation; CHD: Congenital Heart Disease; PCI: Percutaneous Coronary Intervention; CICD: Chronic Ischemic Cardiovascular Disease
ACS including patients with STEMI
Discussion
We presented here our feasibility study of the national comprehensive CVD registry, the way it was developed and implemented. Additionally, we discussed the patients' recruitment methods, follow-up, data collected, and QC measures.
Researchers often use disease registries to understand where a disease management modality stands, for a given medical condition. These critical databases have always been used to help us understand how to deliver quality care and what outcomes are achieved. Furthermore, findings from registries could help policy makers to better assess the magnitude of health-related issues and assist them in setting priorities for interventions, in order to prevent and control the diseases.
CVD is the leading cause of death globally, and specifically in Iran. A registration system for patients with CVD, especially ACS, stroke, HF, and AF could offer valuable information on the course of these diseases, its diagnosis and treatment, acute phases and chronic conditions, medications, in-hospital and out-of-hospital complications, and clinical outcomes.
In the past half-century, major advances have been made in the field of cardiology with the introduction of angiography and coronary artery interventions. In some cases, the effectiveness and safety of the treatment methods were challenged and alternative treatments were proposed, based on the patients’ information through registries.30
As noted earlier, some of the disease registrations are performed through hot pursuit, and some through cold pursuit. Each of these methods has pros and cons. For example, hot pursuit requires more on-site personnel and it is therefore more costly, and prone to miss some information on holidays or when the registration personnel are absent from work. Nevertheless, since this method allows the information to be registered simultaneously with the occurrence of the CV event, it gives a more thorough access to relevant information, and the registration outcome is more complete.
With the cold pursuit, however, registration can be performed months or years later than the occurrence of the CV event and with fewer personnel; but the information derived from previous records and documents may be incomplete.28
In appreciation of a distinct need for real and accessible data on CVD, the European Society of Cardiology (ESC) stated that registries are required for evaluating the epidemic of CVD, the diagnostic, treatment methods, and promotion of the guidelines. Therefore, The EORP was established in 2009 with the aim of creating a better understanding of medical performance, based on the collected data and in order to invite interested countries to join and cooperate.26 Therefore, we decided to join EORP and collaborate in the registry of some types of CVDs in PROVE such as AF, STEMI, CICD, and HF.
PROVE CVD types that have joined EORP use hot pursuit only. However, due to the shortage of personnel, it is currently running on a limited number of centers and it will be gradually run in other centers, when the challenges of the registry are clarified and upon completing the feasibility study.
The CHD questionnaire is short and mostly contains clinical questions; therefore, it is completed by the patients in physicians’ offices and by the on-site personnel at the hospitals.
As for the registration of stroke and ACS, since the MONICA questionnaires were extensive and the number of personnel cooperating with the project was limited, registration has begun in the form of cold pursuit in many years ahead; however as a pilot study, some IUMS-affiliated hospitals have switched to hot pursuit registration.
In many registries, consisting of several diseases, both hot and cold pursuit methods are often used; eg: Global Registry of Acute Coronary Events (GRACE) that uses both methods (hot and cold) pursuits, based on the conditions. The GRACE registry enrolls patients with ACS, like STEMI, non-STEMI, and ACS without biomarker and their risk predictors, as well as in-hospital and six-month outcomes.36
In PROVE, a number of disease registrations are hospital-based, because of the limited number of personnel, or large number of patients, or the lack of specialized clinics, such as CICD, stroke, HF, ACS/MONICA, and STEMI.
A number of diseases’ registration is population-based, for example, registries for AF and CHD. The reason could be either because of the protocol they follow; such as the EORP protocol requires a population-based registration, or because they have detected reliable paths, before proceeding with this type of registration, as the case in CHD.
The collection of long-term follow-up data is often an important component of the disease registries. Follow-up data are often critical to the registry's objectives;37 hence, we followed our patients for longer time periods, after they were discharged from the hospitals.
