Abstract
Purpose
To evaluate associations between sensations of ocular itch and dry eye (DE) symptoms, including ocular pain, and DE signs.
Methods
A cross-sectional study of 324 patients seen in the Miami Veterans Affairs eye clinic was performed. The evaluation consisted of questionnaires regarding ocular itch, DE symptoms, descriptors of neuropathic-like ocular pain (NOP), and evoked pain sensitivity testing on the forehead and forearm, followed by a comprehensive ocular surface examination including corneal mechanical sensitivity testing. Analyses were performed to examine for differences between those with and without subjective complaints of ocular itch.
Results
The mean age was 62 years with 92% being male. Symptoms of DE and NOP were more frequent in patients with moderate-severe ocular itch compared to those with no or mild ocular itch symptoms. With the exception of ocular surface inflammation (abnormal matrix metalloproteinase 9 testing) which was less common in those with moderate-severe ocular itch symptoms, DE signs were not related to ocular itch. Individuals with moderate-severe ocular itch also demonstrated greater sensitivity to evoked pain on the forearm and had higher non-ocular pain, depression, and post-traumatic stress disorders scores, compared to those with no or mild itch symptoms.
Conclusions
Subjects with moderate-severe ocular itch symptoms have more severe symptoms of DE, NOP, non-ocular pain and demonstrate abnormal somatosensory testing in the form of increased sensitivity to evoked pain at a site remote from the eye, consistent with generalized hypersensitivity.
INTRODUCTION
Itch is a common complaint in the general population, affecting between 8 and 38% worldwide.1 It can appear as an idiopathic complaint or in the setting of known conditions such eczema or psoriasis. Itch has also been reported a common complaint in primary Sjogrens syndrome (pSS)2,3, a disease whose hallmark is aqueous tear deficient dry eye (DE). In one study, 10 subjects with pSS and chronic itch were compared to 9 subjects with pSS and no chronic itch.4 Those with itch reported that it was chronic (mean duration ~6 years) and severe (mean 7.7 on a visual analogue scale). Interestingly, xerosis (i.e. dryness) was more common in those with itch compared to those without (9, 90% versus 4, 44%).
In the eye, the classic teaching attributes the presence of itch to allergy. Yet, a recent paper reported that ocular itch associated not only with allergic conjunctivitis (OR=5.0; 95% CI 3.0 to 8.3) but also with DE (OR=2.6; 95% CI 1.7 to 4.1).5 DE, however, is not one disease and includes both DE symptoms (sensations of dryness, pain, and visual disturbances) and signs (decreased tear production, increased evaporation, ocular surface inflammation), which are often disparate.6 It is not known which components of DE (specific symptoms, signs, or both) associate with ocular itch.
To fill this gap, in this study, we evaluate which aspects of DE most closely align with ocular itch, including non-ocular metrics associated with DE in prior studies.7,8 Based on shared neurobiological mechanisms, we hypothesize that ocular itch more closely relates to DE symptoms, especially ocular pain, as compared to DE signs. Data to support this hypothesis comes from the shared role of thermosensitive transient receptor potential (TRP) channel proteins in transmitting multiple sensations (itch, pain, and dryness).9–12 Furthermore, inflammation, a component of DE and skin conditions associated with itch, can alter TRP channel function with increased excitability of peripheral nociceptors.1,11,13 Prolonged peripheral traffic can alter central neurons, with resulting pain amplification expressed as hyperagesia and allodynia.1,14 Finally, DE symptoms and itch are co-morbid with disorders of central processing, namely depression, anxiety, and insomnia.1,7,15,16
METHODS
STUDY POPULATION
Patients with otherwise healthy eyelid and corneal anatomy were prospectively enrolled from the Miami Veterans Affairs eye clinic from October 2013 to August 2016. To study patients with “idiopathic” DE symptoms, we excluded patients with conditions known to underlie DE symptoms including infection, contact lens use, history of refractive, glaucoma, or retina surgery, cataract surgery within the preceding 6 months, use of ocular medications other than artificial tears, human immunodeficiency virus, sarcoidosis, graft-versus-host disease, or collagen vascular diseases. Miami Veterans Affairs Institutional Review Board approved the prospective evaluation of patients. The study was conducted in accordance with the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act. All subjects signed an informed consent form.
DATA COLLECTED
For each individual, demographic information, past ocular and medical history and medication information were collected.
DRY EYE SYMPTOMS
Subjects completed the dry eye questionnaire 5 (DEQ5)17 and the Ocular Surface Disease Index (OSDI).18 The DEQ5 is a validated, 5-item questionnaire that combines patient responses regarding discomfort (frequency and intensity), dryness (frequency and intensity), and watery eyes (frequency) during the past month. DEQ5 scores range from 0 to 22, with higher scores corresponding to greater severity of symptoms. The OSDI is also a validated DE questionnaire that grades symptoms (sensitivity to light, grittiness, soreness, blurred or poor vision), triggers (wind, low humidity, air conditioning), and degree of disability associated with symptoms (limitations in reading, driving, working on the computer, watching television) over a 1 week recall. It is scored on a 0 to 100 scale, with higher scores indicating greater symptoms and disability.
OCULAR ITCH
Individuals were asked to rate the severity of their ocular itch as none, mild, moderate, or severe. This schema was based on the short-form McGill Pain questionnaire which utilizes a similar scale to assess for the presence of sensory and affective pain descriptors.19
OCULAR PAIN
Subjects rated the intensity of their average eye pain over a 1-week period using a numerical rating scale (NRS; 0 for “no pain sensation”, 10 for “the most intense eye pain imaginable”). A modified Neuropathic Pain Symptom Inventory (NPSI) was administered to evaluate neuropathic ocular pain-like symptoms (NPSI-Eye, range 0–100). The modified version replaced the original items regarding allodynia and hyperalgesia in the setting of light touch, pressure, or contact with something cold on the skin with items specific to ocular hyperalgesia and allodynia (namely, eye pain caused or increased by wind, light, and/or heat or cold). The NPSI has been validated as a self-report instrument for assessing neuropathic pain,20 has been used to quantify different aspects of neuropathic pain,20 and has been found to correlate with mechanical and/or thermal allodynia and hyperalgesia assessed using Quantitative Sensory Testing (QST).21
OCULAR SURFACE EVALUATION
The ocular surface evaluation, in the order performed, included (1) tear osmolarity (TearLAB, San Diego, CA, once in each eye); (2) ocular surface inflammation (Inflammadry, Quidel, San Diego, CA presence of matrix metalloprotease 9, once in each eye); (3) tear breakup time (5 μl fluorescein placed, 3 averaged measurements in each eye); (4) corneal epithelial cell disruption via corneal staining (National Eye Institute scale)22, 5 areas of cornea assessed with a score of 0–3 in each, range 0–15); (6) tear production via Schirmer’s strips (mm wetting measured at 5 minutes after placement of proparicaine); and (7) meibomian gland assessment. Eyelid vascularity was graded 0–3 (0 = none; 1 = mild engorgement; 2 = moderate engorgement; 3 = severe engorgement, based on photographs) and meibum quality was graded 0–4 (0 = clear; 1 = cloudy; 2 = granular; 3 = toothpaste; 4 = no meibum extracted).23
DE DISCORDANCE SCORE
Discordance scores were calculated similarly to Vehof et al.24 Values representing DE symptoms (OSDI) and signs (osmolarity, TBUT, corneal staining, Schirmer, eyelid vascularity, meibum quality, more severe value of two eyes) were transformed to a common unit severity score between 0 (representing the most normal value) and 1 (representing the most abnormal value) using linear interpolation. A composite signs severity score was then calculated by averaging the transformed scores of the six DE signs. The DE discordance score was defined as the difference between the transformed score on the OSDI and the transformed score on the composite signs severity score. DE discordance scores could potentially range from −1 (minimal symptoms, maximal signs) to 1 (maximal symptoms, minimal signs). A positive discordance score indicates more symptoms than signs.
