Abstract
Cancer immunotherapy has emerged as treatment of multiple advanced cancer types. Immune checkpoint inhibitors, namely anticytotoxic T-lymphocyte antigen-4 (CTLA-4), antiprogrammed cell death-1 (PD-1), and antiprogrammed cell death-1 ligand 1 (PD-L1) antibodies, have been used for treatment of various cancers. Classified as immune-related adverse events, several endocrinopathies, including hypophysitis, are associated with these agents. Although anti-CTLA-4–induced hypophysitis has been frequently observed, hypophysitis upon use of anti-PD-1 and anti-PD-L1 antibodies is rare. Case 1 is a 65-year-old man presented with a stage IV non-small cell lung cancer (NSCLC) treated with atezolizumab (an anti-PD-L1 antibody) following several inefficacious chemotherapies. After 56 weeks of the treatment, he complained of general malaise and appetite loss, and was diagnosed with adrenal insufficiency. Endocrinological examination revealed isolated adrenocorticotropic hormone (ACTH) deficiency; pituitary magnetic resonance imaging (MRI) showed anterior pituitary atrophy. Hydrocortisone replacement therapy rapidly improved his symptoms and enabled him to continue atezolizumab therapy. Case 2 is a 70-year-old man with a stage IV NSCLC treated with atezolizumab. After 52 weeks of treatment, he was diagnosed with isolated ACTH deficiency. Pituitary MRI revealed no obvious abnormalities in the anterior pituitary. Hydrocortisone replacement therapy was also efficacious. We report two cases of atezolizumab-induced hypophysitis. Both showed isolated ACTH deficiency, suggesting similar clinical characteristics of hypophysitis associated with the use of anti-PD-1 antibodies. These results suggest a caution for the late-onset central adrenal insufficiency associated with hypophysitis in patients treated with anti-PD-L1 antibodies.
Keywords: anti-PD-L1 antibody, atezolizumab, hypophysitis, irAE, isolated ACTH deficiency
We demonstrate two cases of anti–PD-L1 antibody (atezolizumab)-induced hypophysitis characterized by a late-onset isolated ACTH deficiency.
Discovery of immune checkpoint inhibitors (ICIs) represent an important milestone in the modern era of antineoplastic therapy and have been shown to be effective for multiple types of advanced cancer, including malignant melanoma, non-small cell lung cancer (NSCLC), and urothelial cancer. However, these agents are associated with substantial potential toxicities, termed immune-related adverse events (irAEs). In particular, several endocrinopathies, including hypophysitis, thyroid dysfunction, hyperglycemia, and primary adrenal insufficiency, are associated with the use of these agents.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) expressed on T cells, suppresses the function of antigen-presenting cells, and its inhibition by anti-CTLA-4 antibody leads to activation of antigen-presenting cells and inhibition of regulatory T cells [1]. Interestingly, CTLA-4 is also expressed in the pituitary gland, possibly being directly involved in the development of hypophysitis [2]. On the other hand, programmed cell death-1 (PD-1) is expressed on effector cytotoxic T cells (CTLs) where it binds to the programmed cell death-1 ligand 1 (PD-L1) expressed by tumor cells. Generally, tumor cells are able to inactivate and escape from the attack of CTLs by expressing PD-L1.
Hypophysitis induced by ipilimumab, an anti-CTLA-4 antibody, was first reported in 2003 [3], and the number of cases has been markedly increasing. Recent studies demonstrated that approximately 10% to 15% of patients treated with ipilimumab developed hypophysitis; the median onset time after treatment was 9 weeks (range, 5 to 36 weeks) [4]. Along with impairment in the secretion of adrenocorticotropic hormone (ACTH), secretions of thyroid-stimulating hormone (TSH) and luteinizing hormone/follicle-stimulating hormone (LH/FSH) are frequently impaired in the hypophysitis [5].
Anti-PD-1 antibodies, such as nivolumab and pembrolizumab, have induced hypophysitis relatively less frequently (<1%) [5, 6]. Thus far, two cases of nivolumab-induced hypophysitis in patients with melanoma have been reported; both patients developed isolated ACTH deficiency after 39 weeks of the initiation of treatment [7].
