Abstract
Objective: To report cosmetic outcomes and patient satisfaction with ingenol disoxate (LEO 43204) used in a once-daily, three-day field treatment regimen in patients with actinic keratosis. Design: This was a phase II, multicenter, open-label trial (ClinicalTrials.gov: NCT02305888) involving 20 trial sites in the United States. Participants: Patients with between five and 20 clinically typical actinic keratoses lesions on the full face/250cm2 on the chest, 25cm2 to 250cm2 on the scalp, or 250cm2 on the trunk/extremities were included. Measurements: The assessment methods in this study included the examination of global photo-damage at Week 8; a cosmetic outcome questionnaire to evaluate the overall appearance and feel of the skin following treatment at Week 8; and a treatment satisfaction questionnaire for medication (TSQM) to evaluate patient satisfaction with treatment at Week 8. Results: Treatment adherence was high, with 97 percent of patients overall applying the full three-day regimen. Global photo-damage improvement was seen in 66, 69, and 72 percent of patients in the face/chest, scalp, and trunk/extremities groups, respectively. Improved overall appearance of the treatment area was reported by 95, 97, and 80 percent of patients in the face/chest, scalp, and trunk/extremities groups, respectively. In addition, overall feel of the treatment area was reported as improved by 92, 95, and 70 percent of patients in the face/chest, scalp, and trunk/extremities groups, respectively. Overall, the mean scores for all four treatment satisfaction questionnaires for medication domains were high in each treatment group, ranging from 66.7/100 to 91.3/100. In particular, mean scores for global satisfaction were 73.9/100, 79.7/100, 66.7/100 for the face/chest, scalp, and trunk/extremities groups, respectively. Conclusion: Actinic keratosis field treatment with ingenol disoxate provided favorable cosmetic benefits and high treatment satisfaction.
Keywords: Actinic keratosis, ingenol disoxate, cosmetic outcomes, patient satisfaction, field treatment
Actinic keratosis (AK) results from chronic sun exposure and occurs as both subclinically and clinically visible dysplastic lesions in sun-damaged areas of field cancerization.1 AK lesions vary widely from flat and scaly plaques to thick hypertrophic lesions.2 More than 80 percent of AK lesions occur on highly visible areas such as the head, neck, dorsal surface of the hands, and forearms,2 which could have a detrimental impact on skin appearance on top of pre-existing, sun-induced cosmetic impairments. Patients who live with AK often express frustration and embarrassment, as AK lesions can be unsightly, particularly when they are located on the face.3,4 Several studies have demonstrated that AK is associated with a decreased quality of life (QoL);4–6 prolonged treatment durations7 and patient concerns over scarring,8 are contributing factors to this decrease in QoL and are particularly relevant when AKs are on the face. The combined impact of living with AK, the negative effect of AK on cosmetic appearance, and the common interpretation that AK lesions are precursors to skin cancer can have detrimental psychosocial impacts on patients.3–5 The majority of treatments for AK are associated with some level of discomfort, restrictions (e.g., the use of protective clothing and avoidance of sun exposure), and alterations of appearance.9 Between 80 and 90 percent of patients who use topical pharmacotherapy experience local skin responses (LSRs) of erythema, burning, and ulceration.10 In this setting, an understanding of the cosmetic outcomes and patient satisfaction with AK treatments, as well as the impact AK therapy can have on QoL, might help to improve AK treatment efficacy and safety.11,12
Ingenol disoxate (LEO 43204) is an ingenol derivative developed for the field treatment of AK and is often selected for its improved chemical stability over ingenol mebutate, direct cellular cytotoxicity, and ability to induce proinflammatory mediators.13 In Phase II trials, ingenol disoxate has been shown to be effective and well-tolerated as a field treatment for AK when applied once daily for two consecutive days on areas of skin on the face, scalp, and/or chest (measuring between 25–250cm2 depending on anatomical location) and was associated with a significantly higher treatment satisfaction when compared with vehicle gel (p<0.001).14,15 The short-term treatment regimen with ingenol disoxate might positively impact treatment adherence and be perceived more favorably by both patients and physicians,7,8 in contrast with other treatments for AK that are typically used over a period of weeks.
