Abstract
Background
Primary aldosteronism (PA) is the most frequent cause of secondary hypertension. Reported prevalences of PA vary considerably because of a large heterogeneity in study methodology.
Aim
To examine the proportion of patients with PA among patients with newly diagnosed, never treated hypertension.
Design and setting
A cross-sectional study set in primary care.
Method
GPs measured aldosterone and renin in adult patients with newly diagnosed, never treated hypertension. Patients with elevated aldosterone-to-renin ratio and increased plasma aldosterone concentration underwent a saline infusion test to confirm or exclude PA. The source population was meticulously assessed to detect possible selection bias.
Results
Of 3748 patients with newly diagnosed hypertension, 343 patients were screened for PA. In nine out of 74 patients with an elevated aldosterone-to-renin ratio and increased plasma aldosterone concentration the diagnosis of PA was confirmed by a saline infusion test, resulting in a prevalence of 2.6% (95% confidence interval = 1.4 to 4.9). All patients with PA were normokalaemic and 8 out of 9 patients had sustained blood pressure >150/100 mmHg. Screened patients were younger (P<0.001) or showed higher blood pressure (P<0.001) than non-screened patients.
Conclusion
In this study a prevalence of PA of 2.6% in a primary care setting was established, which is lower than estimates reported from other primary care studies so far. This study supports the screening strategy as recommended by the Endocrine Society Clinical Practice Guideline. The low proportion of screened patients (9.2%), of the large cohort of eligible patients, reflects the difficulty of conducting prevalence studies in primary care clinical practice.
Keywords: general practice, hypertension, prevalence, primary aldosteronism
INTRODUCTION
Primary aldosteronism (PA) is the most frequent cause of secondary hypertension. Large variations of its prevalence have been reported, ranging from <1% to 30%.1–5 This variance can be explained by the heterogeneity of studies owing to differences in patient selection, variability in diagnostic procedures, healthcare setting, and region of the world.6 Three aspects determine the clinical relevance of a diagnosis of PA. First, PA carries a high cardiovascular complication rate, independently of the level of blood pressure.7–9 Second, PA requires specific treatment, depending on the underlying subtype: adrenal surgery for an aldosterone-producing adenoma, and a mineralocorticoid receptor antagonist in bilateral adrenal hyperplasia.1 Third, quality of life is adversely affected by PA, and may improve after specific therapy such as an adrenalectomy.10–12 Together with the long delay of 8 years13 in diagnosing PA in hypertensive patients, screening for PA in all patients with newly diagnosed hypertension might be beneficial. However, the Endocrine Society Clinical Practice Guideline does not advocate early screening for PA in patients with new hypertension apart from specific subgroups, such as patients with sustained blood pressure >150/100 mmHg on each of three measurements, and cases of hypertension and spontaneous hypokalaemia.1 The National Institute for Health and Care Excellence guideline advises ‘simply to be aware of signs and symptoms and refer on the basis of a high index of suspicion’, for example, young onset hypertension (aged <40 years).14,15 In the Netherlands, the primary care guideline for hypertension recommends that only patients with hypertension and hypokalaemia and those with therapy-resistant hypertension should be referred on suspicion of secondary hypertension.16 The primary objective of this study was to assess the proportion of patients with PA among patients with newly diagnosed, never treated hypertension presenting at Dutch primary care centres. The secondary objective was to study selection bias in GPs’ referral of patients for screening for PA.17
METHODS
Study setting and design
In this cross-sectional study patients from 55 primary care centres, from the Nijmegen region in the Netherlands, were recruited from 1 August 2013 to 31 December 2015 (further details about recruitment protocol are available from the authors). The screening consisted of two phases. In the first biochemical screening phase, the plasma aldosterone-to-renin ratio (ARR) and plasma aldosterone concentration were determined in patients with newly diagnosed hypertension prior to starting antihypertensive treatment. In the second confirmatory phase, patients with an elevated ARR and elevated plasma aldosterone concentration underwent a saline infusion test (SIT) to verify autonomous aldosterone secretion. The SIT is one of the four confirmation tests that is recommended by the Endocrine Society to confirm or exclude the diagnosis of PA.1 A definite diagnosis of PA was made if sodium loading failed to suppress the plasma aldosterone level. Reporting of this study is in concordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.18,19 This study was approved by the Ethics Committee of the Radboud university medical center and all patients gave informed consent.
How this fits in
Primary aldosteronism (PA) is the most frequent cause of secondary hypertension. Reported prevalences of PA vary considerably because of a large heterogeneity in study methodology. This study reports a prevalence for PA of 2.6% in patients with newly diagnosed hypertension in primary care, which is lower than expected. Scrutiny of the source population of all patients with newly diagnosed, never treated hypertension is essential to detect selection bias.
