ABSTRACT
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection (LRTI) annually affecting >2 million children in the United States <5 years old. In the elderly (>65 years old), RSV results in ∼175,000 hospitalizations annually in the United States with a worldwide incidence of ∼34 million. There is no approved RSV vaccine, and treatments are limited. Recently, a phase 3 trial in the elderly using a recombinant RSV F protein vaccine failed to meet its efficacy objectives, namely, prevention of moderate-to-severe RSV-associated LRTI and reduced incidence of acute respiratory disease. Moreover, a recent phase 3 trial evaluating suptavumab (REGN2222), an antibody to RSV F protein, did not meet its primary endpoint of preventing medically attended RSV infections in preterm infants. Despite these setbacks, numerous efforts targeting the RSV F protein with vaccines, antibodies, and small molecules continue based on the commercial success of a monoclonal antibody (MAb) against the RSV F protein (palivizumab). As the understanding of RSV biology has improved, the other major coat protein, the RSV G protein, has reemerged as an alternative target reflecting progress in understanding its roles in infecting bronchial epithelial cells and in altering the host immune response. In mouse models, a high-affinity, strain-independent human MAb to the RSV G protein has shown potent direct antiviral activity combined with the alleviation of virus-induced immune system effects that contribute to disease pathology. This MAb, being prepared for clinical trials, provides a qualitatively new approach to managing RSV for populations not eligible for prophylaxis with palivizumab.
KEYWORDS: F protein, G protein, RSV, respiratory syncytial virus, monoclonal antibodies, palivizumab
RSV BIOLOGY
The medical need.
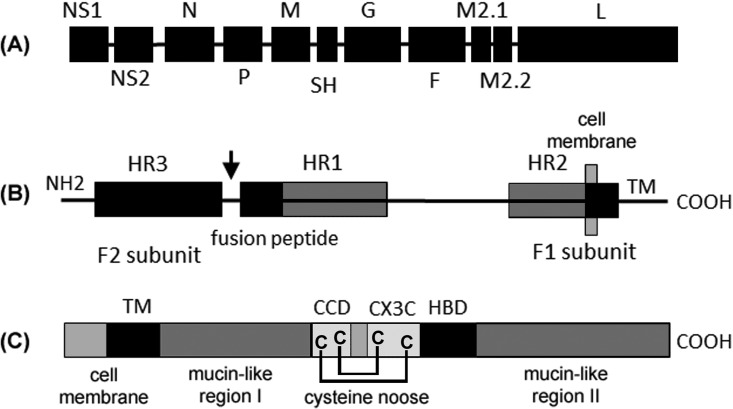
Respiratory syncytial virus (RSV) is a negative-strand RNA virus in the family Pneumoviridae with 10 genes encoding 11 proteins (Fig. 1) that has resisted effective management for >60 years in part because infection does not provide robust immunity. As has been extensively reviewed (1–6), >50% of infants are infected by RSV during their first year, with nearly 5% requiring hospitalization. The only care available for RSV infection is supportive. Preterm infants (gestational age of <29 weeks) have been the focus for prophylaxis with palivizumab, which reduces morbidity but not mortality (1). The RSV F protein is more conserved overall compared to the G protein, and it has been the target for palivizumab and most other pharmacological efforts. However, the G protein has a central conserved domain (CCD) that is nearly invariant across all circulating strains, whose importance has become clearer over the past several years, particularly with regard to the unmet need for a postinfection therapeutic (4).
FIG 1.
RSV genome. (A) Ten genes produce 11 proteins. The M2-2 open reading frame (ORF) is accessed by ribosomes that reinitiate after exiting the M2-1 ORF. The G protein is produced as both membrane-bound and secreted forms via alternative translation start sites. Two antigenic subgroups (A and B) are defined by the hypervariable mucin-like regions of the G protein. (B) RSV F protein (575 amino acids [aa]) is cleaved by furin (at the arrow) to produce the F1 and F2 domains with a conformational change that promotes fusion with cell membranes; the location of heptad repeats (HR), fusion peptide, and transmembrane domain (TM) are shown. (C) The RSV G protein (298 aa) central conserved domain (CCD) includes a conformationally constrained CX3C motif (182-CWAIC-186) that is implicated in infection of lung epithelial cells through binding to CX3CR1, assisted by a heparin binding domain (HBD). The MAb TRL3D3 binds to an epitope within the CCD.