Pre-clinical evaluations of ingenol disoxate suggest a similar dual mode of action to ingenol mebutate,13 which could translate into similar cosmetic benefits in patients with AK; high satisfaction with cosmetic outcome, compared with placebo, use has been reported following field treatment of non-facial AK with ingenol mebutate 0.025% and 0.05% gel for two or three consecutive days.16
Here, we present cosmetic and patient satisfaction outcomes from a Phase II, open-label trial assessing the efficacy and safety of ingenol disoxate applied once daily for three consecutive days on the full face/chest, on the scalp, or on the trunk/extremities. Efficacy and safety results were presented in a separate article.17
METHODS
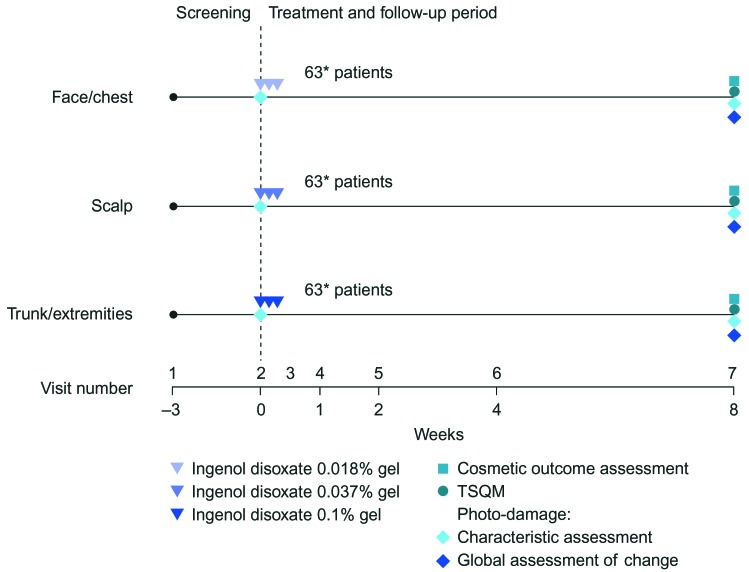
Trial design. This Phase II, multicenter, open-label, eight-week trial evaluated ingenol disoxate (LEO 43204) applied once daily for three consecutive day, on either the full face or approximately 250cm2 of the chest, 25 to 250cm2 of the exposed scalp, or 250cm2 of the trunk or extremities (NCT02305888; Figure 1). Cosmetic and patient satisfaction outcomes were evaluated as additional endpoints and are reported here; the primary and secondary endpoints (safety and efficacy, respectively) are reported in a separate article.17 The protocol was approved by the appropriate independent ethics committee and institutional review boards, and was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation and Good Clinical Practice, and all applicable regulatory requirements. Informed, written consent was obtained from all patients.
FIGURE 1.
Trial design. TSQM: Treatment Satisfaction Questionnaire for Medication
Inclusion and exclusion criteria. Patients were included if they were aged 18 years and older with 5 to 20 clinically typical, visible, discrete AKs within a selected treatment area of sun-damaged skin either on the full face/chest, scalp, or trunk/extremities. In addition, patients could have visible and discrete hyperkeratotic or hypertrophic lesions in the treatment area. Patients were excluded if the selected treatment area was within 5cm of an incompletely healed wound or a suspected basal or squamous cell carcinoma. Other exclusion criteria included prior ingenol mebutate treatment in the selected area, the presence of atypical nonresponsive lesions (unresponsive to cryotherapy on two occasions), or a history of skin conditions that interfere with the evaluation of AKs (such as eczema, unstable psoriasis, or xeroderma pigmentosum).
Treatment. Patients were assigned to receive one of three concentrations of ingenol disoxate gel dependent upon the region to be treated; the face/chest group received 0.018% concentration, the scalp group received 0.037%, or trunk/extremities group received 0.1%. The gel was applied by the patients for three consecutive days. On Day 1, the gel was applied under the supervision of trained research staff, while on Days 2 and 3, the patients self-applied treatment at home.