Participants and recruitment
Eligible patients had newly diagnosed, untreated hypertension and were aged ≥18 years. Hypertension was diagnosed according to the guideline of the European Society of Hypertension.20 In brief, hypertension was diagnosed when: (1) office blood pressure was ≥140/90 mmHg on two or more different encounters within 6 months; (2) home blood pressure measurement (electronic device) was ≥135/85 mmHg; (3) 24-hour ambulatory blood pressure monitoring (ABPM) was ≥130/80 mmHg; or (4) daytime ABPM was ≥135/85 mmHg. Every participating GP was asked to draw a blood sample in eligible patients for measurement of plasma aldosterone and renin. This blood sample was obtained in the morning after the patients had been sitting for 5 minutes. Exclusion criteria were: (prior) use of antihypertensive medication, hypertensive crisis, heart failure class II–IV (according to the New York Heart Association),21 estimated glomerular filtration rate of <45 ml/min/1.73 m2, pregnancy, breast feeding, diabetes mellitus, and presence of severe comorbidity (defined as seriously interfering with diagnostics or possible therapy). Patients who required immediate antihypertensive treatment (according to the GP) received specific medication with minimal effects on renin and aldosterone levels.1
Procedures
From 1 August 2013 to 14 December 2014, plasma aldosterone was measured using the Coat-A-Count aldosterone radioimmunoassay (RIA) from Siemens Medical Solutions Diagnostics. From 15 December 2014 to 31 December 2015, plasma aldosterone was measured by the Active Aldosterone RIA kit from Beckman Coulter. Plasma renin concentration was measured using the DSL-25100 active renin immunoradiometric assay (IRMA) from Diagnostic Systems Laboratories. The cut-off level of the ARR was >40 pmol/mU in combination with a plasma aldosterone of >400 pmol/l. The SIT consisted of intravenous infusion of 2 litres of sodium chloride 0.9% over 4 hours with the patient in the semi-recumbent position. After 4 hours blood was sampled for measurement of aldosterone. Usually, the aldosterone level will decrease after infusion of saline. In case of autonomous aldosterone secretion the negative feedback system is insufficient and aldosterone levels remain too high. A plasma aldosterone concentration of >280 pmol/l was considered as definite PA, while an aldosterone level of <140 pmol/l excluded PA. In case of indeterminate values of 140 to 280 pmol/l the SIT was repeated. If still indeterminate, a diagnosis was reached by consensus after deliberation among clinical experts of the Department of Internal Medicine.
Data collection and processing
Data of all patients with newly diagnosed hypertension were extracted from the Electronic Health Records (EHRs) of the 55 participating centres. The dataset included demographics, clinical characteristics, biochemical test results, prescribed medication, and diagnoses coded according to the International Classification of Primary Care (ICPC).22 Inclusion criteria were applied to select all patients with a new diagnosis of hypertension: ICPC code hypertension (K86 or K87) between 1 August 2013 and 31 December 2015, or, when an ICPC code was not available, elevated blood pressure measurements were included according to the criteria described in the participants and recruitment section (above). Further details of inclusion criteria when an ICPC code was unavailable are available from the authors.
Statistical analysis
Based on a population of nearly 200 000 subjects (from 55 primary care centres), an incidence of hypertension of 0.6% yearly,23,24 and a participation rate of 40%, this study aimed to enrol approximately 1100 patients. Anticipating a prevalence of PA of 5%, at least 931 patients were required to be included to estimate the prevalence of PA with an accuracy of 1.4% and a confidence level of 95%. The statistical package SPSS Statistics version 22.0.0.1 was used to analyse the data. The proportion of patients with PA among patients with newly diagnosed hypertension was calculated by dividing the number of patients with confirmed PA by the number of patients with newly diagnosed hypertension who were screened for PA by using the ARR, plasma aldosterone, and the SIT. The total group of patients with newly diagnosed hypertension was extracted from the EHRs. To study selection bias between the screened and non-screened group, and taking into account the clustering of patients within practices, multilevel analyses25 to calculate P-values were used. To assess if known patient characteristics influenced the referral for biochemical screening of PA, a multivariate multilevel logistic regression analysis with age, blood pressure, and comorbidity was entered into the model. Practice variation for biochemical screening was assessed by the intraclass correlation coefficient (ICC). Independent samples t-tests for all screened patients were used to compare means between the PA and non-PA group, and a bootstrap test was used to corroborate the results.26 Analysis of variance (ANOVA) was used to assess differences in means in case of three groups. Chi-squared tests were used to determine associations between categorical variables. Fisher’s exact test was used in case of small sample size. As renin values showed a skewed distribution median and interquartile ranges (IQR) were calculated, using Mann–Whitney and Kruskal–Wallis tests to compare groups. Patients who did not complete the screening (n=18) were excluded from analyses.