CORNEAL SENSITIVITY
Mechanical detection and pain thresholds of the right central cornea were assessed with a modified Belmonte non-contact aesthesiometer, developed based on the original instrument.25 The tip (0.5 mm diameter) was placed perpendicular to and 4 mm from the surface of the cornea of the right eye. Stimulation consisted of pulses of air at room temperature.26 The method of limits, using ascending series only, was used to measure detection and pain thresholds with highest value for both being 400 mL/min.27
CUTANEOUS SENSITIVITY
Testing was performed over the right forehead, at a site approximately two finger widths above the superior orbital fissure and in line with the pupil, and over the ventral right forearm, on the skin overlying the midpoint between the wrist and cubital fossa. A TSA II (Thermal Sensory Analyzer; Medoc Ltd., Israel) machine was used to assess cold and hot pain thresholds to stimuli delivered via a square thermode (9cm2 surface area). The starting temperature was set at 32 ºC and cooled (or heated) using the software accompanying the machine. Per the ascending method of limits, the probe temperature gradually decreased (for cold pain thresholds at a rate of 2ºC/sec) or increased (for hot pain thresholds, at a rate of 2ºC/sec) until the subject pressed a button (placed in the left hand) to indicate the first moment that he/she perceived pain, or the cut-off temperature was reached (0ºC for cold trials, 50ºC for heat trials). Three trials for cold pain and three trials for hot pain were performed at each test site, with an inter-trial-interval of 45 seconds. Results of the three trials for each modality were averaged and are reported as the absolute change from baseline temperature (32ºC). Thus, higher values for both heat and cold indicate that a greater change in temperature was needed for the subject to report pain. In addition, ratings of pain intensity at threshold for cold pain and hot pain were recorded using a 0 to 100 numerical rating scale, where 0=“no pain” and 100=“the most intense pain imaginable.”
NON-OCULAR PAIN SEVERITY AND MENTAL HEALTH
A NRS for concurrent non-ocular pain was used (“How would you describe the overall intensity of your pain, on average during the last week?” and “How would you describe the overall intensity of your pain, at its worst during the last week?” scale 0–10). Symptoms of PTSD were assessed via the PTSD Checklist – Military Version (score 17–85)28 and symptoms of depression via the Patient Health Questionnaire 9 (score 0–27).29
STATISTICAL ANALYSIS
Individuals were grouped by the presence of itch rated on a 0 to 3 scale, with “0” indicating no itch, “1” indicating mild itch, “2” indicating moderate itch and a “3” indicating severe itch. Statistical analyses were performed using SPSS 22.0 (SPSS Inc, Chicago, IL) statistical package. Student t-test, Mann Whitney U, linear-by-linear association, and Fisher exact tests were used to compare variables of interest between the groups. The sample size of 124 moderate-severe ocular itch patients, 130 patients with mild itch, and 70 without itch provides 80% power to detect ANOVA effect sizes of 0.230, which corresponds to differences seen in QST testing, and more than 95% power to detect effect sizes of 0.3, which corresponds to differences seen in patient reported symptoms.
RESULTS
DEMOGRAPHICS AND CO-MORBIDITIES BY ITCH SUBGROUPS
324 subjects participated in the study (mean age 62 years, 92% men). Of those, 254 individuals reported a sensation of ocular itch: 130 rated the sensation as “mild” in intensity, 79 “moderate”, and 45 “severe”. Full demographic characteristics of the sample, grouped by sensation of itch, are presented in Table 1. Demographics and co-morbidities were similar between groups. A higher proportion of patients with moderate-severe ocular itch were on anxiolytics, antidepressant, and analgesics than their counterparts with no or mild ocular itch.
TABLE 1.
DEMOGRAPHICS AND CO-MORBIDITIES OF STUDY POPULATION BY RESPONSE TO SENSATION OF ITCH
| NO OCULAR ITCH (n=70) | MILD OCULAR ITCH (n=130) | MODERATE-SEVERE OCULAR ITCH (n=124) | P-VALUE | |
|---|---|---|---|---|
| Demograhics | ||||
| Age, years mean (SD) | 62 (9) | 62 (10) | 62 (11) | 0.96 |
| Gender, male, n (%) | 63 (90%) | 118 (91%) | 116 (94%) | 0.35 |
| Race, white, n (%) | 39 (56%) | 62 (48%) | 62 (50%) | 0.52 |
| Ethnicity, Hispanic, n (%) | 18 (26%) | 34 (26%) | 42 (34%) | 0.18 |
| Co-morbidities, n (%) | ||||
| Hypertension | 54 (77%) | 97 (75%) | 84 (68%) | 0.13 |
| Hypercholesterlemia | 40 (57%) | 84 (65%0 | 71 (57%) | 0.82 |
| Diabetes mellitus | 19 (27%) | 42 (32%) | 32 (26%) | 0.69 |
| Sleep apnea | 13 (19%) | 27 (21%) | 30 (24%) | 0.34 |
| BPH | 10 (14%) | 20 (15%) | 26 (21%) | 0.20 |
| Chronic pain (≥3 months) co-morbidities | ||||