Endpoints and assessments. At baseline and Week 8, the investigator performed a visual and tactile clinical evaluation of the extent of photo-damage in the treatment area. The following characteristics of photodamage were evaluated using a 5-point scale of 0 (none) to 4 (extreme): fine wrinkling, coarse wrinkling, mottled pigmentation, roughness, sallowness, skin laxity, and telangiectasia.
The investigator also performed an integrated clinical assessment of the change in photodamage from baseline (i.e., global photo-damage outcome) at Week 8 using a 7-point symmetrical scale from marked worsening (-3) to marked improvement (+3). This assessment was not a summary of the scores from the visual and tactile clinical evaluation of photo damage characteristics described above, although evaluation of these characteristics did serve as a guide for investigators.
The Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4 is comprises 14 questions that evaluate patient satisfaction with treatment across four domains: effectiveness, side effects, convenience, and global satisfaction.18 Scores were transformed for each domain ranging from 0 to 100, with higher scores indicating greater satisfaction. Patients completed the TSQM at Week 8; patients who withdrew or completed the trial prior to Week 8 completed the TSQM at the time of their exit visit.
A cosmetic outcome questionnaire was used to evaluate the change in the overall appearance and overall feel of the skin after treatment. The questionnaire used a 5-point scale for self-reported scoring: 0=much worsened, 1=somewhat worsened, 2=no change, 3=somewhat improved, and 4=much improved. Patients completed the cosmetic outcome questionnaire at Week 8.
Statistical methods. Initially, 18 patients were recruited for each treatment group to determine the safety and tolerability of the treatments up to Day 8 based on dose-limiting events. No formal sample size calculation was performed; the sample size was chosen to obtain an adequate precision for the rate of dose-limiting events as reviewed by the Early Data Review Committee. The sample size for the latter part of the trial (62 patients, including the initial 18) was chosen to obtain the same precision as that seen in other Phase II trials evaluating ingenol disoxate with respect to efficacy endpoints.14,15 The full analysis set (FAS) comprised all patients who received treatment, excluding one patient with AKs on the chest who was assigned to the wrong treatment group and instead received ingenol disoxate 0.1% gel for the treatment of AKs on the trunk/extremities. Investigator and patient-reported outcomes were summarized using descriptive statistics based upon the observed cases in the FAS. For the TSQM questionnaire, scores were summarized at Week 8 for each domain (effectiveness, side effects, convenience, and global satisfaction).
RESULTS
Trial population. A total of 253 patients from 20 trial sites in the United States were enrolled in the trial between March 2015 and May 2015. Of these, 64 patients failed screening and 189 patients were assigned to the treatment groups. In total, 188 patients were included in the FAS, as one patient was excluded due to incorrect dose assignment (face/chest, n=63; scalp, n=63; trunk/extremities, n=62). All but two patients completed the trial; one patient in the scalp group withdrew due to an adverse event (AE) and one patient in the trunk/extremities group was lost to follow-up; patient disposition and baseline characteristics are presented in a separate article.17 Self-reported treatment adherence was high, with 97 percent of patients overall applying the full three-day regimen (face/chest, n=95%; scalp, n=98%; trunk/extremities, n=97%).
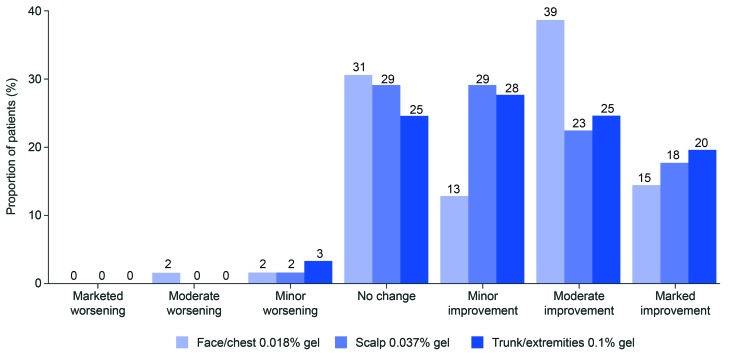
Photo-damage outcome. Global photo-damage improvement was seen in approximately two-thirds of the patients overall (Figure 2); improvements were seen in 66, 69, and 72 percent of patients in the face/chest, scalp, and trunk/extremities groups, respectively. The proportion of patients that had marked or moderate improvements in the face/chest, scalp, and trunk/extremities groups, respectively, were 53, 40, and 44 percent. Moderate worsening was observed in one patient (1.6%) in the face/chest group. Minor worsening was observed in one patient each in the face/chest (1.6%) and scalp (1.6%) groups, and two (3.3%) patients in the trunk/extremities group. No marked worsening was observed in any individuals in any of the three treatment groups.