RESULTS
Characteristics of the total study population
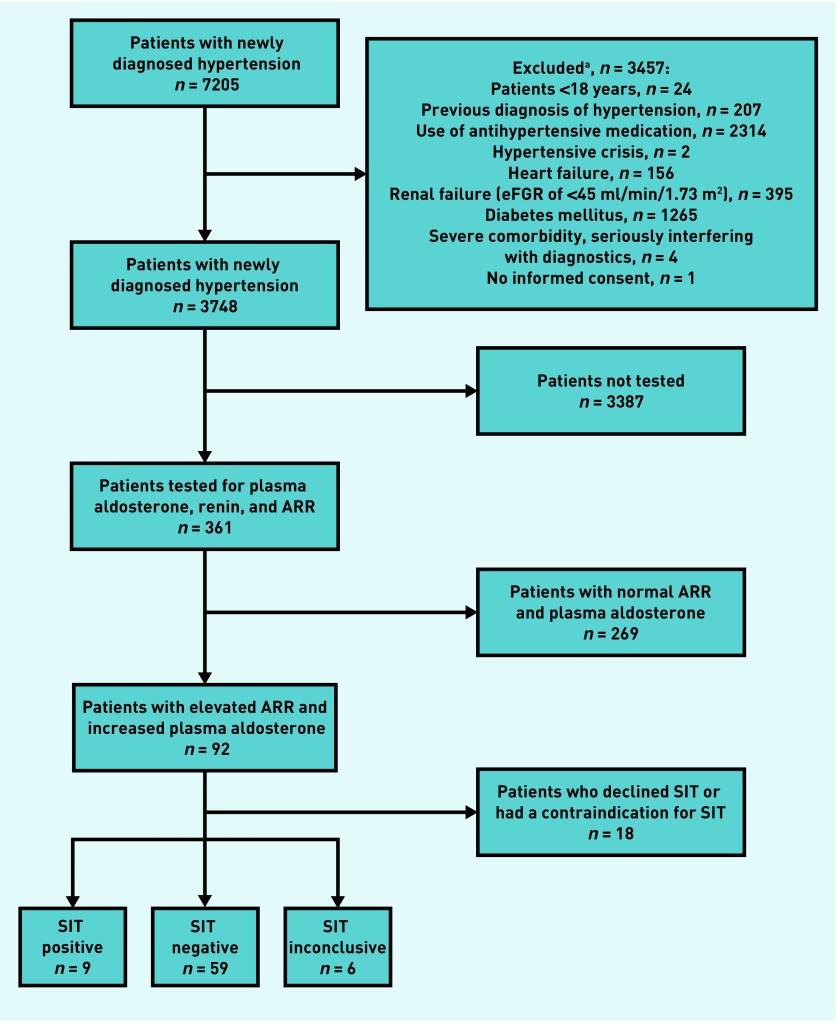
Out of 7205 patients with newly diagnosed hypertension that were identified, 3748 were found to be eligible after application of exclusion criteria (Figure 1). Of these patients, 361 patients (9.6%) were tested by measuring ARR and plasma aldosterone (Figure 1). Eighteen patients did not undergo a SIT for various reasons (further details of reasons are available from the authors). Therefore, 343 completed screening for PA (9.2%). Baseline characteristics between the group that proceeded to a SIT and the group that did not were comparable (baseline characteristics such as participant demographics, blood pressure variables, biochemical parameters, and cardiovascular morbidity are available from the authors).
Figure 1.
Study flowchart. aMultiple patients were excluded by two or more exclusion criteria. ARR = aldosterone-to-renin ratio. eGFR = estimated glomerular filtration rate. SIT = saline infusion test.
Prevalence of primary aldosteronism
Of all 361 biochemically screened patients, 92 (25.5%) showed an elevated ARR in combination with increased plasma aldosterone. Nine of 74 patients who underwent SIT showed insufficient aldosterone suppression, confirming the diagnosis of PA, and 18 patients declined SIT or had contraindication for SIT (Figure 1). Hence, the prevalence of PA in patients with newly diagnosed hypertension was 9 out of 343 or 2.6% (95% CI = 1.4 to 4.9). All nine patients with PA were normokalaemic, and eight of them had sustained blood pressure >150/100 mmHg. As expected, the nine patients with PA had lower values of serum potassium and renin and higher values of plasma aldosterone than patients without PA (Table 1). Six patients had inconclusive test results after the first SIT and refused further diagnostic tests. Based on contextual information discussed at the expert meeting, the diagnosis of PA remained inconclusive. Hypertension in these six patients was treated with a mineralocorticoid receptor antagonist. Of the 74 patients who underwent SIT, 12 patients started immediate antihypertensive treatment after the diagnosis of hypertension (according to the Guideline).1 None of these patients had a positive confirmation test. When comparing the SIT positive group to the SIT negative group, serum potassium and renin levels were significantly lower in the SIT positive group than in the SIT negative group (Table 2).
Table 1.
Baseline characteristics of all screened patients with newly diagnosed hypertension
| Variable | All patients with available data, n/n PA negative/PA positive | PA negative | PA positive | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (male), n (%) | 334/9 | 173 (51.8) | 4 (44.4) | 0.744 |
| Age, years, mean (SD) | 334/9 | 53.4 (11.2) | 54.3 (9.2) | 0.763 |
| BMI, kg/m2, n (%) | 213/9 | 0.498 | ||
| ≤25 | 47 (22.1) | 1 (11.1) | ||
| >25–≤30 | 96 (45.1) | 6 (66.7) | ||
| >30 | 70 (32.9) | 2 (22.2) | ||
| Smoking status, n (%) | 163/9 | 0.404 | ||
| Current | 36 (22.1) | 2 (22.2) | ||
| Former | 55 (33.7) | 1 (11.1) | ||
| Never | 72 (44.2) | 6 (66.7) | ||
|
| ||||
| Blood pressure, mean (SD) | ||||
| Systolic BP, mmHg | 332/9 | 163.8 (13.4) | 158.6 (10.2) | 0.174 |
| Diastolic BP, mmHg | 329/9 | 96.3 (9.9) | 95.3 (9.1) | 0.747 |
| ABPM, mmHg | 47/2 | 154.2 (15.7) | 166.5 (9.2) | 0.282 |
| ABPMday, mmHg | 42/1 | 159.3 (15.6) | 161.0 (−) | – |
| Home BP, mmHg | 2/0 | 160.0 (0) | – | – |
| Heart rate, beats/minute | 192/7 | 75.2 (12.6) | 68.6 (12.8) | 0.224 |