Vaccine hindrances.
For RSV, there are four fundamental vaccination strategies: (i) vaccinate children, (ii) vaccinate adults (19 to 55 years), (iii) vaccinate the elderly, and (iv) vaccinate pregnant woman (2–5). The diminished immune system in the very young and the elderly poses special challenges. Regarding the other vaccine groups, the highly transmissible nature of RSV and the poor immunological memory to natural infection make it difficult to achieve herd immunity. Other obstacles include induction of nonneutralizing antibodies or insufficient titer of neutralizing antibodies, exaggerated Th2-like responses resulting in massive infiltration of inflammatory cells, ineffective priming of CD8 responses, and complement deposition. Moreover, susceptibility varies with factors difficult to manage, such as the level of cocirculating respiratory viruses, including influenza virus and rhinovirus (7). The failure of the Novavax phase 3 vaccine trial (RSV F vaccine) in the elderly (5), despite enrolling nearly 12,000 subjects, is a stark reminder of the obstacles to RSV vaccine development. Novavax is currently in phase 3 testing of the same vaccine in pregnant women. However, persistence of maternal antibodies in the neonate may be too short to achieve reliable protection unless a very high titer of neutralizing antibodies is achieved.
Vaccines that require a cold chain to maintain efficacy have additional obstacles, particularly for global use. Formalin inactivation of whole virus, a technique used to increase stability, caused disease exacerbation upon subsequent natural infection, resulting in two deaths (8), which set back significantly the effort to develop an RSV vaccine for infants. Currently, live-attenuated RSV vaccines remain compelling vaccine candidates for use in infants, while protecting the elderly remains more elusive. Specifically, a structurally stabilized postfusion F protein vaccine has recently failed in phase 2 trials to prevent RSV-associated respiratory illness in the elderly (9). A better understanding of the complex interaction between virus and host, including age-specific factors, is needed for safe and effective RSV vaccine development to proceed (10).
PHARMACOLOGY
Targeting the F protein with a MAb.
Given the obstacles to developing a safe and effective RSV vaccine, providing an optimized MAb with strong virus-neutralizing activity is appealing. The F protein promotes fusion of RSV with the host cell membrane and is essential for infectivity both in vitro and in vivo. Palivizumab, a humanized murine monoclonal antibody (MAb) that inhibits the fusion process, has become widely used for prophylaxis of premature birth infants at high risk of severe RSV disease (1). Although the MAb reduces the incidence of severe disease from ∼10% to less than 5%, widespread use of this costly agent is judged uneconomic (11). Moreover, a phase 2 trial to expand its use to full-term infants as a therapeutic was unsuccessful (12). Although the number of patients enrolled in the study was too small to establish statistical significance (n = 35), all three clinical efficacy endpoints measured were inferior to placebo. Motavizumab, a higher-affinity derivative of palivizumab, reduced hospital admissions in Native American full-term infants (13), but the MAb failed to reach FDA approval because of safety concerns (hives and allergic reactions) that were not offset by any clear superiority to palivizumab (4). Further, escape from palivizumab is easily achieved (14) and may be clinically relevant (15). Escape correlates with a reduced on-rate of the MAb (16), which is important since avoiding escape via higher on-rate risks generating increased off-target reactivity as seen in the protein engineering effort leading to motavizumab (17).
Despite the limitations of palivizumab, its commercial success has led to a variety of products that mimic its pharmacological properties (3, 4, 6). REGN2222 is a biosimilar MAb targeting the F protein. In a recently reported double-blind, placebo-controlled phase 3 study (n = 1,149), healthy preterm infants (gestational age of <36 weeks and <6 months old at the beginning of the study) were treated with one or two doses at 30 mg/kg of body weight; assessment at day 150 failed to show efficacy for the primary endpoint of medically attended RSV infections (http://investor.regeneron.com/releaseDetail.cfm?releaseid=1037184). Other biosimilar efforts are on hold or still preclinical. MEDI8897 is an anti-F protein MAb engineered for longer serum half-life (18). ALX-0171, a 15-kDa “nanobody” with comparable epitope recognition to palivizumab, is being evaluated as an inhaled formulation for postinfection treatment (19).
Other F protein interventions in development.