FIGURE 2.
Global photo-damage outcome assessment at Week 8 by treatment group
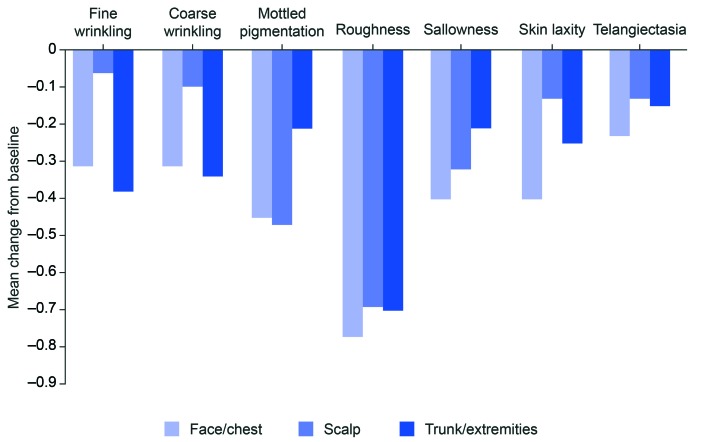
Of the seven individual photo-damage characteristics, the largest mean change from baseline to Week 8 was in roughness (face/chest, -0.77; scalp, -0.69; trunk/extremities, 0.70; Figure 3). Across all treatment groups, the mean change from baseline to Week 8 ranged from -0.40 to 0.06 for fine wrinkling, coarse wrinkling, sallowness, skin laxity, and telangiectasia. For mottled pigmentation, the mean change from baseline to Week 8 was numerically greater in both the face/chest (-0.45) and scalp (-0.47) groups than in the trunk and extremities (0.21) group.
FIGURE 3.
Mean change from baseline to Week 8 in photo-damage assessment scores
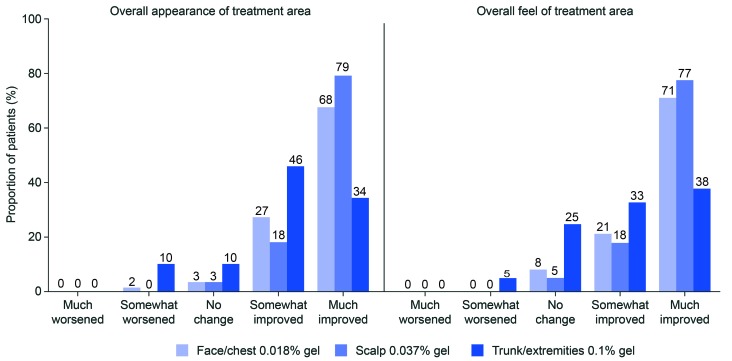
Cosmetic outcome. At Week 8, 95, 97, and 80 percent of patients reported an improved overall appearance of the treatment area in the face/chest, scalp, and trunk/extremities groups, respectively (Figure 4). A much-improved overall appearance of the treatment area was reported by 68, 79, and 34 percent of patients in the face/chest, scalp, and trunk/extremities groups, respectively. The overall feel of the treatment area was also reported as improved by the majority of patients (face/chest, 92%; scalp, 95%; trunk/extremities, 70%; Figure 4). A much-improved feel of the treatment area was reported by 71, 77, and 38 percent in the face/chest, scalp, and trunk/extremities groups, respectively. There were no AEs of scarring. One patient in the face/chest group had an AE of hyperpigmentation that was classed as treatment-related.
FIGURE 4.