|
| ||||
| Biochemical parameters | ||||
| Serum potassium, mmol/l, mean (SD) | 332/9 | 4.43 (0.33) | 4.11 (0.26) | 0.006 |
| Serum sodium, mmol/l, mean (SD) | 332/9 | 141.7 (2.0) | 141.7 (2.6) | 0.951 |
| Serum creatinine, µmol/l, mean (SD) | 332/9 | 78.8 (14.6) | 75.8 (13.1) | 0.510 |
| Plasma aldosterone, pmol/l, mean (SD) | 334/9 | 360.9 (220.5) | 668.4 (118.1) | <0.001 |
| Renin, pmol/l, median (IQR) | 334/9 | 0.78 (0.58 to 1.33) | 0.48 (0.34 to 0.68) | 0.003 |
| ARR, pmol/mU, mean (SD) | 334/9 | 29.8 (22.8) | 105.3 (48.6) | 0.002 |
| Serum glucose, mmol/l, mean (SD) | 301/9 | 5.4 (1.0) | 5.4 (0.5) | 0.750 |
|
| ||||
| Cardiovascular morbidity, n (%) | ||||
| OSAS | 334/9 | 5 (1.5) | 0 | 1.000 |
| Atrial fibrillation | 334/9 | 2 (0.6) | 0 | 1.000 |
| Stroke | 334/9 | 2 (0.6) | 0 | 1.000 |
| Myocardial infarction | 334/9 | 2 (0.6) | 0 | 1.000 |
ABPM = ambulatory blood pressure monitoring. ARR = aldosterone-to-renin ratio. BMI = body mass index. Home BP = home systolic blood pressure. IQR = interquartile range. OSAS = obstructive sleep apnoea syndrome. PA = primary aldosteronism. SD = standard deviation.
Table 2.
Study characteristics of all patients who underwent a saline infusion test
| Variable | Patients with available data, n/n/n SIT+/SIT-/SITi | SIT positive | SIT negative | SIT inconclusive | P-valuea | P-valueb |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Male, n (%) | 9/59/6 | 4 (44.4) | 17 (28.8) | 3 (50.0) | 0.44 | 0.380 |
| Age, years, mean (SD) | 9/59/6 | 54.3 (9.2) | 51.4 (11.2) | 52.8 (4.6) | 0.40 | 0.717 |
| BMI, kg/m2, n (%) | 9/59/6 | 0.65 | 0.165 | |||
| ≤25 | 1 (11.1) | 15 (25.4) | 0 | |||
| >25–≤30 | 6 (66.7) | 27 (45.8) | 6 (100) | |||
| >30 | 2 (22.2) | 17 (28.8) | 0 | |||
| Smoking status, n (%) | 9/58/6 | 0.53 | 0.604 | |||
| Current | 2 (22.2) | 14 (24.1) | 1 (16.7) | |||
| Former | 1 (11.1) | 17 (29.3) | 3 (50.0) | |||
| Never | 6 (66.7) | 27 (46.6) | 2 (33.3) | |||
|
| ||||||
| Blood pressure, mean (SD) | ||||||
| Systolic BP, mmHg | 9/59/6 | 158.6 (10.2) | 163.9 (13.7) | 167.3 (12.5) | 0.20 | 0.417 |
| Diastolic BP, mmHg | 9/59/6 | 95.3 (9.1) | 97.0 (9.8) | 101.5 (10.3) | 0.62 | 0.462 |
| ABPM, mmHg | 2/13/1 | 166.5 (9.2) | 159.8 (18.6) | 171.0 (−) | 0.49 | 0.765 |
| ABPMday, mmHg | 1/12/1 | 161.0 (NA) | 162.8 (19.8) | 175.0 (−) | – | 0.833 |
| Home BP, mmHg | 0/1/0 | – | 160.0 (−) | – | – | – |
| Heart rate, beats/minute | 7/35/5 | 68.6 (12.8) | 76.5 (13.4) | 71.4 (13.6) | 0.17 | 0.313 |
|
| ||||||
| Biochemical parameters | ||||||
| Serum potassium, mmol/l, mean (SD) | 9/58/6 | 4.11 (0.26) | 4.38 (0.39) | 4.3 (0.2) | 0.02 | 0.121 |
| Serum sodium, mmol/l, mean (SD) | 9/58/6 | 141.7 (2.6) | 142.0 (2.1) | 141.3 (2.1) | 0.74 | 0.749 |
| Serum creatinine, µmol/l, mean (SD) | 9/58/6 | 75.8 (13.1) | 79.4 (13.5) | 88.2 (14.3) | 0.45 | 0.216 |
| Aldosterone, pmol/l, mean (SD) | 9/59/6 | 668.4 (118.1) | 592.3 (244.2) | 494.0 (94.5) | 0.15 | 0.345 |
| Renin, pmol/l, median (IQR) | 9/59/6 | 0.48 (0.34 to 0.68) | 0.67 (0.55 to 0.78) | 0.55 (0.38 to 0.62) | 0.04 | 0.023 |
| ARR, pmol/mU, mean (SD) | 9/59/6 | 105.3 (48.6) | 61.1 (19.9) | 75.9 (41.3) | 0.03 | <0.001 |
| Serum glucose, mmol/l, mean (SD) | 9/56/5 | 5.4 (0.5) | 5.1 (0.8) | 5.2 (0.5) | 0.27 | 0.665 |
|
| ||||||
| Cardiovascular morbidity, n (%) | ||||||
| OSAS | 9/59/6 | 0 | 0 | 0 | – | – |
| Atrial fibrillation | 9/59/6 | 0 | 0 | 0 | – | – |
| Stroke | 9/59/6 | 0 | 1 (1.7) | 0 | 1.00 | – |
| Myocardial infarction | 9/59/6 | 0 | 0 | 0 | – | – |
P-value: comparison of SIT positive and SIT negative.