Treatment with the anti-PD-L1 antibody, atezolizumab, has been reported to cause type 1 diabetes [8] and is suspected of causing adrenal insufficiency in only one patient with HIV infection after 36 weeks of the initiation of treatment [9]. The patient was asymptomatic and showed decreased serum cortisol level with normal pituitary function; the adrenal insufficiency resolved without any intervention. Therefore, the involvement of hypophysitis remains unclear in this case. Here, we report two cases of atezolizumab-induced hypophysitis.
1. Case Reports
A. Case 1
A patient (aged 61 years) was diagnosed with NSCLC, for which he received chemotherapy. After a year, metastatic lesions in the brain and pancreas were detected, and Cyber Knife radiosurgery was performed. However, the hypothalamus and pituitary were not exposed to radiation. Second-line chemotherapy was also provided but was in vain. Therefore, intravenous treatment with atezolizumab (1200 mg), every 3 weeks, was started. Since then, the metastatic lesions have been stable, indicating that atezolizumab was efficacious. After 19 doses of atezolizumab (56 weeks from the initiation of therapy), the patient (now aged 65 years) complained of general malaise, appetite loss, and diarrhea. The laboratory data showed more eosinophils (14.0%), and endocrinological examinations revealed that morning serum ACTH and cortisol levels were 3.5 pg/mL and 0.2 µg/dL, respectively; he was admitted to our hospital. Provocative test for the anterior pituitary function demonstrated a normal response in growth hormone, prolactin, and TSH, and the basal LH/FSH and testosterone levels were preserved (Table 1). In contrast, the response of ACTH and cortisol was blunted to the insulin tolerance test (Table 1). Magnetic resonance imaging of the pituitary gland showed anterior pituitary atrophy (Fig. 1). Accordingly, we identified the cause of isolated ACTH deficiency to be atezolizumab-induced hypophysitis. Treatment with hydrocortisone replacement (15 mg/d) was initiated, after which rapid improvement in the general condition was observed and he was able to continue atezolizumab treatment. Twenty-four months after the diagnosis of hypophysitis, ACTH deficiency remains and the replacement therapy has been continued.
Table 1.
Endocrinological Data
| Case 1 | Case 2 | Normal Range | ||
|---|---|---|---|---|
| GH | (ng/mL) | 0.10 | 0.19 | |
| Peak GH | (ng/mL) | 7.8 | 16.1 | |
| ACTH | (pg/mL) | 4.3 | 13.7 | 7.7–63.1 |
| Peak ACTH | (pg/mL) | 5.3 | 22.8 | |
| Cortisol | (μg/dL) | 0.3 | 4.9 | |
| Peak cortisol | (μg/dL) | 0.3 | 5.7 | |
| TSH | (μIU/mL) | 1.9 | 1.6 | 0.4–4.9 |
| Peak TSH | (μIU/mL) | 4.6 | 6.6 | |
| PRL | (ng/mL) | 7.4 | 11.2 | 3.6–12.8 |
| Peak PRL | (ng/mL) | 70.9 | 150.8 | |
| LH | (μIU/mL) | 16.6 | 27.2 | 0.8–5.7 |
| FSH | (μIU/mL) | 6.9 | 53.3 | 0.7–1.5 |
| IGF-1 | (ng/mL) | 104.0 | 66.0 | |
| SD score | −0.9 | −1.9 | ||
| f-T4 | (ng/dL) | 1.0 | 1.2 | 0.7–1.5 |
| Testosterone | (ng/mL) | 5.9 | 5.4 | 2.9–9.5 |
| DHEA-S | (μg/dL) | NA | 166.0 | 240–2440 (age 61–70 y, male) |
| TPOAb | (IU/mL) | Negative | Negative | |
| TgAb | (ng/mL) | Negative | Negative | |
| TRAb | (IU/L) | Negative | Negative | |
| GADAb | (U/mL) | Negative | Negative | |
| IA-2Ab | (U/mL) | NA | Negative | |
| PAb | NA | Negative |
Provocative test for the evaluation of the pituitary function was performed by using insulin (0.025 unit/kg), TRH (200 μg). The hydrocortisone replacement therapy was performed before the provocative test and was discontinued 1 day before the test.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate, GADAb, antiglutamic acid decarboxylase antibody; GH, growth hormone; IA2Ab, anti-insulinoma-associated protein-2 antibody; IGF-I, insulin-like growth factor-I; NA, not applicable; PAb, antipituitary cell antibody; PRL, prolactin; TgAb, antithyroglobulin antibody; TPOAb, antithyroid peroxidase antibody; TRAb, TSH receptor antibody.