Different registries in PROVE suggested different times and intervals for the follow-up of patients. Those who use cold pursuit method like stroke and HF have periodical follow-up in a short interval, in order to avoid loss of patients and the associated information. Other registries have follow-up periods in line with their own protocols.
Limitations and strengths: To the best of our knowledge, to date, no comprehensive registry has been established in Iran or in the Eastern Mediterranean region, including as many types of CVD, as in PROVE. We believe that PROVE could help to fill the gap on CVD effective management, prevention, and control, by providing valuable data.
Furthermore, our joint collaboration with EORP will allow a larger pool of data to be compared, and in addition, to obtain scientific knowledge on an international scope. The continuous QC, all throughout the initiation and implementation of PROVE, is a point of strengths for this initiative.
However, this program suffers from some limitations, such as lack of continuous funding source, lack of a proper infrastructure for implementation, less committed clinicians to collaborate, and limited hospitals to participate without expecting financial incentives.
Conclusion
PROVE development and implementation as a feasibility study was successful. While the implementation was initiated in Isfahan, scale-up pilot study at the national level, has been started. This registry can generate a valuable data pool to be used for purposes, such as improving the current CVD management in participating centers and at a national level, filling the gaps in preventative care, establishing effective treatment and disease control guidelines, and eventually local and international research.
Acknowledgments
This project is funded by the undersecretary of Research and Technology of the Iranian Ministry of Health and Medical Education, the Isfahan Cardiovascular Research Institute, the Espadan Association of Heart Health Research, and the Iranian Network of Cardiovascular Research. The authors would like to express their gratitude to the large PROVE team members and the personnel at Isfahan Cardiovascular Research Institute for their cooperation and assistance. Project registration code in IUMS is 93121.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Amiri Z, Farazmand A, Tolooei MH. Causes of hospitalization of elderly people in hospitals in Rasht in 2000. J Guilan Univ Med Sci. 2002;11(42):28–32. In Persian. [Google Scholar]
- 2.Hatami H, Razavi SM, Eftekhar Ardebili H, Majlesi F, Syednozady M, Parizadeh MG. Book comprehensive public health. Tehran, Iran: Arjmand Publications; 2004. p. 1524. In Persian. [Google Scholar]
- 3.Akbari M. According ten years of the Islamic Republic of Iran on the international goals on Population and Development. Tehran, Iran: Ministry of Health and Medical Education; 2004. pp. 14–21. In Persian. [Google Scholar]
- 4.Kathleen Mahan l, Escott-Stump S. Krause's food & nutrition therapy. Philadelphia, PA: Saunders/Elsevier; 2008. p. 1021. [Google Scholar]
- 5.Sarraf-Zadegan N, Sayed-Tabatabaei FA, Bashardoost N, Maleki A, Totonchi M, Habibi HR, et al. The prevalence of coronary artery disease in an urban population in Isfahan, Iran. Acta Cardiol. 1999;54(5):257–63. [PubMed] [Google Scholar]
- 6.Sarraf-Zadegan N, Boshtam M, Rafiei M. Risk factors for coronary artery disease in Isfahan, Iran. Eur J Public Health. 1999;9(1):20–6. [Google Scholar]
