Abstract
Skin cancer is the most common type of cancer with increasing incidence rate and public health burden. Solar Ultraviolet (UV) radiation causes an array of damaging cellular and molecular events that eventually lead to the development of skin cancer. Despite increased awareness about sun protection, the exposure rate remains high with less than 15% of men and 30% of women using sunscreen on a regular basis. Therefore, there is an imperative need for the development of novel preventive approaches. Skin cancer chemoprevention using phytochemicals either as dietary supplements or by topical applications has gained considerable attention due to their low toxicity, availability, and anti-carcinogenic properties. Tea, the second most commonly consumed beverage in the world, is a rich source of promising phytochemicals known as polyphenols. In this review, we discuss the findings of various in vitro, in vivo and human studies signifying the chemopreventive effects of tea polyphenols against UVB-induced skin cancer. This is accomplished by exploring the role of tea polyphenols in DNA repair, inflammation, oxidative stress, signaling pathways, and epigenetics. Finally, this review discusses a variety of innovative delivery methods that enhance the photochemopreventive effects of tea polyphenols against skin cancer.
Introduction
Human skin, the largest organ of the body serves as a protective barrier against solar ultraviolet (UV) radiation. UV radiation reaching the surface of the earth is divided into two types based on wavelength: UVA and UVB. UVA radiation, the long wave (320 to 400 nm), makes up approximately 95% of the UV radiation. It can penetrate deep into the dermis, where it induces the generation of reactive oxygen species (ROS). These ROS can damage DNA, proteins, lipids, and alter cell signaling pathways eventually leading to photocarcinogenesis (1–3). UVB radiation, the short wave (290 to 320 nm), comprises merely 5% of the UV radiation. It is absorbed in the epidermis by cellular DNA causing direct DNA damage, inflammation, deregulation of cellular signaling pathways and photocarcinogenesis (4, 5).
Skin cancer is the most common form of cancer. It is divided into two subcategories: nonmelanoma skin cancer (NMSC) and melanoma. NMSC is comprised of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). NMSC has become a rising public health concern owing to its increasing incidence. More than 5 million new cases of NMSC are diagnosed each year in the U.S. leading to a significant economic burden (6). In 2011, the annual cost of skin cancer treatment averaged $8.1 billion. In comparison, the annual cost averaged $3.6 billion in 2006, a sharp increase of 126.2% (7). In view of these murky statistics, it is necessary to advance effective preventive measures.
Chemoprevention through the dietary consumption of phytochemicals is one of the potential preventive measures. Phytochemicals have received a great attention in the scientific community due to their low toxicity, low cost, human acceptance, and considerable effectiveness (8, 9). Polyphenols are one of those promising phytochemicals against UV-induced skin cancer. They are considered photoprotective agents due to their antioxidant, anti-inflammatory, anti-proliferative and anti-carcinogenic properties (4, 10).
Polyphenols are found in tea, which is a hot water infusion of leaves of Camellia sinensis, a plant of the Theaceae family. It is the second most consumed beverage throughout the world, after water. Tea is divided into subtypes based on the processing. White tea consists of minimally processed young leaves. Green tea is minimally processed mature leaves, while Oolong tea is semi-fermented and black tea is fully fermented. Almost 78% of the tea production worldwide is black tea, followed by green tea at 20%. Oolong tea and white tea constitutes about 2% of tea production (11). Green tea is an abundant source of polyphenols, also known as catechins. They generally account for 30–42% of the dry solids in brewed green tea. There are four major classes of green tea polyphenols (GTPs): (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatchin-3-gallate (EGCG) (Figure 1). The most abundant among these polyphenols is EGCG (50–80%) (8). The black tea polyphenols content is different from that of the green tea due to the degree of oxidation during processing. It mainly contains the following polyphenols: thearubigins, theaflavins, flavonols and catechins (Fig. 1) (12).
Fig. 1.