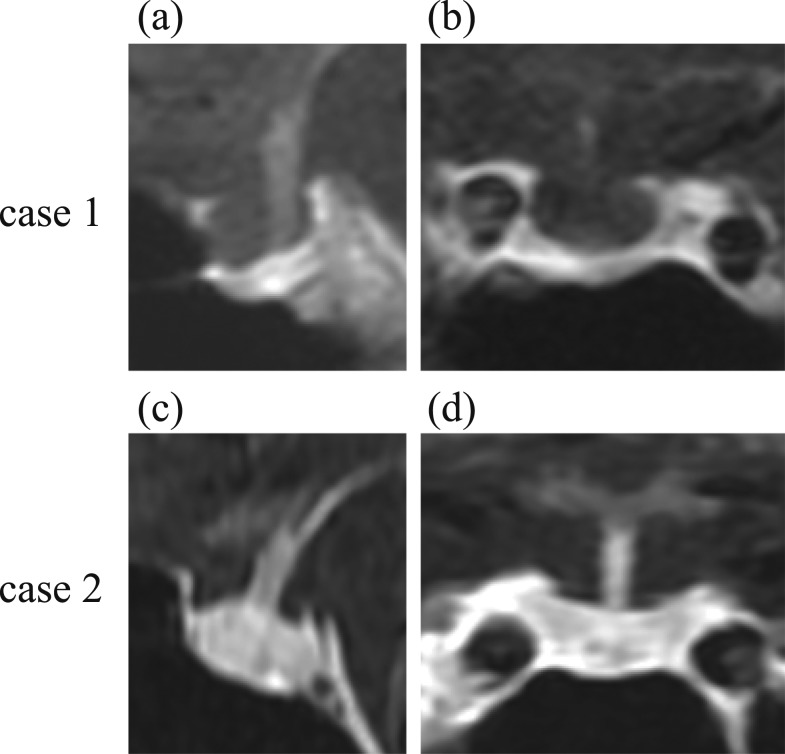
Figure 1.
Pituitary magnetic resonance imaging with gadolinium enhancement. Case 1 showed atrophy in the anterior pituitary; case 2 showed no obvious abnormalities in the anterior pituitary grand.
B. Case 2
A 68-year-old man was diagnosed with NSCLC with multiple metastases, including bilateral lung, multiple lymph nodes, left adrenal gland, and vertebral metastasis. After being administered atezolizumab (1200 mg) every 3 weeks, the patient’s condition became stable. After 18 doses (52 weeks from initiation of therapy), the patient (now aged 70 years) complained of appetite loss and general malaise. The number of eosinophils (7.5%) was increased, and morning serum ACTH and cortisol levels were 13.7 pg/mL and 4.9 µg/dL, respectively (Table 1). Upon provocative test for pituitary function, a blunted response of ACTH and cortisol to the insulin tolerance test was observed. However, responses of other anterior pituitary hormones were not impaired (Table 1). Pituitary magnetic resonance imaging revealed no obvious abnormalities in the anterior pituitary (Fig. 1). His symptoms rapidly resolved after the hydrocortisone replacement therapy (15 mg/d). The case was identified as that of ACTH deficiency caused by atezolizumab-induced hypophysitis. After 13 months of the hypophysitis diagnosis, ACTH deficiency remains and the replacement therapy has been continued.