| Number chronic pain conditions, mean (SD) | 2.3 (1.5) | 2.4 (1.6) | 3.0 (1.5) | 0.001 |
| Number chronic pain locations, mean (SD) | 3.7 (2.9) | 3.7 (2.9) | 4.6 (3.2) | 0.03 |
| Headache, n (%) | 22 (31%) | 40 (31%) | 50 (40%) | 0.23 |
| Back Pain, n (%) | 50 (71%) | 83 (64%) | 93 (75%) | 0.15 |
| Muscle Pain, n (%) | 23 (33%) | 48 (37%) | 60 (48%) | 0.06 |
| Tendonitis, n (%) | 11 (17%) | 30 (25%) | 31 (27%) | 0.33 |
| Sciatica, n (%) | 20 (29%) | 28 (22%) | 28 (23%) | 0.51 |
| Arthritis, n (%) | 30 (43%) | 56 (43%) | 64 (52%) | 0.19 |
| Post-Surgical Pain, n (%) | 15 (21%) | 28 (22%) | 27 (22%) | 0.99 |
| Diabetic Neuropathy, n (%) | 12 (17%) | 25 (19%) | 20 (16%) | 0.81 |
| Trigeminal Neuralgia, n (%) | 2 (3%) | 6 (5%) | 6 (5%) | 0.79 |
| Medications, n (%) | ||||
| Anxiolytic | 26 (37%) | 51 (39%) | 65 (52%) | 0.02 |
| Antidepressant | 30 (43%) | 49 (38%) | 67 (54%) | 0.06 |
| Anti-histamine | 15 (21%) | 23 (18%) | 29 (23%) | 0.61 |
| Analgesics | 34 (49%) | 70 (54%) | 87 (71%) | 0.001 |
SD=standard deviation; n=number in each group; BPH=benign prostatic hypertrophy
DE SYMPTOMS AND OCULAR SURFACE SIGNS BY ITCH
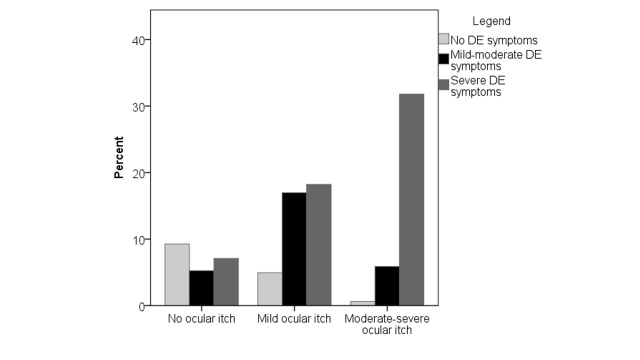
All DE symptoms, including neuropathic-like ocular pain symptoms, were higher in patients with moderate-severe ocular itch compared to their counterparts with no or mild ocular itch (Table 2). In fact, the frequency of severe DE symptoms increased with increased degree of ocular itch (Figure 1, p<0.0005). However, DE signs were not related to itch, with the exception of ocular inflammation (via matrix metalloproteinase (MMP) 9 detection) which was less common in those with moderate-severe ocular itch, although the difference did not reach statistical significance. When evaluating DE discordance scores, those with moderate-severe ocular itch had higher discordance scores (mean 0.35 standard deviation (SD) 0.28) compared to individuals without (0.11 SD 0.26) or with mild (0.17 SD 0.24) ocular itch (Figure 2). Clinically, this indicates that the group of individuals with moderate-severe ocular itch had a higher degree of DE symptoms compared to signs than individuals without or with mild itch.
TABLE 2.
DRY EYE SYMPTOMS AND OCULAR SURFACE EXAMINATION IN STUDY POPULATION BY ITCH SUBGROUPS
| MEAN (SD) | NO ITCH (n=70) | MILD ITCH (n=130) | MODERATE-SEVERE ITCH (n=124) | P-VALUE |
|---|---|---|---|---|
| Dry eye symptoms | ||||
| DEQ5 (range 0–22) | 7.9 (6.1) | 10.7 (4.4) | 14.1 (3.4) | <0.0005 |
| OSDI (range 0–100) | 25.1 (25.4) | 29.5 (21.5) | 47.4 (24.2) | <0.0005 |
| Ocular pain | ||||
| Pain intensity averaged past week (range 0–10) | 2.1 (2.7) | 2.7 (2.3) | 4.7 (2.4) | <0.0005 |
| Hot-burning pain (range 0–10) | 1.4 (2.6) | 2.3 (2.6) | 4.3 (3.2) | <0.0005 |
| Sensitivity to wind (range 0–10) | 1.6 (2.8) | 1.7 (2.5) | 4.5 (3.4) | <0.0005 |
| Sensitivity to light (range 0–10) | 2.5 (3.7) | 2.3 (2.7) | 4.5 (3.3) | <0.0005 |
| NPSI-Eye (range 0–100) | 12.7 (17.5) | 15.3 (16.2) | 33.8 (23.7) | <0.0005 |
| Ocular surface findings | ||||
| Tear osmolarity*, mOsm/L | 308 (19) | 305 (14) | 305 (16) | 0.39 |
| Tear film breakup time*, seconds | 10.0 (5.2) | 9.9 (4.0) | 9.6 (4.9) | 0.79 |
| Corneal staining* (range 0–15) | 2.4 (3.0) | 1.8 (2.3) | 1.8 (2.2) | 0.23 |
| Schirmer’s test*, mm of moisture | 11.9 (7.4) | 14.4 (7.6) | 14.2 (8.2) | 0.54 |
| Eyelid vascularity* (range 0–3) | 0.52 (0.70) | 0.60 (0.76) | 0.65 (0.79) | 0.18 |
| Meibum quality* (range 0–4) | 1.7 (1.3) | 1.9 (1.2) | 2.0 (1.2) | 0.14 |
| Ocular surface inflammation in either eye, n (%)† | 23 (43%) | 39 (42%) | 29 (30%) | 0.08 |
| Corneal evoked mechanical sensitivity, mL/min | ||||
| Corneal detection threshold** | 96 (45) | 93 (44) | 87 (44) | 0.38 |
| Corneal pain threshold** | 238 (109) | 218 (99) | 219 (112) | 0.46 |
DEQ5=Dry Eye Questionnaire; OSDI=Ocular Surface Disease Index questionnaire; NPSI-Eye= neuropathic pain symptom inventory modified for the eye;
represents value from more severely affected eye;
ocular surface inflammation is expressed as a percentage and not a mean;
tested in the right eye only.
FIGURE 1.
Bar graph demonstrating that subjects with increasing ocular itch severity also had increasing dry eye symptoms severity.
FIGURE 2.
Box plot demonstrating that patients with moderate-severe ocular itch had a greater discordance between symptoms and sign (higher symptoms, lower signs) compared to those without or with mild ocular itch.
SYSTEMIC PROFILES BY ITCH SUBGROUPS
Patient reported non-ocular pain and mental health indices were higher in those with moderate-severe ocular itch compared to those with no or mild itch. (Table 3) Individuals with moderate-severe ocular itch had lower hot pain thresholds at a site remote from the eye (forearm) indicating increased cutaneous sensitivity.
TABLE 3.