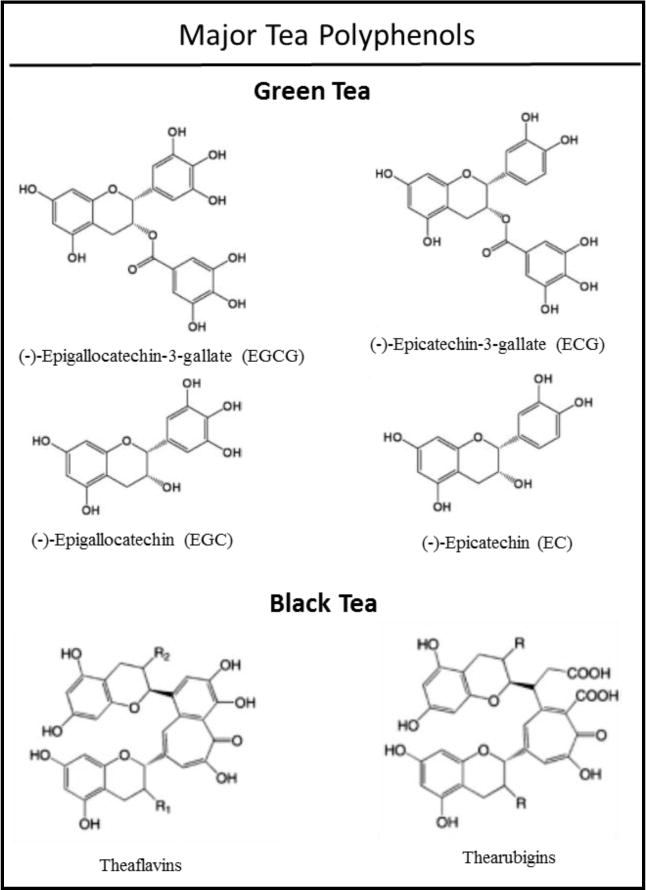
Major tea polyphenols in green and black tea
The focus of this review is to summarize studies on the photoprotective effects of tea polyphenols against UV-induced DNA damage, inflammation, oxidative stress, alterations in cell signaling pathways and epigenetics changes that play a pivotal role in photocarcinogenesis (Table 1, Fig. 2).
Table 1.
Chemopreventive properties of tea polyphenols in skin cancer
| Mechanisms/Activities | Reference |
|---|---|
|
| |
| Inhibition of UVB-mediated DNA damage by IL-12 dependent functional NER mechanism | (20,21) |
| Reduction in UVB-mediated DNA damage through IL-12 dependent induction of DNA repair | (22) |
| Reduction in UVB-mediated formation of CPD+ cells | (19) |
| Inhibition of UVB-mediated increase in COX-2, PGE2, cyclin D1, TNFα, IL-6, IL-1β. | (18) |
| Inhibition of UVB-mediated increase production of PGE2, leukocytes infiltration and myeloperoxidase activity | (27) |
| Inhibition of UVB-mediated phosphorylation of MAPK and activation of NFκB | (39,40) |
| Suppression of UVB-induced depletion of glutathione peroxidase, catalase, and glutathione. Inhibition of UVB-induced LPO and protein oxidation | (33,36) |
| Inhibition of UVB-mediated increase in H2O2 and NO production | (33) |
| Reduction in UVB-mediated phosphorylation of JNK and p38 | (43) |
| Inhibition of UVB-induced AP-1 activation | (45) |
| Inhibition of UVB-induced PI3K signaling | (46) |
| Inactivation of β-catenin signaling | (54) |
| Suppression of p53 expression and apoptosis in UVB irradiated human skin | (59) |
| Inhibition of UVB-induced MMP-1, MMP-8 and MMP-13 production, activation of ASK-1 and phosphorylation of MAPK | (42) |
| Reduction in expression of MMP-3 | (51) |
| Reduction in the gene expression of DNMT1, DNMT3a and DNMT3b | (65) |
| Reduction in Bmi-1 and Ezh2 level, induction in caspase-9, -8 and -3 cleavage, induction in Bax expression and inhibition of Bcl-xL expression | (78) |
| Reduction in the level of PcG proteins including Ezh2, eed, Suz12, Mel18 and Bmi-1 | (79) |
Fig. 2.
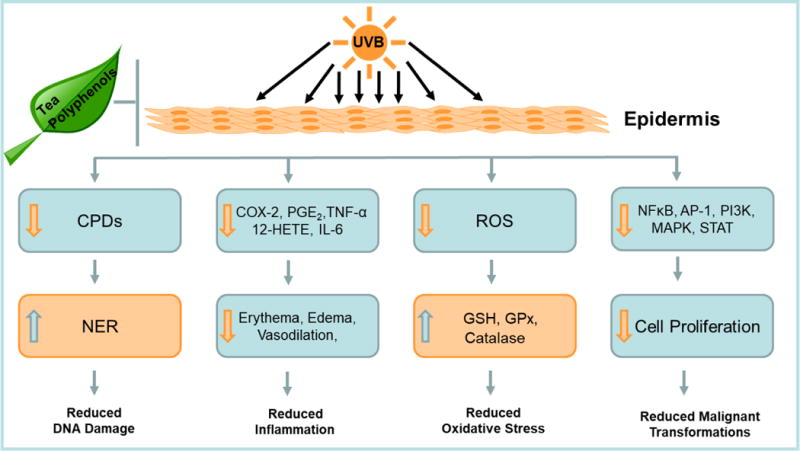
Inhibition of UVB-induced skin cancer by tea polyphenols
Tea polyphenols reduce UVB-mediated DNA damage by enhancing DNA repair mechanism
UVB radiation causes direct DNA damage in skin by inducing the formation of cyclobutane pyrimidine dimers (CPDs), which can initiate mutations if not repaired. CPDs are the most common, most harmful, and least rapidly repaired pre-mutagenic photoproducts formed in mammalian cells (13). Cells repair the damaged DNA through the nucleotide excision repair (NER) mechanism, which removes dimers and seals nicks in DNA. NER is an essential protective mechanism; its importance is highlighted by the enhanced mutagenicity and increased risk for photocarcinogenesis in NER-deficient xeroderma pigmentosum patients (14). Persistent CPDs that are not repaired by NER, or that undergo error-prone repair during replication, can form C→T or CC→TT transitions. These types of mutations are considered “UV signature mutations” and are commonly observed in tumor suppressor genes (15). Therefore, the prevention of the formation of CPDs is accomplished by the use of sunscreens that can block UVB radiation, and enhancing the repair of CPDs through NER may protect against the development of skin cancer.