Vaccine efforts focused on the F protein are continuing, as recently reviewed (2–5), including MEDI-559, a cold-passaged, live-attenuated RSV, and MEDI-534, a human-bovine chimeric parainfluenza virus construct expressing the RSV F protein. GSK3389245A is a vaccine based on an adenovirus vector to stimulate a T cell response. DS-Cav1 and GSK3003891A are stabilized prefusion forms of the F protein (Fig. 1). MEDI-7510 is a recombinant F protein vaccine in the postfusion conformation; it failed to show efficacy in a phase 2 trial in the elderly (n = 1,894) despite being immunogenic (9). Preclinical efforts exploring the utility of stabilized postfusion F protein are continuing (4).
Although the first small molecule fusion inhibitor compound, BTA9881, was terminated when the phase 1 clinical results did not meet the desired safety margin (6), several compounds with similar activity are still being pursued, including TMC-353121 (an improved-pharmacokinetics version of JNJ-2408068), AK-0529, RFI-641, and BMS-433771 (4). GS-5806 has shown efficacy in a phase 2a challenge model (attenuated virus in healthy adults); however, it also showed evidence of escape mutations (4).
Targeting intracellular viral proteins.
The efficacy of palivizumab is linked to neutralization of RSV replication by blocking cell entry. Other routes to blocking replication have been explored that involve targeting viral proteins expressed intracellularly (3). ALN-RSV01 is an RNA interference (RNAi) construct to the N (nucleoprotein) gene that showed initial signs of efficacy in a live-virus challenge model (Memphis 37 strain) in healthy adults. It was dropped after missing the primary endpoint, namely, a reduction in progressive bronchiolitis obliterans syndrome at 180 days in lung transplant patients with confirmed RSV infection (20). RSV604 is a small molecule targeting the N protein, which reached phase 2 in bone marrow transplant patients but was discontinued due to variability in oral absorption (4). Another approach to blocking replication is inhibition of the viral polymerase (L protein), exemplified by ALS-8174 (4).
Targeting the G protein with a MAb.
The G protein is one of two major RSV envelope proteins. The F protein is generally conserved, and its deletion abolishes RSV infectivity; loss of the more variable G protein varies in effect from some inhibition of replication (21) to full prevention of replication (22, 23). The G protein has attracted increasing attention to address the need for RSV prophylaxis in healthy infants through an entire RSV season and for postinfection treatment. Building on a promising vaccine over a decade ago that showed efficacy in mice and immunogenicity in humans (24), three features have emerged that define the G protein as an attractive target. First, there is a small central conserved domain (CCD) that is highly conserved (4). Although variation in the hypervariable domains flanking the CCD increases in response to immune system pressure (25), the CCD itself remains highly conserved. Second, the CCD is essential for infectivity in vivo and mediates attachment to airway epithelial cells (26–28). Third, the CCD has a CX3C chemokine motif implicated in alteration of the host immune response (29).
The G protein's role in RSV pathology.
Under some circumstances, RSV is known to bias the immune response toward a Th2 phenotype (30). A role for the innate immune response affecting RSV disease severity has also been suggested by observations of increased disease severity in patients with certain polymorphisms of either Toll-like receptor 4 (TLR4) (31) or the CX3C chemokine receptor (32). The G protein modulates neonatal regulatory B lymphocytes (nBreg cells) to produce immunosuppressive interleukin-10 (IL-10), and the frequency of RSV-infected nBreg cells in the neonate respiratory tract is predictive of acute bronchiolitis severity (33). It is worth noting that the cotton rat provides a useful model to study MAbs against the F protein because it is more permissive for viral replication than mice; however, the cotton rat is an imperfect model for understanding the pathological host response in humans (34).
An important unmet need is for a post-RSV treatment since conventional anti-inflammatory agents have failed to provide clinical benefit (35). Nonclinical studies using anti-G protein MAbs targeting the CCD motif have shown efficacy as a postinfection treatment (33, 34, 36, 37). In a mouse model using RSV strain A2, a murine anti-G protein MAb (131-2G) administered at day 3 postinfection reduced the influx of inflammatory cells into the airways, with a pronounced effect at day 5 that was sustained to day 14 (36). A murine anti-F protein MAb (143-6C) had no such effect. In a similar model, using RSV line 19F (known to cause increased airway hyperreactivity and mucus hyperproduction in mice), an anti-G protein MAb improved breath distension of peripheral arteries (pulse oximetry) (37). These studies emphasize the anti-inflammatory activity of MAbs targeting the RSV G protein CCD. Additional studies have boosted this therapeutic rationale by comparison of an anti-G protein IgG to an F(ab′)2 construct. Both were able to suppress airway inflammation, but only the intact IgG was able to reduce viral load, consistent with the complement-dependent activity of a different MAb against a similar epitope (38). This experiment established that the anti-inflammatory effect is not just a result of reduced viral load (39).
