Abstract
Several recent studies have suggested that compounds known as endocrine-disrupting chemicals (EDCs) can promote obesity by serving as ligands for nuclear receptors, including the peroxisome proliferator–activated receptor γ (PPARγ) and the glucocorticoid receptor (GR). Thiazolidinedione insulin sensitizers, which act as ligands for PPARγ, also interact with and regulate the activity of the mitochondrial pyruvate carrier (MPC). We evaluated whether several EDCs might also affect MPC activity. Most of the EDCs evaluated did not acutely affect pyruvate metabolism. However, the putative endocrine disruptors tributyltin (TBT) and tolylfluanid (TF) acutely and markedly suppressed pyruvate metabolism in isolated mitochondria. Using mitochondria isolated from brown adipose tissue in mice with adipocyte-specific deletion of the MPC2 protein, we determined that the effect of TF on pyruvate metabolism required MPC2, whereas TBT did not. We attempted to determine whether the obesogenic effects of TF might involve MPC2 in adipose tissue. However, we were unable to replicate the published effects of TF on weight gain and adipose tissue gene expression in wild-type or fat-specific MPC2 knockout mice. Treatment with TF modestly enhanced adipogenic gene expression in vitro but had no effect on GR activation or phosphorylation in cultured cells. These data suggest that TF may affect mitochondrial pyruvate metabolism via the MPC complex but also call into question whether this compound affects GR activity and is obesogenic in mice.
The effects of TF on the activity of the MPC were found to inhibit the activity of this carrier.
Endocrine-disrupting chemicals (EDCs) are environmental compounds that may affect human health by mimicking or inhibiting endogenous endocrine hormones. EDCs have been linked to impaired cognitive development, sexual hormonal abnormalities, anatomical deformations, and cancer (1–3). Recently, studies conducted in vitro and in rodent models have suggested that some EDCs may promote obesity, and these compounds have been termed “obesogens” (4, 5). The obesogenic effects are believed to be mediated by these compounds serving as ligands for the nuclear receptor transcription factors that regulate adipocyte differentiation and lipid metabolism. Specifically, these compounds are believed to activate the peroxisome proliferator–activated receptor γ (PPARγ) or the glucocorticoid receptor (GR) to drive the expression of genes that promote lipid storage in adipocytes and thus cause increased adiposity (6–8). However, the concept that EDCs are important contributors to human obesity is controversial and difficult to test (9).
Nuclear receptors play a critical role in adipogenesis and lipogenesis in adipocytes. Administration of synthetic ligands for GR are well known to stimulate weight gain and adipose tissue expansion through enhanced lipogenesis (10). PPARγ is often referred to as a master regulator of adipocyte differentiation and is required for this process to occur (11, 12). Although the identity of the endogenous ligands for PPARγ is still debated (13), the insulin-sensitizing thiazolidinedione (TZD) class of antidiabetic drugs are highly potent ligands for this receptor (14). Interestingly, a number of PPARγ-independent and nongenomic effects of TZDs have been noted. It has become apparent that TZDs interact with the mitochondrial pyruvate carrier (MPC) complex in the inner mitochondrial membrane and suppress transport of pyruvate into the mitochondrial matrix (15–17). However, the effects of TZDs on the MPC occurred within minutes, suggesting a direct, nongenomic effect on pyruvate transport into mitochondria. This suggested an interesting dual level of regulation because activation of PPARγ has been known for some time to transcriptionally induce PDK4 (18, 19), which inhibits pyruvate oxidation.
The MPC comprises two proteins, MPC1 and MPC2, which are both required for pyruvate transport to occur (20–22). Moreover, genetic deletion of either MPC1 or MPC2 destabilizes the MPC complex and leads to degradation of the remaining MPC protein (16, 17, 20–22). Recent work has shown that the interaction between the MPC and TZDs is direct and that the acute effects of TZDs on mitochondrial pyruvate metabolism require an intact MPC complex (16). The structural basis for the interaction between TZDs and the MPC is not clear but is likely explained by some similarity in the binding pocket of PPARγ and the MPC.
Given the interaction between synthetic PPARγ ligands and the MPC, we sought to determine whether endogenous compounds reported to be natural ligands for PPARγ might also interact with the MPC complex. Herein we show that most reported endogenous or environmental PPARγ ligands do not directly affect mitochondrial pyruvate metabolism even at supraphysiologic concentrations. However, two identified EDCs, tributyltin (TBT) and tolylfluanid (TF), suppressed mitochondrial pyruvate transport and metabolism in a matter of minutes at low μM concentrations. We determined that the effect of TF on pyruvate metabolism required the expression of MPC2, whereas TBT did not. However, we were unable to replicate the published effects of TF on weight gain and adipose tissue metabolic gene expression in wild-type (WT) or fat-specific MPC2 knockout mice. These data suggest that TF may affect mitochondrial pyruvate metabolism via the MPC complex but also call into question whether this compound universally acts as an obesogen in mice.
Materials and Methods
Materials
We obtained 1-oleoyl-sn-glycero-2,3-cyclic-phosphate (LPA) (Avanti Lipids Polar, Alabaster, AL) dissolved in DMSO, 10-nitrolinoleate (LNO2) (Cayman Chemical, Ann Arbor, MI) dissolved in ethanol, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC) (Avanti Lipids Polar) dissolved in ethanol, and 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) (Cayman Chemical) dissolved in DMSO. Other compounds were purchased from Sigma-Aldrich (St. Louis, MO), including triphenyl phosphate (TPP) dissolved in DMSO, bisphenol A (BPA) dissolved in ethanol, TF dissolved in ethanol, and TBT dissolved in ethanol. For all experiments, compounds were compared with matched vehicle solutions for the appropriate diluent.
Isolation of mitochondria from mouse brown adipose tissue for assessment of pyruvate metabolism
Adult female C57BL6/J mice (8 weeks old) were maintained on a 12-hour dark/light cycle and given ad libitum access to food. Mice were euthanized with CO2. The brown adipose tissue (BAT) was excised from the mice, and mitochondria were isolated from BAT by differential centrifugation. Briefly, BAT was washed, minced, and homogenized using a Wheaton Dounce tissue grinder in ice-cold buffer containing 250 mM sucrose, 10 mM Tris, 1 mM EDTA, and 0.5% fatty acid–free bovine serum albumin (BSA) (pH 7.4). The homogenate was centrifuged at 1000 g for 10 minutes at 4°C to pellet unbroken cells and nuclei. Subsequently, the supernatant was centrifuged at 10,000 g for 10 minutes at 4°C to pellet mitochondria. The pellet was then resuspended and washed twice in the same buffer as above without BSA. The final pellet was reconstituted in a minimal amount of assay buffer.
Mitochondrial respiration assays for assessment of pyruvate and succinate use
To assess mitochondrial pyruvate use, isolated mitochondria were resuspended in mitochondrial respiration solution (MIR05: 0.5 mmol/L EGTA, 3 mmol/L MgCl2, 60 mmol/L K-lactobionate, 20 mmol/L taurine, 10 mmol/L KH2PO4, 20 mmol/L HEPES, 110 mmol/L sucrose, and 1 g/L BSA [pH 7.1]). Protein concentration was determined using the BCA method per the manufacturer’s instructions (Thermo Fisher Scientific). Mitochondria (50 µg protein) were placed in 2 mL of MIR05 in the Oxygraph-2k chamber (OROBOROS Instruments, Innsbruck, Austria) at 37°C for the measurement of O2 flux. Mitochondrial respiration was initiated by the addition of substrates (5 mM pyruvate and 2 mM malate) followed by the addition of 1 mM ADP to stimulate state 3 respiration. Subsequently, vehicle (matched to the diluent of each compound) or test compound at the desired concentrations were added to allow the assessment of the effects of compounds on pyruvate metabolism. At the end of measurement, 10 mM succinate was added to allow assessment of the effects of compounds on succinate oxidation. Initial studies added compounds at a final concentration of 25 µM, and for the TF and TBT dose responses, concentrations of 0.1 to 25 µM of these compounds were used.
