Abstract
Background
Patients resuscitated from cardiac arrest have brain and cardiac injury. Recent animal studies suggest that the administration of sodium nitrite after resuscitation from 12 minutes of asystole limits acute cardiac dysfunction and improves survival and neurologic outcomes. It has been hypothesized that low doses of IV sodium nitrite given during resuscitation of out of hospital cardiac arrest (OHCA) will improve survival. Low doses of sodium nitrite (e.g., 9.6 mg of sodium nitrite) are safe in healthy individuals, however the effect of nitrite on blood pressure in resuscitated cardiac arrest patients is unknown.
Methods
We performed a single-center, pilot trial of low dose sodium nitrite (1 or 9.6 mg dose) vs. placebo in hospitalized out-of-hospital cardiac arrest patient to determine whether nitrite administration reduced blood pressure and whether whole blood nitrite levels increased in response to nitrite administration.
Results
This is the first reported study of sodium nitrite in cardiac arrest patients. Infusion of low doses of sodium nitrite in comatose survivors of OHCA (n=7) compared to placebo (n=4) had no significant effects on heart rate within 30 minutes after infusion (70+ 20 vs. 78 ± 3 beats per minute, p=0.18), systolic blood pressure (103 ± 20 vs 108 ± 15 mmHg, p=0.3), or methemoglobin levels (0.92 ± 0.33 vs. 0.70 ± 0.26, p=0.45). Serum nitrite levels of 2–4 μM were achieved within 15 min of a 9.6 mg nitrite infusion.
Conclusions
Low dose sodium nitrite does not cause significant hemodynamic effect in patients with OHCA, which suggests that nitrite can be delivered safely in this critically ill patient population. Higher doses of sodium nitrite are necessary in order to achieve target serum level of 10 μM.
Introduction
Out-of-hospital cardiac arrest (OHCA) is a common and debilitating public health problem.1 Existing treatments for OHCA combine cardiopulmonary resuscitation (CPR) and early defibrillation by bystanders or first responders with advanced cardiac life support by emergency medical services (EMS) providers that include CPR, defibrillation and intravenous drugs, and finally, post-resuscitation care in hospital.2–4 Neurologic injury is a major cause of morbidity and mortality in these patients with most resuscitated victims never regaining consciousness.5–8 Despite advances in resuscitation, as many as 70% of those who have circulation restored after OHCA die before hospital discharge.3,9 Safe and effective therapies that improve post-resuscitation outcomes after cardiac arrest are urgently needed.
Therapeutic delivery of nitrite during ischemia or at time of reperfusion is cytoprotective in animal models of cardiac arrest. In a rodent model of cardiac arrest, a single low dose of nitrite (50 μM; 1.85 μM/kg) given intravenously (IV) during resuscitation significantly improved survival (19/25, 76% vs. 12/25, 48%, p value = 0.033) as compared to placebo.10 Optimal cardioprotection after experimental myocardial infarction has noted when blood levels of sodium nitrite close to 12 μM were achieved11 and optimal neuroprotection after cardiac arrest noted at blood levels close to 19 μM.12 These data suggest that low doses of sodium nitrite (~15–25 mg IV) would be sufficient to achieve therapeutic (10–20 μM) blood levels in humans. Numerous animal studies within experimental models of cardiac arrest, myocardial infarction and stroke10–17 demonstrate the potential of nitrite as a therapy during resuscitation to reduce cardiac and neurologic injury and improve survival.
Despite the promise of nitrite therapy in animal models of cardiac arrest, human studies of this are limited. Infusion of 75 mg of IV sodium nitrite over 25 min resulted in a mean decrease of 10 mmHg in blood pressure in healthy patients18 and ~17.5 mg delivered over 5 minutes resulted in a mean reduction of blood pressure of 4 mmHg in heart failure patients.19 However, the time period after successful resuscitation from OHCA is often associated with hemodynamic instability, therefore we sought to determine whether sodium nitrite may be given without significant reduction in blood pressure. We performed a single-center clinical study of sodium nitrite (1–9.6 mg) IV vs. identically appearing placebo in patients resuscitated from OHCA and transported to hospital to assess whether IV administration of sodium nitrite is associated with significant hypotension in this critically ill population.