In normal human bronchial epithelial (NHBE) cells infected by RSV, TLR4 signaling was reduced, an effect linked to increased SOCS3, which suppresses antiviral interferons (IFNs) (40). Treatment with an anti-G protein MAb (131-2G) counteracted the immune-modifying nature of the RSV G protein leading to enhanced IFN, whereas an anti-F protein MAb depressed the IFN response below the mock infection control level. A similar effect was observed in plasmacytoid dendritic cells, where mutation of the G protein CCD prevented IFN suppression with an anti-G protein F(ab′)2 antibody, emulating the phenotype of the G protein mutation (41). A finding showing a preponderance of IFN-λ1 (IL-29) in lower airway samples from RSV-infected infants suggests that IFN-λ1 is the principal IFN responding to RSV infection in infants with severe disease (42).
G protein provides a favorable target.
Interventions that reduce viral replication are important and have represented the vast majority of RSV control efforts (43). However, suppression of replication does not specifically address a key feature of RSV, namely, alteration of the host's immune system resulting in airway inflammation (4). An early attempt to target the CCD of the G protein with a recombinant protein vaccine (BBG2Na) showed a moderate ability to induce neutralizing antibodies in healthy, young adults (24). Low immunogenicity of the CCD is a prominent feature of the virus (25). A more direct route to targeting the G protein CCD is use of a MAb. Building on foundational work using murine hybridomas (36), the most advanced preclinical candidate is TRL3D3, a native human antibody that binds the CCD motif with low picomolar affinity (38). As epitope conservation implies essential functionality, escape mutants are less likely than for therapeutics targeting other viral proteins or domains of the G protein.
An important feature of the G protein is an alternative translation initiation site that leads to secretion of ∼15% of the protein beginning 6 h following RSV infection and well before the appearance of progeny virus (44). A mutation that prevents production of the soluble G protein improved the efficacy of a polyclonal serum against the F protein (45), and this mutation has been incorporated into a live-attenuated vaccine candidate (46). Neutralization of soluble proteins typically requires higher affinity than for membrane-bound proteins to avoid prolonging the serum half-life that results in increased exposure of tissues to the factor (47). Achieving high affinity uniformly in a diverse population is difficult for a vaccine, and the RSV G protein CCD is particularly difficult to target since it is poorly immunogenic. One approach to achieving a vaccine against both A and B strains has been to create a fusion peptide comprising CCD peptides from both strains, with promising efficacy in mice, although the affinity of the induced MAbs has not yet been studied (23). In another recent study (48), recombinant G protein ectodomain induced protective responses in cotton rats, although the immunodominant epitopes were in the highly variable N- and C-terminal regions (Fig. 1).
The TRL3D3 MAb, whose affinity is high enough to neutralize the soluble G protein, has also shown direct antiviral activity with improved potency over palivizumab in mice (38). Consistent with the ex vivo effects of MAbs against RSV F or G proteins on IFN production, treatment with palivizumab was associated with increased airway inflammation in a mouse model, whereas TRL3D3 suppressed it (49). The lack of robust, long-lasting immunity following RSV infection results in frequent reinfection. Early infection, when the immune system is immature predisposes to asthma-like symptoms in childhood (1, 49, 50). To model the effect of infection when the immune system is immature in a mouse entails initial exposure to RSV as a neonate followed by secondary exposure at 6 weeks. Prophylaxis with TRL3D3 at the primary infection provided markedly improved lung function upon secondary infection, whereas palivizumab provided no such improvement (49). Supplemental IFN at the neonate sensitization step substantially reduced perivascular inflammation and mucus hyperproduction upon reinfection (51). Consistent with these results, palivizumab has no effect on reinfection rate or disease severity (3).