Generation of fat-specific MPC2-deficient mice
Mpc2 floxed mice have been previously described (16) and were crossed with the adiponectin promoter–driven Cre-expressing mouse (23) to create fat-specific (FS)-Mpc2−/− mice. Littermate Mpc2 fl/fl mice not expressing Cre were used as controls.
TF feeding studies
We conducted a TF feeding study using FS-Mpc2−/− mice and littermate controls to replicate the effects of TF on obesity and metabolic parameters observed in the literature (24) and to determine whether MPC2 is involved in these effects. Male FS-Mpc2−/− mice and littermate controls were randomly assigned to receive ad libitum access to chow diet (2016 Teklad Global Diet, Envigo Teklad, Madison, WI) containing 21.0% protein, 68.6% carbohydrate, and 10.4% fat (by % kcal) or the same diet containing TF at 100 ppm as described (24). TF was added at the time of manufacturing (Envigo Teklad). The animals were maintained on these diets for 16 weeks. At the end of the feeding period, mice were euthanized by CO2 inhalation. Plasma and metabolic tissues were harvested, flash frozen in liquid nitrogen, and stored at −80°C. All animal procedures were approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis.
Blood glucose measures
For the TF feeding study, after 15.5 weeks of TF exposure, mice were held without food for 6 hours followed by intraperitoneal injection of glucose (1 g/kg body weight). Glucose concentrations were measured in the blood samples from the tail veins using a blood glucose meter (One Touch Ultra 2; Lifescan Europe, Zug, Switzerland). The measurements were taken immediately before injection (time = 0), and at 15, 30, 60, 90, and 120 minutes after injection.
Assessments of plasma insulin, free fatty acids, total ketones, triglyceride, and cholesterol
Plasma total ketones and nonesterified fatty acids were measured using enzymatic assays (Wako Diagnostics, Mountain View, CA). Plasma triglyceride and cholesterol were measured using Infinity colorimetric assay kits (Thermo Fisher Scientific, St. Peters, MO). Plasma insulin was measured using an immunoassay (Singulex, Alameda, CA) by the Core Laboratory of the Diabetes Research Center at Washington University.
Western blotting analyses
Tissues, mitochondria, or cells were lysed in ice-cold lysis buffer. Proteins were separated on Criterion gel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. The blots were blocked in 5% (w/v) BSA in Tris-buffered saline with 0.05% (v/v) Tween-20 for 1 hour and incubated in primary antibody in 5% BSA in Tris-buffered saline with 0.05% (v/v) Tween-20 overnight. Antibodies against MPC1, MPC2 (gifts of Michael Wolfgang, Johns Hopkins University), VDAC (ab15895; Abcam, Cambridge, MA), tubulin (T5168; Sigma, St. Louis, MO), pSer211 GR (4161; Cell Signaling Technology, Beverly, MA), and total GR (3660; Cell Signaling Technology) were used. Secondary antibodies (926-32210 and 926-32211; LI-COR, Lincoln, NE) were incubated for 1 hour, and then blots were visualized using the LI-COR imaging system.
mRNA isolation and real-time quantitative polymerase chain reaction analyses
Total RNA was isolated using RNAzol method (RNA-bee; Tel-test, Alvin, TX). cDNA was synthesized using a reverse transcription kit (Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain reaction was performed using SYBR Green with validated primers and an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). The relative expression of the selected genes was normalized to the reference gene 36B4.
Mouse embryonic fibroblast cell culture and adipocyte differentiation experiments
Immortalized mouse embryonic fibroblasts (MEFs) expressing PPARγ were cultured as previously described (25). Differentiation was initiated 2 days after confluency was reached. Specifically, for differentiation, cells were incubated in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 167 nM porcine insulin, 0 to 100 nM dehydrocorticosterone, and 0.5 mM isobutylmethylxanthine. After 3 days, the medium was aspirated, and the cells were cultured for 2 days in DMEM plus 10% FBS and 167 nM insulin. The cells were then maintained in DMEM plus 10% FBS until use.
TF dose-response studies in MDA-kb2 cells
MDA-kb2 cells are derived from a human breast cancer cell line expressing the firefly luciferase under control of a mouse mammary tumor virus promoter containing response elements for both GRs and androgen receptors [MDA-kb2 (ATCC CRL-2713)]. Cells were plated in quadruplicate and treated with vehicle (control), 1 to 1000 nM of dexamethasone (DEX), 1 to 1000 nM TF, or 1 to 1000 nM of DEX with 1 μM TF in media containing 10% FBS (charcoal-stripped) for 22 hours followed by measurement of luminescence.
Effect of TF on GR phosphorylation and gene expression in differentiated MEFs
Differentiation in WT MEFs (+PPARγ) was initiated 3 days after reaching confluence. Specifically, for differentiation, cells were incubated in DMEM containing 10% FBS, 10 µg/mL bovine insulin, 1 μM DEX, and 0.5 mM isobutylmethylxanthine. After 3 days, the medium was aspirated, and the cells were cultured for 3 days in DMEM plus 10% FBS and 10 µg/mL insulin. The cells were maintained in DMEM plus 10% FBS for 2 days, and then the medium was aspirated and replaced with DMEM plus 10% FBS (charcoal-stripped) for 1 day. For Western blotting, cells were treated for 1 hour with DEX (100 nM), TF (1 µM), or RU486 (1 µM) followed by harvesting protein in ice-cold lysis buffer. For gene expression, cells were treated for 6 hours with DEX (100 nM), TF (1 µM), or both compounds followed by harvesting protein in ice-cold lysis buffer.
Statistical analysis
All data are presented as mean ± standard error of the mean and were analyzed by Student t test or analysis of variance where appropriate. A P value <0.05 was considered significant.