Methods
This study was approved by the University of Washington Institutional Review Board and was registered with clinicaltrial.gov (NCT01178359). Adult survivors of OHCA admitted to a single participating receiving hospital were eligible to be enrolled in this study if the patient was successfully resuscitated and survived to ICU admission, had IV access, and if consent could be obtained from legal next-of-kin. Patients were excluded if they had traumatic etiology of arrest, known history of dialysis dependent kidney disease, a pre-existing “do not resuscitate” order in place, required vasopressor or inotropic support for myocardial dysfunction, had PaO2 < 90 mmHg on an FiO2 of 1.0 or had a systolic blood pressure (BP) < 90 mmHg and/or mean arterial pressure (MAP) < 60 mmHg. Finally, patients were excluded if the nitrite (or placebo) infusion could not be delivered within 12 h after cardiac arrest.
In the initial phase of this study, four patients were randomized in a double-blinded manner, 3:1 ratio to sodium nitrite (1 mg in 100 mL normal saline) or 100 mL saline placebo infused IV over 5 minutes. In the second phase, four patients received 9.6 mg IV nitrite and two received placebo. A third phase was planned, 14.5 mg IV nitrite, however this phase was not completed due to completion of the funding period for the study. One patient in this 14.5 mg group received placebo.
Since sodium nitrite has never been administered in the post resuscitation period, we therefore utilized an ascending dose study design with a placebo control arm. A sodium nitrite dose of 2–3 μmol/kg was found to be beneficial in mouse cardiac arrest model10 so we sought to test this dose in patients with OHCA. Furthermore, these doses were not associated with a significant decrease in mean arterial blood pressure in human studies.20 The 9.6 mg dose corresponds to 2 μmol/kg and 14.5 mg dose corresponds to 3 μmol/kg (assuming a 70 kg patient). Since, the blood pressure effects of sodium nitrite during the post resuscitation period were unknown, we selected the first dose to be 10 times lower than target dose and then titrated the dose upward with a goal to monitor for significant hypotension.
Plasma was obtained at baseline (within five minutes prior to initiation of drug infusion) and 15, 30, 60 min after infusion start for measurement of nitrite levels triiodide reduction followed by ozone chemiluminescence with an NO analyzer (GE Analytic, Boulder, Colorado). Plasma S-nitrosothiol measurements were made following reaction of plasma with acidified sulfaniliamide for 3 minutes before reductive chemiluminescence.21 Plasma cyclic guanosine monophosphate (cGMP) levels were measured using a commercial enzyme immunoassay (Cell Signaling, Danvers, MA) based on the manufacturer’s instructions. Methemoglobin levels were measured by cooximetry of whole blood at the same time points. Blood pressure measured by automated cuff and heart rate (measured by telemetry) were recorded before infusion, every minute during infusion, and every five minutes for an additional 25 minutes followed by every 15 min until 2 h. Planned stopping criteria were the occurrence of systolic BP < 90 mmHg on two consecutive measurements separated by 1 minute or a decrease in systolic BP > 15 mmHg on two consecutive measurements or increase in heart rate > 10 beats per min for more than one minute. In the higher dose groups, additional planned stopping criteria are systolic BP < 90 mmHg or a decrement of > 15 mmHg in more than 3/4 of the patients in the 9.6 or 14 mg dosing group. Additional adverse events sought included the occurrence of hypoxia (oxygen saturation < 90%), hypotension requiring the use of vasopressors any subsequent time during hospitalization, recurrent CA, atrial or ventricular arrhythmias, infection (specifically pneumonia or sepsis) or the occurrence of seizures within the first 120 min after dose administration.