SUMMARY
The RSV F protein has historically been favored as a target for vaccine and preventive intervention. As summarized in Table 1, four F protein vaccines and three MAbs or MAb analogs are currently in clinical trials with a corresponding predominance in the catalog of preclinical agents in development (43). However, from an efficacy perspective, the G protein central conserved domain is an increasingly compelling target. Antibodies to this site combine (i) complement-mediated antiviral activity, (ii) blockade of airway epithelial cell infection, and (iii) anti-inflammatory activity. The nearly invariant sequence reduces escape potential, a significant advantage compared to targeting the F protein (14). From a safety perspective, a reduced ability of the G protein to modify the host immune response may provide unique efficacy as a postinfection treatment. The development path for a treatment is more practical than for a prophylactic agent, with RSV detection kits (52) expected to facilitate adoption of novel therapeutic agents.
TABLE 1.
RSV interventions in developmenta
| Name | Classification | Target | Stage |
|---|---|---|---|
| RSV F Vaccine (Novavax) | Vaccine (nanoparticle) | F protein | Phase 3 |
| MEDI-534 | Vaccine (PIV3 vector) | F protein | Phase 2 |
| GSK3389245A | Vaccine (adenovirus vector) | F protein | Phase 2 |
| MEDI-7510 | Vaccine | F protein (postfusion) | Phase 2 |
| DPX-RSV | Vaccine | SH protein (epitope) | Phase 1 |
| CX3C-LbL-NP | Vaccine (nanoparticle) | G protein (epitope) | Preclinical |
| REGN-2222 | MAb | F protein | Phase 3 |
| MEDI-8897 | MAb | F protein | Phase 2 |
| ALX-0171 | Nanobody | F protein | Phase 2 |
| TRL3D3 | MAb | G protein | Pre-INDb |
| GS-5806 | Small molecule | F protein | Phase 2 |
| JNJ-53,718,678 | Small molecule | F protein | Phase 2 |
| BTA-C585 | Small molecule | F protein | Phase 2 |
| AK-0529 | Small molecule | F protein | Phase 2 |
| AK-0529 | Small molecule | F protein | Phase 2 |
| VP-14637 | Small molecule | F protein | Phase 1 |
| RFI-641 | Small molecule | F protein | Preclinical |
| TMC-353121 | Small molecule | F protein | Preclinical |
| BMS-433771 | Small molecule | F protein | Preclinical |
| RSV604 | Small molecule | N protein | Phase 2 |
| ALN-RSV01 | RNAi | N protein | Phase 2 |
| ALS-8176 | Small molecule | L protein | Phase 2 |
ACKNOWLEDGMENTS
R.T. thanks the Georgia Research Alliance. U.P. thanks the Wellcome Trust (grant 108818/Z/15/A), UK Biotechnology and Biological Sciences Research Council (grant BB/P004040/1), and the Public Health Service HSC R&D Division, Northern Ireland (grant COM/5237/15). P.O. thanks the National Institute for Health Research (NIHR) for providing a Senior Investigator Award, Imperial's Health Protection Research Unit (HPRU) in Respiratory Infection, and the Imperial Biomedical Research Centre (BRC) at Imperial College Healthcare NHS Trust. L.K. thanks NIAID (grant 1R44AI122360-02). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health (U.K.).
We thank Lia Haynes at the CDC (USA) and Larry J. Anderson at Emory University for advice, efforts, and support.
Biographies

Ralph A. Tripp is Professor and Georgia Research Alliance Chair of Vaccine and Therapeutic Development in the Department of Infectious Diseases at University of Georgia (UGA). He joined UGA in 2004 following 6 years at the U.S. Centers for Disease Control and Prevention. His translational research focus for 30 years has been on interventions to protect humans from established and emerging respiratory viruses. His lab investigates the mechanisms of immunity, inflammation, and disease pathogenesis to understand the functional differences between innate and adaptive immune responses that facilitate vaccine and antiviral therapeutic protocols. Dr. Tripp has published >200 peer-reviewed articles, is an inventor on 12 issued U.S. patents, has written numerous book chapters on virology and/or immunology, and has edited several such books.