Results
TBT and TF directly reduce mitochondrial pyruvate metabolism
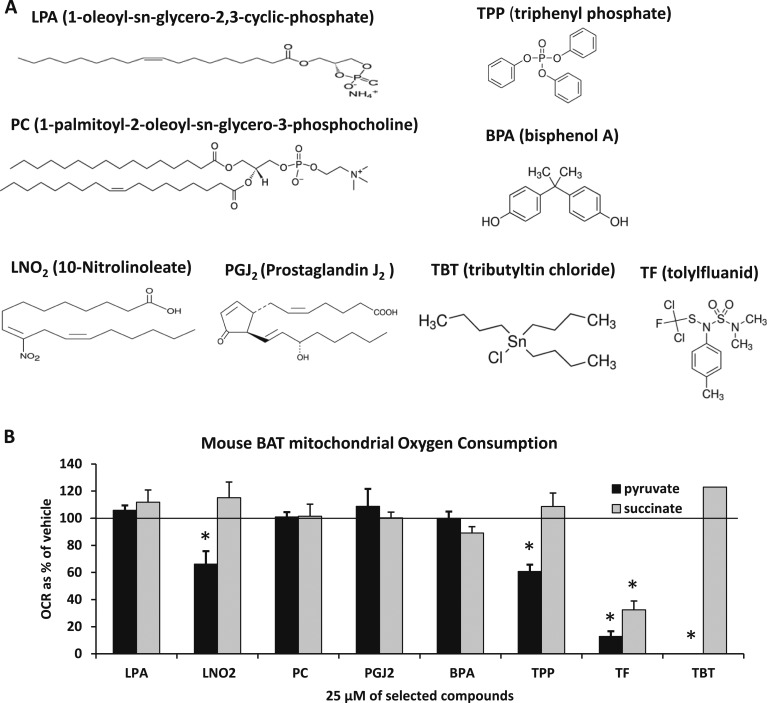
To evaluate whether endogenous or environmental compounds believed to act as ligands for nuclear receptors affect mitochondrial pyruvate metabolism by interacting with the MPC, we isolated mitochondria from BAT, which contains abundant amounts of MPC1 and MPC2 (26), and assessed mitochondrial oxygen consumption after the addition of these compounds in the presence of pyruvate or succinate as metabolic substrates. We evaluated LPA, LNO2, PC, and PGJ2 (Fig. 1A), which are purported to be endogenous PPARγ ligands (27–30). We began by assessing the effects of these compounds at 25 μM, which is far in excess of their abundance in cells. However, the addition of LPA, PC, or PGJ2 did not affect respiration of BAT mitochondria in the presence of pyruvate or succinate even at these supraphysiologic concentrations (Fig. 1B). The addition of LNO2 reduced pyruvate-stimulated respiration modestly but significantly. We also evaluated the effects of BPA, TPP, TBT, or TF, which are EDCs believed to mediate effects via PPARγ or potentially the GR (24, 31–33). It was determined that BPA did not affect respiration and that TPP only modestly suppressed respiration. TBT and TF markedly suppressed pyruvate metabolism at this very high concentration, but TF also reduced succinate-mediated respiration at 25 μM. The effect of these compounds on isolated mitochondria was immediate (within minutes), suggesting that the effects were direct and nongenomic.
Figure 1.
TBT and TF suppress mitochondrial respiration on pyruvate. (A) Structures of PPARγ ligands LPA, LNO2, PC, PGJ2, TPP, BPA, TBT, and TF. (B) The effects of PPARγ ligands on mitochondrial pyruvate and succinate use. A concentration of 25 µM of the selected compounds was used in the mitochondrial respiration assay. Oxygen consumption rates are expressed as percentage of vehicle control. *Statistical significance (P < 0.05) as compared with vehicle control. For LPA, PGJ2, and TPP, DMSO was used as the vehicle control. For LNO2, PC, BPA, TF, and TBT, ethanol was used as the vehicle control.
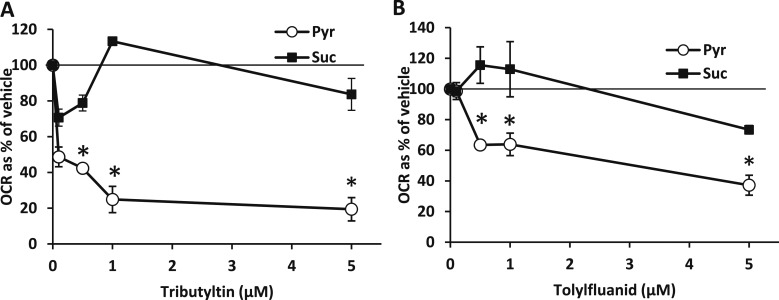
We next examined the effects of various doses of TBT and TF on pyruvate-mediated respiration to determine if physiologically relevant exposures would suppress pyruvate-mediated respiration. TBT suppressed respiration in the presence of pyruvate but not succinate and affected respiration at doses as low as 100 nM (Fig. 2A). TF suppressed pyruvate metabolism at 500 nM and did not affect succinate-mediated respiration at concentrations of 5 μM or lower (Fig. 2B). Thus, these compounds suppress pyruvate-mediated respiration in isolated mitochondria at concentrations consistent with their ability to serve as nuclear receptor ligands.
Figure 2.
Dose-response curves for the effects of TBT and TF on respiration. (A) The effects of TBT (100 nM, 500 nM, 1 µM, and 5 µM) on mitochondrial pyruvate and succinate use in mouse BAT. Data are presented as oxygen consumption rate (OCR) as a percentage of vehicle control (ethanol). *Statistical significance (P < 0.05) as compared with vehicle control. (B) The effects of TF (100 nM, 500 nM, 1 µM, and 5 µM) on mitochondrial pyruvate and succinate use in mouse BAT. Data are presented as OCR as a percentage of vehicle control (ethanol). *Statistical significance (P < 0.05) as compared with vehicle control.
TF requires MPC2 for its effects on pyruvate metabolism
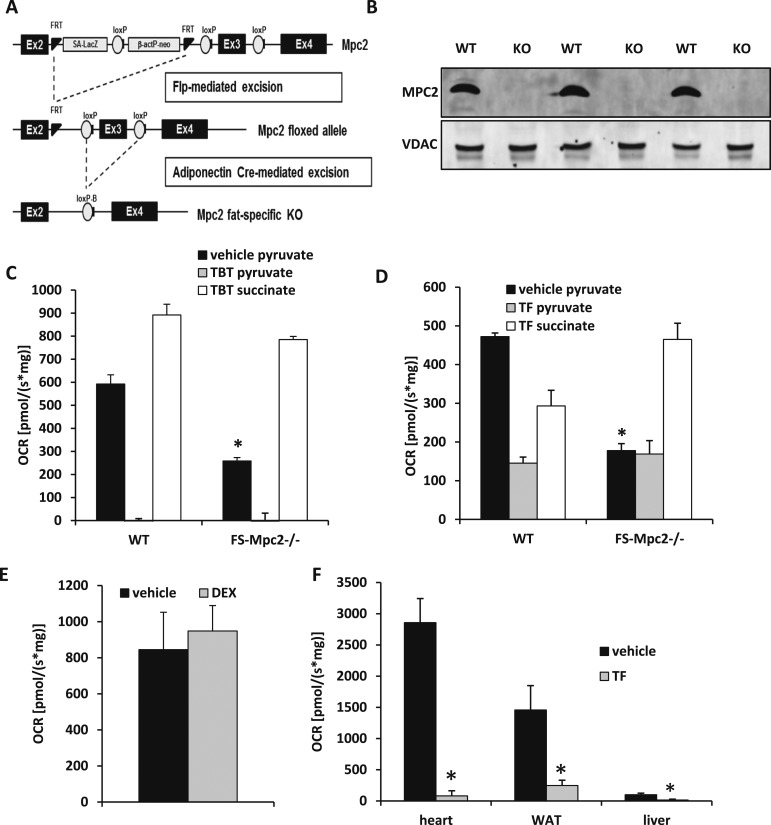
To determine whether the effects of TBT and TF on mitochondrial pyruvate metabolism required MPC2, we generated mice with adipocyte-specific deletion of MPC. Using previously described mice with a “floxed” Mpc2 allele (16, 34–36), we crossed them with mice expressing Cre recombinase in adipocytes (Fig. 3A). The resulting mice lacked expression of Mpc2 in all adipocytes, including BAT (Fig. 3B). It should also be noted that loss of MPC2 destabilizes the MPC complex and results in a double MPC1/MPC2 knockout. Fat-specific Mpc2−/− mice (FS-Mpc2−/− mice) were viable and phenotypically indistinguishable from littermate WT mice at baseline (data not shown). Mitochondria were isolated from BAT of WT and FS-Mpc2−/− mice, and the effects of TF and TBT on pyruvate and succinate metabolism were assessed. Predictably, the oxygen consumption by mitochondria from FS-Mpc2−/− mice in the presence of pyruvate was significantly reduced compared with WT mitochondria at baseline (Fig. 3C). Interestingly, TBT still suppressed pyruvate-mediated respiration in both WT and FS-Mpc2−/− mitochondria (Fig. 3C), suggesting that the suppression of pyruvate metabolism likely occurs at another step in the pathway. However, the ability of TF to suppress respiration in the presence of pyruvate was lost in mitochondria lacking MPC2 (Fig. 3D). Thus, TF affects mitochondrial pyruvate metabolism in an MPC2-dependent manner, whereas TBT has MPC-independent effects.
