Abstract
Immunoglobulin light chain amyloidosis (AL) is a plasma cell disorder characterized by overproduction and deposition of monoclonal immunoglobulin (Ig) light chains (LC) or variable region fragments as amyloid fibrils in various organs and tissues. Much clinical evidence indicates that patients with AL amyloidosis sustain cardiomyocyte impairment and suffer from oxidative stress. We seek to understand the underlying biochemical pathways whose disruption or amplification during sporadic or sustained disease states leads to harmful physiological consequences and to determine the detailed structures of intermediates and products that serve as signposts for the biochemical changes and represent potential biomarkers. In this study, matrix-assisted laser desorption/ionization mass spectrometry provided extensive evidence for oxidative post-translational modifications (PTMs) of an amyloidogenic Ig LC protein from a patient with AL amyloidosis. Some of the tyrosine residues were heavily mono- or di-chlorinated. In addition, a novel oxidative conversion to a nitrile moiety was observed for many of the terminal aminomethyl groups on lysine side chains. In vitro experiments using model peptides, in-solution oxidation, and click chemistry demonstrated that hypochlorous acid produced by the myeloperoxidase - hydrogen peroxide - chloride system could be responsible for these and other, more commonly observed modifications.
Keywords: Amyloid, oxidative stress, myeloperoxidase (MPO), hypochlorous acid (HOCl), tyrosine, lysine, nitrile, MALDI-MS, tandem MS
1. Introduction
Immunoglobulin (Ig) light chain amyloidosis (AL) is the most frequent form of systemic amyloidosis in America (1). The disease is a plasma cell dyscrasia characterized by overproduction and tissue deposition of monoclonal Ig light chain (LC) proteins as amyloid fibrils rich in β-sheet structure (2). These amyloid deposits are usually found in the extracellular space and can disrupt normal cellular and tissue functions. In contrast to Alzheimer disease where the deposits primarily occur within the brain, the deposits in AL amyloidosis are found in multiple sites throughout the body (3–5). Cardiovascular complications are especially common in patients with AL amyloidosis, with congestive heart failure being the primary cause of death. There is much evidence that amyloidogenic Ig LC proteins can compromise cardiomyocyte functions, with an increase in cellular oxidative stress (6,7). Infusion of LCs from patients with cardiac amyloidosis into isolated mouse hearts can cause oxidative stress (8). Increased oxidative stress may also be responsible for multi-organ dysfunction in AL amyloidosis. Compared to healthy control subjects, AL patients tend to have elevated levels of circulating oxidized proteins (6,7).
Oxidative stress is usually associated with a burst of reactive oxygen species (ROS). Excess ROS can cause damage to almost all biomolecules that have important physiological functions, including proteins, lipids and DNA, and it is now believed to be responsible for many diseases (9–11).
There are multiple sources for ROS, both exogenous and endogenous (9). Ultraviolet light and environmental toxins play important roles as exogenous factors. In vivo, neutrophils are a major source for ROS. Under conditions of inflammation, neutrophils can not only generate hydrogen peroxide (H2O2), but also release the heme peroxidase enzyme myeloperoxidase (MPO) (12–14). Under physiological conditions, MPO can convert H2O2 and chloride (Cl−) into hypochlorous acid (HOCl). HOCl is a strong oxidizing and chlorinating agent that reacts with many functional groups. Neutrophil/MPO is a key element of our defense system for killing bacteria. However, it can also cause problems to the host. For example, MPO oxidation of low-density lipoprotein (LDL) turns it into a form more likely to be taken up by macrophages, and thus increasing the risk of cardiovascular diseases (15). In the case of renal failure, infiltration of neutrophils aggravates the situation by destroying normal cells and tissues with its oxidizing agents (16,17). It is thus important to identify proper markers for detection of high levels of HOCl to aid in the assessment and treatment of conditions that are fostered by oxidative stress.
For peptides and proteins, HOCl reacts with both the peptide bonds and the amino acid side chains. Reactions with peptide bonds can finally lead to the fragmentation and degradation of peptides and proteins. Among the amino acid side chains, HOCl reacts fastest with the sulfur-containing residues, namely cysteine and methionine (18). It can also react with other side chains, including those of tyrosine, lysine and the free N-terminal α-amino group (18,19). Chlorination of the aromatic ring of the tyrosine side chain produces 3-chlorotyrosine or even 3,5-dichlorotyrosine (20,21). Our laboratory previously found low levels of monochlorinated tyrosine residues in amyloidogenic kappa LC proteins (22). Di-chlorination of tyrosine residues in vivo has not been reported perhaps due to their low concentrations. HOCl also reacts readily with amine groups, present on lysine side chains or the free N-terminus, forming chloramines, which are usually unstable and may undergo further reactions (23,24).
In this report, we have investigated the post-translational modifications (PTMs) of amyloidogenic LC proteins purified from a urine sample of patient with AL amyloidosis. We found that this LC protein had more extensive and more diverse modifications at tyrosine and lysine residues than we had previously encountered, despite having analyzed dozens of samples of this type over the last fifteen years. The extent of these modifications suggested that this patient had a very high level of oxidative stress.
We determined that some of the tyrosine residues in the Ig LC proteoforms were mono- and/or di-chlorinated and that the side chains of many lysine residues had been converted to nitrile groups. In vitro studies with the MPO-H2O2-Cl− system and model peptides showed the same reaction products. MALDI-MS peptide mapping of tryptic digests of the Ig LC proteins from urine and plasma samples, and analysis of the end product analogs that were generated in vitro from model peptides, were critical for detecting the sequence variations and PTMs and for following the chemistry.