PAIN ELSEWHERE IN THE BODY (NON-OCULAR), MENTAL HEALTH INDICES, AND CUTANEOUS SENSITIVITY IN THE STUDY POPULATION BY ITCH
| NO ITCH (n=70) | MILD ITCH (n=130) | MODERATE-SEVERE ITCH (n=124) | P-VALUE | |
|---|---|---|---|---|
| Patient reported symptoms | ||||
| Non-ocular pain intensity, averaged over past week (range 0–10) | 3.7 (3.0) | 4.5 (2.9) | 5.8 (2.7) | <0.0005 |
| PTSD checklist Military Version (range 17–85) | 35 (20) | 35 (17) | 48 (20) | <0.0005 |
| Depression via PHQ9 (range 0–27) | 7.1 (8.1) | 7.2 (7.0) | 12.5 (8.2) | <0.0005 |
| Cutaneous sensitivity over forehead* | ||||
| Cold pain threshold, change from 32°C | 18.5°C (10.8) | 19.1°C (9.4) | 16.9°C (10.7) | 0.48 |
| Pain intensity rating at cold pain threshold | 37.5 (26.4) | 39.7 (25.0) | 41.5 (25.1) | 0.75 |
| Hot pain threshold, change from 32°C | 12.0°C (4.1) | 11.9°C (6.9) | 11.7°C (4.6) | 0.95 |
| Pain intensity rating at hot pain threshold | 46.8 (27.6) | 46.8 (26.2) | 47.0 (25.8) | 0.99 |
| Cutaneous sensitivity over forearm* | ||||
| Cold pain threshold, change from 32°C | 19.2°C (10.2) | 20.2°C (9.5) | 16.7°C (10.4) | 0.14 |
| Pain intensity rating at cold pain threshold | 32.7 (28.9) | 38.2 (26.4) | 38.6 (25.3) | 0.54 |
| Hot pain threshold, change from 32°C | 12.4°C (4.7) | 12.7°C (4.2) | 10.5°C (4.8) | 0.02 |
| Pain intensity rating at hot pain threshold | 46.3 (27.9) | 46.6 (25.5) | 46.7 (27.1) | 0.99 |
SD=standard deviation; PTSD= post-traumatic stress disease; PHQ=Patient Health questionnaire;
information available in 162 patients as only a sub-set of population underwent quantitative sensory testing
DISCUSSION
The current study evaluated the relationship between various DE symptoms, signs, and somatosensory findings by severity of ocular itch. We found that patients with moderate-severe ocular itch had more severe DE symptoms including those consistent with NOP with no differences in ocular surface measures. Additionally, moderate to severe ocular itch associated with increased cutaneous pain sensitivity, a higher discordance between symptoms and ocular surface signs, and higher non-ocular pain and mental health complaints. This suggests that various ocular sensations, which can include dryness, itch, and pain (e.g. burning, aching, and tenderness) have shared underlying mechanisms. Consequently, the remaining discussion will focus on potential commonalties (and unique differences) between these sensations and their translation to DE management.
THE ETIOLOGY OF ITCH, CHRONIC PAIN AND DE
Itch and pain are related but distinct sensations. In acute disease, mechanisms underlying itch and pain are often disparate, while in chronic disease, shared mechanisms underlie the pathophysiology. Similar considerations apply to sensations of dryness. Below we discuss how chronic sensations are driven by disorders of the nervous system prompted by immune dysfunction (Table 4).31
TABLE 4.
SIMILARITIES BETWEEN CHRONIC ITCH AND PAIN AND ITS POTENTIAL IMPLICATIONS IN DRY EYE (DE).
| SIMILARITIES BETWEEN CHRONIC ITCH AND PAIN | ITCH SPECIFIC | RELEVANCE TO DE | |
|---|---|---|---|
| Symptoms | Unpleasant, source of morbidity, affect quality of life | Itch evokes scratching while pain induces withdrawal | Chronic sensations of dryness often accompanied by other unpleasant sensations (pain, itch) |
| Co-morbidities | Depression and anxiety common co-morbidities | Depression and anxiety common co-morbidities | |
| Inflammation | Inflammation induces and maintains sensitization | IL-31 more specific to itch | Both cellular and soluble mediators elevated in tears and ocular surface in DE |
| Other mediators | Nerve growth factor involved | Nerve growth factor increased in the tears of individuals with DE | |
| Peripheral Transmission and Sensitization | Both involve increased responsiveness of peripheral nociceptive neurons, with changes in TRP function | G protein-coupled receptors (Mrgpr and PAR2) involved in non-histaminic mediated chronic itch. | Inflammation and high osmolarity, components of DE, sensitize corneal peripheral nerves, likely through changes in TRP function. |
| Central Transmission and Sensitization | Both involve increased CNS excitatory and decreased inhibitory signal transmission | Gastrin-releasing peptide (GRP) receptor expressing neurons essential for itch transmission. Imbalance between μ and κ opioids receptors in chronic itch. | Enhanced temporal summation and persistent aftersensations in patients with DE symptoms imply central sensitization |
| Higher order processing | Multiple areas of brain involved, both sensory and affective. Both sensations are not a direct measure of input | Precuneus involved. | |
| Supporting cells | Interaction between nerves with epithelial, immune and glial cells occurs in peripheral and central nervous system | Corneal epithelial cells interact and support corneal nerves in a manner similar to Schwann cells | |
| Treatment | Both respond in some degree to neuropathic pain medication | Anti-IL-4, IL-13, and IL-31, butorphanol (kappa opioid agonist) have shown success in chronic itch | Anti-neuropathic pain medication can be considered as an adjuvant to ocular surface optimization in those with a suspected neuropathic component to DE symptoms (whether sensations of itch, pain, or dryness) |
DE=dry eye; IL=interleukin; TRP= Transient receptor potential channels; CNS=central nervous system; PAR=Proteinase-activated receptor
PERIPHERAL INFLAMMATION
Inflammation is an important component of chronic pain, itch, and DE.
Peripheral inflammation and chronic pain
Injury to epithelial cells leads to the release of various mediators such as adenosine 5′-triphosphate (ATP), histamine, serotonin, bradykinin, and prostaglandin (PG) E2 which stimulate peripheral nerves to release inflammatory mediators such as substance P and calcitonin gene-related peptide (CGRP).32 These mediators co-activate resident antigen presenting cells and recruit additional immune cells to the site of injury.33–35 Immune cells release additional soluble mediators such as tumor necrosis factor (TNF) α and interleukin (IL) 1β that promote sensitization of nociceptors via gene expression and modulation of ion channels. In addition, nerve growth factor is secreted by keratinocytes and can cause sprouting and reorganization of nerves.