Ultan F. Power is a Reader in Molecular Virology at Queen's University Belfast (QUB), Northern Ireland, where he has been a researcher for 13 years. He received his Ph.D. from the National University of Ireland and was a postdoctoral fellow at St Jude Children's Research Hospital. He was previously Head of Virology and Senior Scientist at Institut de Recherche Pierre Fabre, where his RSV vaccine work was awarded the Prix Galien. At QUB, he has developed models of RSV infection based on well-differentiated primary pediatric airway epithelial cell cultures, the primary targets of RSV infection. Using these models has provided novel insights into RSV biology, including tentative identification of gene biomarkers of severe RSV disease in infants. Improved understanding of RSV pathogenesis in humans provides the basis for identifying novel therapeutic targets and for authentic preclinical models to test novel anti-RSV therapeutics. He has published >60 peer-reviewed articles.

Peter J. M. Openshaw is Professor of Experimental Medicine at Imperial College London and an Honorary Physician in the Department of Respiratory Medicine of the Imperial College NHS Trust. He was appointed President of the British Society for Immunology in 2013, the first clinician to lead the organization. He trained at Guy's Hospital, the Brompton and the Royal Postgraduate Medical School before Ph.D. training. His research focus is on the immunology of the lung, viral lung disease, vaccination, and immunopathogenesis of viral disease. He received the Chanock Award for lifetime contribution to RSV research in 2012 and is the inaugural President of the International RSV Society (2017). He is interim Chair of NERVTAG (a DH advisory committee on outbreaks of pandemic potential) and Theme Lead for Infection on Imperial's Biomedical Research Centre. He now leads a network supporting human volunteer challenge to accelerate vaccine development (HIC-Vac, 2017–2021).

Lawrence M. Kauvar is the founder and VP Chief Scientist of Trellis Bioscience, a company focused on discovery and development of native human antibodies against infectious disease and cancer targets. He previously played the same role at Telik, a company focused on small molecules for cancer. He received his B.A. from Harvard and his Ph.D. from Yale and pursued postdoctoral studies at Caltech and UC San Francisco. The primary focus of his industrial research for the past 25 years has been on development of innovative drug discovery assay technologies that combine high-throughput empirical methods with computer intensive data reduction. Dr. Kauvar has published >40 peer-reviewed articles and is an inventor on >60 issued U.S. patents. Trellis monoclonal antibody candidates progressing towards an IND include ones to treat RSV, CMV, influenza, and bacterial drug resistance associated with biofilm formation.
REFERENCES
- 1.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. 2014. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 134:e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 2.Broadbent L, Groves H, Shields MD, Power UF. 2015. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza Other Respir Viruses 9:169–178. doi: 10.1111/irv.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello HM, Ray WC, Chaiwatpongsakorn S, Peeples ME. 2012. Targeting RSV with vaccines and small molecule drugs. Infect Disord Drug Targets 12:110–128. doi: 10.2174/187152612800100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorquera PA, Tripp RA. 2017. Respiratory syncytial virus: prospects for new and emerging therapeutics. Expert Rev Respir Med 11:609–615. doi: 10.1080/17476348.2017.1338567. [DOI] [PubMed] [Google Scholar]
- 5.Rezaee F, Linfield DT, Harford TJ, Piedimonte G. 2017. Ongoing developments in RSV prophylaxis: a clinician's analysis. Curr Opin Virol 24:70–78. doi: 10.1016/j.coviro.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z, Pan Y, Jiang S, Lu L. 2013. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses 5:211–225. doi: 10.3390/v5010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wishaupt JO, van der Ploeg T, de Groot R, Versteegh FG, Hartwig NG. 2017. Single- and multiple viral respiratory infections in children: disease and management cannot be related to a specific pathogen. BMC Infect Dis 17:62. doi: 10.1186/s12879-016-2118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 9.Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Takas T, Levin MJ, Falsey AR. 23 September 2017. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis doi: 10.1093/infdis/jix503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. 2014. Immunity to RSV in early-life. Front Immunol 5:466. doi: 10.3389/fimmu.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meissner HC, Kimberlin DW. 2013. RSV immunoprophylaxis: does the benefit justify the cost? Pediatrics 132:915–918. doi: 10.1542/peds.2013-2449. [DOI] [PubMed] [Google Scholar]