2. Materials and methods
2.1. Patient samples
The serum and urine samples were obtained from a 65- year-old female patient who was evaluated for AL amyloidosis at the Amyloidosis Center at Boston University School of Medicine and Boston Medical Center. Informed consent for data and collection of samples was obtained from the patient at presentation, with the permission of the Boston Medical Center Institutional Review Board. The diagnosis of AL amyloidosis was made using standard criteria (5). Amyloid deposits were identified by Congo red staining of renal tissue biopsy and abdominal fat aspirate under light microscopy and polarized light. The load of amyloid tissue deposits on a scale from 0 to 4+ was estimated to be 2+ for kidney and 1+ for abdominal fat tissue. Bone marrow biopsy demonstrated 10–15% plasma cells with lambda LC restriction. Major organ involvement included renal and cardiac along with primary and secondary organ involvements were renal and cardiac, respectively, along with gastrointestinal and soft tissue involvement. The disease was characterized by a very aggressive course and the patient has died within one year after her first symptom. The samples discussed here were obtained two months prior to patient death.
The Ig LC variable region gene was cloned and sequenced from bone marrow sample in Alan and Sandra Gerry Amyloid Research Laboratory using methodology previously described(25). The LC protein sequence was deduced from bone marrow cDNA and found to be IGLV2-23 by the International Immunogenetics Information System nomenclature (http://www.imgt.org/).
2.2. Materials
Ultrapure water was used throughout the experiments except where otherwise stated. Trifluoroacetic acid (TFA), formic acid (FA), hydrogen peroxide (H2O2), phosphate buffered saline (PBS), acetonitrile (ACN), 2-propanol, methanol and ethyl acetate were obtained from Fisher Scientific (Pittsburgh, PA). Other chemicals, if not specified, were from Sigma-Aldrich (St. Louis, MO).
MPO from human leukocytes was also obtained from Sigma-Aldrich. Trypsin Gold was purchased from Promega (San Luis Obispo, CA). Synthetic peptides were produced and purified by GenScript (Piscataway, NJ) with three different sequences: peptide A - VLIYEDFKR; peptide B - VLIYEDFRR; peptide C - VLIFEDFKR. All three peptides were acetylated at the N-terminus and amidated at the C-terminus; the purity was ≥ 98 %.
2.3. LC protein purification from urine sample
Urinary LC protein purification was carried out according to a protocol we have published earlier, with minor modifications (22). In brief, the urine sample was dialyzed against deionized H2O in dialysis membrane tubing (Spectrum Laboratories, Rancho Dominguez, CA) with molecular weight cut-off 12–14,000 Da, through three separate changes. Following dialysis, aliquots of the urine sample were lyophilized and stored at −20 °C until further use. Lyophilized raw urine was reconstituted with PBS buffer and passed through four Hi-Trap Blue columns (GE Healthcare Bio-Sciences, Piscataway, NJ) to remove albumin. The albumin-depleted sample was again dialyzed in dialysis membrane tubing with molecular weight cut-off 6–8,000 Da and lyophilized. Further purification employed a Sephacryl S-200 (GE Healthcare Bio-Sciences, Piscataway, NJ) size exclusion column in PBS buffer. Fractions were collected, dialyzed and lyophilized. Purified samples were assessed for heterogeneity and the presence of LC proteoforms by SDS-PAGE and Western blot analyses.
2.4. FLC protein purification from serum sample and 2D gel electrophoresis
Circulating free LC (FLC) protein was isolated from serum by immunoprecipitation following a published method (26) with modifications. The Direct IP kit, purchased from Pierce (Rockford, IL), contained Aminolink Plus™ agarose beads and was used as described in the manufacturer’s detailed handling procedure. Sheep anti-human lambda free LC antibody (Bethyl, Montgomery, TX) was coupled to the agarose beads (380 μg antibody/100 μl beads slurry, a ratio higher than the minimum recommended). After coupling, any unbound antibodies were washed away and the remaining reactive sites were blocked. For immunoprecipitation, 100 μl of the suspension of coupled beads was used for analysis of 40 μl of serum. The flow-through after coupling to the beads and the precipitated protein suspension were subjected to SDS-PAGE (NuPAGE® bis-tris precast 4–12% gradient gel, 1.0 mm thick, Invitrogen, Grand Island, NY) to check for the coupling efficiency and the presence of LC proteins.
The immunoprecipitated proteins were first dialyzed against ultrapure water at −4 °C overnight. Subsequently, samples were subjected to 2D SDS-PAGE gel electrophoresis using a detailed protocol published elsewhere (27). In summary, a 17-cm IPG strip with pH range from 3 to 10 (ReadyStrip™, Bio-Rad, Hercules, CA) was used for first dimension separation. For the second dimension, the IPG strip was overlaid to a 1.00-mm-thick PAGE gel with a gradient of 8–16% Tris-Glycine (0.375M Tris-HCl, pH 8.8 for gel buffer, no SDS) (Jule, Milford, CT). After these steps, the gel was washed and stained with a mass spectrometry compatible Coomassie stain reagent (GelCode® Blue from Pierce). Gels were stored at −4 °C. Within a week, spots of interest were selectively removed and subjected to in-gel digestion.
2.5. In-solution and in-gel protein digestion
For in-solution protein digestion, purified and lyophilized urinary LC protein was first reconstituted with 50 mM ammonium bicarbonate buffer at a final concentration of 0.5–1 μg/μL. The protein solution was then incubated with 5 mM dithiothreitol (DTT) at 55 °C for 1 hour. After the mixture was cooled to room temperature, the proteins were alkylated by treatment with 2-iodoacetamide (2AA) at a concentration of 10 mM. The reaction was carried out in the dark at room temperature for half an hour. Excess 2AA was quenched by addition of more DTT to yield the final concentration of 20 mM. Protein digestions were carried out with the protease at a weight ratio of 1:50 (protease: protein), incubating overnight at 37 °C. The next day, protein digests were dried on a SpeedVac (Savant SC110A, ThermoFisher Scientific, Waltham, MA) for future use.