Peripheral inflammation and chronic itch
Similar mechanisms have been found in itch. In the skin, mast cells and keratinocytes interact and sensitize peripheral neurons. Specifically T cells release specific cytokines, IL-2 and IL-31, which have been involved with itch.31
Relevance to dry eye
Ocular surface inflammation is an important component in DE. Many mediators implicated in non-ocular pain and itch, such as TNFα, IL1, IL636, 37, PGE238, MMP-939, serotonin40, and NGF41 are elevated in the tears of patients with DE. Cellular inflammation also plays a role in DE with T cells detected more frequently in the conjunctivae of DE patients.42 The enhanced soluble and cellular inflammatory milieu described on the ocular surface in DE likely promotes peripheral sensitization in a similar fashion to that seen elsewhere in the body (Figure 3). Interestingly, both DE and non-ocular pain have been found to have a hereditary component43, 44 with genetic polymorphisms in pro-inflammatory genes (e.g. IL1 and IL6R) associated with DE in a Korean population.45
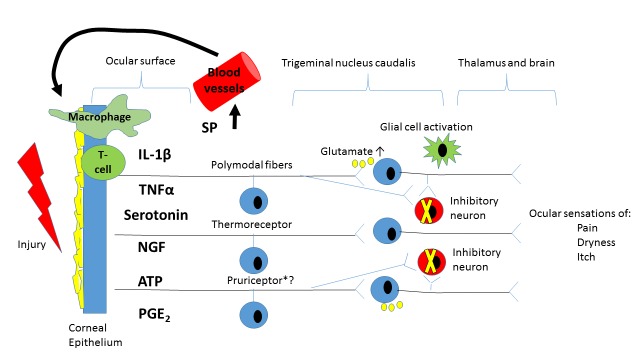
FIGURE 3.
Diagram showing potential shared pathways between the ocular sensations of itch, pain, and dryness. Of note, it is not known whether itch is conveyed by dedicated pruriceptors or whether itch is encoded by nociceptive fibers (e.g. polymodal afferents) that express specific pruriceptors. After injury, ocular surface epithelial cells secrete a variety of inflammatory mediators (e.g. prostaglandin E2 (PGE2), serotonin, adenosine triphosphate (ATP) that activate peripheral nociceptors. Activated nociceptors secrete substance P (SP) which recruits inflammatory cells (T cells and macrophages) into the injured area. Inflammatory cells secrete various cytokines (tumor necrosis factor α (TNFα) and interleukin-1β (IL-1 β), among others) which alter the ocular surface environment (e.g. increased osmolarity). These ocular surface alterations contribute to peripheral sensitization with increased responsiveness to external stimuli and spontaneous activation. Persistent peripheral traffic leads to amplified central nervous system processing (e.g. central sensitization) including augmented excitatory and decreased inhibitory transmission. These changes lead to a variety of unpleasant ocular surface sensations including itch, pain, and dryness, which are often co-morbid to each other.
NGF=nerve growth factor
Neuropathic pain
Both nociceptive and neuropathic mechanisms likely play a role in chronic itch and DE. Nociceptive pain (defined as pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors46) is driven by chronic ocular surface abnormalities that are seen in many DE sub-types (pSS, graft versus host disease). With time, inflammation, trauma, and chronic activation of nociceptors can lead to permanent changes in nociceptor structure and function (i.e. sensitization) that can occur both peripherally and/or centrally, thus adding a neuropathic component to the pain (defined as a pain due to a lesion or disease of the somatosensory system46).
Peripheral sensitization
Immune cells are in close proximity to peripheral nerves and their interactions can lead to peripheral sensitization (defined as reduced threshold and increased responsiveness of peripheral nociceptive neurons in response to stimulation of their receptive fields46).
Peripheral sensitization and chronic pain
Inflammation sensitizes peripheral nerves by enhancing the function of ion channels, including TRP channels (TRPV1 senses heat, chemicals, and abnormal pH and TRPM8 senses cooling) and voltage-gated sodium (Nav1.7–1.9), calcium, and potassium channels through phosphorylation and translation of new channels.47 Electrophysiologically, sensitized nerves demonstrate enhanced transduction (generation of an action potential) and conduction (propagation of the action potential).32 Spontaneous peripheral nerve activity may develop in several locations including adjacent uninjured afferent fibers and damaged axonal endings. Neuroma formation is a common finding in patients with pain in the setting of traumatic peripheral nerve injuries.48 Sodium channel activity has been found to be altered in neuromas, leading to modified sodium currents and abnormal ectopic firings.49
Peripheral sensitization and chronic itch
In the skin, specialized mechanically insensitive (chemo-) nociceptor (CMi) fibers are responsible for the transmission of histaminergic itch and play a role in neuronal sensitization and inflammation.34, 50–52 However, most chronic itch conditions do not respond to anti-histamines nor exhibit signs of CMi involvement. In fact, similar to chronic pain, chronic itch signaling is thought to occur via a non-histaminergic polymodal nociceptors mediated pathway. Unique nociceptors have been described in mediating chronic itch defined by the presence of specific G protein-coupled receptors (PAR231 and Mas-related G protein-coupled receptors (Mrgpr) A3+53).
Relevance to dry eye
In order to understand the role of peripheral sensitization in DE, it is important to first review normal corneal anatomy and physiology. The cornea is innervated by the ophthalmic branch of the trigeminal nerve. Nerves enter the peripheral cornea in a radial fashion and lose their myelin sheath approximately 1 mm from the limbus.54 The nerves continue to branch and eventually turn 90 degrees towards the corneal epithelium.54, 55 The terminal nerve endings interdigitate between the epithelial cells and reach past them so as to sense the ocular surface environment.56 Corneal nerves are primarily unmyelinated C fibers with myelinated Aδ fibers present to a lesser extent.11 The 3 most prevalent corneal nociceptors are the Aδ mechanoreceptors (approximately 20%) which respond to indentations of the corneal surface, the polymodal nociceptors (approximately 70%) which respond to near-noxious mechanical stimuli, chemical and thermal stimuli, and endogenous inflammatory mediators, and C-fiber cold thermoreceptors (approximately 10%) that are temperature sensitive.11
The location of corneal nociceptors on the ocular surface makes them susceptible to damage. Both external insults, such as surgery and adverse environmental conditions (air pollution, low humidity), and their effects on the ocular surface (increased osmolarity, inflammation) can injure corneal nerves. In support of this, in vivo confocal microscopy has demonstrated corneal nerve alterations in individuals with Sjogrens syndrome57 and with a history of corneal surgery58, including neuroma formation.59 In animal models, both polymodal neurons and cold thermoreceptors in the cornea can be sensitized, the former with inflammatory mediators and the latter after lacrimal gland transection and with hyperosmolarity.11, 60, 61
CENTRAL SENSITIZATION
Prolonged changes in peripheral nerves can alter the function of central nerves, leading to central sensitization (defined as increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input46). Features of central sensitization have been found in individuals with chronic itch, pain, and DE.
Central sensitization and chronic pain
The neuronal changes leading to central sensitization are similar to those seen in peripheral sensitization, and include altered gene expression, signaling cascades (new synapses and rewiring of cortical circuits), inflammatory mediators, and altered ion channels.62 Both enhancement of excitatory neurons and blocking of inhibitory neurons occur on a central level leading to pain amplification and a perception of pain that is disproportional to the peripheral stimulus (e.g. hyperalgesia and allodynia).63 Within the brain, sensations of pain (and itch) are comprehensively processed by multiple regions involved in sensation and emotion (somatosensory cortex, amygdala, hippocampus, and hypothalamus).