P-value: comparison of SIT positive and SIT negative and SIT inconclusive. ABPM = systolic ambulatory blood pressure monitoring. ABPMday = daytime systolic ambulatory blood pressure monitoring. ARR = aldosterone-to-renin ratio. BMI = body mass index. Home BP = home systolic blood pressure. IQR = interquartile range. OSAS = obstructive sleep apnoea syndrome. SD = standard deviation. SIT = saline infusion test. SITi = saline infusion test with inconclusive result. Systolic BP = office systolic blood pressure.
Screened versus non-screened patients
Screened patients were younger, had higher blood pressure, and had higher serum potassium levels than non-screened patients. More patients in the non-screened group suffered from stroke (Table 3). Multivariate multilevel logistic regression analysis showed an independent effect on biochemical screening of age, systolic blood pressure, and previous stroke (P<0.001, P<0.001, P = 0.016, respectively. Referral for biochemical screening was more likely in younger patients (odds ratio [OR] 0.96, 95% CI = 0.95 to 0.97), and in patients with higher blood pressure (OR 1.06, 95% CI = 1.05 to 1.07). Patients who suffered from stroke had a lower chance to be referred for screening (OR 0.17, 95% CI = 0.04 to 0.72) (further details of multivariate multilevel logistic regression analysis of patient characteristics that may influence referral for screening for PA are available from the authors). Intraclass correlation coefficient was 0.22, which indicates a considerable variation in referral for screening among centres.
Table 3.
Baseline characteristics of all patients with newly diagnosed hypertension
| Variable | Patients with available data, n/n screened/non-screened | Screened | Non-screened | P-valuea |
|---|---|---|---|---|
| Demographics | ||||
| Male, n (%) | 343/3387 | 177 (51.6) | 1586 (46.8) | 0.146 |
| Age, years | 343/3387 | 53.4 (11.1) | 58.5 (13.4) | <0.001 |
| BMI, kg/m2, n (%) | 222/1377 | 0.133 | ||
| ≤25 | 48 (21.6) | 383 (27.8) | ||
| >25–≤30 | 102 (45.9) | 576 (41.8) | ||
| >30 | 72 (32.4) | 418 (30.4) | ||
| Smoking status, n (%) | 172/597 | 0.841 | ||
| Current | 38 (22.1) | 135 (22.6) | ||
| Former | 56 (32.6) | 218 (36.5) | ||
| Never | 78 (45.3) | 244 (40.9) | ||
|
| ||||
| Blood pressure, mean (SD) | ||||
| Systolic BP, mmHg | 341/2880 | 163.6 (13.3) | 156.3 (11.8) | <0.001 |
| Diastolic BP, mmHg | 338/2880 | 96.3 (9.9) | 89.8 (9.5) | <0.001 |
| ABPM, mmHg | 49/229 | 154.7 (15.6) | 147.0 (12.9) | 0.001 |
| ABPMday, mmHg | 43/204 | 159.4 (15.4) | 152.1 (12.8) | 0.003 |
| Home BP, mmHg | 2/12 | 160.0 (0) | 144.0 (5.2) | – |
| Heart rate, beats/minute | 199/1659 | 75.0 (12.6) | 74.5 (12.1) | 0.742 |
|
| ||||
| Biochemical parameters | ||||
| Serum potassium, mmol/l, mean (SD) | 341/1785 | 4.42 (0.34) | 4.37 (0.37) | 0.044 |
| Serum sodium, mmol/l, mean (SD) | 341/1685 | 141.7 (2.0) | 141.9 (2.1) | 0.084 |
| Serum creatinine, µmol/l, mean (SD) | 341/1996 | 78.8 (14.6) | 78.9 (15.2) | 0.871 |
| Aldosterone, pmol/l, mean (SD) | 343/0 | 369.0 (223.8) | – | – |
| Renin, pmol/l, median (IQR) | 343/0 | 0.77 (0.58 to 1.31) | – | – |
| ARR, pmol/mU, mean (SD) | 343/0 | 31.8 (26.6) | – | – |
| Serum glucose, mmol/l, mean (SD) | 310/1786 | 5.43 (1.01) | 5.45 (0.83) | 0.546 |
|
| ||||
| Cardiovascular morbidity, n (%) | ||||
| OSAS | 343/3387 | 5 (1.5) | 41 (1.2) | 0.714 |
| Atrial fibrillation | 343/3387 | 2 (0.6) | 52 (1.5) | 0.166 |
| Stroke | 343/3387 | 2 (0.6) | 156 (4.6) | 0.003 |
| Myocardial infarction | 343/3387 | 2 (0.6) | 32 (0.9) | 0.597 |
P-value calculated by univariate multilevel analyses. ABPM = systolic ambulatory blood pressure monitoring. ABPMday = daytime systolic ambulatory blood pressure monitoring. ARR = aldosterone-to-renin ratio. BMI = body mass index. Home BP = home systolic blood pressure. IQR = interquartile range. OSAS = obstructive sleep apnoea syndrome. PA = primary aldosteronism. SD = standard deviation.