The Ig LC protein was also further purified by SDS-PAGE (Supplementary Fig. S1), using a 4–12% gradient gel (same as above). The band with an apparent molecular weight slightly more than 25 kDa, presumed to contain monomeric LC, was removed for in-gel protein digestion that employed a published method (28) with modifications. If reduction and alkylation had not been performed beforehand, the SDS-PAGE gel bands were incubated with DTT solution after destaining and washing; afterwards, they were washed again and incubated with 2AA. The DTT and 2AA solutions used in this protocol had the same concentrations as those used above for in-solution digestion. Protein digestion with Trypsin Gold was facilitated by the addition of ProteaseMAX™ Surfactant (Promega), following the commercial instructions, to achieve more efficient elution of the larger peptides. No special extraction step was needed. The protein digests were dried on a SpeedVac and stored at −80 °C prior to analysis.
2.6. In vitro oxidation of model peptides and purification of the products
2.6.1. In vitro oxidation
Each synthetic peptide (10 nmol) was resuspended in 20 μL PBS buffer and oxidized via sequential additions of MPO (0–2 pmol) and H2O2 (0–20 nmol). The H2O2 was added in several aliquots, at 3–5 minute intervals, with thorough shaking. The mixtures were incubated at 37 °C for 5 to 20 minutes, depending on the amount of H2O2 used. The reaction mixture was taken to dryness on a SpeedVac and stored at −80 °C for downstream applications.
For large-scale oxidation reactions, NaClO was used (molar ratio, peptide: NaClO = 1:40). This process proved to have reaction characteristics similar to the MPO-H2O2-Cl− system (29).
2.6.2. HPLC separation
Peptide reaction products with MPO-H2O2-Cl− were purified with C18 ZipTips® (Millipore, Billerica, MA), lyophilized, reconstituted in buffer A (1% ACN/99% H2O/0.1% TFA) and loaded to an Agilent HPLC (1200 series, Agilent Technologies, Santa Clara, CA) equipped with a VydacR C18 column (5 μm, 2.1 mm × 250 mm) (GraceR, Deerfield, IL). Buffer B was made of 99% ACN/1% H2O/0.1% TFA. Flow rate was kept at constant at 0.2 ml/min. Separation was done with a gradient elution: 0–2 min, 1%–3% buffer B; 2–40 min, 3% – 70% buffer B; 43–46 min, 70% – 90% buffer B; 46–49 min, 90% - 3% buffer B.
Peptide reaction products with NaClO were separated with the same procedure with a few modifications made for the larger quantity. The product mixture was desalted with Sep-Pak C18 cartridges (Waters, Milford, MA) before being lyophilized. The C18 column was Adsorbosphere HS (7 μm, 10 mm × 250 mm) from Alltech Associates (Deerfield, IL). The flow rate was 3.5 ml/min. The elution gradient was as follows: 0–7 min, 1% –3 % buffer B; 7 – 22 min, 3% – 40% buffer B; 22 – 45 min, 40% – 70% buffer B; 45 – 48 min, 70% – 90 % buffer B; 48 – 51 min, 90% – 90% buffer B; 51 – 55 min, 90% - 3% buffer B.
Both columns were used at room temperature with detection at 214 nm. Fractions were collected manually.
2.7. DNPH test for aldehyde group
A saturated methanol solution of 2,4-dinitrophenylhydrazine (DNPH) was freshly prepared. The supernatant was then diluted 1:1 with ultrapure water. Ten microliters of this solution was added to each dried HPLC fraction, and then 0.2 μL HCl (12 N) was added. The mixture was incubated at room temperature for 1 hour and the products were dried on a SpeedVac and stored for future analysis.
2.8 Click chemistry test for nitrile group
The click chemistry reaction between the nitrile group on the peptide and NaN3 was performed according to protocols published in the literature (30,31) with modifications for small-scale reaction. The HPLC fraction containing the peptide of interest was resuspended in 120 μL water and 2-propanol (volume ratio, 2: 1) solution. The solution was transferred to a glass reaction vial and the reagent NaN3 and catalyst ZnBr2 were added at a molar ratio of 4:1. The amount of NaN3 was roughly 2–20 equivalents of the peptide. The mixture was incubated at 80 °C for 24 hours. Finally, 0.2 μL 12N HCl was added to the mixture, and the products were then dried on a SpeedVac for further analysis.
2.9 Mass spectrometry: MS and tandem MS/MS analyses
Each sample was desalted with a C18 ZipTip® before any mass spectrometric analysis.
The products from protease digests of the patient light chain proteins and synthetic peptide samples, including the HPLC fractions and reaction products, were first analyzed by MALDI-TOF MS with the ultrafleXtreme™ MS system (Bruker Daltonics, Billerica, MA). For Ig LC samples, MALDI-TOF MS spectra were recorded with an ultrafleXtreme™ MALDI-TOF/TOF MS (Bruker Daltonics), operated in the positive ion, reflectron mode. This instrument is equipped with a Smartbeam Nd/YAG laser and spectra were recorded for irradiation at 355 nm, using 2,5-dihydroxybenzoic acid as the matrix. Mass spectra shown were summed over 500–1500 laser shots.