Cosmetic outcome at Week 8
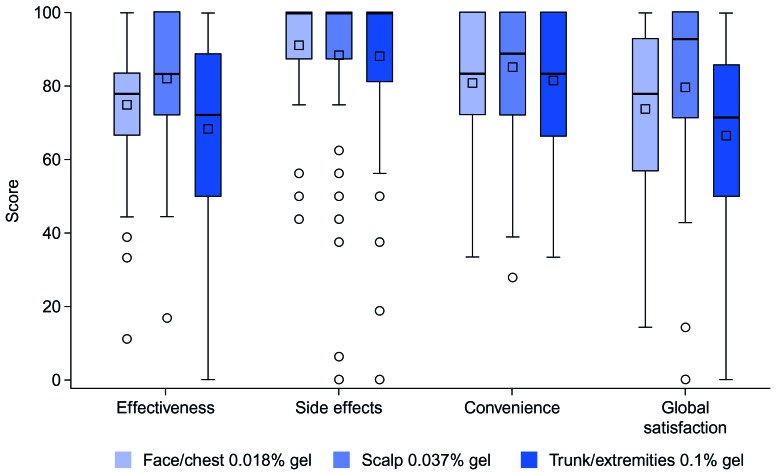
Patient satisfaction (TSQM). Overall, mean scores for all four TSQM domains were high in each treatment group, ranging from 66.7/100 to 91.3/100 (Figure 5). Mean scores for side effects and for convenience were similar across treatment groups, ranging from 88.2/100 to 91.3/100 and from 80.9/100 to 85.3/100, respectively. For effectiveness, higher mean scores were observed in the face/chest (74.9/100) and scalp (82.2/100) groups than in the trunk/extremities (68.4/100) group; similar results were observed for global satisfaction (face/chest, 73.9/100; scalp, 79.7/100; trunk/extremities, 66.7/100).
FIGURE 5.
TSQM at Week 8
DISCUSSION
AK is caused by chronic sun exposure and predominantly occurs in highly visible regions such as the face, thus having a detrimental impact on cosmetic appearance, QoL, and existing sun damage.2–4, 6 This Phase II trial evaluated the cosmetic outcomes and patient satisfaction with ingenol disoxate gel using a once-daily treatment regimen for three consecutive days on the face/chest, scalp, or the trunk/extremities of patients with AK.
Global photo-damage and cosmetic outcome. Improvement in investigator-assessed photo-damage was observed in approximately two-thirds of the patients in this trial, and approximately half were assessed as having marked or moderate improvement. This was supported by the proportion of patients in the face/chest, scalp, and trunk/extremities groups self-reporting a much-improved overall appearance (68%, 79%, and 34%, respectively) and feel (71%, 77%, and 38%, respectively); it should be noted that more patients reported improved cosmetic outcomes in the face/chest and scalp groups than in the trunk/extremities group. It is also worth noting that approximately 15 percent of patients each in the face/chest and scalp groups and 26 percent of patients in the trunk/extremities group had hyperkeratotic/hypertrophic lesions at baseline.17 These lesions are typically difficult to treat and yet patients scored highly in terms of investigator- and patient-reported cosmetic outcomes. Patient satisfaction with cosmesis in this trial might also reflect inclusion criteria that specified that patients have AK lesions within an area of sun-damaged skin; ingenol disoxate might be treating underlying sun damage in addition to cosmetically unpleasant AK lesions. Interestingly, the proportion of patients who reported overall appearance or feel as much-improved exceeded rates of complete clearance of AK lesions (AKCLEAR 100).17 Patients and clinicians should discuss the balance between efficacy and cosmetic outcome when choosing their treatment; for example, cryotherapy is associated with high efficacy but also hypopigmentation and scarring.19
Comparisons of cosmesis between treatments for AK can be difficult, as cosmetic outcomes vary substantially between trials.12 However, a meta-analysis of 84 randomized trials evaluating 24 treatments for AK found that, generally, imiquimod treatments and photodynamic therapy resulted in better cosmetic outcomes than did cryotherapy and 5-fluorouracil (5-FU) treatments.12 However, it is important to note that the tools used for assessment of cosmetic outcomes were not harmonized for comparison in this meta-analysis. High patient satisfaction with cosmetic outcome has been observed following field treatment of nonfacial AK lesions with ingenol mebutate 0.025% and 0.05% gels for two or three consecutive days as compared with treatment with vehicle gel (p<0.0001).16 This is in line with subsequent reports of improvements in skin texture and the absence of scarring, hypopigmentation, or hyperpigmentation with ingenol mebutate treatment.20,21 In addition, ingenol mebutate has beneficial features in terms of clearing mottled pigmentation and reducing tactile roughness, with authors also reporting a noteworthy effect on signs of skin aging.22
A trial evaluating 5-FU 5% applied to the full face for two weeks assessed photoaging characteristics on a scale of 0 to 9, including coarse wrinkling, fine wrinkling, and mottled hyperpigmentation. The overall global photoaging severity score was shown to be significantly improved from baseline at Week 24 (p<0.05); however, treatment was associated with skin irritation and unappealing cosmetic outcomes during treatment.23 No scarring was observed in the present trial, which is commonly associated with aggressive treatments such as strong chemical peels;24 interestingly, cosmetic outcomes reported with light-to-moderate trichloroacetic acid chemical peels are comparable with the cosmetic outcomes in the present trial.25