A study has found that the topical application of green tea or white tea extracts to human skin offered protection from UV damage. The protective effects of the tea extracts were not due to direct UVB absorption or sunscreen effects (16). Topical application of GTPs to human skin prevented UVB-induced CPDs in both the dermis and epidermis (17). Another study that focused on EGCG found that this polyphenol promotes the repair of UVB-induced CPDs. Skin samples from mice obtained after UVB exposure showed a significant time-dependent decline in the number of CPDs in the EGCG-treated mice compared to untreated mice. In addition, a similar effect was observed with orally administered GTPs in mice (18).
The observed enhanced DNA repair was followed by further investigation to determine the effect of GTPs on NER. It was found that mice treated orally with GTPs had an increased mRNA expression of NER-related genes such as XPA, XPC, and RPA1 upon UVB exposure compared to untreated mice. This study suggests that oral GTPs induce certain NER-related genes in response to UVB exposure (19). NER-deficient cells with mutated XPA−/− from xeroderma pigmentosum patients were used to study the extent of NER involvement in GTP-induced DNA repair. After UVB exposure, the number of CPDs was significantly reduced in XPA+/+ cells treated with GTPs but not in treated XPA−/− cells. The study on XPA−/− mice also resulted in similar findings. This indicates that GTP-induced DNA repair requires a functional NER mechanism (19).
Interleukin-12 (IL-12), an immunoregulatory cytokine, has been extensively studied for its anti-tumor activities. It is known to mediate DNA repair through the NER enzymes. Therefore, its role in photoprotection of skin cancer by GTPs and EGCG was examined using IL-12 knockout (KO) mouse models. Topical application of EGCG prevented photocarcinogenesis in wildtype mice, but not in IL-12 KO mice (20). In fact, the UVB-induced DNA damage in the IL-12 KO mice treated with EGCG did not differ from that of the untreated IL-12 KO mice. Oral administration of GTPs demonstrated similar results in mice (18). However, when EGCG-treated IL-12 KO mice were injected subcutaneously with IL-12, the number of UVB-induced CPDs were significantly reduced (21). These findings in murine models suggest that the rapid repair of UVB-induced CPDs by EGCG and GTPs was mediated via IL-12.
Schwarz et al. (22) studied the photoprotective effects of GTPs in normal human epidermal keratinocytes (NHEK). GTPs induced the expression of IL-12 in a dose-dependent manner in NHEK, while preventing apoptosis and reducing DNA damage after UVB exposure. In addition, the reduction of UVB-induced apoptosis in human keratinocytes exposed to GTPs was almost completely reversed by the addition of an anti-IL-12 antibody. These results give strong support to the theory that EGCG and GTPs may exert their chemopreventive effects on UVB-induced skin damage via IL-12 (22).
Tea polyphenols reduce UVB-mediated inflammation and oxidative stress
Skin exposure to UVB radiation results in inflammation that manifest as erythema and edema due to increased vasodilation and vascular permeability. Prolonged UVB exposure leads to chronic inflammation that plays a role in initiation, promotion, and progression of skin cancer (4, 10, 23). Absorbed UVB radiation activates phospholipase A2 enzyme, which releases arachidonic acid from phospholipids in the cell membrane. Arachidonic acid is converted into eicosanoids such as prostaglandins (PGE) and 12-hydroxyeicosatetraenoic acid (12-HETE) by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes, respectively. COX-2 is the rate limiting enzyme in arachidonic acid metabolism, and its expression is upregulated after UVB exposure leading to an increased level of PGE. Exposure of human keratinocytes to UVB radiation resulted in increased COX-2 level (24).