Tandem MS analysis used to determine or confirm sequence or modification information was performed with several instruments. An electrospray ionization (ESI) ion trap instrument, the amaZon™ ETD (Bruker Daltonics), was used with collision-induced-dissociation (CID) activation. LC-MS/MS analysis was performed with an LTQ-Orbitrap™ MS (ThermoFisher Scientific) coupled with an Advion TriVersa NanoMate™ system (Advion Biosciences, Ithaca, NY). The higher-energy C-trap dissociation (HCD) activation method was used throughout these experiments and all mass spectra were measured with the Orbitrap. These data sets were analyzed with Proteome Discoverer 3.0 (Thermo Scientific) using the SEQUEST search engine and the results were checked manually.
A solariX™ 12-T Qh Fourier-transform (FT) MS (Bruker Daltonics) was used for tandem MS measurements on samples introduced by nanoelectrospray. Both CID and electron capture dissociation (ECD) activation were employed as complementary dissociation methods.
3. Results
3.1. Analysis of urinary LC
3.1.1. Sequence coverage and N-terminal cyclization of urinary LC
Tryptic peptides from in-solution digestion of the urinary LC protein were first subjected to peptide mass fingerprinting using MALDI-TOF MS (Fig. 1). Full sequence coverage of the protein was achieved, by combining data from in-solution and in-gel digestion of the protein with Trypsin Gold (data not shown). The N-terminal peptide (residues 1–47) was detected in the LTQ-Orbitrap™, but its tandem mass spectrum showed only a few fragments. The data indicated that the N-terminal glutamine was converted to pyroglutamate, and this was confirmed by recording the CID MS/MS in the amaZon™ ion trap instrument (Supplementary Fig. S2). The amino acid sequence determined by mass spectrometry was consistent with the LC sequence deduced from analysis of the bone marrow cDNA.
Fig. 1. MALDI-TOF mass spectrum of tryptic peptides from in-solution digestion of the LC sample extracted from the patient’s urine.

Laser power used was 50%. Not all peaks identified by tandem MS have been labeled. Peaks assigned to some of the chlorinated peptides are labeled. The measured mass for the lower abundance peptide assigned to residues [69–93]*, [M + H]+ m/z 2721.15, suggests that one cysteine was carbamidomethylated and the other cysteine was present in the dehydro form. Sequence coverage based only on this spectrum is underlined in the scheme. Full sequence coverage for this LC was achieved after multiple protease digestions, peptide mapping and tandem MS analysis. T: trypsin autolysis peak.
3.1.2. Tyrosine chlorination of urinary LC
The products from the tryptic digestion of the urinary LC protein included peptides with mass differences of 34 Da or 68 Da, which had isotopic distributions (theoretical patterns not shown) consistent with those calculated for the assigned mono-/di-chlorinated peptides. Fig. 2 (A) shows an example of the peaks in the MALDI-TOF mass spectrum that corresponded to multiple forms of the peptide assigned to contain residues 48–63. Further analysis with LC-MS/MS using the LTQ-Orbitrap™ instrument enabled the determination that chlorine was substituted at tyrosine 51 (Fig. 2 (B)). Dichlorination of tyrosine 51 was also detected (Fig. 2 (C)).
Fig. 2. Tyrosine 51 of the patient’s urinary LC was (B) chlorinated and (C) di-chlorinated.

(A) Expansion of the region m/z 1960–2050 in Fig. 1, to show peaks for unmodified and possibly modified forms of the peptide containing residues [48–63]. (B) Tandem MS spectrum for mono-chlorinated peptide with residues [48–55], [M + 2H]2+ m/z 520.7870. (C) Tandem MS spectrum for di-chlorinated peptide with residues [48–63], [M + 4H]4+ m/z 509.9923. In-gel tryptic digest of the spot corresponding to the LC monomer was analyzed by LC-MS/MS on an LTQ-Orbitrap™ with HCD activation.
For this LC protein, a total of three tyrosine residues, Y51, Y176, and Y195, were found partially chlorinated (Figs. S3 and S5); tyrosine 51 and 176 were found to be present in both mono- and di-chlorinated forms. The peptide with monochlorinated tyrosine 195 was observed with LC-MS, but not on by MALDI-TOF MS, perhaps due to the very low quantity of that peptide.
3.1.3. Lysine modification on urinary LC
In the MALDI-TOF mass spectra obtained for the patient’s urinary LC, low abundance peaks were observed at positions 4 Da below the [M + H]+ peaks that could be assigned to several peptides that contained lysine residues. Tandem MS spectra with HCD fragmentation and detection in the Orbitrap™ showed a molecular weight loss of 4.0300 Da for tryptic peptide [154–170] ([M + 2H]2+ m/z 837.9309) (Fig. 3). According to the fragmentation observed in the tandem MS spectrum, the mass shift was most likely due to a modification at the residue Lys160.
Fig. 3. Lysine residues of the patient’s LC were modified.

The urinary proteins were separated by SDS-PAGE and the band corresponding to the monomer form of the LC protein was in-gel digested with Trypsin Gold. The tandem mass spectrum ([M + 2H]2+, m/z 835.9159) recorded during LC-MS/MS on the LTQ-Orbitrap™ with HCD activation showed a mass deficit of −4.0300 Da at lysine residue 160 of the LC protein.
More possible modifications at lysine residues with a mass difference of −4 Da were observed in the LC-MS/MS spectra, including K55, K106, K114, K160, K170, K175, K190, K193 and K208 (See Supplementary Figs. S3 and S4 for the assignments of modifications on K106, K190 and K208).