Differences were analyzed using Wilcoxon rank-test using the statistical package SPSS (Version 11, Chicago, IL). For the comparison of the sodium nitrite group with other cardiac arrest patients admitted during the study period (i.e. contemporaneous controls not enrolled in the study), we used the Chi-square statistic for categorical variables and the independent samples t-test for continuous ones. For the comparison of the sodium nitrite and placebo groups, we used the Chi-square statistic for categorical variables and the Wilcoxon Rank Sum Test for continuous ones, given the very small sample size. Two-tailed paired tests were performed with the significance level was set at 0.05. All values presented are mean values ± standard deviation.
Results
Four hundred and ninety six survivors of out-of-hospital cardiac arrest were admitted to Harborview Medical Center between January 11, 2010 and Dec 31, 2015. The 11th patient was enrolled on Nov 30, 2015. The trial remained open to enrollment through April 30, 2016, however, no additional patients were enrolled during this period.
Enrollment for this study was limited since the majority of patients did not meet enrollment criteria (Figure 1). Since the infusion protocol had to be completed within the first 12 h after cardiac arrest a significant majority of the admitted patients did not qualify for enrollment since written consent could not be obtained within a 6–8 h time window after cardiac arrest (n=215). Legal next-of-kin were difficult to locate or could not arrive at the hospital in a timely manner. In addition, if family members arrived at the hospital it was difficult to approach family members since both medical and nursing staff needed to update family members or occasionally the medical staff did not think it would be appropriate to approach family members. Some of these patients had emergent coronary or cardiac procedures, which also made it difficult to start the study in a timely manner. Finally, study staff was only available for enrollment 40 h during the week so many subjects were missed if cardiac arrest patients were admitted at night or during the weekends.
Figure 1. Study flow diagram.
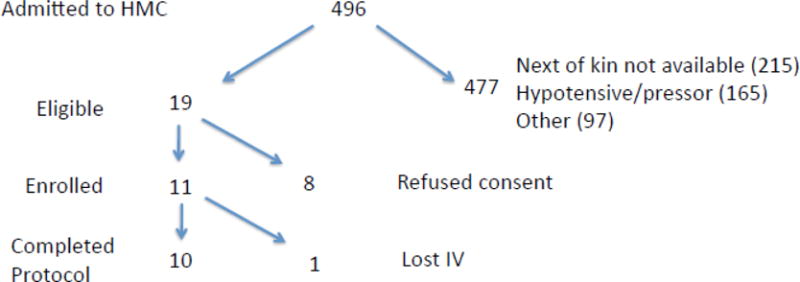
Diagram describing the number of patients screened, enrolled, and completing the protocol.
Other admitted cardiac arrest patient were excluded due to hypotension, use of vasopressors, or need for dialysis, FiO2 of 1 with PaO2 of less than 90 mmHg on arterial blood gas (ABG) (n=262).
Study staff approached 19 patient’s family members and 11 were enrolled and randomized in the study with ten completing the full protocol. One patient, who was randomized to placebo within the second [9.6 mg] dosing group, did not complete the IV infusion since vascular access was lost during the infusion.
The average age of the enrolled patients was 53 years and the average weight was 80.6 ± 13.3 kg (Table 1). Baseline characteristics of the 11 patients who were enrolled are presented in Table 1 along with characteristics of admitted patients who were not enrolled (n=496).
Table 1.