PGE2 is known to have vasodilatory effects and the ability to increase proliferation rate (25). It has been linked with tumorigenesis and metastatic growth as evident by its overexpression in advanced BCC and SCC tissue samples (26). Katiyar et al. (27) investigated the role of EGCG as an anti-inflammatory agent in the prevention of photocarcinogenesis. Skin biopsies were obtained from human volunteers who were exposed to UVB with or without topical treatment of EGCG. It was found that topical treatment of EGCG to human skin significantly reduced erythema compared to untreated skin. In addition, COX activity was reduced in EGCG treated individuals as evident by the reduction in the levels of PG metabolites including PGE2. A study was conducted on healthy human volunteers to determine whether GTPs and its constituents EGCG, EC, and EGC could effectively limit UV-induced photodamage (28). Areas of skin were pretreated with equal concentrations of 5% GTP and its constituents before UV exposure. Among the individual polyphenolic fractions, EGCG and ECG, both with a galloyl group at the 3 position, were most effective in protecting from UV-induced erythema, while, EGC and EC had little effect. In addition, skin treated with GTPs reduced the number of sunburn cells, protected epidermal Langerhans cells from UV damage, and reduced UV-induced DNA damage (28). Another study focused on the efficacy of orally administered GTPs in preventing inflammation. Human volunteers were exposed to UVB before and after treatment with GTPs for 12 weeks. Erythema was significantly reduced in post-treatment exposure compared to pre-treatment exposure. In addition, the concentration of 12-HETE, a potent leukocyte chemoattractant, was reduced in post-treatment compared to pre-treatment exposure (29). Treatment of chronically UVB exposed mice with orally administered GTPs resulted in a reduction in proinflammatory markers (COX-2 and PGE2), and proinflammatory cytokines (TNF-α, IL-1β and IL-6) (18).
After UVB exposure, leukocytes mostly neutrophils, infiltrate the exposed area due to increased vasodilation and vascular permeability. The increased vasodilation is largely attributed to nitric oxide (NO) formation by nitric oxide synthase (iNOS), an enzyme induced by UVB radiation (30). The infiltrating leukocytes generate ROS, which are produced by NADPH oxidase and myeloperoxidase (MPO) (31). A study has shown that treatment of C3H/HeN mice with EGCG inhibited UVB-induced infiltration of leukocytes and MPO activity (32). In addition, EGCG was found to significantly reduce MPO activity in UVB exposed human skin (27). Topical application of EGCG to human skin also effectively reduced the production of NO and hydrogen peroxide (H2O2) (33).
ROS oxidize cell molecules causing oxidative stress, which leads to damaging cellular consequences (23, 34). Cells have innate mechanisms to protect from ROS by inducing endogenous ROS detoxifying enzymes and antioxidant molecules. However, these enzymes/molecules are usually depleted by UVB exposure leading to cellular oxidative damage (4, 23, 34). Tea polyphenols are potent ROS scavengers that can protect the cell from oxidative damage (34). In fact, they are more potent antioxidants than vitamin C and E (35). Therefore, their role as antioxidants was investigated in SKH-1 hairless mice post UVB exposure. Vayalil et al. (36) have shown that topical application of GTPs or EGCG in hydrophilic ointment USP to mice before single or multiple UVB exposures prevented UVB-mediated depletion of glutathione (GSH), glutathione peroxidase (GPx) and catalase. In addition, administration of GTP in drinking water inhibited single or multiple UVB exposure-mediated depletion of antioxidant. Furthmore, treatment of human skin with EGCG prior to UVB radiation exposure restored UVB-mediated decrease in the levels of GSH and GPx enzyme activity (33). Huang et al. (37) demonstrated that ECG possesses photoprotective effects against UV-induced damage. ECG inhibited UV-induced keratinocyte death, and ECG was found to have a similar potency to EGCG. ECG inhibited H2O2 formation in UV irradiated HaCaT keratinocytes, suggesting that ECG can act as a free radical scavenger. Because ECG reduced cell damage mediated by H2O2 and hypoxanthine-xanthine oxidase, the scavenging effect of ECG was further confirmed.
Tea polyphenols downregulate UVB-induced signaling pathways
UVB radiation causes activation of several cellular signaling pathways involved in cell proliferation, inflammation and photocarcinogenesis (5, 38). Studies have shown that exposure of NHEK and mouse skin to UVB radiation resulted in the phosphorylation of mitogen activated protein kinases (MAPK) and activation of nuclear factor kappa B (NFκB) that are involved in oxidative stress, inflammation and skin cancer (39–41). Pretreatment of NHEK with EGCG inhibited UVB-mediated MAPK phosphorylation and activation of NFκB signaling pathway, suggesting a photocheompreventive effect of EGCG (39, 40). Topical application of GTP to SKH-1 hairless mice also resulted in inhibition of UVB-mediated activation of NFκB and IKKα, as well as phosphorylation and degradation of IKBα. By altering the NFκB signaling pathway, GTP acts as a protective agent against photocarcinogenesis (41). A study on human dermal fibroblasts found that EGCG inhibited UVB-induced phosphorylation of MAPK (42). In another study, kim et al. (43) utilized living skin equivalents consisting of epidermal keratinocytes and dermal fibroblasts to study the effects of EGCG on UVB-mediated phosphorylation of MAPK. The living skin equivalents resemble human skin, comprising of organized basal, spinous, granular and cornified epidermal layers (44). Treatment of the living skin equivalents with EGCG reduced UVB-mediated phosphorylation of JNK and p38 (43). One study used a hydrophilic cream based formulation of GTP which was applied to SKH-1 hairless mouse skin before UVB exposure. This resulted in inhibition of UVB-induced phosphorylation of MAPK proteins (36). By decreasing the activation of these signaling pathways, EGCG and GTP may be used to inhibit UVB-induced skin damage and photocarcinogenesis.