Central sensitization and chronic itch
Central sensitization is also a component of chronic itch. In the spinal cord, MrgprA3+ neurons connect with gastrin-releasing peptide (GRP) receptor (GRPR) expressing neurons that are essential for itch transmission. In line with central sensitization, increased responses to PAR-2 agonists and up-regulation in GRP have been found in chronic itch. An imbalance of opioid receptors has also been described in chronic itch. Interestingly, activation of the μ opioid receptor decreases pain but increases itch while activation of the κ opioid receptor inhibits both itch and pain.31
Relevance to dry eye
Clinical evidence of a role for central sensitization in DE comes from several lines of evidence. First, allodynia and hyperalgesia are frequent complaints in individuals with DE (for example, individuals report pain/sensitivity with wind and light)64 and there is often a disconnect between symptoms and signs of disease.24 Second, some individuals with DE symptoms report persistent pain after topical anesthesia65, a treatment that quiets the firing of peripheral nociceptors. Finally, individuals with DE symptoms (as a group) have increased sensitivity to stimuli as tested by quantitative sensory testing (QST) on the forearm66, signaling generalized hypersensitivity. Furthermore, specific QST metrics, such as the finding of increased temporal summation and persistent aftersensations, further support central mechanisms underlying some portion of DE symptoms.8 Considering anatomy, peripheral nerves leave the cornea and first synapse in the Vi/Vc and Vc/C1 regions within the trigeminal nucleus caudalis. Similar to spinal dorsal horn neurons, second-order neurons in these regions receive both innocuous and noxious sensory information. Sensitization has been demonstrated in trigeminal nucleus caudalis neurons as demonstrated by increased sensitivity to ocular stimulus and increased convergent input from periocular skin in models of lacrimal gland resection, uveitis and photokeratitis.67–69 As in chronic non-ocular pain, decreased inhibition from higher brain centers likely also plays a role through inhibition of GABA receptor activity.70
Supporting cells
Many cells (epithelial, glial) support peripheral and/or central nerves and are involved in both the maintenance of health and disease.
The role of supporting cells in chronic pain
In the peripheral nervous system, Schwann cells and epithelial cells interact with nerves. In the central nervous system, glial cells such as microglia, astrocytes, and oligodendrocytes modulate the function of neurons. For example, microglia have been found to rapidly activate in response to peripheral nerve injury32, and subsequently produce a variety of pro-excitatory inflammatory mediators such as TNFα, IL1β, IL18, and PGE2.71 Astrocytes also become activated after nerve injury and subsequently lose their ability to maintain appropriate levels of potassium and glutamate (a powerful excitatory neurotransmitter) extracellularly, resulting in neuronal hyperexcitability.32 In fact, overstimulation of the N-methyl-D-aspartate (NMDA) glutamate receptor is an important component of central sensitization.71 Interestingly, sex related differences (chronic pain more frequent in females) have been observed in the pain phenotype which may be explained mechanistically. For example, one mechanism of central sensitization is the release of brain-derived neurotrophic factor (BDNF) which leads to alterations in potassium/chloride and a decreased strength of inhibitory transmission. In males, BDNF was released in response to microglia activation while in females, release required involvement of adaptive immune cells.63
The role of supporting cells in chronic itch
Similar to pain, activation of glial cells plays an important role in chronic itch, with release of the cytokines TNF-α and IL-1β and BDNF. Again as in chronic pain, these mediators lead to enhanced excitatory transmission and decreased inhibition in spinal cord neurons.
Relevance to dry eye
Recent data suggest that the corneal epithelium plays a similar role to Schwann cells in interacting with corneal nerves, both in maintaining normal homeostasis and in responding to acute injury.56 For example, corneal epithelial cells have been found to phagocytize distal axon fragments within hours of corneal nerve crush wounds and are likely the source of some of the inflammatory mediators found in DE. Similar to chronic pain, there is a gender differential in DE with females having a higher frequency of disease.72
TREATMENT
Integrating the information presented above, the take home point is that many individuals with chronic DE symptoms (sensations of dryness, pain, or itch) have a neuropathic component, and as such treatments that target neuropathic pain can be considered as an adjuvant to ocular surface optimization in cases of recalcitrant and severe symptoms, in consultation with the appropriate specialists.
Treatments of chronic pain
There is no standardized algorithm for the treatment of patients with neuropathic pain; selected therapies depend on pain severity, underlying pathophysiology, and systemic co-morbidities. However, alpha 2 delta ligand anti-epileptics (e.g. gabapentin; pregabalin) are often used as first line agents. Serotonin-norepinephrine reuptake inhibitors (e.g. duloxetine; venlafaxine) are often used as second line agents or as first line agents in patients with concomitant musculoskeletal pain or depression. Tricyclic anti-depressants (e.g. nortriptyline, amitriptyline) are usually not first line agents primarily due to their side effect profile. Based on response to treatment, combination therapies (antiepileptics and antidepressants) can be used in cases where monotherapy provides partial relief. Depending on the etiology of pain (e.g. post herpetic neuralgia), topical agents (lidocaine; capsaicin) can be used as first line therapies or as parts of a multimodal treatment plan. Short courses of corticosteroids or other anticonvulsants (topiramate, lamotrigine, carbamazepine, etc) are also used in specific circumstances. In addition, depending on pain severity, some opioids (e.g. tramadol) can be used in selected patients, in conjunction with the therapies above. However, the benefits of opioids should be weighed against their side effects, which among others may include addiction and hyperalgesia.73 Other measures, such as nerve stimulation or blocks, can be used as adjuvants or if there are specific indications, such as for neuropathic pain related to a specific nerve. In addition, delivering all these therapies in a multidisciplinary approach is important. Specifically, cognitive behavioral therapy focusing on coping mechanisms is vital when managing patients with chronic neuropathic pain.74
Treatments of chronic itch
Drug therapies that target neuropathic pain have also been effective in itch, such as gabapentinoids and anti-depressants. Anti-NGF therapies are being investigated as a treatment for both modalities as well. Based on the specific cytokines involved in chronic itch, anti- IL-4 IL-13, and IL-31 have also been tested in patients with atopic eczema.1 Kappa opioid agonists, such as butorphanol (which is a partial agonist of the κ-opioid receptor and has antagonist activity of the μ-opioid receptor), have also been found effective in reducing itch.75
Relevance to dry eye
DE treatments that target the ocular surface also have an effect on corneal nerves. For example, artificial tears and ointments protect the ocular surface and provide a barrier between nerves and the environment. Anti-inflammatories (corticosteroids, cyclosporine, lifitigrast) and oral antibiotics (doxycycline) decrease ocular surface stress and inflammation with a beneficial effect on nerves. However, many patients have persistent symptoms on current therapies, especially those with NOP complaints.76 As such, other treatments need to be considered in those whose persistent symptoms are thought to be mediated in part by neuropathic mechanisms. For example, autologous serum tears may have a beneficial effect on corneal nerve structure and function, perhaps through the actions of NGF. In one retrospective study of 16 patients with corneal pain, improved photosensitivity and corneal nerve anatomy were noted with autologous serum tear use.59 In patients with centrally mediated pain, systemic therapies can be considered. We have anecdotally used gabapentin and pregabalin in such patients and have found beneficial effects in many patients at relatively high doses (gabapentin: 900–1200 mg 3 times daily; pregabalin 150 mg 2 times daily). A case report described the use of a trigeminal nerve stimulator and intrathecal catheter delivery of bupivacaine and fentanyl in a patient with ocular pain after refractive surgery as a proof of concept that strategies useful in non-ocular pain may be applied to ocular pain in appropriate individuals.77 More studies are needed to assess which anti-neuropathic therapies, in what combinations, will be most beneficial.