Comparison of baseline and follow-up characteristics: enrolled sodium nitrite study vs all others admitted to Harborview Medical Center 2010–2015
| Characteristic | Enrolled sodium nitrite or placebo (n=11) |
Others admitted (n=496) |
P |
|---|---|---|---|
| Age (years)(median, 25th, 75th percentile) | 55 (44–61) | 55 (44–65) | 0.68a |
| Women | 3 (27%) | 140 (28%) | 0.95b |
| Race | 0.38b | ||
| Caucasian | 9 (82%) | 315 (63%) | |
| African American | 1 (9%) | 89 (18%) | |
| Native American | 1 (9%) | 10 (2%) | |
| Asian | — | 62 (12%) | |
| Other | — | 16 (3%) | |
| Unknown | — | 4 (1%) | |
| Cardiac arrest before EMS arrival | 11 (100%) | 412 (83%) | 0.14b |
| Witnessed arrest | 9 (82%) | 295 (60%) | 0.14b |
| Bystander CPR | 4 (36%) | 219 (44%) | 0.61b |
| Shockable rhythm | 9 (82%) | 174 (35%) | 0.001b |
| Weight (kg)(median, 25th, 75th percentile) | 80 (71–88) | 80 (68–93)) | 0.99a |
by independent samples t-test
by Chi-square
EMS emergency medical services CPR cardiopulmonary resuscitation
The first four enrolled patients received 1 mg sodium nitrite or placebo (three received nitrite, one received placebo) and the next six patients received 9.6 mg sodium nitrite or placebo (four received nitrite, one received placebo, one placebo did not complete infusion). In Table 2, baseline characteristics between those who received sodium nitrite vs. placebo are presented and 6 (86%) patients survived to discharge who received nitrite whereas 3 (75%) who received placebo was discharged alive.
Table 2.
Comparison of baseline and follow-up characteristics by randomization group
| Characteristic | Sodium nitrite (n=7) |
Placebo (n=4) |
P |
|---|---|---|---|
| Age (yrs)(median, 25th, 75th percentile) | 54 (43–65) | 57 (44–60) | 0.64* |
| Women | 2 (29%) | 1 (25%) | 0.90** |
| Caucasian | 5 (71%) | 4 (100%) | 0.24** |
| Shockable rhythm | 6 (86%) | 3 (75%) | 0.66** |
| Weight (kg)(median, 25th, 75th percentile) | 75 (68–87) | 84 (73–94) | 0.34* |
| Witnessed arrest | 6 (86%) | 3 (75%) | 0.66** |
| Bystander CPR | 4 (57%) | 3 (75%) | 0.55** |
| Discharged alive | 6 (86%) | 3 (75%) | 0.66** |
by Wilcoxon Rank Sum Test
by Chi-square
CPR cardiopulmonary resuscitation