UVB exposure also leads to activation of activator protein-1 (AP-1), a transcription factor, which mediates cell proliferation, inflammation, extracellular matrix damage, and skin tumorigenesis (5). Pretreatment of JB6 mouse epidermal cell line with theaflavins or EGCG led to inhibition of UVB-induced AP-1 activity and ERK phosphorylation (45). The effects of tea polyphenols (EGCG or theaflavins) were also investigated on UVB-mediated activation of the PI3K signaling pathway that play an important role in UVB-induced skin carcinogenesis. Pretreatment of mouse epidermal JB6 Cl 41 cells with tea polyphenols inhibited UVB-mediated activation of PI3K, Akt and p70 S6-K (46). By inhibiting these signal transduction pathways, tea polyphenols may be used to inhibit UVB-induced skin carcinogenesis.
Signal transducer and activator of transcription (STAT) modulates several important signal transduction pathways involved with stress, inflammation, and photocarcinogenesis. STAT1 becomes phosphorylated by MAPK proteins. One study demonstrated that UVB radiation induces phosphorylation of STAT1 in mouse epidermal JB6 cells. Further, treatment of these cells with theaflavins or EGCG resulted in inhibition of UVB-induced STAT1 phosphorylation as well as inhibition of ERK and JNK activation (47). By inhibiting phosphorylation of these signal transduction proteins, tea polyphenols may be used as chemopreventive agents against skin cancer. A keloid is a benign skin tumor that extends beyond the borders of the original wound. Several signaling pathways are involved in keloid formation, and keloid fibroblasts contain increased levels of Smad2/3, STAT3, and p38 phosphorylation. Studies have found that suppression of the STAT3 pathway inhibits keloid extracellular matrix production (48). A study on normal and keloid fibroblasts found that EGCG exerts numerous inhibitory effects including decreased proliferation and migration of keloid fibroblasts, inhibition of the STAT3 signaling pathway, and decreased growth and collagen production. The study found that using a JAK2/STAT3 inhibitor and a STAT3 siRNA, which inhibits STAT3 expression, led to decreased proliferation, migration, and collagen production by keloid fibroblasts (49). Therefore, EGCG may be used as a protective agent as well as a treatment option for keloids.
MicroRNAs are small non-coding RNA molecules that function by regulating RNA silencing and gene expression and have been found to play an important role in skin cells. One study found that pretreatment of UVB-exposed normal human dermal fibroblasts with EGCG resulted in a photoprotective effect by regulating specific miRNAs that are involved in gene transcription and cell survival (50). Pretreatment of human dermal fibroblasts with EGCG led to a pronounced suppression of collagen degradation, inhibition of UVB-induced collagenases, MMP-1, -8, and -13 (42). A study on hairless mice found that green, white, and black tea water extracts reduced UVB-mediated epidermal thickness, increased collagen and elastic fiber content, and decreased expression of MMP-3 which normally degrades collagen (51).
The Wnt/β-catenin pathway regulates genes involved in cell proliferation and has been associated with skin cancer (52, 53). Treatment of A431 and SCC13 skin cancer cells with EGCG resulted in inhibition of β-catenin signaling, leading to decreased inflammatory mediators, decreased cell cycle regulatory proteins, and decreased cell survival (54). These findings suggest the role of EGCG as a protective agent against skin carcinogenesis.
Tea polyphenols modulate UVB-mediated apoptosis
The p53 tumor suppressor gene acts as a guardian of the genome by regulating cell cycle, inducing growth arrest, and apoptosis. For example, p53 plays a role in upregulating Bax, a pro-apoptotic protein, and decreasing the levels of Bcl2, an anti-apoptotic protein (55). Mutations in this tumor suppressor gene lead to cells entering the cell cycle without undergoing DNA repair and becoming resistant to apoptosis (56). UVB-exposed SKH-1 hairless mouse skin exhibits mutant p53 positive patches. However, oral feeding of green tea in drinking water to SKH-1 hairless mice inhibited UVB-induced formation of mutant p53 positive patches. Green tea exerts it chemopreventive effect against UVB radiation by inducing apoptosis in early precancerous lesions (57). Another study on SKH-1 hairless mice found that UVB exposure followed by topical application of EGCG led to decreased nonmalignant and malignant skin tumors and increased apoptosis via increased expression of caspase 3 in areas of epidermal dysplasia. By inducing apoptosis in tumors and inhibiting tumor proliferation, EGCG may be used as photoprotective agent against UVB-induced skin carcinogenesis (58).