Patient satisfaction (TSQM). Patient satisfaction with treatment, as measured by the TSQM, was high in all four domains, with higher scores observed for effectiveness and global satisfaction in the face/chest and scalp groups. The high patient satisfaction was supported by the low drop-out rate and high treatment adherence observed in this trial.
Similarly, previous dose-finding trials evaluating once-daily ingenol disoxate for two consecutive days as a field treatment for AK have demonstrated significantly higher global treatment satisfaction and effectiveness scores (as measured by TSQM) in comparison with those seen with the use of vehicle gel (p<0.001).14,15 In addition, the results in the present trial, which evaluated a larger treatment area, are similar to the patient satisfaction data reported in ingenol mebutate trials; the TSQM was used in four Phase III trials evaluating two- and three-day regimens in areas measuring up to 25cm2. Significantly greater global satisfaction and satisfaction with effectiveness were observed for ingenol mebutate as compared with vehicle use (p<0.001).11
High patient satisfaction with ingenol mebutate and ingenol disoxate might reflect the low impact of treatment on QoL. In a prospective pilot study, patients treated for their AK had significant improvements in QoL three weeks after treatment with ingenol mebutate and were not affected by the presence of side effects.26 Conversely, Hanke et al27 reported a correlation between LSRs and health-related QoL (HRQoL) in patients treated with ingenol mebutate after cryotherapy; however, the impact of treatment-related LSRs on HRQoL was reported as small, manageable, and short-lasting; HRQoL ultimately improved beyond baseline two weeks after application of ingenol mebutate. Given the effectiveness and patient satisfaction of a short-term treatment with ingenol mebutate,11,28 similar dosing strategies were employed in this study17 and in other studies examining ingenol disoxate.14,15
With regard to other topical treatments, patients treated with 5-FU have reported high treatment satisfaction; however, treatment satisfaction with 5-FU can be affected by dissatisfaction with side effects. In a trial of 20 patients treated with 5-FU 5% cream for two weeks, 75 percent of patients reported being very satisfied with treatment, despite 63 percent of patients reporting that their treatments were very uncomfortable.23 Side effects associated with 5-FU treatment, such as pain, erythema, and unsightliness, might be unacceptable for many patients, which could lead to premature discontinuation of treatment.29 A comparison between 5-FU 5% cream and ingenol mebutate 0.015% gel as treatments for AK found no difference in most of the side effects not evaluated in the LSR score (such as pruritus, pain, and headache).30 However, side effects encompassed in the LSR score (erythema, flaking/scaling, crusting, and swelling) lasted more than twice as long in patients treated with 5-FU in comparison with in patients treated with ingenol mebutate; this was demonstrated in the significantly greater LSR area under the curve, despite similar peak composite LSR intensities. The mean pain intensity per visit was five times greater in the 5-FU treatment group compared with such in the ingenol mebutate treatment group.30
Treatment regimen. The ingenol disoxate treatment regimen evaluated in this trial was short in comparison with other field therapies for AK, which can range from several weeks to several months.31–33 In studies evaluating two- or three-day ingenol mebutate regimens treating AK over a 25cm2 area, data suggest that short treatment duration contributes to a low impact on QOL26, 27 and a rate of more than 98 percent for adherence to therapy.28 These data are in line with the self-reported treatment adherence observed in this trial. Treating larger areas in this trial with ingenol disoxate compared to previous trials evaluating ingenol mebutate resulted in comparably high patient satisfaction. This may, in part, be attributed to the similar short treatment durations.28 Of note, treatment of a 250cm2 area of skin with ingenol disoxate did not result in any new safety or LSR findings compared to ingenol mebutate treatments done over a 25cm2 area.17,28 Other AK treatments, such as imiquimod, have a dose-dependent increase in risk of systemic side effects, such as flu-like symptoms,34 which could impact patient satisfaction.