Neither the 1 mg or 9.6 mg nitrite dose resulted in a significant decrease in MAP (Fig 2C,D) or increase in heart rate (Fig 2E,F). Although the 1 mg dose resulted in minimal increases in blood nitrite levels (Figure 2A), the 9.6 mg dose increased blood nitrite levels as high as 4 μM within 10 min of infusion end (Figure 2B). In two patients who received a dose of 9.6 mg there was minimal change in whole blood nitrite levels (patients B1 and B3; Figure 2B) similar to the two patients who received placebo.
Figure 2. Nitrite infusion did not affect hemodynamics.
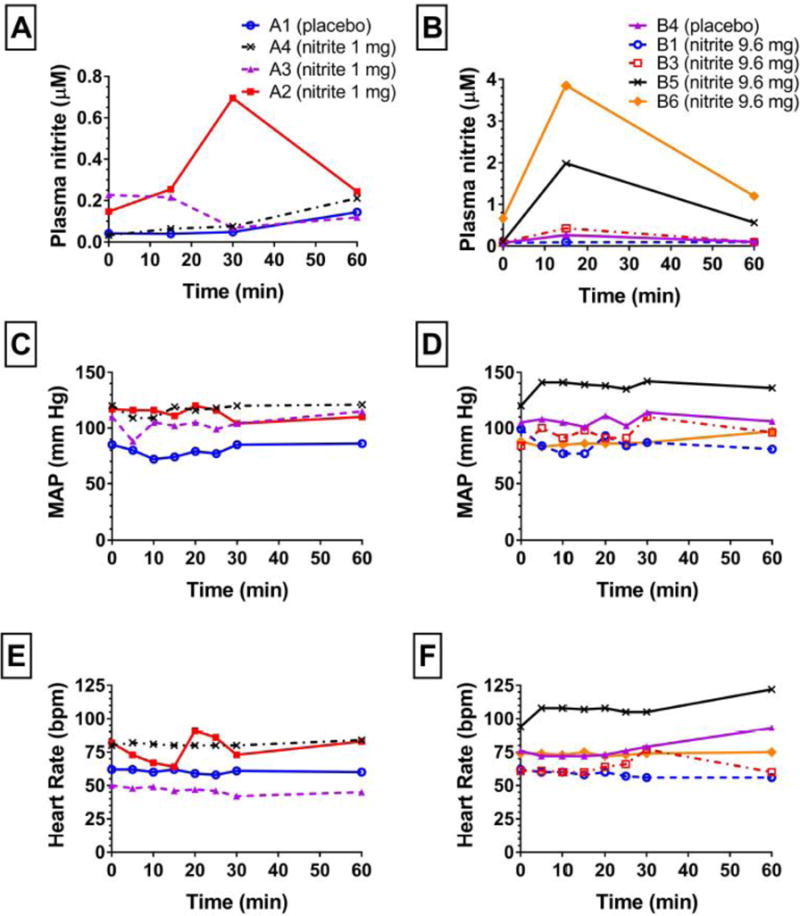
(A,B) Plasma nitrite levels, (C,D) mean arterial pressure (MAP) and (E,F) heart rate (HR) reported in beats per minute (bpm) are shown in subjects receiving 1 (A,C,E) or 9.6 (B,D,F) mg of sodium nitrite infused over first 5 minutes beginning at 0 minutes. Of the 4 patients in the 1 mg dosing group 3 received 1 mg nitrite and 1 received placebo. Of the 6 patients in the 9.6 mg group, 4 received 9.6 mg and 1 received placebo, 1 did not complete the protocol (loss of IV). One patient in the 14.6 mg group received placebo (data not shown). Key shows subject identifications codes and drug assignments; group A received 1 mg and group B 9.6 mg sodium nitrite.
We had sufficient plasma to measure S-nitrosothiols and cGMP in 4 patients (Figure 3) who received nitrite (n=3) or placebo (n=1). The patient who received placebo had the lowest increase in plasma nitrite (relative to baseline) and minimal increases in S-nitrosothiols and cGMP. All patients who received nitrite had an increase in S-nitrosothiols (Figure 3B) although this was not correlated with the change in plasma nitrite level (Figure 3A). Only one patient receiving nitrite had an increase in cGMP (patient B3; Figure 3C). Interestingly this patient had the lowest increase in plasma nitrite.
Figure 3. Nitrite levels do not correlate with increased in S-nitrosothiols or cGMP.
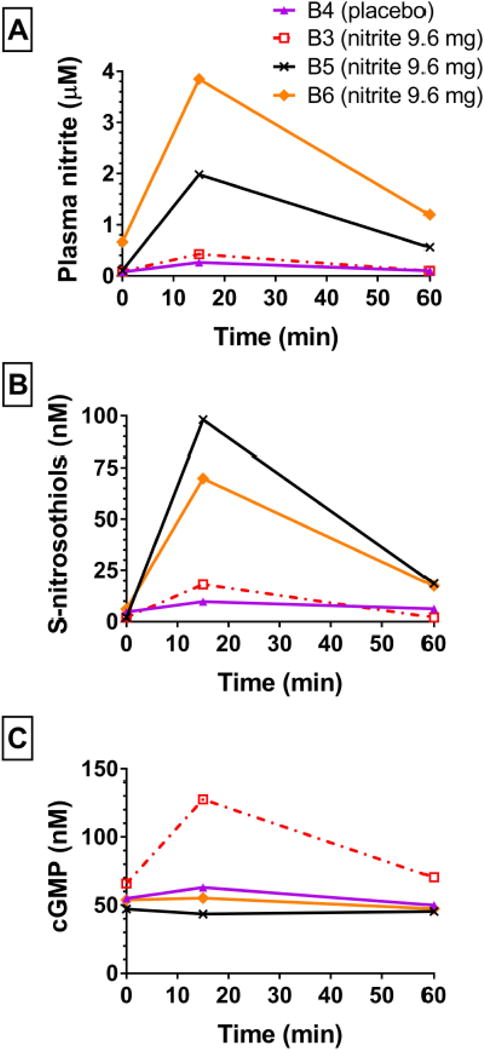
Increases in plasma nitrite (A) did not correlate with increases in plasma S-nitrosothiols (B) which rose in all subjects receiving active drug. Only one subject (B3) had a rise in cGMP (C) which did not correspond with the rise in plasma nitrite level. Key shows subject identifications codes and drug assignments.