3.1.4. Other urinary LC modifications
Additional oxidative modifications of the urinary LC were observed. For example, tryptophan 189 oxidation products were detected (Supplementary Fig. S5). Cysteines 90 and/or 91 were also modified (Fig. 1, Supplementary Fig. S6). In addition to reduction and alkylation side products, evidence for the oxidative modification of cysteine 90 to the dehydro form was observed in the MALDI-MS and LC-MS/MS spectra shown in Fig. 1 and Fig. S6. Although the α, β unsaturated thiol would be subject to carbamidomethylation, such a product would be unstable and likely lose the CAM group under the conditions of MALDI-MS.
Digestions of the non-reduced, non-alkylated proteoforms of the LC were also performed and the products were analyzed using the methods described above. No further PTMs were found (data not shown).
3.2. In vitro investigations of oxidative modifications to model peptides
3.2.1. In vitro study of tyrosine chlorination in model peptides
Tyrosine residues can be chlorinated by HOCl. Myeloperoxidase (MPO) is the only human enzyme which can produce HOCl under physiological conditions. To investigate this reaction in vitro, peptides that contained portions of the LC sequence were synthesized and subjected to similar oxidative conditions.
Synthetic peptide A (VLIYEDFKR, corresponding to the amyloid LC protein residues 48–56, with both terminals modified to avoid reaction at these sites (calc. [M + H]+ m/z 1223.6783) was incubated with MPO and H2O2 in PBS buffer. This led to formation of mono-/di-chlorination products (peaks [M + H]+ m/z 1257.66 and 1291.62, respectively). H2O2 alone was not sufficient to initiate the reaction (Fig. 4 (A)). The CID MS/MS spectrum obtained for the mono-chlorinated species measured with the solariX™ FT-MS identified the component as the monochlorinated product (Supplementary Fig. S7). However, close examination of the MS/MS spectra obtained for the dichlorinated species revealed multiple possibilities (Supplementary Fig. S7). Fig. S7 compares tandem FT-MS spectra of precursors (a) [M + 2H]2+ m/z 629.32±2 and (b) [M + 2H] 2+ m/z 646.30±2. While an un-shifted y5+ peak was only detected in (a), peaks shifted by 34 Da (y5*+) and 68 Da (y5**+) were detected in (b). These data indicated that the lysine residue was not chlorinated in (a) and at least monochlorinated in (b). In (b), considering the shifted and un-shifted peaks for b6+, the tyrosine residue was present in both the mono-chlorinated and unmodified forms. The two added chlorine atoms in (b) species [M + H]+ m/z 1291.62 in Fig. 4 (A) could both be located the lysine side chain (as chloramine RNCl2) or one on could be located on lysine and the other on tyrosine.
Fig. 4. MALDI-TOF mass spectra for treated synthetic peptides.

(A) Synthetic peptide AcVLIYEDFKR-NH2 (peptide A) ([M + H]+ m/z 1223.70). Its incubation with MPO-H2O2-Cl− produced chlorinated species while H2O2 alone could not. Replacement of the lysine residue with arginine (peptide B) ([M + H]+ m/z 1251.71) to avoid chlorination at the lysine side chain could still lead to formation of modifications during chlorination, e.g., to produce the peak at [M + H]+ m/z 1219.66. (B) Incubation of synthetic peptide AcVLIFEDFKR-NH2 (peptide C, [M + H]+ m/z 1207.65) with the MPO-H2O2-Cl− system generated the peak which exhibited the decrease of 4 Da. A low abundance peak corresponding to the chlorinated peptide ([M + H]+ m/z 1241.62) was also observed.
To avoid interference from the lysine side chain, an analog of synthetic peptide B was synthesized with K replaced by R (calc. [M + H]+ m/z 1251.6844), so that a basic residue still contributed positive charge but it bore a side chain less reactive with HOCl. Under the same reaction conditions with respect to MPO and H2O2, fewer chlorinated products were formed from this peptide (Fig. 4 (A)).
3.2.2. In vitro study of lysine oxidation in model peptides
Synthetic peptide A, when reacted with HOCl, also yielded a peak in the mass spectrum for [M + H]+ m/z 1219.66 Da, a value 4 Da less than the neighboring unmodified peptide peak (Fig. 4 (A)). Tandem MS spectra recorded with using the solariX™ FT-MS (both ECD and CID), indicated that the mass shift was due to an unknown modification on the lysine residue (data not shown).
Therefore, we prepared synthetic peptide C with Y converted to F (calc. [M + H]+ m/z 1207.6834) to determine whether MPO-H2O2-Cl− could be responsible for the novel modification at lysine residues. This time, we were still able to detect the modified peptide (Fig. 4 (B)). The reaction mixtures were separated on a C18 column. Compared to the results obtained with unreacted peptides or peptides incubated with H2O2 alone, the data obtained after HOCl treatment exhibited two additional peaks. MALDI-TOF mass spectra showed clear separation of three species (Fig. 5). From these data and the accurate mass differences determined on the solariX™ MS (data not shown), it was then possible to assign the modifications on the products b and c as an aldehyde group (lysine oxidized to allysine) and a nitrile group, respectively.
Fig. 5. (A) Separation of the reaction products of the synthetic peptide C with MPO-H2O2-Cl− gave three well-separated peaks.

Separation was done on an Agilent 1200 series HPLC. The expanded scale is also shown. (B) MALDI-TOF mass spectra for these three species. The spectra revealed a as the unmodified peptide, b as the reaction product with 1-Da loss and c as the reaction product with 4-Da loss. Scaling up the reaction and separating the products by HPLC also yielded these three fractions.
To verify this assignment, five mg of synthetic peptide C were reacted with a large excess of NaOCl at pH 7.4. Under these conditions, NaOCl exists as a mixture of HOCl and OCl−, making it an inexpensive substitute for MPO-H2O2 –Cl−. The product mixture was separated on a semi-preparative C18 column, and yielded the same fractions a, b and c. In this way, relatively large amounts of the oxidation products from the peptide standards were prepared and purified.