Figure 3.
TF, but not TBT, inhibits pyruvate respiration in an MPC2-dependent manner. (A) Mpc2 floxed mice were crossed with the adiponectin promoter–driven Cre-expressing mouse to create FS-Mpc2−/− mice. (B) Western blotting confirmed MPC2−/− in BAT from FS-Mpc2−/− mice. (C) The effects of TBT (5 µM) on BAT mitochondrial pyruvate and succinate use from WT and FS-Mpc2−/− mice. Sequential addition of pyruvate/ADP (black), TBT (gray), and succinate (white). Upon addition of TBT, both WT and FS-Mpc2−/− mitochondria decreased oxygen consumption rate (OCR) compared with their perspective pyruvate/ADP addition. *Indicates P < 0.05 versus WT vehicle. (D) The effects of TF (5 µM) on mitochondrial pyruvate and succinate use from WT and FS-Mpc2−/− mice. Sequential addition of pyruvate/ADP (black), TBT (gray), and succinate (white). Upon addition of TF, FS-Mpc2−/− mitochondria did not alter OCR; however, WT mitochondria decreased OCR compared with its pyruvate/ADP addition. *Indicates P < 0.05 versus WT vehicle. (E) The effects of DEX (5 µM) on mitochondrial pyruvate–stimulated respiration. Data are presented as OCR [pmol/(s⋅mg)]. Ethanol was used as the vehicle control. (F) The effects of TF (5 µM) on mitochondrial pyruvate–mediated respiration in mitochondria isolated from a variety of tissues. *Indicates P < 0.05 versus vehicle. Data are presented as OCR [pmol/(s⋅mg)]. Ethanol was used as the vehicle control. KO, knockout.
TF has been reported to elicit metabolic effects by affecting GR signaling in adipocytes (7, 25). Although TZDs, which are PPARγ ligands, have been linked to suppressed MPC activity, there was no evidence linking GR ligands to MPC complex activity. We therefore assessed whether DEX also directly regulated mitochondrial pyruvate metabolism in isolated mitochondria. We found that 5 μM DEX did not affect mitochondrial respiration with pyruvate (Fig. 3E). TF suppressed pyruvate-stimulated respiration by mitochondria from a variety of tissues, including heart, WAT, and liver, although the relative rate of respiration varied dramatically across these tissue mitochondria (Fig. 3F).
Feeding TF fails to induce obesity or affect glucose tolerance
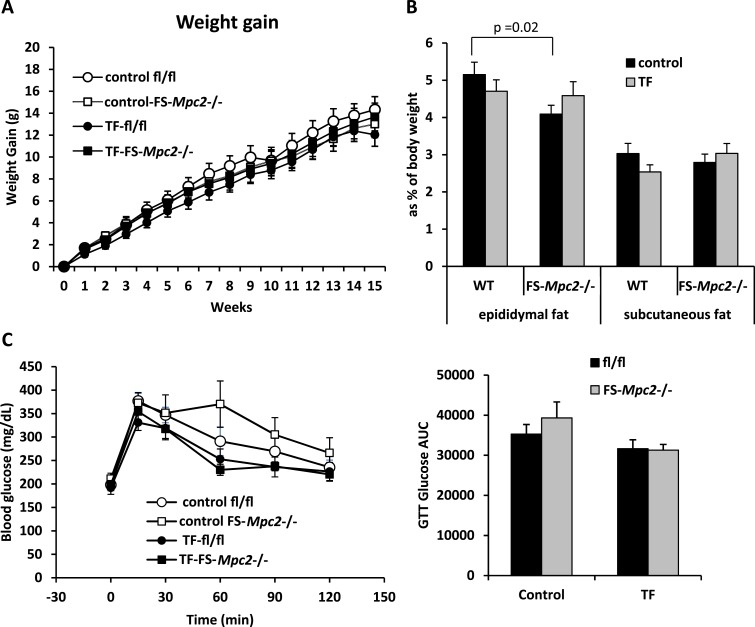
Feeding TF to male mice increased weight gain and glucose intolerance in a previous study (24). To determine whether this response involved adipose tissue MPC2, we fed TF to WT and FS-Mpc2−/− mice at a concentration of 100 ppm (100 mg/kg diet) in rodent chow, which is the concentration used by Regnier et al. (24). Unexpectedly, in our study, there was no effect of TF on body weight gain compared with control chow in either WT or FS-Mpc2−/− mice (Fig. 4A). TF also had no effect on adiposity as measured by epididymal and subcutaneous adipose tissue weight (Fig. 4B). Glucose tolerance tests conducted after 15.5 weeks on diet failed to detect an effect of TF on glucose tolerance in mice of either genotype (Fig. 4C). Analysis of plasma collected at time of death found no effect of genotype or TF treatment on the plasma concentrations of insulin, total ketones, triglycerides, free fatty acids, or cholesterol (Table 1).
Figure 4.
TF does not increase adiposity or cause glucose intolerance in mice. (A) WT and FS-Mpc2−/− mice were randomly assigned to receive a chow diet or the same diet fortified with 100 ppm TF for 16 weeks (n = 9 control fl/fl; n = 10 control FS-Mpc2−/−; n = 13 TF-fl/fl; n = 11 TF-FS-Mpc2−/−). Body weight was measured weekly. Weight gain was plotted over time from 0 to 16 weeks. (B) At the end of the study, epididymal and subcutaneous fats were harvested and weighed. Data are presented as percentage of body weight. (C) TF-fed mice did not develop glucose intolerance. A glucose tolerance test was performed at 15.5 weeks of the study by intraperitoneal injection of glucose (1 g/kg) with serial blood glucose measured for 120 minutes. Glucose tolerance was measured as the area under the curve of glucose over time for 120 minutes.
Table 1.
Plasma Characteristics in Control or TF-Treated Mice
| Plasma Characteristics |
Control |
TF |
||
|---|---|---|---|---|
| fl/fl | FS-Mpc2−/− | fl/fl | FS-Mpc2−/− | |
| Insulin, pg/mL | 1263 ± 282 | 913 ± 171 | 1288 ± 358 | 1644 ± 315 |
| Total ketones, mM | 0.58 ± 0.19 | 0.52 ± 0.07 | 0.72 ± 0.18 | 0.82 ± 0.18 |
| Triglyceride, mg/dL | 132 ± 14 | 125 ± 10 | 118 ± 16 | 109 ± 7 |
| Free fatty acids, mM | 0.97 ± 0.16 | 1.03 ± 0.09 | 0.84 ± 0.16 | 0.91 ± 0.07 |
| Cholesterol, mg/dL | 125 ± 13 | 145 ± 10 | 124 ± 11 | 189 ± 42 |
No significant differences were detected (n = 8 per group).