Both nitrite doses were well tolerated. Baseline methemoglobin levels were 0.76 ± 0.17 vs. 0.60 ± 0.2 respectively for nitrite and placebo groups and changed minimally in response to nitrite or placebo infusion (0.92 ± 0.33 vs. 0.70 ± 0.26). In the 11 enrolled patients, we recorded no events of hypoxia, vasopressor requirements, or ventricular or atrial arrhythmias during the 120 minute monitoring period after study drug infusion. None of the enrolled patients met stopping criteria during the study.
Discussion
In this first clinical study of sodium nitrite for OHCA, we found no significant hemodynamic effect of either 1 or 9.6 mg of IV nitrite vs. placebo and that an infusion of 9.6 mg of IV nitrite over 5 minutes increased plasma levels of nitrite as high as 4 μM within 15 min. Nitrite infusion (9.6 mg) was associated with increases in plasma S-nitrosothiols and cGMP though these increases were neither uniform nor readily predicted by the plasma nitrite level.
Nitric oxide synthase (NOS) synthesizes nitric oxide (NO), a gaseous molecule from L-arginine and oxygen. NO oxidation yields nitrate (NO3−) and nitrite (NO2−), which can also accumulate in the body via dietary sources.22 Nitrate from dietary or endogenous sources is reduced in the body to nitrite, which can be further reduced to NO particularly in the setting of ischemia and reperfusion.20 Inhaled NO has been proposed as a delivery method to reduce ischemic reperfusion injury during myocardial infarction,23 liver transplantation24 and to improve outcomes during resuscitation from cardiac arrest.25 An alternative method to increase systemic NO levels is to infuse sodium nitrite. There has been interest in the use of sodium nitrite to reduce reperfusion injury and several small clinical studies of nitrite during myocardial ischemia26 or infarction,27 peripheral arterial disease,28 and heart failure19 have been published.
The post-ischemic protective effects of NO are believed to be mediated by activation of soluble guanylate cyclase (sGC) and S-nitros(yl)ation of critical cysteines of mitochondrial complex I.29–31 In the setting of myocardial ischemia and reperfusion, S-nitrosation of Cys39 of the ND3 subunit of mitochondrial complex I has been demonstrated to be a reversible post-translational modification which results in transient inhibition of electron flow with dramatic reductions in reperfusion mitochondrial oxidative burst.32 Several S-nitrosating agents have been demonstrated to provide protection from ischemic injury through this mechanism including nitrite.10,17,33–35 Importantly, the ability of nitrite to provide protective signaling against reperfusion injury through S-nitrosation is not shared by many NO donors.36 In the setting of experimental cardiac arrest, S-nitrosation mechanisms appears more important than sGC activation.17 Our study is the first to demonstrate that IV nitrite is capable of increasing both plasma cGMP (via sGC activation) and S-nitrosothiols (Figure 3). Larger humans studies are needed to characterize the factors, which influence signaling through each pathway. Thus nitrite provides the broad advantages of targeted NO delivery with pluripotent protective signaling through activation of sGC and S-nitrosation. In addition nitrite is FDA approved (for cyanide poisoning) and has a good human safety profile in a number of cardiovascular diseases.18,19,27,28,37
Although not designed to assess formal PK, this study suggests that the 9.6 mg sodium nitrite dose can produce significant C15min plasma nitrite elevations to 2–4 μM. Baseline plasma nitrite levels varied considerably (mean ± standard deviation: 0.163 ± 0.197 μM). It should be noted that these baseline levels in resuscitated cardiac arrest patients were significantly lower than baseline plasma nitrite recently measured by the same lab using the same methods in healthy human volunteers38 (0.324 ± 0.099; Mann Whitney U test p-value = 0.0014). This may represent the whole body depletion of nitrite, which occurs during global ischemia well documents in animal studies.10,11 A target of 10 μM within 10–15 min of infusion, suggested by preclinical studies to be the optimal target for heart and brain protection following cardiac arrest, therefore will require a dose higher than 9.6 mg to achieve. Our preliminary modeling suggests a nitrite doses of 25 mg or higher may be necessary. This would represent the highest dose of nitrite given in the setting of human cardiac arrest or any other ischemia-reperfusion injury and clearly requires further phase I testing to confirm safety. This study is presently ongoing (clinicaltrials.gov identifier: NCT02987088).