Fraction b was reacted with DNPH and a peak with a mass shift of 180 Da was observed (Fig. 6 (A)). The unmodified peptide failed to react with DNPH. This confirmed the presence of an oxidized form of the lysine side chain (formation of allysine). This type of lysine oxidation product was also detected in the amyloid LC protein (Supplementary Fig. S4(C)).
Fig. 6. Identification of fractions b and c. Derivatization of the fractions obtained by separation of the mixture from the scaled-up reactions.
(A) MALDI-TOF mass spectrum of the product mixture of fraction b after reaction with DNPH showed a peak with the 180-Da mass shift expected for the reaction of an aldehyde groups with DNPH. The peak labeled with “*” corresponds to a side product that contains a nitroso group. It should also be noticed that the reaction of fraction b with DNPH also yielded peaks [M + H]+ m/z 1203.70 and 1207.72. Apparently, fraction b was not stable and underwent further reaction or was converted back. (B) Fraction c was incubated with click chemistry reagent NaN3 and catalyst ZnBr in water and 2-propanol solution (volume ratio 2:1) at 80 °C for 24 hours. Its MALDI-TOF mass spectrum shown here indicates the formation of a product with a 43-Da mass shift, consistent with formation of the cyclic product from addition of the azide to a nitrile group in fraction c. Here also, the reaction conditions caused some conversion of the initial product back to the unmodified peptide.
Fraction c was subjected to a “click” reaction with NaN3 (Fig. 6 (B)). The anticipated cyclic product with a 43-Da mass increase was detected. These results confirmed that HOCl treatment could produce a nitrile group from lysine side chain oxidation.
3.3. Analysis of serum FLC sample
A serum sample from the same patient, collected at the same clinical visit, was also analyzed. FLC proteins were pulled down from 40 μl of the serum sample and separated by 2D SDS-PAGE (Fig. 7(A)). Following in-gel digestion, the tryptic peptides were first analyzed by MALDI-TOF MS (Fig. 7(B)) followed by LC-MS/MS for detailed identification of the peaks. Full LC sequence coverage was obtained by combining both types of data (not shown). N-terminal cyclization at Gln was found. Tryptophan oxidation was observed. However, no tyrosine chlorination or lysine oxidation products were detected.
Fig. 7. The FLC protein was immunoprecipitated from serum with sheep-anti-human free LC antibody and analyzed by 2D SDS-PAGE gel electrophoresis and MALDI-TOF MS.
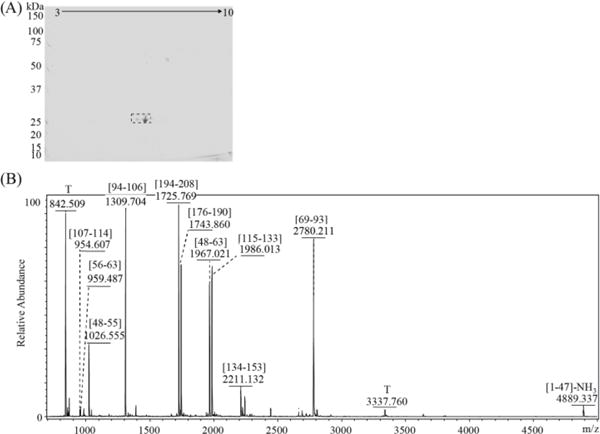
The protein was subjected to 2D-PAGE separation (A), which showed significant spots in the region slightly above 25 kDa. The spots were excised and digested with trypsin. The MALDI-TOF mass spectrum for the darkest spot is shown in (B). T: trypsin autolysis pea
4. Discussion
Oxidative stress and different oxidants are responsible for the phenotypes characterized for many diseases (10,32,33). Patients with amyloidogenic LC-related cardiomyopathy or heart involvement cardiovascular diseases are known to suffer from significant levels of oxidative stress (6,7). Further, upon exposure to amyloidogenic LCs, both rodent tissues as well as human coronary artery endothelial cells have been shown to develop responses and injuries consistent with resulting from oxidative stress (8,34). In the present study, we found that an amyloidogenic urinary Ig LC protein was extensively oxidatively modified. This patient had renal and cardiac involvement and apparently endured a high level of oxidative stress.
This report documents the first observation of a human protein with di-chlorinated tyrosine residues. Furthermore, it also describes the discovery of a novel nitrile product from oxidation of the lysine side chain. These modifications may arise from the oxidizing and chlorinating reactivities of the MPO-H2O2-Cl− system. MPO is a heme enzyme that is released by stimulated neutrophils (12,14). As a peroxidase, it can oxidize the target substrates into radical intermediates. The MPO-H2O2 system plays an important role that leads to the formation of ROS, including the conversion of chloride ions into the powerful oxidant HOCl (13).
In vitro, HOCl can react with proteins and cause side chain modifications and amino bond cleavages (18,19,35). The lysine side chain carries a primary amine, whose oxidation products are chloramines (RNHCl, RNCl2). Under physiological conditions, the reaction rate constant is smaller than that with the free N-terminal amine. To investigate this chemistry, we used synthetic peptides that were acetylated at the N-terminals. Incubation of HOCl with tyrosine residues generates chlorinated aromatic rings. Synthetic peptide A has both tyrosine and lysine residues. Its oxidation by HOCl (Fig. 4) led to mono-chlorination of tyrosine, di-chlorination of lysine or mixed di-chlorination products. These results agree with previous reports that the lysine side chain reacts faster than the tyrosine side chain under the same physiological conditions (18).