- 12.Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, Buckingham S, Vernacchio L, Ambrosino DM. 1998. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis 178:1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, Millar EV, Jensen KM, Harris BS, Reid R, Moulton LH, Losonsky GA, Karron RA, Santosham M, Respiratory Syncytial Virus Prevention Study Group . 2015. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 15:1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 14.Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. 2010. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin Infect Dis 51:185–188. doi: 10.1086/653534. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Q, McAuliffe JM, Patel NK, Palmer-Hill FJ, Yang CF, Liang B, Su L, Zhu W, Wachter L, Wilson S, MacGill RS, Krishnan S, McCarthy MP, Losonsky GA, Suzich JA. 2011. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis 203:674–682. doi: 10.1093/infdis/jiq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Schief WR, Crowe JE Jr. 2014. Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology 454-455:139–144. doi: 10.1016/j.virol.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Pfarr DS, Losonsky GA, Kiener PA. 2008. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol 317:103–123. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, Moldt B, Khan A, Svabek C, McAuliffe JM, Wrapp D, Patel NK, Cook KE, Richter BWM, Ryan PC, Yuan AQ, Suzich JA. 2017. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 9:eaaj1928. doi: 10.1126/scitranslmed.aaj1928. [DOI] [PubMed] [Google Scholar]
- 19.Detalle L, Stohr T, Palomo C, Piedra PA, Gilbert BE, Mas V, Millar A, Power UF, Stortelers C, Allosery K, Melero JA, Depla E. 2015. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother 60:6–13. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb J, Zamora MR, Hodges T, Musk AW, Sommerwerk U, Dilling D, Arcasoy S, DeVincenzo J, Karsten V, Shah S, Bettencourt BR, Cehelsky J, Nochur S, Gollob J, Vaishnaw A, Simon AR, Glanville AR. 2016. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant 35:213–221. doi: 10.1016/j.healun.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Teng MN, Collins PL. 1998. Identification of the respiratory syncytial virus proteins required for formation and passage of helper-dependent infectious particles. J Virol 72:5707–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuentes S, Klenow L, Golding H, Khurana S. 2017. Preclinical evaluation of bacterially produced RSV-G protein vaccine: strong protection against RSV challenge in cotton rat model. Sci Rep 7:42428. doi: 10.1038/srep42428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Chang J. 2017. Universal vaccine against respiratory syncytial virus A and B subtypes. PLoS One 12:e0175384. doi: 10.1371/journal.pone.0175384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus AD, de Groot R, Corvaia N, Beck A, Bouveret-Le-Cam N, Bonnefoy JY. 2001. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis 184:1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 25.Grad YH, Newman R, Zody M, Yang X, Murphy R, Qu J, Malboeuf CM, Levin JZ, Lipsitch M, DeVincenzo J. 2014. Within-host whole-genome deep sequencing and diversity analysis of human respiratory syncytial virus infection reveals dynamics of genomic diversity in the absence and presence of immune pressure. J Virol 88:7286–7293. doi: 10.1128/JVI.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong KI, Piepenhagen PA, Kishko M, DiNapoli JM, Groppo RP, Zhang L, Almond J, Kleanthous H, Delagrave S, Parrington M. 2015. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS One 10:e0130517. doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, Walsh EE, Peeples ME. 2015. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog 11:e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortjens B, Yasuda E, Yu X, Wagner K, Claassen YB, Bakker AQ, van Woensel JBM, Beaumont T. 2017. Broadly reactive anti-respiratory syncytial virus G antibodies from exposed individuals effectively inhibit infection of primary airway epithelial cells. J Virol 91:e02357-16. doi: 10.1128/JVI.02357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chirkova T, Lin S, Oomens AG, Gaston KA, Boyoglu-Barnum S, Meng J, Stobart CC, Cotton CU, Hartert TV, Moore ML, Ziady AG, Anderson LJ. 2015. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen Virol 96:2543–2556. doi: 10.1099/vir.0.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, Wenzel SE, Moore ML, Peebles RS Jr, Ray A, Ray P. 2012. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tal G, Mandelberg A, Dalal I, Cesar K, Somekh E, Tal A, Oron A, Itskovich S, Ballin A, Houri S, Beigelman A, Lider O, Rechavi G, Amariglio N. 2004. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis 189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 32.Amanatidou V, Sourvinos G, Apostolakis S, Tsilimigaki A, Spandidos DA. 2006. T280M variation of the CX3C receptor gene is associated with increased risk for severe respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 25:410–414. doi: 10.1097/01.inf.0000214998.16248.b7. [DOI] [PubMed] [Google Scholar]