The length of enrollment in this trial also highlights the difficulty in enrolling resuscitated cardiac arrest patients for clinical studies. The time window to contact next-of-kin and obtain informed consent overlapped with a difficult time from both a patient and family’s standpoint. Almost 50% of resuscitated cardiac arrest patients admitted to Harborview were excluded from the study due to inability to obtain consent. Much of the hypothesized benefit of sodium nitrite is lost if administered long after resuscitation (i.e. after reperfusion injury has taken place). With this in mind, during the consent process, we could not list a direct theoretical benefit to the subject. Despite this, over 50% of subject’s family members agreed to participate in this study.
Limitations
The patient population enrolled as highlighted by table 1 suggests some differences between the OHCA population and those in the study. Exclusion criteria were stringent for safety reasons since this study represents the first-in-man study of the use of sodium nitrite for OHCA. Thus, the enrolled population may not be as critically ill as the general OHCA population. This is a small clinical study to ascertain whether the administration of sodium nitrite is associated with a large or significant effect on blood pressure, and is not powered to detect smaller changes in hemodynamics or other possible side effects. A much larger phase 1 study is currently under way to answer these questions.
This study represents the first study of sodium nitrite in patients with OHCA. Although the number of subjects was very small, we found that doses of nitrite of 1 or 9.6 mg did not reduce blood pressure or increase heart rate compared to placebo and blood levels of 2–4 μM can be achieved with a 9.6 mg dose. Furthermore we find evidence that nitrite can increase plasma SNO and cGMP both of which have been independently linked to protection against reperfusion injury. These findings suggest that even in this critically ill patient population, sodium nitrite can be administered safely in low doses, however additional study is warranted.
Acknowledgments
Data Safety and Monitoring: Michael A. Chen, MD PhD, Pathmaja Paramsothy, MD, MS, Division of Cardiology, University of Washington
Funding Source: Medic One Foundation, Seattle, WA (FK), UL1RR025014 Institute of Translational Health Sciences (FK), NIH/NHLBI R01HL129722 (FK, GN), NIH/NINDS K08NS069817 (CD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: clinicaltrials.gov Identifier: NCT01178359
Conflict of Interest:
None
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 3.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 4.Mccarty K, Nichol G, Chikani V, et al. Early Withdrawal of Post-Arrest Care After Therapeutic Hypothermia in Victims of Out-of-Hospital Cardiac Arrest. Circulation. 2010;122:A232. [Google Scholar]
- 5.Longstreth WT., Jr Brain resuscitation after cardiopulmonary arrest. Acta Anaesthesiol Belg. 1988;39(3):115–119. [PubMed] [Google Scholar]
- 6.Longstreth WT, Jr, Copass MK, Dennis LK, Rauch-Matthews ME, Stark MS, Cobb LA. Intravenous glucose after out-of-hospital cardiopulmonary arrest: a community-based randomized trial. Neurology. 1993;43(12):2534–2541. doi: 10.1212/wnl.43.12.2534. [DOI] [PubMed] [Google Scholar]
- 7.Raichle ME. The pathophysiology of brain ischemia. Ann Neurol. 1983;13(1):2–10. doi: 10.1002/ana.410130103. [DOI] [PubMed] [Google Scholar]
- 8.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 9.Aufderheide TP, Kudenchuk PJ, Hedges JR, et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods part 1: rationale and methodology for the impedance threshold device (ITD) protocol. Resuscitation. 2008;78(2):179–185. doi: 10.1016/j.resuscitation.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezfulian C, Shiva S, Alekseyenko A, et al. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120(10):897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezfulian C, Alekseyenko A, Dave KR, et al. Nitrite Therapy is Neuroprotective and Safe in Cardiac Arrest Survivors. Nitric Oxide. 