CONCLUSION
In conclusion, ingenol disoxate 0.018%, 0.037%, or 0.1% gel applied once-daily for three consecutive days on the full face or up to 250cm2 on the chest, on 25 to 250cm2 of the scalp, or on 250cm2 of the trunk or extremities, respectively, resulted in favorable cosmetic benefits from both physician and a patient perspectives. High adherence and patient treatment satisfaction were observed with these treatments.
ACKNOWLEDGMENTS
The authors would like to acknowledge Louise Prince, PhD, and Patrick Griffin, MSc, of iMed Comms, an Ashfield Company, part of UDG Healthcare plc, for medical writing support that was funded by LEO Pharma in accordance with Good Publication Practices (GPP3). The authors thank the investigators who participated in this study.
REFERENCES
- 1.Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol. 2013;169(3):502–518. doi: 10.1111/bjd.12420. [DOI] [PubMed] [Google Scholar]
- 2.Berman B, Amini S. Pharmacotherapy of actinic keratosis: an update. Expert Opin Pharmacother. 2012;13(13):1847–1871. doi: 10.1517/14656566.2012.716039. [DOI] [PubMed] [Google Scholar]
- 3.Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18(1):53–58. doi: 10.1080/09546630601028737. [DOI] [PubMed] [Google Scholar]
- 4.Tennvall GR, Norlin JM, Malmberg I, et al. Health related quality of life in patients with actinic keratosis—an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes. 2015;13(1):111. doi: 10.1186/s12955-015-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmann S, Vinding GR, Christensen KB, Jemec GB. Assessing the influence of actinic keratosis on patients’quality of life: The AKQOL questionnaire. Br J Dermatol. 2013;168(2):277–283. doi: 10.1111/bjd.12036. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA topical tretinoin chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61(2):207–215. doi: 10.1016/j.jaad.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27(6):538–545. doi: 10.1080/09546634.2016.1178372. [DOI] [PubMed] [Google Scholar]
- 8.Shergill B, Zokaie S, Carr AJ. Non-adherence to topical treatments for actinic keratosis. Patient Prefer Adherence. 2013;8:35–41. doi: 10.2147/PPA.S47126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockfleth E, Ferrandiz C, Grob JJ, et al. Development of a treatment algorithm for actinic keratoses: a European consensus. Eur J Dermatol. 2008;18(6):651–659. doi: 10.1684/ejd.2008.0514. [DOI] [PubMed] [Google Scholar]
- 10.Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 Suppl 1):S28–S38. doi: 10.1016/j.jaad.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72(5):816–821. doi: 10.1016/j.jaad.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415. doi: 10.1002/14651858.CD004415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertelsen M, Stahlhut M, Grue-Sorensen G, et al. Ingenol disoxate: a novel 4-isoxazolecarboxylate ester of ingenol with improved properties for treatment of actinic keratosis and other non-melanoma skin cancers. Dermatol Ther (Heidelb). 2016;6(4):599–626. doi: 10.1007/s13555-016-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss J, Ulrich M, Bukhalo M, et al. A seamless phase I/II dose-finding trial assessing ingenol disoxate (LEO 43204) for field treatment of actinic keratosis on the scalp. Br J Dermatol. 2017;176(6):1456–1464. doi: 10.1111/bjd.15304. [DOI] [PubMed] [Google Scholar]
- 15.Bourcier M, Stein Gold L, Guenther L, et al. A dose finding trial with a novel ingenol derivative (ingenol disoxate; LEO 43204) for field treatment of actinic keratosis on full face or 250 cm2 on the chest. J Dermatolog Treat. 2017;28(7):652–658. doi: 10.1080/09546634.2017.1303568. [DOI] [PubMed] [Google Scholar]