- 7.Esteghamati A, Abbasi M, Alikhani S, Gouya MM, Delavari A, Shishehbor MH, et al. Prevalence, awareness, treatment, and risk factors associated with hypertension in the Iranian population: The national survey of risk factors for noncommunicable diseases of Iran. Am J Hypertens. 2008;21(6):620–6. doi: 10.1038/ajh.2008.154. [DOI] [PubMed] [Google Scholar]
- 8.Sarrafzadegan N, AminiNik S. Blood pressure pattern in urban and rural areas in Isfahan, Iran. J Hum Hypertens. 1997;11(7):425–8. doi: 10.1038/sj.jhh.1000448. [DOI] [PubMed] [Google Scholar]
- 9.Roohafza H, Sadeghi M, Shirani S, Bahonar A, Mackie M, Sarafzadegan N. Association of socioeconomic status and life-style factors with coping strategies in Isfahan Healthy Heart Program, Iran. Croat Med J. 2009;50(4):380–6. doi: 10.3325/cmj.2009.50.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarrafzadegan N, Kelishadi R, Baghaei A, Hussein SG, Malekafzali H, Mohammadifard N, et al. Metabolic syndrome: An emerging public health problem in Iranian women: Isfahan Healthy Heart Program. Int J Cardiol. 2008;131(1):90–6. doi: 10.1016/j.ijcard.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Kelishadi R, Sadri G, Tavasoli AA, Kahbazi M, Roohafza HR, Sadeghi M, et al. Cumulative prevalence of risk factors for atherosclerotic cardiovascular diseases in Iranian adolescents: IHHP-HHPC. J Pediatr (Rio J) 2005;81(6):447–53. doi: 10.2223/JPED.1418. [DOI] [PubMed] [Google Scholar]
- 12.Fars News Agency. Heart diseases are responsible for 38 percent of deaths in Iran [Online]. 2006. Available from: URL: http://www.farsnews.net/newstext.php?nn=8507010512.
- 13.Sarrafzadegan N, Talaei M, Sadeghi M, Kelishadi R, Oveisgharan S, Mohammadifard N, et al. The Isfahan cohort study: Rationale, methods and main findings. J Hum Hypertens. 2011;25(9):545–53. doi: 10.1038/jhh.2010.99. [DOI] [PubMed] [Google Scholar]
- 14.Talaei M, Sarrafzadegan N, Sadeghi M, Oveisgharan S, Marshall T, Thomas GN, et al. Incidence of cardiovascular diseases in an Iranian population: The Isfahan Cohort Study. Arch Iran Med. 2013;16(3):138–44. [PubMed] [Google Scholar]
- 15.Design & Implementing of health information system: Ministry of health and medical education-deputy of research & technology [Online]. 2005. Available from: URL: http://www.allhealth.org/publications/health_information_technology/health_information_technology_toolkit.asp.
- 16.Iowa Department of Public Health. Disease registry issue brief [Online]. 2010. Available from: URL: https://idph.iowa.gov/Portals/1/Files/OHCT/Disease%20Registry%20Issue%20Brief-%20Executive%20Summary.pdf.
- 17.World Health Organization, Office of Cardiovasculr Diseases. MONICA Manual. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 18.Oveisgharan S, Shirani S, Ghorbani A, Soltanzade A, Baghaei A, Hosseini S, et al. Barthel index in a Middle-East country: Translation, validity and reliability. Cerebrovasc Dis. 2006;22(5-6):350–4. doi: 10.1159/000094850. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini S, Baghbanian P. Assessment of predictive factors of death from myocardial infarction at 28 days. Research in Medical Sciences. 2003;8:98–102. [Google Scholar]
- 20.Sarrafzadegan N, Oveisgharan S, Toghianifar N, Hosseini S, Rabiei K. Acute myocardial infarction in Isfahan, Iran: hospitalization and 28th day case-fatality rate. ARYA Atheroscler. 2007;5(3):495–9. [Google Scholar]
- 21.Oveisgharan S, Sarrafzadegan N, Shirani S, Hosseini S, Hasanzadeh P, Khosravi A. Stroke in Isfahan, Iran: Hospital admission and 28-day case fatality rate. Cerebrovasc Dis. 2007;24(6):495–9. doi: 10.1159/000110418. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadian Hafshejani A, Sarrafzadegan N, Baradaran Attar Moghaddam HR, Hosseini S, Hosseini S. Gender difference in determinants of short-term survival of patients with acute myocardial infarction in Isfahan, Iran. J Isfahan Med Sch. 2012;30(209):1611–22. [Google Scholar]