A study on living skin equivalents found that p53 expression was increased following UVB irradiation, and pretreatment with EGCG resulted in decreased UVB-induced p53 expression (43). A study on human subjects was done in which body lotion containing green tea extract was applied to UVB-exposed human skin. The green tea extract was found to inhibit expression of p53 and therefore prevent apoptosis. The proposed mechanism for this decrease in p53 expression and apoptosis by green tea extract may be due to reduce oxidative stress and also through anti-inflammatory properties (59).
Another marker and regulator of apoptosis is the Fas ligand system. Fas is a death receptor that can become activated by UV radiation and lead to the induction of apoptosis (60). A study on living skin equivalents found that UVB irradiation induced Fas expression, but pretreatment with EGCG resulted in inhibition of UVB-induced expression of Fas (43). This suggests another mechanism by which EGCG may be used in the prevention of UVB-induced human skin damage.
Tea Polyphenols modulate UVB-mediated epigenetic changes
Epigenetic modifications are heritable changes superimposed on the primary DNA sequence. Though many occur spontaneously, some epigenetic modifications are influenced by environmental factors. They may cause changes in gene expression and regulation without altering DNA sequence. In recent years, epigenetic regulatory mechanisms such as DNA methylation, histone modifications, chromatin regulators and microRNAs were found to have a significant role in the initiation and progression of skin cancer (61, 62).
Skin exposure to UVB radiation leads to a significant alteration of epigenetic status. Several studies have shown the overall effects of UVB radiation on epigenetic regulators (63–66). For instance, a frequent epigenetic defect in UVB induced skin cancer seems to be the hypermethylation of tumor suppressor genes and global hypomethylation (67–70). Malignancies with anomalous methylation patterns appear to show significant alterations in their DNA methyltransferases (DNMTs) activity (71–73). DMNTs catalyze the transfer of the methyl group to DNA. Therefore, the inhibition of DNMTs activity in cancer cells can aid in the reversal of gene hypermethylation and subsequently mend the expression of various tumor suppressor genes.
Epigenetic modifications are potentially reversible during cancer initiation and development. Therefore, current research is focused on the epigenetic approaches to cancer therapy and prevention. Quite a few epigenetically active synthetic molecules like DNMTs and histone deacetylase inhibitors are currently being pursued for treatment of various cancers (74, 75). Considering that many of these synthetic inhibitors displayed adverse side effects and are expensive, bioactive phytochemicals and their epigenetic targets have emerged as the focus of research for skin cancer prevention and therapy (76). Recent research has unraveled the potential of dietary polyphenols in inducing chemoprevention based on their capacity to modulate epigenetic alterations in cancer cells. This appears to be a promising prospect from a medical perspective.
Topical treatment of EGCG significantly inhibited UVB induced global DNA hypomethylation pattern in chronically UVB-exposed mouse skin (77). In addition, EGCG treatment reduced tumor incidence, tumor multiplicity, and tumor size. These findings suggest high protection against photocarcinogenesis by EGCG through the alteration of epigenetic pathways. Nandakumar et al. (65) showed EGCG to be an epigenetic modulator that reduced the global DNA methylation levels in human epidermoid carcinoma A431 cells. EGCG decreased the activity of DNMTs as well as mRNA and protein expression of DNMT1, DNMT3a, and DNMT3b in A431 cells. EGCG also decreased methylation of lysine 9 of histone H3, allowing for the transcription of tumor suppressor genes. Furthermore, EGCG treatment increased the mRNA expression of previously silenced tumor suppressor genes such as p16INK4a and Cip1/p21.
Polycomb group proteins such as B-cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1) and enhancer of zeste homolog 2 (Ezh2) are known to be epigenetic regulators of gene expression that enhance cell proliferation and survival. Studies by Eckert group (78, 79) showed that EGCG treatment reduced skin tumor cell survival by inhibiting the expression of Bmi-1 and Ezh2. RECK is a tumor suppressor gene that acts as MMP inhibitor and reduced RECK protein levels correlate with increased metastasis. EGCG was seen to partially reverse the hypermethylation status of the tumor suppressor gene RECK and significantly enhance the expression level of RECK mRNA in oral squamous cell carcinoma cells. In addition, treatment with EGCG inhibited MMP-2 and MMP-9 levels in these cells. These observations suggest a key role of EGCG in suppressing cell invasion through demethylation of MMP inhibitors like RECK (80).