2. Discussion
Although hypophysitis is generally rare, increased use of ICI for treating advanced cancers has led to increased reports of ICI-induced hypophysitis. ICI evoke immune responses not only toward neoplastic cells, but also toward the body’s own tissues, including endocrine organs. Therefore, irAEs are commonly observed in patients treated with ICI, with hypophysitis being common in these conditions. Among ICI, anti-CTLA-4 antibodies frequently (up to 17%) induce hypophysitis, whereas it is relatively less frequent (<1%) with use of anti-PD-1 antibodies. Further, no clear case of hypophysitis induced by anti-PD-L1 antibodies had been reported thus far. In this paper, we have reported two cases of anti-PD-L1 antibody (atezolizumab)-induced hypophysitis.
Although underlying mechanisms for ICI-induced hypophysitis remain unclear, enhanced immune responses caused by ICI have been suggested to play a pivotal role. In a case of autopsy-proven hypophysitis resulting from tremelimumab (anti-CTLA-4 antibody), it was speculated that the antibody binds to the CTLA-4 protein expressed on anterior pituitary cells and initiates a series of cytopathic immune reactions, mainly through CD4+ T cells and CD20+ B cells that infiltrate the pituitary grand [5]. In an experimental model in which the anti-CTLA-4 antibody was administered to mice, a focal infiltration of lymphocytes and macrophages was observed in the pituitary gland along with complement activation and associated cytotoxic reactions against the anterior pituitary cells [2].
We have shown a late onset of anti-PD-L1 antibody-induced hypophysitis compared with that induced by an anti-CTLA-4 antibody. It is speculated that expression of CTLA-4 in the pituitary may induce a direct rapid and severe response. It seems the clinical characteristics of hypophysitis induced by anti-PD-1 and anti-PD-L1 antibodies are similar based on the limited number of the reported cases including our cases. Interestingly, both cases demonstrated an isolated ACTH deficiency that may be compatible with the fact that corticotroph is mostly impaired in general hypophysitis [10]. In contrast, in anti-CTLA-4 antibody-induced hypophysitis, it has been reported that not only ACTH but also TSH, LH, and FSH secretion is impaired. It is interesting to investigate the expression of PD-L1 and PD-L2 in the pituitary cells, particularly in corticotroph, to further understand the pathophysiology of anti-PD-L1 antibody-induced hypophysitis.
In conclusion, we have demonstrated two cases of atezolizumab-induced hypophysitis. These data draw the attention of a late-onset adrenal insufficiency caused by hypophysitis in patients treated with anti-PD-L1 antibody.
Acknowledgments
Financial Support: This work was partially supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education Science, Sports, and Culture 23591354 (to Y.I.), and 16H05332, 17K19684, and 26670459 (to. Y.T.); the Japan Agency for Medical Research and Development (to J.P.); Japan Agency for Medical Research and Development 17bm0804012h0001; Uehara Memorial Foundation (to Y.T.); and the Naito Foundation (to Y.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- CTL
- cytotoxic T cell
- CTLA
- cytotoxic T-lymphocyte antigen
- FSH
- follicle-stimulating hormone
- ICI
- immune checkpoint inhibitor
- irAE
- immune-related adverse event
- LH
- luteinizing hormone
- NSCLC
- non-small cell lung cancer
- PD-1
- antiprogrammed cell death-1
- PD-L1
- programmed cell death-1 ligand 1
- TSH
- thyroid-stimulating hormone.
References and Notes
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 3.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21(4):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitajima K, Ashida K, Wada N, Suetsugu R, Takeichi Y, Sakamoto S, Uchi H, Matsushima T, Shiratsuchi M, Ohnaka K, Furue M, Nomura M. Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab. Jpn J Clin Oncol. 2017;47(5):463–466. [DOI] [PubMed] [Google Scholar]
- 8.Hickmott L, De La Peña H, Turner H, Ahmed F, Protheroe A, Grossman A, Gupta A. Anti-PD-L1 atezolizumab-induced autoimmune diabetes: a case report and review of the literature. Target Oncol. 2017;12(2):235–241. [DOI] [PubMed] [Google Scholar]
- 9.Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, Eron JJ; AIDS Clinical Trials 5326 Study Team . Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis. 2017;215(11):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26(5):599–614. [DOI] [PubMed] [Google Scholar]