2012;26(4):241–250. doi: 10.1016/j.niox.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104(48):19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez FM, Shiva S, Vincent PS, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung KH, Chu K, Ko SY, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37(11):2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 17.Dezfulian C, Kenny E, Lamade A, et al. Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest. Journal of neurochemistry. 2016;139(3):419–431. doi: 10.1111/jnc.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejam A, Hunter CJ, Tremonti C, et al. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod JOM, Arif S, Mukadam M, et al. Short-Term Intravenous Sodium Nitrite Infusion Improves Cardiac and Pulmonary Hemodynamics in Heart Failure Patients. Circulation: Heart Failure. 2015;8(3):565–571. doi: 10.1161/CIRCHEARTFAILURE.114.001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 21.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1–2):93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neye N, Enigk F, Shiva S, et al. Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive care medicine. 2012;38(8):1381–1391. doi: 10.1007/s00134-012-2605-1. [DOI] [PubMed] [Google Scholar]
- 24.Lang JD, Jr, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117(9):2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minamishima S, Kida K, Tokuda K, et al. Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation. 2011;124(15):1645–1653. doi: 10.1161/CIRCULATIONAHA.111.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram TE, Fraser AG, Bleasdale RA, et al. Low-dose sodium nitrite attenuates myocardial ischemia and vascular ischemia-reperfusion injury in human models. J Am Coll Cardiol. 2013;61(25):2534–2541. doi: 10.1016/j.jacc.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi N, Neil C, Bruce M, et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur Heart J. 2014;35(19):1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohler ER, 3rd, Hiatt WR, Gornik HL, et al. Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: safety, walking distance and endothelial function. Vasc Med. 2014;19(1):9–17. doi: 10.1177/1358863X13515043. [DOI] [PubMed] [Google Scholar]
- 29.Benhar M, Stamler JS. A central role for S-nitrosylation in apoptosis. Nat Cell Biol. 2005;7(7):645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 30.Mannick JB, Hausladen A, Liu L, et al. Fas-induced caspase denitrosylation. Science. 1999;284(5414):651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 31.Melino G, Bernassola F, Knight RA, Corasaniti MT, Nistico G, Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388(6641):432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 32.Chouchani ET, Methner C, Nadtochiy SM, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19(6):753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chouchani ET, Methner C, Nadtochiy SM, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19(6):753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadtochiy SM, Burwell LS, Ingraham CA, et al. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. Journal of molecular and cellular cardiology. 2009;46(6):960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alencar JL, Lobysheva I, Chalupsky K, et al. S-nitrosating nitric oxide donors induce long-lasting inhibition of contraction in isolated arteries. J Pharmacol Exp Ther. 2003;307(1):152–159. doi: 10.1124/jpet.103.052605. [DOI] [PubMed] [Google Scholar]
- 37.Ingram TE, Fraser AG, Bleasdale RA, et al. Low-Dose Sodium Nitrite Attenuates Myocardial Ischemia and Vascular Ischemia-Reperfusion Injury in Human Models. Journal of the American College of Cardiology. 2013;61(25):2534–2541. doi: 10.1016/j.jacc.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Dezfulian C, Taft M, Corey C, et al. Biochemical signaling by remote ischemic conditioning of the arm versus thigh: Is one raise of the cuff enough? Redox biology. 2017;12:491–498. doi: 10.1016/j.redox.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