Topical- and Nano-formulations of tea polyphenols to enhance photochemoprevention
GTPs are highly unstable and can become oxidized in the environment, resulting in decreased activity and cutaneous bioavailability. Therefore, special topical formulations can be used to inhibit GTPs deactivation expanding their pharmacological potential. Li et al. (81) emulsified GTPs with carboxymethyl cellulose sodium (CMC-Na) to assess the protective effects of the formulation on UVB-exposed hairless mouse skin. The addition of CMC-Na was found to enhance the stabilization of GTPs. This formulation also resulted in the prevention of various harmful effects of UVB on the skin such as inflammatory cell infiltration, increased skin thickness, reduction of antioxidant enzymes, and lipid peroxidation (81). In addition, sunlight exposure presents a challenge to topical formulations due to its ability to induce the decomposition of EGCG. Therefore, topical creams made of oil-in-water emulsions containing EGCG and co-antioxidants such as vitamin C and α-lipoic acid were tested as a potential method to overcome sunlight induced decompensation. Vitamin C and α-lipoic acid were found to prevent photodecomposition of EGCG by increased photostabilization. In addition, these agents increased the antioxidant activity of EGCG (82).
Another popular approach is to enzymatically enhance the activity of GTPs. For instance, tannin acyl hydrolase, also known as tannase, can be used to hydrolyze polyphenols such as EGCG. Tannase cleaves EGCG to gallic acid and EGC which is known to have a greater antioxidant activity than EGCG. A formula of tannase-converted green tea extract was tested for its photoprotective effects against UVB-induced oxidative stress. This formulation resulted in a significant increase in the reduced form of glutathione. In addition, there was inhibition of H2O2 production, decreased lipid peroxidation, delayed onset of wrinkling, and decreased photoaging (83).
The hydrophilic properties of some GTPs including EGCG presents a great barrier to its delivery through the skin. Therefore, studying innovative drug delivery systems has become a great area of interest. The use of emulsion formulations containing propylene glycol caprylate and polyoxyethylene hydrogenated castor oil for the delivery of polyphenols such as EG, ECG, EGC, and EGCG were found to have an enhanced skin permeation. In addition, the photoprotective effects of emulsion formulations of these polyphenols resulted in an increased cell viability of UVB-exposed EPI-200 and HEK cells (84). Another study used self-double emulsifying drug delivery system containing long chain solid lipids and macadamia oil to deliver EGCG in a transdermal manner. Compared to using an EGCG aqueous solution, this formulation resulted in increased skin diffusion and retention of EGCG (85). The use of lipid based nano-carriers such transfersomes to deliver hydrophilic molecules is another delivery method that has been recently pursued. Avadhani et al. (86) have developed a nano-transfersomal formulation containing EGCG and hyaluronic acid (HA) to permeate the skin and provide antioxidant and anti-aging effects. Transfersomes were made by using hydration and high-pressure homogenization, and then were applied to HaCaT cells. Transfersomes containing EGCG and HA exhibited ROS-scavenging properties, suppression of MMP-2 and MMP-9, and increased skin permeability compared to EGCG alone (86). In addition, core/shell fiber meshes are becoming increasingly popular for applications in drug delivery. In one study, EGCG was loaded into hyaluronic acid/poly(lactic-co-glycolic acid) (HA/PLGA) core/shell fiber meshes to investigate the effectiveness of this formulation as a skin tissue engineering scaffold for the regeneration of skin. The structure of the fiber meshes had three-dimensional interconnected pores, and the EGCG was evenly distributed throughout the shell. Then EGCG release from the formulation was sustained via controlled diffusion and PLGA degradation. It was found that HA/PLGA loaded with EGCG exhibited an increased attachment to human dermal fibroblasts. These results suggest the potential for EGCG-loaded HA/PLGA core/shell fiber meshes to be used for skin regeneration and skin tissue engineering scaffolds (87).
Conclusions and Future Perspectives
The in vitro, in vivo, and human studies unequivocally demonstrated the potential benefits of tea polyphenol in chemoprevention of UVB-induced skin cancer. Tea polyphenols can induce DNA repair, reduce inflammation and oxidative stress, down regulate UVB-induced signaling pathways, and reverse UVB-induced epigenetic changes. In addition, a significant number of these studies have successfully demonstrated the mechanism through which those photoprotective benefits occur. Based on these promising findings, the addition of tea polyphenols into topical products such as sunscreens and creams may enhance protection against the development of UVB-induced skin cancer by reducing the medical and economic burdens of this disease. In order to attain a practical use of tea polyphenols on a commercial scale, the challenges of its delivery must be eliminated by optimizing the dosing and the delivery methods. In addition, we must focus on developing more stable and potent analogs of tea polyphenols.
Acknowledgments
This work was supported by NIH Grants (1R21CA173043 and 1R03CA212798) and ACS-IRG (IRG-60-001-53).