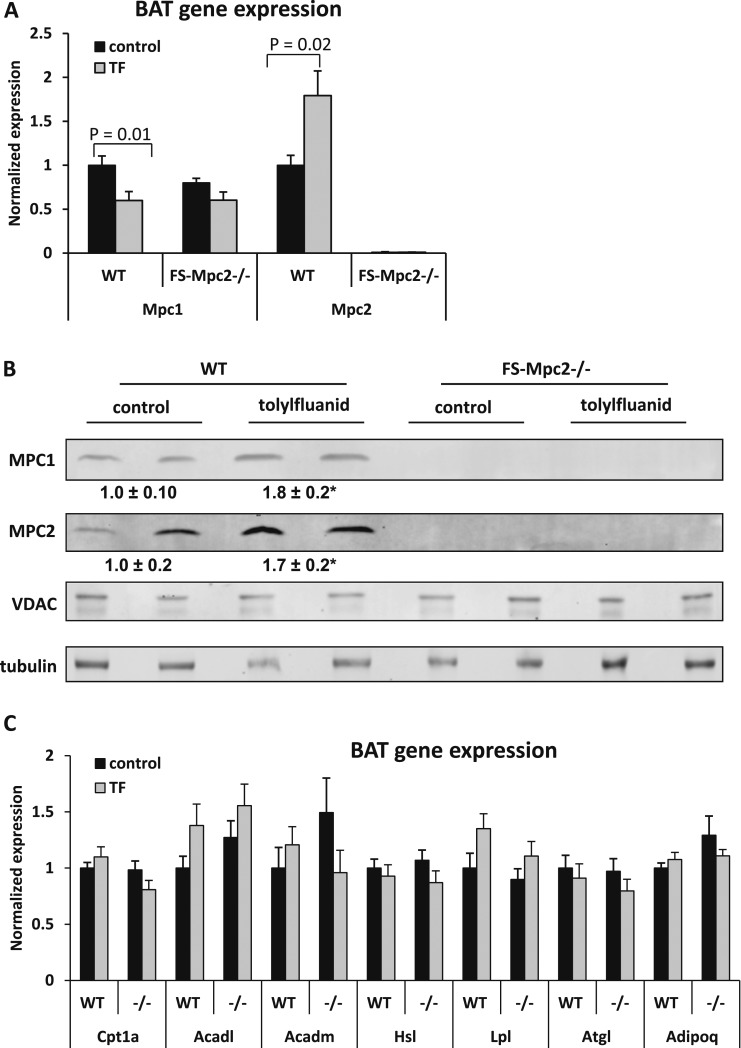
We also assessed the expression of various genes of interest in BAT of WT and FS-Mpc2−/− mice fed TF-containing chow or control chow. Interestingly, Mpc2 expression was induced by feeding TF to WT mice (Fig. 5A). This resulted in a significant increase in BAT MPC2 protein content (Fig. 5B). Conversely, the expression of Mpc1 was suppressed by TF in WT mice (Fig. 5A), although MPC1 protein abundance was increased by TF (Fig. 5B). The expression of several genes encoding enzymes involved in fatty acid oxidation (Cpt1a, Acadl, and Acadm), which have been reported to be reduced by TF feeding (24), was not affected in this study in mice of either genotype (Fig. 5C). There was also no effect of TF in mice of either genotype on the expression of various genes encoding lipolytic enzymes and adiponectin (Hsl, Lpl, Atgl, and Adipoq), which were reported to be suppressed in white adipose tissue by TF feeding (Fig. 5C). The effects of TF on the expression of these genes in epididymal adipose tissue were also minimal and not dependent on genotype, except for Acadm, which was reduced by TF (Supplemental Fig. 1 (45KB, TIF) ).
Figure 5.
TF increases BAT MPC1 and MPC2 content. (A) Gene expression from BAT of TF-fed and control diet–fed mice (n = 7 control fl/fl; n = 10 control FS-Mpc2−/−; n = 12 TF-fl/fl; n = 11 TF-FS-Mpc2−/−). (B) MPC1 and MPC2 protein expression in BAT of TF and control diet–fed WT and FS-Mpc2−/− mice. The expression of MPC1 and MPC2 proteins was standardized to the voltage dependent anion channel (VDAC) expression, and the WT-control was set as 100%. *Statistical significance between the WT-control and WT-TF groups. For MPC1, P = 0.04; for Mpc2, P = 0.02. (C) Gene expression from BAT of TF-fed and control diet–fed mice (n = 7 control fl/fl; n = 10 control FS-Mpc2−/−; n = 12 TF-fl/fl; n = 11 TF–FS-Mpc2−/−). Acadl, long chain–specific acyl-CoA dehydrogenase; Acadm, medium chain–specific acyl-CoA dehydrogenase; Adipoq, adiponectin; Atgl, adipose triglyceride lipase; Cpt1a, carnitine palmitoyltransferase I; Hsl, hormone-sensitive lipase; Lpl, lipoprotein lipase.
TF does not affect triacylglycerol accumulation in embryonic fibroblasts
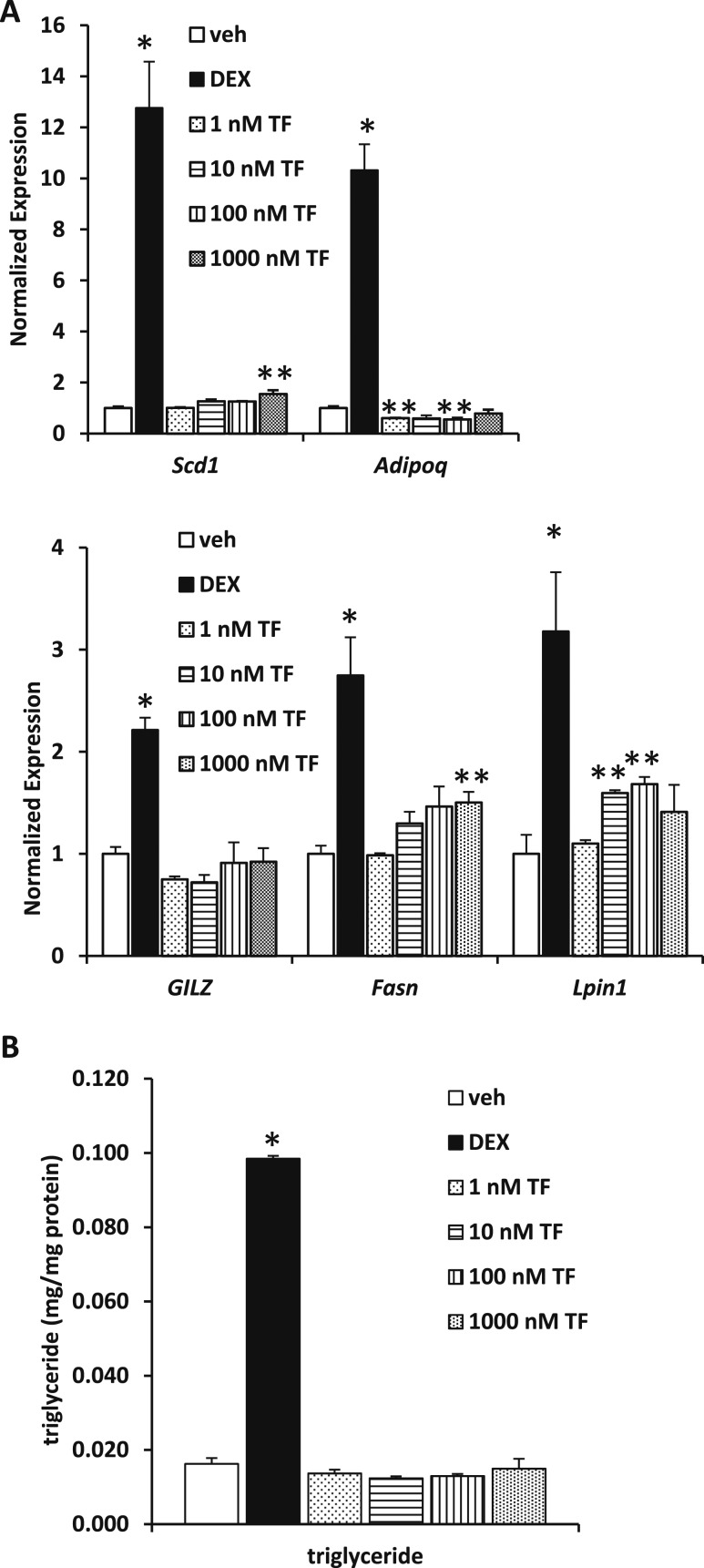
We examined the effect of TF on adipogenic markers in MEFs stimulated to differentiate. Many of these genes are known to be GR targets and to be regulated by TF, including Scd1, Adipoq, glucocorticoid-induced leucine zipper (GILZ), Fasn, and Lpin1. As previously reported, Adipoq expression was suppressed by TF (Fig. 6A). High concentrations of TF modestly, but significantly, increased Scd1, Fasn, and Lpin1 but failed to induce GILZ even though DEX produced a significant increase (Fig. 6A). Moreover, treatment with TF did not enhance triglyceride accumulation in these cells, whereas Dex administration elicited a strong effect (Fig. 6B). Thus, in this system, TF modestly enhanced the effects of adipogenic cocktail on MEF gene expression but did not affect triglyceride accumulation.