- 23.Mohammadian Hafshejani A, Baradaran H, Sarrafzadegan N, Asadi Lari M, Ramezani A, Hosseini S, et al. Predicting factors of short-term survival in patients with acute myocardial infarction in Isfahan using a cox regression model. Iran J Epidemiol. 2012;8(2):39–47. [Google Scholar]
- 24.Mohammadian Hafshejani A, Baradaran Attar Moghaddam HR, Sarrafzadegan N, Bakhsi Hafshejani FA, Hosseini S, AsadiLari M, et al. Evaluation of short-term survival of patients with acute myocardial infarction and the differences between the sexes in Isfahan and Najaf Abad between (1999-2008). Razi J Med Sci. 2012;19(95):25–34. [Google Scholar]
- 25.Mohammadian-Hafshejani A, Baradaran-AttarMoghaddam H, Sarrafzadegan N, AsadiLari M, Roohani M, Allah-Bakhsi F, et al. Secular trend changes in mean age of morbidity and mortality from an acute myocardial infarction during a 10-year period of time in Isfahan and Najaf Abad. J Shahrekord Univ Med Sci. 2013;14(6):101–14. [Google Scholar]
- 26.European Society of Cardiology. EURObservational Research Programme [Online]. 2016. Available from: URL: https://www.escardio.org/portal/site/Escardio/menuitem.c9f480f01a3ca18798f54de7202031ca/?vgnextoid=5c4407daabb2b410VgnVCM1000004e03a8c0RCRD&vgnextchannel=2f468124b9945510VgnVCM1000005303a8c0RCRD&vgnextfmt=default&vgnextlocale=EN.
- 27.Noncommunicable Diseases and Mental Health, World Health Organization. STEPS-stroke: The WHO STEP wise approach to stroke surveillance. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 28.Giampaoli S, Hammar N, Adany R, De PC. Population-based register of stroke: Manual of operations. Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 3):S23–S41. doi: 10.1097/01.hjr.0000277987.10473.6f. [DOI] [PubMed] [Google Scholar]
- 29.Ringelstein EB, Meckes-Ferber S, Hacke W, Kaste M, Brainin M, Leys D. European stroke facilities survey: The German and Austrian perspective. Cerebrovasc Dis. 2009;27(2):138–45. doi: 10.1159/000177922. [DOI] [PubMed] [Google Scholar]
- 30.Cardiovascular Research Institute. Persian Registry Of cardioVascular disease (PROVE) [Online]. 2015. Available from: URL: http://prove.mui.ac.ir.
- 31.Jonsson A, Edner M, Alehagen U, Dahlstrom U. Heart failure registry: A valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12(1):25–31. doi: 10.1093/eurjhf/hfp175. [DOI] [PubMed] [Google Scholar]
- 32.Laothavorn P, Hengrussamee K, Kanjanavanit R, Moleerergpoom W, Laorakpongse D, Pachirat O, et al. Thai acute decompensated heart failure registry (Thai ADHERE). CVD Prevention & Control. 2010;5(3):89–95. [Google Scholar]
- 33.Huffman DA. A method for the construction of minimum-redundancy codes. Proceedings of the IRE. 1952;40(9):1098–101. [Google Scholar]
- 34.Golin MJ, Kenyon C, Young NE. Huffman coding with unequal letter costs.. Proceedings of the 34th Annual ACM Symposium on Theory of Computing;; 2002 May 19-21; New York, NY. [Google Scholar]
- 35.Arts DG, De Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: A literature review, case study, and generic framework. J Am Med Inform Assoc. 2002;9(6):600–11. doi: 10.1197/jamia.M1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA. The global registry of acute coronary events, 1999 to 2009-GRACE. Heart. 2010;96(14):1095–101. doi: 10.1136/hrt.2009.190827. [DOI] [PubMed] [Google Scholar]
- 37.Gliklich RE, Dreyer NA, Leavy MB. Registries for evaluating patient outcomes: A user's guide. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]