DISCUSSION
Summary
In this primary care study the prevalence for primary aldosteronism in patients with newly diagnosed hypertension is 2.6%. This number is lower than reported prevalences from other primary care studies so far. The low number of screened patients (9.2%) of the large cohort of eligible patients reflects the difficulty of studying prevalence of primary aldosteronism in primary care clinical practice, when the daily routine of GPs collides with a study protocol.
Strengths and limitations
This study set out to screen for PA in patients with newly diagnosed hypertension in a primary care setting. In many countries, the initial diagnosis of hypertension is predominantly made by GPs in primary care centres where there is no referral bias as is the case in prevalence studies from referral centres. A strength of this research was that specific subgroups in which PA may be more or less prevalent were excluded, such as patients with diabetes mellitus27–29 and patients with a hypertensive crisis.30 Another strong aspect of this study was that all patients were not treated with antihypertensive drugs at the time of inclusion or any time before. This minimises any confounding effects of these drugs on plasma aldosterone and renin.1 Finally, a strong feature of the research was that the total source population was examined for incomplete screening and possible selection bias by digital scrutiny of the EHRs.31 Concerning the reliability and validity, the ARR is generally considered the best first-line screening test for hypertensive patients in whom there is clinical suspicion of PA.32,33 Yet, the ARR as an exploratory screening test has its limitations as it is influenced by many factors.34,35 Selection bias was assessed by the use of ICPC codes from EHR data. These ICPC codes depend on the quality of recording. As GPs may not always assign an ICPC code for hypertension,36 the patients with elevated blood pressure measurements without an ICPC code were also included. This improved case finding, but may have been too sensitive as the number of patients with newly diagnosed hypertension (n = 3748) was higher than expected (further details about baseline characteristics of patients with newly diagnosed hypertension according to ICPC code versus patients without an ICPC code are available from the authors).
Comparison with existing literature
In this primary care study the proportion of patients with PA in patients with newly diagnosed hypertension was 2.6%. This is lower than reported in previous primary care studies that performed a confirmation test in at least half of biochemically screened patients, ranging from 3.2% to 11.5%.37–46 The two studies that restricted their study population to patients with newly diagnosed hypertension found a prevalence of 5.5% and 6.0%.40,45 Two other studies included only normokalaemic patients with hypertension and established prevalences of 3.2% and 12.7%.41,43 Several explanations for the low prevalence in this research have to be considered. First, studies vary considerably in their methods and screening cut-offs. In this research a relatively low cut-off value for the ARR of >40 pmol/ mU was deliberately used with the aim to miss as few PA patients as possible.47,48 To prevent too many false-positive test results due to low renin hypertension, the criterion of a minimum plasma aldosterone level of 400 pmol/l was added.49 However, the cut-off for the SIT in this study was quite strict. In 26 patients an elevated ARR was found without an increased plasma aldosterone. These patients did not undergo a SIT and this might have also contributed to the low prevalence seen in this study. In addition, 18 eligible patients did not undergo the SIT and six other patients had inconclusive test results (Figure 1). Under the theoretical assumption that these 24 patients might have PA, the virtual prevalence would be maximally 9.1% (33/361). However, this possibility is unlikely. Screened patients were younger and had higher blood pressures as compared with non-screened patients. This indicates that GPs intuitively followed the screening recommendation of the Endocrine Society Guideline1, which recommends screening of patients with blood pressure >150/100 mmHg. In addition, for unknown reasons, GPs were less likely to perform biochemical screening in patients with newly diagnosed hypertension who suffered from a stroke. Apparently, GPs screened patients with a higher a priori chance of having PA and may have missed patients with a lower chance of having PA. It is therefore conceivable that this selective screening has contributed to an underestimation of the real PA prevalence. In addition, GPs might consider stroke as ‘severe comorbidity’, which was an exclusion criteria for participation in this research. The present study illustrates that, despite a straightforward clinical protocol, an unbiased selection of patients for screening for PA is very hard to achieve in a primary care setting. The protocol in this research, intentionally designed to minimise bias, is apparently not compatible with routine daily practice in primary care.17,50 This is reflected in the discrepancy between the a priori calculated sample size and the number of included patients in this research. Although the planned number of screened patients was not reached, nonetheless a prevalence estimate was achieved with a narrow confidence margin (95%, CI = 1.4 to 4.9). Because the definition of hypertension may differ between countries, for example, the UK and the Netherlands,15,16 and population characteristics may vary, the denominator in the prevalence estimate may also differ between countries. Moreover, the authors’ experience in the current research raises the question of whether similar potential selection bias might have confounded previously published primary care studies on the prevalence of PA. To assess the prevalence of PA more precisely and to circumvent selection bias, rigorous screening of all patients with newly diagnosed hypertension is required. Employing a computerised algorithm without involvement of the GP in the selection process might be a better screening strategy, for example, a pop-up in the screen when an elevated blood pressure is added for the second time or when an ICPC code for hypertension is entered.