Figure 6.
TF does not enhance adipogenesis in embryonic fibroblasts. (A) The effects of 3 days of exposure to TF (1, 10, 100, and 1000 nM) or DEX (100 nM) on the expression of adipogenic gene expression markers after MEF differentiation into adipocytes. The expression of stearoyl-CoA desaturase-1 (Scd1), adiponectin (Adipoq), glucocorticoid-induced leucine zipper (GILZ), fatty acid synthase (Fasn), and lipin (Lpin)1 was measured using quantitative polymerase chain reaction. *Statistical significance (P < 0.05) as compared with vehicle control. (B) Triglyceride content of MEF cells treated with vehicle (ethanol), TF (1, 10, 100, and 1000 nM), or DEX (100 nM) for 3 days during differentiation was quantified. *Statistical significance (P < 0.05) as compared with vehicle control. **Statistical significance (P < 0.05) as compared to both vehicle control and DEX treated groups.
TF fails to alter GR activity or phosphorylation in vitro
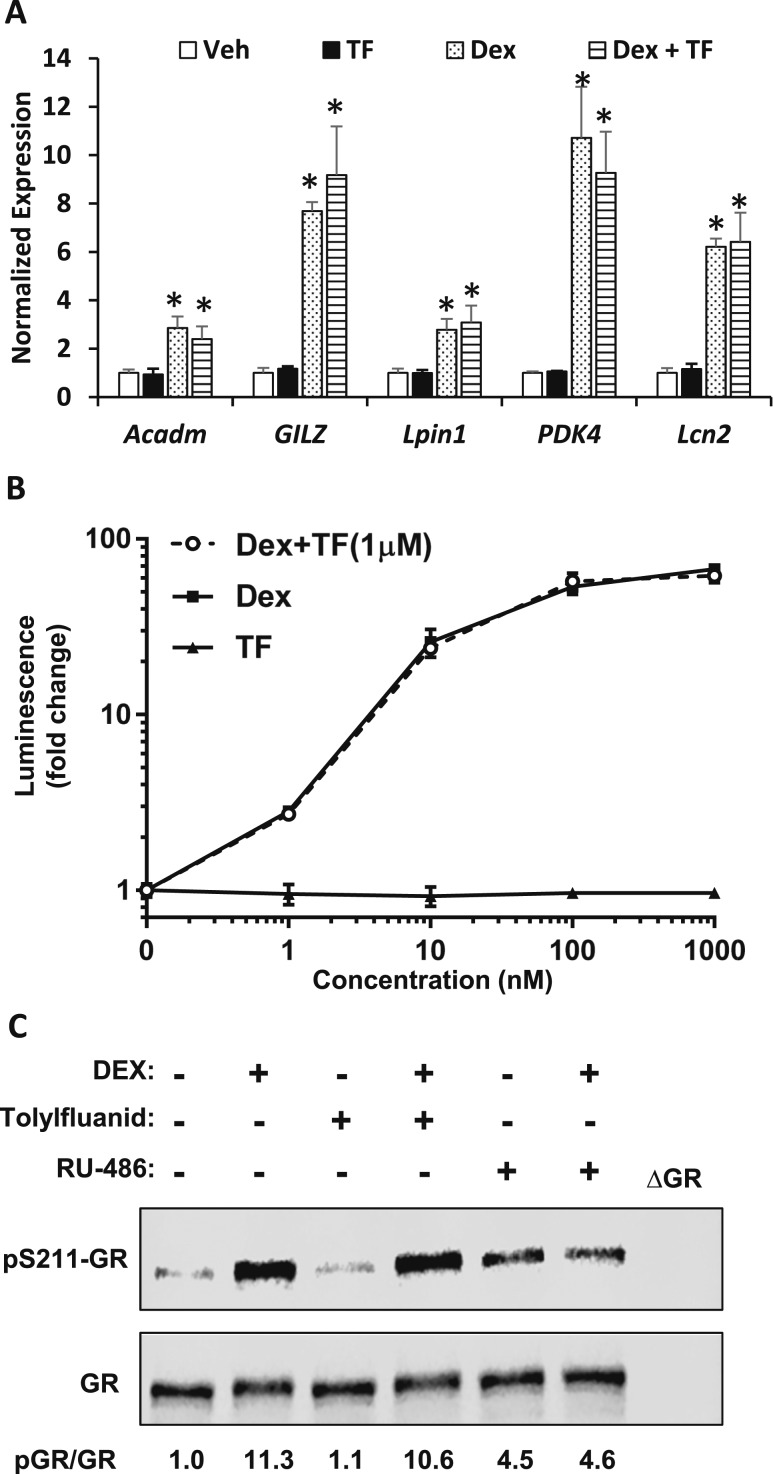
To evaluate the acute effects of TF on adipocyte GR signaling, MEFs were differentiated and then treated for 6 hours with TF (1 µM), DEX (100 nM), or both compounds and compared with vehicle controls (ethanol). The expression of known GR target genes (Acadm, GILZ, Lpin1, PDK4, and Lcn2) was not affected by treatment with TF (Fig. 7A). The expression of these genes was induced by DEX treatment, and the effect of DEX was neither enhanced nor impaired by cotreatment with TF (Fig. 7A).
Figure 7.
TF fails to affect GR activity or phosphorylation. (A) The effects of TF and/or DEX on GR target gene expression in immortalized MEFs. Cells were plated and treated in media containing 10% FBS (charcoal-stripped) for 22 hours before stimulation with TF (1 μM), DEX (100 nM), or TF plus DEX at those doses. The expression of medium chain–specific acyl-CoA dehydrogenase, glucocorticoid-induced leucine zipper (GILZ), lipin (Lpin)1, PDK4, and lipocalin 2 (Lcn)2 was measured using quantitative polymerase chain reaction. *Statistical significance (P < 0.05) as compared with vehicle control (ethanol). (B) TF dose response in MDA-kb2 cells. Cells were plated in quadruplicate and treated in media containing 10% FBS (charcoal-stripped) for 22 hours. Cells were treated with the indicated concentrations of DEX, TF, or DEX plus TF (1 μM). Data are expressed as firefly luminescence divided by renilla luminescence. (C) The effects of TF (1 μM), RU-486 (1 μM), and/or DEX (100 nM) on GR phosphorylation in differentiated MEFs.