The mechanism for tyrosine aromatic ring chlorination is still not clear. Winterbourn and Kettle proposed that, rather than direct chlorination of the aromatic ring, chloride atoms can be delivered directly from a chloramine to a tyrosyl residue (20). They suggested that the nearby presence of a lysine residue might make tyrosine chlorination reaction proceed faster. For the LC protein characterized in this study, a total three tyrosine residues were found partially chlorinated, Y51, Y176, and Y195; these tyrosines were located three, zero, and one amino acid(s) apart from a lysine residue, respectively. However, Y88 and Y89, which immediately precede two cysteine residues, were not chlorinated. This may be due to steric hindrance effects. Furthermore, no chlorinated lysine residues were observed in the amyloid LC, whose sequence would have allowed chlorine atoms to be transferred to a tyrosine residue. Perhaps some other mechanism is involved in the in vivo action of HOCl.
The lysine residue, with a primary amine-containing side chain, is very chemically active. In vivo, it can be oxidized to 2-aminoadipic acid and allysine. The latter can be also produced by lysyl oxidase in the extracellular matrix (36,37). Oxidation of LDL with HOCl can cause lysine oxidation and the subsequent protein aggregation (15). Lysine mono-chloramine, an oxidation product of HOCl, is a transient intermediate. It reacts more rapidly with HOCl than with lysine and is converted into di-chloramine (24). It is possible that, because of excess HOCl, chlorinated lysine residues were further modified into carbonyls and nitriles in the case described here. Stelmaszynska and Zgliczynski have reported that the final products of dipeptide chlorination mediated by HOCl in vitro were N-(2-oxoacyl) amino acids and nitriles (38). However, in their report, the chloramines were formed at the N-terminal amino group and the nitriles were released when di-chloramines were decomposed with HCl. The nitrile bond was formed between the N-terminal nitrogen atom and the connecting carbon atom. Lysosphingolipids treated with HOCl can also produce nitriles (39).
Generation of a nitrile by oxidation of the side chain of a lysine residue in a protein has not previously been reported. Furthermore, this is the first report of evidence for in vivo nitrile formation detected in clinically-derived patient samples.
The mono-chlorinated tyrosine residue has been used as a biomarker for exposure of tissues to HOCl (40,41). We propose that di-chlorination of tyrosine and formation of a nitrile group on a lysine side chain, as observed in this study, should also be evaluated as biomarkers for exceptionally high levels of oxidative stress.
The serum sample of the same patient, collected at the same visit as the urine sample, was also analyzed. However, with the method used here, no similar modifications of the tyrosine and lysine residues were found. This may due to the low concentration of proteins with modified amino acids in the circulation. Furthermore, since epitope for FLC antibodies is located in the LC constant domain, it is quite possible that some LC protein species with truncated C-termini (i.e., missing either a portion or all of the constant domain) were not recognized and precipitated. More work needs to be done to determine the precise locations and conditions that govern exposure of the amyloidogenic LC protein to HOCl.
Another observed modification, N-terminal pyroglutamate formation from glutamine, observed for both the urinary LC and the serum FLC, can happen in vivo or in vitro, in the presence or absence of enzyme. Further modifications of the amyloid LC, including the more common types of oxidation at tryptophan, methionine and cysteine residues, could have occurred in vivo or may have been introduced as artifacts during sample handling.
5. Conclusions
In summary, we report here the definition of two novel in vivo oxidative modifications, di-chlorination of tyrosine residues and nitrile formation from lysine residues, detected as PTMs to an amyloidogenic Ig LC protein that we extracted from the urine of a patient with AL amyloidosis and analyzed using a combination of several mass spectrometry approaches. The assignments were validated with in vitro transformations of model peptides. Both protein PTMs may result from exceptionally high bursts of activity in the MPO-H2O2-Cl− oxidizing system and thus should be further investigated as potential markers for oxidative stress.
Supplementary Material
Acknowledgments
The authors are grateful to our former colleague, Dr. Yang Mao, presently located at the Copenhagen Center for Glycomics, Univ. of Copenhagen, for helpful discussions.
This research was supported by the US National Institutes of Health grants P41 RR010888/GM104603, S10 RR020946, S10 RR025082, and S10 OD010724 to C.E.C., and by the Wildflower Foundation and the Boston University Amyloid Research Fund.
Abbreviations
- Ig
Immunoglobulin
- AL
immunoglobulin light chain amyloidosis
- LC
light chain
- FLC
reactive oxygen species
- ROS
free light chain
- MPO
myeloperoxidase
- LDL
low-density lipoprotein
- DTT
dithiothreitol
- IAA
2-iodoacetamide
- DNPH
2,4-dinitrophenylhydrazine
- MALDI
matrix-assisted laser desorption/ionization
- ESI
electrospray ionization
- CID
collision-induced-dissociation
- HCD
higher-energy C-trap dissociation
- FT
Fourier-transform
- ECD
electron capture dissociation
- PTM
post-translational modification
Appendix A. Supporting Information
Supplementary data, primarily additional MS and tandem MS spectra, associated with this article can be found in the online version at …
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors do not have any financial/commercial conflicts of interest.