Implications for practice
As previous studies have shown that hypokalaemia is only present in a minority of PA patients, it should be noted that all PA patients in the current study were normokalaemic. This re-emphasises that the absence of hypokalaemia as a reliable clinical marker to exclude PA should be considered obsolete.51
A diagnosis of PA has enormous consequences, both on patients’ wellbeing and on healthcare logistics and costs. Early screening followed by adequate treatment may not only improve quality of life, but it may also be cost-saving.52 Although the low prevalence, as found in this study, does not support indiscriminate screening of all new hypertensive patients for PA, all patients in this research project would have been missed following the current primary care guideline. In contrast, if the recommendations of the Endocrine Society Guideline had been used, eight of the nine PA patients would have been picked up as they had a sustained blood pressure of >150/100 mmHg. It might be, therefore, reasonable to adopt the recommendations of the Endocrine Society Guideline also in the field of primary care, so that the detection rate of PA may be improved.1
Acknowledgments
The authors wish to thank all GPs, their staff, and the patients for taking part in this study. They also wish to thank the primary-care-based diagnostic centre Stichting Huisartsenlaboratorium Oost, Jenneke van Happen, Tessa Schmits, Jasper Maters, Lea Peters, José Donkers, Hans Peters, and Waling Tiersma (deceased) for their help in data acquisition. They especially thank Carel Bakx (deceased) and Mark van der Wel, who were involved in the starting phase of the study.
Funding
A grant for this study was obtained from the Radboudumc by Jaap Deinum.
Ethical approval
The study protocol was approved by the Medical Review Ethics Committee Region Arnhem-Nijmegen, reference: NL40133.091.12.
Provenance
Freely submitted, externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 2.Jansen PM, Boomsma F, van den Meiracker AH, Dutch ARRAt investigators Aldosterone-to-renin ratio as a screening test for primary aldosteronism — the Dutch ARRAT study. Neth J Med. 2008;66(5):220–228. [PubMed] [Google Scholar]
- 3.Plouin PF, Amar L, Chatellier G. Trends in the prevalence of primary aldosteronism, aldosterone-producing adenomas, and surgically correctable aldosterone-dependent hypertension. Nephrol Dial Transplant. 2004;19(4):774–777. doi: 10.1093/ndt/gfh112. [DOI] [PubMed] [Google Scholar]
- 4.Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies — a review of the current literature. Horm Metab Res. 2012;44(3):157–162. doi: 10.1055/s-0031-1295438. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun D, Nishizaka M, Zaman A. Low prevalence of white-coat hypertension in subjects with resistant hypertension and hyperaldosteronism. J Hypertens. 2004;22:S206. [Google Scholar]
- 6.Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826–2835. doi: 10.1210/jc.2016-1472. [DOI] [PubMed] [Google Scholar]
- 7.Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 9.Rizzoni D, Paiardi S, Rodella L, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J Clin Endocrinol Metab. 2006;91(7):2638–2642. doi: 10.1210/jc.2006-0101. [DOI] [PubMed] [Google Scholar]
- 10.Künzel HE, Apostolopoulou K, Pallauf A, et al. Quality of life in patients with primary aldosteronism: gender differences in untreated and long-term treated patients and associations with treatment and aldosterone. J Psychiatr Res. 2012;46(12):1650–1654. doi: 10.1016/j.jpsychires.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Sukor N, Kogovsek C, Gordon RD, et al. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2010;95(3):1360–1364. doi: 10.1210/jc.2009-1763. [DOI] [PubMed] [Google Scholar]
- 12.Dekkers T, Prejbisz A, Kool LJ, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739–746. doi: 10.1016/S2213-8587(16)30100-0. [DOI] [PubMed] [Google Scholar]
- 13.Dhanjal TS, Beevers DG. Delay in the diagnosis of Conn’s syndrome: a single-center experience over 30 years. Hypertension. 2008;52(3):e22. doi: 10.1161/HYPERTENSIONAHA.108.117697. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence . Hypertension in adults: diagnosis and management. CG127. London: NICE; 2016. https://www.nice.org.uk/guidance/cg127 (accessed 15 Dec 2017). [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence . Hypertension in adults: diagnosis and management. CG127. London: NICE; 2011. [Google Scholar]
- 16.Collaboration Group Cardiovascular Risk Management NHG-Standard Cardiovascular Risk Management (Second review) General Practitioner Act. 2012;55(1):14–28. [Google Scholar]
- 17.Nilsson G, Mooe T, Söderström L, Samuelsson E. Use of exercise tests in primary care: importance for referral decisions and possible bias in the decision process; a prospective observational study. BMC Fam Pract. 2014;15:182. doi: 10.1186/s12875-014-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 21.Criteria Committee of the New York Heart Association . Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th edn. Boston, MA: Little, Brown & Co; 1994. [Google Scholar]
- 22.Lamberts H, Wood M, Hofmans-Okkes IM. International primary care classifications: the effect of fifteen years of evolution. Fam Pract. 1992;9(3):330–339. doi: 10.1093/fampra/9.3.330. [DOI] [PubMed] [Google Scholar]
- 23.Westert GP, Schellevis FG, de Bakker DH, et al. Monitoring health inequalities through general practice: the Second Dutch National Survey of General Practice. Eur J Public Health. 2005;15(1):59–65. doi: 10.1093/eurpub/cki116. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden MW, Westert GP, de Bakker DH, Schellevis FG. Second national study on diseases and operations in the GP practice: complaints and conditions in the population and in general practice. Utrecht/Bilthoven: NIVEL/RIVM; 2004. [Google Scholar]