We also assessed whether TF could activate a GR-driven luciferase reporter system in MDA-kb2 cells (37). Using this read out, TF failed to activate GR in vitro at any dose, whereas DEX markedly increased reporter activity (Fig. 7B). Again, cotreatment with TF (1 μM) did not affect the response to DEX on reporter activity. The phosphorylation of GR at serine 211 is also reported to be stimulated by TF treatment (7). However, we failed to detect an increase in GR phosphorylation in response to TF; nor did TF attenuate the ability of DEX to stimulate GR phosphorylation, which was robust (Fig. 7C). GR phosphorylation in response to DEX could be reduced by treatment with RU-486, a GR partial agonist. Altogether, these data indicate an inability to replicate the published effects of TF on GR signaling.
Discussion
The mitochondrial pyruvate carrier is a gatekeeper at the intersection of cytosolic pyruvate synthesis and the further metabolism of pyruvate in the mitochondrial matrix. Therefore, this can be a critical nodal point for controlling intermediary metabolism. However, the factors that regulate flux through the MPC are incompletely understood, and it remains to be determined whether and how covalent modification and allosteric regulation are important regulatory mechanisms. Although theoretical, control by allostery is plausible given the accumulating data suggesting that MPC activity can be modulated by synthetic compounds that act as ligands for nuclear receptors and affect intermediary metabolism.
We explored the possibility that other compounds, including endogenous PPARγ ligands, might also serve as regulators of MPC activity. However, we were unable to detect a class effect of these putative ligands on MPC activity even at concentrations of 25 μM, suggesting that the structural diversity of these compounds does not lend itself to MPC binding. Thus, it does not appear that these endogenous lipids are acute allosteric modulators of MPC activity. The evidence implicating various hydrophobic lipids as bona fide ligands for PPARγ is controversial because physiological concentrations of these lipids are usually very low, and activation of the receptor often occurs at much higher levels. Also, many of these putative ligands likely exist as components of membranes insoluble in the aqueous phase, and the accessibility for nuclear receptors is unclear.
Like the endogenous PPARγ ligands, EDCs are believed to activate PPARγ or GR in adipocytes and have been linked to developing obesity. However, the concept that environmental compounds are contributing to the current obesity epidemic is contrversial (38). As mentioned above, it can be questioned whether these compounds are present in the cell in concentrations sufficient to activate PPARγ (39, 40). Moreover, it remains unclear why these ligands produce obesity yet render the mice insulin resistant when synthetic PPARγ ligands produce increased adiposity and insulin sensitivity (14, 41, 42). Of the PPARγ ligands tested, only TBT produced an immediate drop in pyruvate respiration at physiologic concentrations, and the effect was quite robust. Subsequently, it was determined that the effect of TBT did not require intact MPC2, suggesting that this acute effect is, at least in part, mediated at some other step in pyruvate metabolism, such as the oxidation of pyruvate. Additional work is needed to clarify this mechanism.
On the other hand, TF is believed to mediate its obesogenic effects by activating the glucocorticoid receptor (7), which is logical because pharmacologic administration of glucocorticoids increases adiposity and produces insulin resistance (43, 44). However, in the current study we were unable to replicate the obesogenic effects of TF in male mice (24). In our hands, the only observable effect of the drug was to stimulate an increase in the expression of MPC2 mRNA and protein in BAT and WAT, which occurred for reasons that are not clear. In eWAT, Acadm expression was decreased, which was observed previously (24). There are several potential explanations for the lack of effect on body weight, including potential differences in the genetic backgrounds of mice, effects of distinct institutional microbiomes, higher variability in our knockout strain, or other unrecognized factors. Moreover, in our work TF was incorporated into the standard mouse chow for our facility (Teklad 2016), which contains different ratios of fat, protein, and carbohydrates compared with Teklad 2018, which was used in the previous study. The two diets also contain different amounts of phytoestrogens, which are known to affect body weight in mice. The previous study demonstrated a modest effect on body weight (∼2 g) and detected a significant difference by using a large number of mice (n = 28) (24). In our studies with 9 to 13 mice per group, there were no significant differences in body weight, adiposity, other metabolic parameters, or adipose tissue gene expression.
We also did not detect many of the reported in vitro effects of TF on adipogenesis or GR signaling in adipocytes. We did see a modest effect of TF in stimulating Scd1, Fasn, and Lpin1 at higher doses in the adipogenesis experiments, although triacylglycerol accumulation was not affected. Previous work has shown both stimulatory (7) and inhibitory (45) effects on GR reporter activity, whereas we saw no effect. In our study, TF also did not activate GR in luciferase reporter assays or affect GR phosphorylation. We also did not detect a competitive or synergistic effect of TF with DEX in these assays. In our GR-focused studies, we used charcoal-stripped FBS, whereas the previous studies seem to have used regular FBS (7, 25). In addition, previous work on the effects of TF on GR phosphorylation and gene expression in vitro was conducted with isolated fat pads, and it is possible that the presence of other cell types affects the response to TF. Recent work used TF obtained from Fluka (St. Louis, MO) (7, 25). Fluka was formerly a subsidiary of Sigma Chemical Co. but no longer distributes this compound after being acquired by another entity. We conducted studies with multiple orders of TF from Sigma, but it is unclear whether this is the same source as the previous studies. We also likely used TF from different lots of the drug given the number of years that have passed. Thus, there are several minor differences that could affect the outcome of the studies. However, these discrepancies and the lack of effect in our study remain to be clarified.
In summary, the EDC TF acutely affects pyruvate metabolism in isolated mitochondria via an MPC2-dependent mechanism. These acute effects include an inhibition of mitochondrial pyruvate import, leading to reduced mitochondrial oxidation of this substrate. These data provide evidence of the direct effects of this EDC on mitochondrial metabolism. However, despite several previous papers linking TF to modulation of GR activity, no effect of TF on obesity or GR activity was detected in our study. Thus, the physiological consequences of the observed interaction between TF and the MPC or GR remain unclear.
Acknowledgments
Financial Support: This work was funded by National Institutes of Health Grants R01 DK104735 (to B.N.F.) and R01 DK106083 (to C.A.H.). The Core Services of the Diabetes Research Center (Grant P30 DK020579) and the Nutrition Obesity Research Center (Grant P30 DK56341) at the Washington University School of Medicine also supported this work. K.S.M. was supported by the Diabetes Research Postdoctoral Training Program fellowship (Grant T32 DK007120) and is now supported by Grant K99 HL136658. A.M.H. is supported by Grant 17-IBS-109 from the American Diabetes Association.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Mitochondrial pyruvate carrier 1 (MPC1) | QGGRLINYEMSKRPSA | MPC1 | Gift from Michael Wolfgang | Rabbit | 1:1000 | |
| Mitochondrial pyruvate carrier 2 (MPC2) | WRYNQELKSKGIQ | MPC2 | Gift from Michael Wolfgang | Rabbit | 1:1000 | |
| Voltage-dependent anion channel (VDAC) | VDAC1/Porin | Abcam, ab15895 | Rabbit; polyclonal | 1:1000 | AB_2214787 | |
| α-Tubulin | “Sarkosyl-resistant filaments and sperm axonemes” | Tubulin | Sigma Aldrich, T5168 | Mouse; monoclonal | 1:1000 | AB_477579 |
| Phosphorylated glucocorticoid receptor | “Residues surrounding Ser211 of human GR” | Phospho-glucocorticoid receptor (Ser211) | Cell Signaling Technology, 4161 | Rabbit; polyclonal | 1:1000 | AB_2155797 |
| Glucocorticoid receptor | “Residues surrounding Leu378 of human GR” | Glucocorticoid receptor (D8H2) XP | Cell Signaling Technology, 3660 | Rabbit; monoclonal | 1:1000 | AB_11179215 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- BAT
- brown adipose tissue
- BPA
- bisphenol A
- BSA
- bovine serum albumin
- DEX
- dexamethasone
- DMEM
- Dulbecco’s modified Eagle medium
- EDC
- endocrine-disrupting chemical
- FBS
- fetal bovine serum
- GR
- glucocorticoid receptor
- LNO2
- 10-nitrolinoleate
- LPA
- 1-oleoyl-sn-glycero-2-3-cyclic-phosphate
- MEF
- mouse embryonic fibroblast
- MPC
- mitochondrial pyruvate carrier
- PC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- PPARγ
- peroxisome proliferator–activated receptor γ
- TBT
- tributyltin
- TF
- tolylfluanid
- TPP
- triphenyl phosphate
- TZD
- thiazolidinedione
- WT
- wild-type.