- 33.Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain PO, Schandene L, Casartelli N, Rameix-Welti MA, Herve PL, Deriaud E, Beitz B, Ripaux-Lefevre M, Miatello J, Lemercier B, Lorin V, Descamps D, Fix J, Eleouet JF, Riffault S, Schwartz O, Porcheray F, Mascart F, Mouquet H, Zhang X, Tissieres P, Lo-Man R. 2017. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 46:301–314. doi: 10.1016/j.immuni.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor G. 2017. Animal models of respiratory syncytial virus infection. Vaccine 35:469–480. doi: 10.1016/j.vaccine.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi XY, Jiang SJ, Wang J, Wang JP. 2011. Effect of glucocorticoid in mice of asthma induced by ovalbumin sensitisation and RSV infection. Asian Pac J Allergy Immunol 29:176–180. [PubMed] [Google Scholar]
- 36.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. 2009. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis 200:439–447. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 37.Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2015. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 483:117–125. doi: 10.1016/j.virol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, Tripp RA, Walsh EE, Keyt BA, Kauvar LM. 2009. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 183:6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 39.Boyoglu-Barnum S, Gaston KA, Todd SO, Boyoglu C, Chirkova T, Barnum TR, Jorquera P, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2013. A respiratory syncytial virus (RSV) anti-G protein F(ab′)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J Virol 87:10955–10967. doi: 10.1128/JVI.01164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. 2009. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a Toll-like receptor pathway. Viral Immunol 22:147–161. doi: 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- 41.Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. 2013. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol 87:13466–13479. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villenave R, Broadbent L, Douglas I, Lyons JD, Coyle PV, Teng MN, Tripp RA, Heaney LG, Shields MD, Power UF. 2015. Induction and antagonism of antiviral responses in respiratory syncytial virus-infected pediatric airway epithelium. J Virol 89:12309–12318. doi: 10.1128/JVI.02119-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giersing BK, Karron RA, Vekemans J, Kaslow DC, Moorthy VS. 14 March 2017. Meeting report: WHO consultation on respiratory syncytial virus (RSV) vaccine development, Geneva, 25-26 April 2016. Vaccine doi: 10.1016/j.vaccine.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 44.Hendricks DA, McIntosh K, Patterson JL. 1988. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol 62:2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bukreyev A, Yang L, Collins PL. 2012. The secreted G protein of human respiratory syncytial virus antagonizes antibody-mediated restriction of replication involving macrophages and complement. J Virol 86:10880–10884. doi: 10.1128/JVI.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stobart CC, Rostad CA, Ke Z, Dillard RS, Hampton CM, Strauss JD, Yi H, Hotard AL, Meng J, Pickles RJ, Sakamoto K, Lee S, Currier MG, Moin SM, Graham BS, Boukhvalova MS, Gilbert BE, Blanco JC, Piedra PA, Wright ER, Moore ML. 2016. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun 7:13916. doi: 10.1038/ncomms13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabrizi M, Bornstein GG, Suria H. 2010. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J 12:33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng J, Hotard AL, Currier MG, Lee S, Stobart CC, Moore ML. 2015. Respiratory syncytial virus attachment glycoprotein contribution to infection depends on the specific fusion protein. J Virol 90:245–253. doi: 10.1128/JVI.02140-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J, Takeda K, Wang M, Zeng W, Jia Y, Shiraishi Y, Okamoto M, Dakhama A, Gelfand EW. 2014. Effects of anti-G and anti-F antibodies on airway function after respiratory syncytial virus infection. Am J Respir Cell Mol Biol 51:143–154. doi: 10.1165/rcmb.2013-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelfand EW. 2012. Development of asthma is determined by the age-dependent host response to respiratory virus infection: therapeutic implications. Curr Opin Immunol 24:713–719. doi: 10.1016/j.coi.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cormier SA, Shrestha B, Saravia J, Lee GI, Shen L, DeVincenzo JP, Kim YI, You D. 2014. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol 88:9350–9360. doi: 10.1128/JVI.00818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eboigbodin KE, Moilanen K, Elf S, Hoser M. 2017. Rapid and sensitive real-time assay for the detection of respiratory syncytial virus using RT-SIBA. BMC Infect Dis 17:134. doi: 10.1186/s12879-017-2227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]