- 25.Snijders TA, Bosker RJ. Multilevel analysis An introduction to basic and advanced multilevel analysis. London: Sage Publications; 1999. [Google Scholar]
- 26.Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton, London: Chapman and Hall/CRC; 1993. [Google Scholar]
- 27.Jefic D, Mohiuddin N, Alsabbagh R, et al. The prevalence of primary aldosteronism in diabetic patients. J Clin Hypertens (Greenwich) 2006;8(4):253–256. doi: 10.1111/j.1524-6175.2005.05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee JJ, Khoo CM, Thai AC, et al. Type 2 diabetic patients with resistant hypertension should be screened for primary aldosteronism. Diab Vasc Dis Res. 2010;7(1):6–13. doi: 10.1177/1479164109350556. [DOI] [PubMed] [Google Scholar]
- 29.Umpierrez GE, Cantey P, Smiley D, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care. 2007;30(7):1699–1703. doi: 10.2337/dc07-0031. [DOI] [PubMed] [Google Scholar]
- 30.Börgel J, Springer S, Ghafoor J, et al. Unrecognized secondary causes of hypertension in patients with hypertensive urgency/emergency: prevalence and co-prevalence. Clin Res Cardiol. 2010;99(8):499–506. doi: 10.1007/s00392-010-0148-4. [DOI] [PubMed] [Google Scholar]
- 31.Biermans MC, Spreeuwenberg P, Verheij RA, et al. Striking trends in the incidence of health problems in the Netherlands (2002–05). Findings from a new strategy for surveillance in general practice. Eur J Public Health. 2009;19(3):290–296. doi: 10.1093/eurpub/ckn130. [DOI] [PubMed] [Google Scholar]
- 32.Tiu SC, Choi CH, Shek CC, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90(1):72–78. doi: 10.1210/jc.2004-1149. [DOI] [PubMed] [Google Scholar]
- 33.Raizman JE, Diamandis EP, Holmes D, et al. A renin-ssance in primary aldosteronism testing: obstacles and opportunities for screening, diagnosis, and management. Clin Chem. 2015;61(8):1022–1027. doi: 10.1373/clinchem.2015.242990. [DOI] [PubMed] [Google Scholar]
- 34.Young WF. Minireview: primary aldosteronism — changing concepts in diagnosis and treatment. Endocrinology. 2003;144(6):2208–2213. doi: 10.1210/en.2003-0279. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger MH, Fineberg NS. The diagnosis of primary aldosteronism and separation of two major subtypes. Arch Intern Med. 1993;153(18):2125–2129. [PubMed] [Google Scholar]
- 36.Hripcsak G, Knirsch C, Zhou L, et al. Bias associated with mining electronic health records. J Biomed Discov Collab. 2011;6:48–52. doi: 10.5210/disco.v6i0.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon RD, Ziesak MD, Tunny TJ, et al. Evidence that primary aldosteronism may not be uncommon: 12% incidence among antihypertensive drug trial volunteers. Clin Exp Pharmacol Physiol. 1993;20(5):296–298. doi: 10.1111/j.1440-1681.1993.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 38.Loh KC, Koay ES, Khaw MC, et al. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab. 2000;85(8):2854–2859. doi: 10.1210/jcem.85.8.6752. [DOI] [PubMed] [Google Scholar]
- 39.Mosso L, Carvajal C, González A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161–165. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 40.Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193–202. doi: 10.1291/hypres.27.193. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51(2):386–394. doi: 10.1373/clinchem.2004.041780. [DOI] [PubMed] [Google Scholar]
- 42.Westerdahl C, Bergenfelz A, Isaksson A, et al. High frequency of primary hyperaldosteronism among hypertensive patients from a primary care area in Sweden. Scand J Prim Health Care. 2006;24(3):154–159. doi: 10.1080/02813430600830931. [DOI] [PubMed] [Google Scholar]
- 43.Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20(2):129–136. doi: 10.1038/sj.jhh.1001948. [DOI] [PubMed] [Google Scholar]
- 44.Fogari R, Preti P, Zoppi A, et al. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res. 2007;30(2):111–117. doi: 10.1291/hypres.30.111. [DOI] [PubMed] [Google Scholar]
- 45.Westerdahl C, Bergenfelz A, Isaksson A, et al. Primary aldosteronism among newly diagnosed and untreated hypertensive patients in a Swedish primary care area. Scand J Prim Health Care. 2011;29(1):57–62. doi: 10.3109/02813432.2011.554015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811–1820. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GL, Chapman AB, Boerwinkle E, et al. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clin Chem. 2002;48(11):1919–1923. [PubMed] [Google Scholar]
- 48.Jansen PM, van den Born BJ, Frenkel WJ, et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J Hypertens. 2014;32(1):115–126. doi: 10.1097/HJH.0b013e3283656b54. [DOI] [PubMed] [Google Scholar]
- 49.Seiler L, Rump LC, Schulte-Mönting J, et al. Diagnosis of primary aldosteronism: value of different screening parameters and influence of antihypertensive medication. EurJ Endocrinol. 2004;150(3):329–337. doi: 10.1530/eje.0.1500329. [DOI] [PubMed] [Google Scholar]
- 50.Lebeau JP, Cadwallader JS, Vaillant-Roussel H, et al. General practitioners’ justifications for therapeutic inertia in cardiovascular prevention: an empirically grounded typology. BMJ Open. 2016;6(5):e010639. doi: 10.1136/bmjopen-2015-010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 52.Lubitz CC, Economopoulos KP, Sy S, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circ Cardiovasc Qual Outcomes. 2015;8(6):621–630. doi: 10.1161/CIRCOUTCOMES.115.002002. [DOI] [PMC free article] [PubMed] [Google Scholar]