STUDY LIMITATIONS
Several study limitations must be considered when interpreting these results. First, the study sample consisted of United States veterans, the majority of whom are older males, and thus our results may not be generalized to other populations. However, it is encouraging that population of older male veterans had comparable levels of ocular pain complaints, systemic co-morbidities, and somatosensory dysfunction as a population of British woman.66, 78, 79 Second, not all sub-types of DE were included in the study. Specific sub-types excluded were those with Sjogren’s syndrome, graft-versus-host disease, a history of corneal refractive surgery, glaucoma medication associated ocular surface disease, and contact lens were. As such, we can only comment on the relationship between ocular itch and DE symptoms in those without the above ocular conditions. Third, limitations on our quantitation of ocular itch included using a 4 point scale and not capturing the temporal course of the itch (acute versus chronic). Fourth, all measurements were taken on one day and the retest reliability of our metrics is not known. Fifth, confocal microscopy was not available on the majority of individuals and therefore we cannot evaluate the relationship between nerve anatomy and ocular itch.
STUDY CONCLUSIONS
Despite these limitations, this study demonstrated that moderate-severe ocular itch associates with other ocular symptoms including sensations of dryness and NOP complaints. Mechanistically, patients with all 3 ocular sensations displayed evidence of chronic non-ocular pain and altered pain processing suggestive of central sensitization (e.g. hyperalgesia) and more severe mental health complaints (e.g. co-morbid depression and anxiety). This suggests that chronic eye symptoms, whether reported as dryness, itch, and/or pain, have shared underlying mechanisms that when severe may involve generalized neuronal dysfunction. This knowledge is of importance to eye care professionals as identifying patients with a neuropathic component to their DE symptoms (whether pain, itch, or dryness) will allow for individualized treatment algorithms in which therapies that address sensitization can be added to those that target the ocular surface. More research is needed on which therapies, whether topical or systemic, that will be most effective in this patient population.
ACKNOWLEDGEMENTS
Funding/Support: The financial and material support of this paper comes from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, NIH NIDCR RO1 DE022903 (Dr. Levitt), and the Department of Anesthesiology, Perioperative Medicine, and Pain Management, University of Miami Miller School of Medicine, Miami, FL.
Financial Disclosures: None
Contributions of Authors:
Design and conduct of the study; AG
Collection, management, analysis, and interpretation of the data: AG, WF
Preparation, review, or approval of the manuscript: AG, LS, WF, RL, KDS, GY
Other Acknowledgments: None
REFERENCES
- 1.Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments. F1000Res. 2016:5. doi: 10.12688/f1000research.8659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haber JS, Valdes-Rodriguez R, Yosipovitch G. Chronic Pruritus and Connective Tissue Disorders: Review, Gaps, and Future Directions. Am J Clin Dermatol. 2016 Oct;17(5):445–449. doi: 10.1007/s40257-016-0201-9. [DOI] [PubMed] [Google Scholar]
- 3.Bernacchi E, Amato L, Parodi A, et al. Sjogren’s syndrome: a retrospective review of the cutaneous features of 93 patients by the Italian Group of Immunodermatology. Clin Exp Rheumatol. 2004 Jan-Feb;22(1):55–62. [PubMed] [Google Scholar]
- 4.Valdes-Rodriguez R, Rowe B, Lee HG, et al. Chronic Pruritus in Primary Sjogren’s Syndrome: Characteristics and Effect on Quality of Life. Acta Derm Venereol. 2017 Mar 10;97(3):385–386. doi: 10.2340/00015555-2524. [DOI] [PubMed] [Google Scholar]
- 5.Stull C, Valdes-Rodriguez R, Shafer BM, et al. The prevalence and characteristics of chronic ocular itch: a cross-sectional survey. Itch. 2017;2(1):e4. doi: 10.1097/itx.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galor A, Feuer W, Lee DJ, Florez H, Venincasa VD, Perez VL. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013 Feb;54(2):1426–1433. doi: 10.1167/iovs.12-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012 Aug;154(2):340–346 e342. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Galor A, Levitt RC, McManus KT, et al. Assessment of Somatosensory Function in Patients With Idiopathic Dry Eye Symptoms. JAMA Ophthalmol. 2016 Nov 01;134(11):1290–1298. doi: 10.1001/jamaophthalmol.2016.3642. [DOI] [PubMed] [Google Scholar]
- 9.Shim WS, Tak MH, Lee MH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007 Feb 28;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010 Dec;16(12):1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 11.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004 Mar;78(3):513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981 Dec;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013 Feb;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalangara JP, Galor A, Levitt RC, Felix ER, Alegret R, Sarantopoulos CD. Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye? Pain Med. 2016 Apr;17(4):746–755. doi: 10.1093/pm/pnv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labbe A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013 Nov;97(11):1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 16.Galor A, Seiden BE, Park JJ, et al. The Association of Dry Eye Symptom Severity and Comorbid Insomnia in US Veterans. Eye Contact Lens. 2017 Jan 06; doi: 10.1097/ICL.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010 Apr;33(2):55–60. doi: 10.1016/j.clae.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000 May;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 19.Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire. J Pain. 2012 Dec;13(12):1250–1257. doi: 10.1016/j.jpain.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004 Apr;108(3):248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005 May;115(1–2):29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007 Apr;5(2):108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson A, Bron AJ, Korb DR. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;(52):2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of Discordance between Symptoms and Signs in Dry Eye Disease. Ophthalmology. 2016 Dec 23; doi: 10.1016/j.ophtha.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999 Feb;40(2):513–519. [PubMed] [Google Scholar]
- 26.Situ P, Simpson TL, Fonn D. Eccentric variation of corneal sensitivity to pneumatic stimulation at different temperatures and with CO2. Exp Eye Res. 2007 Sep;85(3):400–405. doi: 10.1016/j.exer.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Spierer O, Felix ER, McClellan AL, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Invest Ophthalmol Vis Sci. 2016 Feb;57(2):617–625. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornestad AG, Schweinle A, Elhai JD. Measuring secondary traumatic stress symptoms in military spouses with the posttraumatic stress disorder checklist military version. J Nerv Ment Dis. 2014 Dec;202(12):864–869. doi: 10.1097/NMD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 29.Sidebottom AC, Harrison PA, Godecker A, Kim H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch Womens Ment Health. 2012 Oct;15(5):367–374. doi: 10.1007/s00737-012-0295-x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical Power analysis for the behavioral science. In: Cohen J, editor. Statistical Power analysis for the behavioral science. Second ed. New Jersey: Lawrence Erlbaum associates; 1988. [Google Scholar]