References
- 1.Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, Tillitt DE, Vom Saal FS, Rosenfeld CS. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen Comp Endocrinol. 2015;214:195–219. [DOI] [PubMed] [Google Scholar]
- 2.Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. [DOI] [PubMed] [Google Scholar]
- 3.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Muñoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6, Suppl):S50–S55. [DOI] [PubMed] [Google Scholar]
- 5.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1-2):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira-Fernandes A, Vanparys C, Hectors TL, Vergauwen L, Knapen D, Jorens PG, Blust R. Unraveling the mode of action of an obesogen: mechanistic analysis of the model obesogen tributyltin in the 3T3-L1 cell line. Mol Cell Endocrinol. 2013;370(1-2):52–64. [DOI] [PubMed] [Google Scholar]
- 7.Neel BA, Brady MJ, Sargis RM. The endocrine disrupting chemical tolylfluanid alters adipocyte metabolism via glucocorticoid receptor activation. Mol Endocrinol. 2013;27(3):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyssimachou A, Santos JG, André A, Soares J, Lima D, Guimarães L, Almeida CM, Teixeira C, Castro LF, Santos MM. The mammalian “obesogen” tributyltin targets hepatic triglyceride accumulation and the transcriptional regulation of lipid metabolism in the liver and brain of zebrafish. PLoS One. 2015;10(12):e0143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legler J. An integrated approach to assess the role of chemical exposure in obesity. Obesity (Silver Spring). 2013;21(6):1084–1085. [DOI] [PubMed] [Google Scholar]
- 10.Chapman AB, Knight DM, Ringold GM. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J Cell Biol. 1985;101(4):1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. [DOI] [PubMed] [Google Scholar]
- 12.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. [DOI] [PubMed] [Google Scholar]
- 13.Varga T, Czimmerer Z, Nagy L.. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270(22):12953–12956. [DOI] [PubMed] [Google Scholar]
- 15.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci USA. 2013;110(14):5422–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, Burgess SC, Finck BN. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 2015;22(4):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS, Wolfe CL, Wheeler JS, Coulter KR, Kilkuskie PM, Gracheva E, Korshunova Y, Trusgnich M, Karr R, Wiley SE, Divakaruni AS, Murphy AN, Vigueira PA, Finck BN, Kletzien RF. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT): relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8(5):e61551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes. 2008;57(9):2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogue A, Spire C, Brun M, Claude N, Guillouzo A.. Gene expression changes induced by PPAR gamma agonists in animal and human liver. PPAR Res. 2010:325183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–96. [DOI] [PubMed] [Google Scholar]
- 22.McCommis KS, Finck BN. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J. 2015;466(3):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S, Kong X, Rosen ED. Adipocyte-specific transgenic and knockout models. Methods Enzymol. 2014;537:1–16. [DOI] [PubMed] [Google Scholar]
- 24.Regnier SM, Kirkley AG, Ye H, El-Hashani E, Zhang X, Neel BA, Kamau W, Thomas CC, Williams AK, Hayes ET, Massad NL, Johnson DN, Huang L, Zhang C, Sargis RM. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology. 2015;156(3):896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring). 2010;18(7):1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR, Kletzien RF, Burgess SC, Finck BN. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Reports. 2014;7(6):2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281(6):3398–3407. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton CM, Mashek DG, Wang S, Nagle CA, Cline GW, Thuillier P, Leesnitzer LM, Li LO, Stimmel JB, Shulman GI, Coleman RA. Lysophosphatidic acid activates peroxisome proliferator activated receptor-γ in CHO cells that over-express glycerol 3-phosphate acyltransferase-1. PLoS One. 2011;6(4):e18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA. 2005;102(7):2340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. [DOI] [PubMed] [Google Scholar]
- 31.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor γ/retinoid X receptor pathway. Mol Pharmacol. 2005;67(3):766–774. [DOI] [PubMed] [Google Scholar]
- 32.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect. 2014;122(11):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, Balaguer P, Zalko D. Characterization of novel ligands of ERα, Erβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci. 2011;122(2):372–382. [DOI] [PubMed] [Google Scholar]
- 34.Lam WY, Becker AM, Kennerly KM, Wong R, Curtis JD, Llufrio EM, McCommis KS, Fahrmann J, Pizzato HA, Nunley RM, Lee J, Wolfgang MJ, Patti GJ, Finck BN, Pearce EL, Bhattacharya D. Mitochondrial Pyruvate Import Promotes Long-Term Survival of Antibody-Secreting Plasma Cells. Immunity. 2016;45(1):60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCommis KS, Hodges WT, Bricker DK, Wisidagama DR, Compan V, Remedi MS, Thummel CS, Finck BN. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Mol Metab. 2016;5(8):602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCommis KS, Hodges WT, Brunt EM, Nalbantoglu I, McDonald WG, Holley C, Fujiwara H, Schaffer JE, Colca JR, Finck BN. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology. 2017;65(5):1543–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson VS, Bobseine K, Lambright CR, Gray LE Jr. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002;66(1):69–81. [DOI] [PubMed] [Google Scholar]
- 38.Safe S, Li X. Endocrine disruption: Relevance of experimental studies in female animals to human studies. Current Opinion in Toxicology. 2017;3:12–19. [Google Scholar]
- 39.Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, Vanparys C. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8(10):e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. [DOI] [PubMed] [Google Scholar]
- 41.Huang JV, Greyson CR, Schwartz GG. PPAR-γ as a therapeutic target in cardiovascular disease: evidence and uncertainty. J Lipid Res. 2012;53(9):1738–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perriello G, Pampanelli S, Di Pietro C, Brunetti P; Italian Pioglitazone Study Group . Comparison of glycaemic control over 1 year with pioglitazone or gliclazide in patients with Type 2 diabetes. Diabet Med. 2006;23(3):246–252. [DOI] [PubMed] [Google Scholar]
- 43.Shpilberg Y, Beaudry JL, D’Souza A, Campbell JE, Peckett A, Riddell MC. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis Model Mech. 2012;5(5):671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perelló M, Moreno G, Gaillard RC, Spinedi E. Glucocorticoid-dependency of increased adiposity in a model of hypothalamic obesity. Neuroendocrinol Lett. 2004;25(1-2):119–126. [PubMed] [Google Scholar]
- 45.Johansson M, Johansson N, Lund BO. Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin Pharmacol Toxicol. 2005;96(4):309–315. [DOI] [PubMed] [Google Scholar]