References
- 1.Gertz MA. The classification and typing of amyloid deposits. Am J Clin Pathol. 2004;121:787–789. doi: 10.1309/TR4L-GLVR-JKAM-V5QT. [DOI] [PubMed] [Google Scholar]
- 2.Sipe JD. Amyloidosis. Annu Rev Biochem. 1992;61:947–975. doi: 10.1146/annurev.bi.61.070192.004503. [DOI] [PubMed] [Google Scholar]
- 3.Falk RH, Comenzo RL, Skinner M. Medical progress - The systemic amyloidoses. New Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 4.Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75–84. doi: 10.1136/hrt.2009.190405. [DOI] [PubMed] [Google Scholar]
- 5.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 6.Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao RL. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 7.Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, Schlundt B, Phillips SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. International Journal of Cardiology. 2010;145:67–68. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao RL, Jain M, Teller P, Connors LH, Ngoy S, Skinner M, Falk RH, Apstein CS. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104:1594–1597. [PubMed] [Google Scholar]
- 9.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 12.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2010;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 15.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 17.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins CL, Davies MJ. Reaction of HOCl with amino acids and peptides: EPR evidence for rapid rearrangement and fragmentation reactions of nitrogen-centred radicals. J Chem Soc, Perk Trans. 1998;2:1937–1946. [Google Scholar]
- 20.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of Tyrosyl Residues in Peptides by Myeloperoxidase and Human Neutrophils. J Biol Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 21.Kettle AJ. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996;379:103–106. doi: 10.1016/0014-5793(95)01494-2. [DOI] [PubMed] [Google Scholar]
- 22.Connors LH, Jiang Y, Budnik M, Theberge R, Prokaeva T, Bodi KL, Seldin DC, Costello CE, Skinner M. Heterogeneity in primary structure, post-translational modifications, and germline gene usage of nine full-length amyloidogenic kappa1 immunoglobulin light chains. Biochemistry. 2007;46:14259–14271. doi: 10.1021/bi7013773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nightingale ZD, Lancha AH, Jr, Handelman SK, Dolnikowski GG, Busse SC, Dratz EA, Blumberg JB, Handelman GJ. Relative reactivity of lysine and other peptide-bound amino acids to oxidation by hypochlorite. Free Radic Biol Med. 2000;29:425–433. doi: 10.1016/s0891-5849(00)00262-8. [DOI] [PubMed] [Google Scholar]
- 24.Joo SH, Mitch WA. Nitrile, aldehyde, and halonitroalkane formation during chlorination/chloramination of primary amines. Environ Sci Technol. 2007;41:1288–1296. doi: 10.1021/es0612697. [DOI] [PubMed] [Google Scholar]
- 25.Prokaeva T, Spencer B, Kaut M, Ozonoff A, Doros G, Connors LH, Skinner M, Seldin DC. Soft tissue, joint, and bone manifestations of AL amyloidosis: Clinical presentation, molecular features, and survival. Arthritis Rheum. 2007;56:3858–3868. doi: 10.1002/art.22959. [DOI] [PubMed] [Google Scholar]
- 26.Lavatelli F, Brambilla F, Valentini V, Rognoni P, Casarini S, Di Silvestre D, Perfetti V, Palladini G, Sarais G, Mauri P, Merlini G. A novel approach for the purification and proteomic analysis of pathogenic immunoglobulin free light chains from serum. Biochim Biophys Acta. 2011;1814:409–419. doi: 10.1016/j.bbapap.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Lavatelli F, Perlman DH, Spencer B, Prokaeva T, McComb ME, Théberge R, Connors LH, Bellotti V, Seldin DC, Merlini G, Skinner M, Costello CE. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Mol Cell Proteomics. 2008;7:1570–1583. doi: 10.1074/mcp.M700545-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman DH, Berg EA, O’Connor PB, Costello CE, Hu JM. Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc Natl Acad Sci U S A. 2005;102:9020–9025. doi: 10.1073/pnas.0502138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of oxidant to hypochlorite. Biochim Biophys Acta, Gen Subj. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 30.Demko ZP, Sharpless KB. An expedient route to the tetrazole analogues of α-amino acids. Org Lett. 2002;4:2525–2527. doi: 10.1021/ol020096x. [DOI] [PubMed] [Google Scholar]
- 31.Demko ZP, Sharpless KB. Preparation of 5-substituted 1H-tetrazoles from nitriles in water. J Org Chem. 2001;66:7945–7950. doi: 10.1021/jo010635w. [DOI] [PubMed] [Google Scholar]
- 32.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Migrino RQ, Truran S, Gutterman DD, Franco DA, Bright M, Schlundt B, Timmons M, Motta A, Phillips SA, Hari P. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol. 2011;301:H2305–H2312. doi: 10.1152/ajpheart.00503.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkins LC, Pattison ID, Davies JM. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 36.Pinnell SR, Martin GR. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci USA. 1968;61:708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith-Mungo LI, Kagan HM. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 38.Stelmaszyńska T, Zgliczynski JM. N-(2-oxoacyl)amino acids and nitriles as final products of dipeptide chlorination mediated by the myeloperoxidase/H2O2/Cl− system. Eur J Biochem. 1978;92:301–308. doi: 10.1111/j.1432-1033.1978.tb12748.x. [DOI] [PubMed] [Google Scholar]
- 39.Brahmbhatt VV, Hsu FF, Kao JL, Frank EC, Ford DA. Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chem Phys Lipids. 2007;145:72–84. doi: 10.1016/j.chemphyslip.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Buss IH, Senthilmohan R, Darlow BA, Mogridge N, Kettle AJ, Winterbourn CC. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: Association with adverse respiratory outcome. Pediatr Res. 2003;53:455–462. doi: 10.1203/01.PDR.0000050655.25689.CE. [DOI] [PubMed] [Google Scholar]
- 41.Sochaski MA, Jarabek AM, Murphy J, Andersen ME. 3-Chlorotyrosine and 3,5-dichlorotyrosine as biomarkers of respiratory tract exposure to chlorine gas. J Anal Toxicol. 2008;32:99–105. doi: 10.1093/jat/32.1.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