- 16.Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acai Dermatol. 2009;60(6):934–943. doi: 10.1016/j.jaad.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Siegel DM, Tyring S, Nahm WK, et al. Three-day field treatment with ingenol disoxate (LEO 43204 for actinic keratosis: a phase II trial. Manuscript in Preparation. 2017. [PMC free article] [PubMed]
- 18.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanoue J, Do T, Goldenberg G. Therapies for actinic keratosis with a focus on cosmetic outcomes. Cutis. 2015;96(3):165–172. 193. [PubMed] [Google Scholar]
- 20.Erlendsson AM, Karmisholt KE, Haak CS, et al. Topical corticosteroid has no influence on inflammation or efficacy after ingenol mebutate treatment of grade I to III actinic keratoses (AK): A randomized clinical trial. J Am Acad Dermatol. 2016;74(4):709–715. doi: 10.1016/j.jaad.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Cantisani C, Paolino G, Corsetti P, et al. Evaluation of ingenol mebutate efficacy for the treatment of actinic keratosis with antera 3D camera. Eur Rev Med Pharmacol Sci. 2015;19(1):92–97. [PubMed] [Google Scholar]
- 22.Braun SA, Gerber PA. Cosmetic effects of ingenol mebutate gel in the treatment of field-cancerized photodamaged skin. Dermatol Surg. 2015;41(11):1328–1329. doi: 10.1097/DSS.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 23.Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145(6):659–666. doi: 10.1001/archdermatol.2009.97. [DOI] [PubMed] [Google Scholar]
- 24.Fulton JE, Porumb S. Chemical peels: their place within the range of resurfacing techniques. Am J Clin Dermatol. 2004;5(3):179–187. doi: 10.2165/00128071-200405030-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for non-melanoma skin cancer prophylaxis. Arch Dermatol. 2006;142(8):976–982. doi: 10.1001/archderm.142.8.976. [DOI] [PubMed] [Google Scholar]
- 26.Jubert-Esteve E, del Pozo-Hernando LJ, Izquierdo-Herce N, et al. Quality of life and side effects in patients with actinic keratosis treated with ingenol mebutate: a pilot study. Actas Dermo-Sifiliográficas. 2015;106(8):644–650. doi: 10.1016/j.ad.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Hanke WC, Norlin JM, Mark Knudsen K, et al. Quality of life in treatment of AK: treatment burden of ingenol mebutate gel is small and short lasting. J Dermatolog Treat. 2016;27(5):450–455. doi: 10.3109/09546634.2016.1160024. [DOI] [PubMed] [Google Scholar]
- 28.Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 29.Ostertag JU, Quaedvlieg PJ, van der Geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38(8):731–739. doi: 10.1002/lsm.20379. [DOI] [PubMed] [Google Scholar]
- 30.Samorano LP, Torezan LA, Sanches JA. Evaluation of the tolerability and safety of a 0.015% ingenol mebutate gel compared to 5% 5-fluorouracil cream for the treatment of facial actinic keratosis: a prospective randomized trial. J Eur Acad Dermatol Venereol. 2015;29(9):1822–1827. doi: 10.1111/jdv.13063. [DOI] [PubMed] [Google Scholar]
- 31.Valeant Pharmaceutical. Efudex® (fluorouracil) topical solutions and creams. Prescribing information. 2015.
- 32.Graceway Pharmaceuticals. Zyclara® (imiquimod) cream, 3.75%. Prescribing information. 2010.
- 33.Pharmaderm. SolarazeTM (diclofenac) gel, 3%. Prescribing information. 2011.
- 34.Cantisani C, Lazic T, Richetta AG, et al. Imiquimod 5% cream use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6(1):65–69. doi: 10.2174/187221312798889301. [DOI] [PubMed] [Google Scholar]