- 31.Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013 Dec;465(12):1671–1685. doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016 Nov 04;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutwald J, Goebeler M, Sorg C. Neuropeptides enhance irritant and allergic contact dermatitis. The Journal of investigative dermatology. 1991;96:695–698. doi: 10.1111/1523-1747.ep12470630. [DOI] [PubMed] [Google Scholar]
- 34.Weidner C, Klede M, Rukwied R, et al. Acute effects of substance P and calcitonin gene-related peptide in human skin--a microdialysis study. J Invest Dermatol. 2000;115(6):1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 35.Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2014 Mar;141(3):314–327. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 37.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009 Feb;147(2):198–205 e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shim J, Park C, Lee HS, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012 Nov;119(11):2211–2219. doi: 10.1016/j.ophtha.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chhadva P, Lee T, Sarantopoulos CD, et al. Human Tear Serotonin Levels Correlate with Symptoms and Signs of Dry Eye. Ophthalmology. 2015 Aug;122(8):1675–1680. doi: 10.1016/j.ophtha.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012 Jul;23(4):296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- 42.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002 Aug;43(8):2609–2614. [PubMed] [Google Scholar]
- 43.Vehof J, Wang B, Kozareva D, Hysi PG, Snieder H, Hammond CJ. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci. 2014 Sep 23;55(11):7278–7283. doi: 10.1167/iovs.14-15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: recent advances. J Med Genet. 2012 Jan;49(1):1–9. doi: 10.1136/jmedgenet-2011-100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Na KS, Mok JW, Kim JY, Joo CK. Proinflammatory gene polymorphisms are potentially associated with Korean non-Sjogren dry eye patients. Mol Vis. 2011;17:2818–2823. [PMC free article] [PubMed] [Google Scholar]
- 46.IASP Taxonomy. [Accessed June, 2017]. https://www.iasp-pain.org/Taxonomy.
- 47.Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009 Jul;144(1–2):187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Davis G, Curtin CM. Management of Pain in Complex Nerve Injuries. Hand Clin. 2016 May;32(2):257–262. doi: 10.1016/j.hcl.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003 Aug 01;550(Pt 3):921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmelz M, Schmidt R, Bickel a, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(20):8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003 May;89(5):2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 52.Schmelz M, Michael K, Weidner C, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2000;11(3):645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- 53.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013 Feb;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003 May;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 55.Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005 Jul;24(5):608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 56.Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S. Corneal epithelial cells function as surrogate schwann cells for their sensory nerves. Glia. 2016 Nov 23; doi: 10.1002/glia.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabbriellini G, Baldini C, Varanini V, et al. In vivo confocal scanning laser microscopy in patients with primary Sjogren’s syndrome: A monocentric experience. Mod Rheumatol. 2015 Jul;25(4):585–589. doi: 10.3109/14397595.2014.979523. [DOI] [PubMed] [Google Scholar]
- 58.Li M, Niu L, Qin B, et al. Confocal comparison of corneal reinnervation after small incision lenticule extraction (SMILE) and femtosecond laser in situ keratomileusis (FS-LASIK) PLoS One. 2013;8(12):e81435. doi: 10.1371/journal.pone.0081435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015 Jul;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014 Aug;155(8):1481–1491. doi: 10.1016/j.pain.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 61.Kurose M, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol. 2013 Jul;110(2):495–504. doi: 10.1152/jn.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011 Mar;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science. 2016 Nov 04;354(6312):578–584. doi: 10.1126/science.aaf8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalangara JP, Galor A, Levitt RC, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens. 2017 May;43(3):192–198. doi: 10.1097/ICL.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017 Jan 18; doi: 10.1136/bjophthalmol-2016-309658. [DOI] [PubMed] [Google Scholar]
- 66.Vehof J, Kozareva D, Hysi PG, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013 Oct;131(10):1304–1308. doi: 10.1001/jamaophthalmol.2013.4399. [DOI] [PubMed] [Google Scholar]
- 67.Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005 Dec;94(6):3815–3825. doi: 10.1152/jn.00616.2005. [DOI] [PubMed] [Google Scholar]
- 68.Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience. 2010 Aug 11;169(1):455–462. doi: 10.1016/j.neuroscience.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain. 2015 May;156(5):942–950. doi: 10.1097/j.pain.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirata H, Okamoto K, Bereiter DA. GABA(A) receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol. 2003 Nov;90(5):2837–2849. doi: 10.1152/jn.00544.2003. [DOI] [PubMed] [Google Scholar]
- 71.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999 Jun 5;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 72.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007 Apr;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 73.Gong K, Jasmin L. Sustained Morphine Administration Induces TRPM8-Dependent Cold Hyperalgesia. J Pain. 2016 Nov 12; doi: 10.1016/j.jpain.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attal N. Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneap Minn) 2012 Feb;18(1):161–175. doi: 10.1212/01.CON.0000411564.41709.2d. [DOI] [PubMed] [Google Scholar]
- 75.Phan NQ, Lotts T, Antal A, Bernhard JD, Stander S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol. 2012 Sep;92(5):555–560. doi: 10.2340/00015555-1353. [DOI] [PubMed] [Google Scholar]
- 76.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016 Jun;100(6):745–749. doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 77.Hayek SM, Sweet JA, Miller JP, Sayegh RR. Successful Management of Corneal Neuropathic Pain with Intrathecal Targeted Drug Delivery. Pain Med. 2016 Jul;17(7):1302–1307. doi: 10.1093/pm/pnv058. [DOI] [PubMed] [Google Scholar]
- 78.Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014 Dec;98(12):1712–1717. doi: 10.1136/bjophthalmol-2014-305201. [DOI] [PubMed] [Google Scholar]
- 79.Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, Nibourg SA, Hammond CJ. Clinical Characteristics of Dry Eye Patients With Chronic Pain Syndromes. Am J Ophthalmol. 2016 Jun;166:203–204. doi: 10.1016/j.ajo.2016.03.023. [DOI] [PubMed] [Google Scholar]