Footnotes
DR FARRUKH AFAQ (Orcid ID : 0000-0001-8612-0879)
Conflicts of Interest: The authors declare no conflicts of interest
References
- 1.Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–335. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 2.McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol. 2008;60:969–976. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- 3.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Montes de Oca MK, Pearlman RL, McClees SF, Strickland R, Afaq F. Phytochemicals for the prevention of photocarcinogenesis. Photochem Photobiol. 2017;93:956–974. doi: 10.1111/php.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 6.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 7.Guy GP, Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 9.DiMarco-Crook C, Xiao H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu Rev Food Sci Technol. 2015;6:505–526. doi: 10.1146/annurev-food-081114-110833. [DOI] [PubMed] [Google Scholar]
- 10.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui IA, Afaq F, Adhami VM, Ahmad N, Mukhtar H. Antioxidants of the beverage tea in promotion of human health. Antioxid Redox Signal. 2004;6:571–582. doi: 10.1089/152308604773934323. [DOI] [PubMed] [Google Scholar]
- 13.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vink AA, Roza L. Biological consequences of cyclobutane pyrimidine dimers. J Photochem Photobiol B. 2001;65:101–104. doi: 10.1016/s1011-1344(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y, Ananthaswamy HN. Molecular mechanisms of photocarcinogenesis. Front Biosci. 2002;7:765–783. doi: 10.2741/matsumur. [DOI] [PubMed] [Google Scholar]
- 16.Camouse MM, Domingo DS, Swain FR, et al. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp Dermatol. 2009;18:522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 17.Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clin Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- 18.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res (Phila) 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 21.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12. Photochem Photobiol. 2008;84:350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 23.Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 25.Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderveen EE, Grekin RC, Swanson NA, Kragballe K. Arachidonic acid metabolites in cutaneous carcinomas. Evidence suggesting that elevated levels of prostaglandins in basal cell carcinomas are associated with an aggressive growth pattern. Arch Dermatol. 1986;122:407–412. doi: 10.1001/archderm.122.4.407. [DOI] [PubMed] [Google Scholar]
- 27.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- 28.Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes LE, Darby G, Massey KA, Clarke KA, Dew TP, Farrar MD, Bennett S, Watson RE, Williamson G, Nicolaou A. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br J Nutr. 2013;110:891–900. doi: 10.1017/S0007114512006071. [DOI] [PubMed] [Google Scholar]
- 30.Suschek CV, Mahotka C, Schnorr O, Kolb-Bachofen V. UVB radiation-mediated expression of inducible nitric oxide synthase activity and the augmenting role of co-induced TNF-alpha in human skin endothelial cells. J Invest Dermatol. 2004;123:950–957. doi: 10.1111/j.0022-202X.2004.23422.x. [DOI] [PubMed] [Google Scholar]
- 31.Björnsdottir H, Welin A, Michaëlsson E, Osla V, Berg S, Christenson K, Sundqvist M, Dahlgren C, Karlsson A, Bylund J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med. 2015;89:1024–1035. doi: 10.1016/j.freeradbiomed.2015.10.398. [DOI] [PubMed] [Google Scholar]
- 32.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–726. (2001) [PubMed] [Google Scholar]
- 33.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012;67:1013–1024. doi: 10.1016/j.jaad.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Intra J, Kuo SM. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal caco-2 cells. Chem Biol Interact. 2007;169:91–99. doi: 10.1016/j.cbi.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 37.Huang CC, Fang JY, Wu WB, Chiang HS, Wei YJ, Hung CF. Protective effects of (−)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296:473–481. doi: 10.1007/s00403-005-0540-5. [DOI] [PubMed] [Google Scholar]
- 38.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 40.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 41.Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 42.Bae JY, Choi JS, Choi YJ, Shin SY, Kang SW, Han SJ, Kang YH. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem Toxicol. 2008;46:1298–1307. doi: 10.1016/j.fct.2007.09.112. [DOI] [PubMed] [Google Scholar]
- 43.Kim SY, Kim DS, Kwon SB, Park ES, Huh CH, Youn SW, Kim SW, Park KC. Protective effects of EGCG on UVB-induced damage in living skin equivalents. Arch Pharm Res. 2005;28:784–790. doi: 10.1007/BF02977343. [DOI] [PubMed] [Google Scholar]
- 44.Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura M, Ma WY, Huang C, Yang CS, Bowden GT, Miyamoto K, Dong Z. Inhibition of ultraviolet B-induced AP-1 activation by theaflavins from black tea. Mol Carcinog. 2000;28:148–155. [PubMed] [Google Scholar]
- 46.Nomura M, Kaji A, He Z, Ma WY, Miyamoto K, Yang CS, Dong Z. Inhibitory mechanisms of tea polyphenols on the ultraviolet B-activated phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2001;276:46624–46631. doi: 10.1074/jbc.M107897200. [DOI] [PubMed] [Google Scholar]
- 47.Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–342. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]
- 48.Lim CP, Phan TT, Lim IJ, Cao X. Stat3 contributes to keloid pathogenesis via promoting collagen production, cell proliferation and migration. Oncogene. 2006;25:5416–5425. doi: 10.1038/sj.onc.1209531. [DOI] [PubMed] [Google Scholar]
- 49.Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh C, You S. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol. 2008;128:2429–2441. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 50.An IS, An S, Park S, Lee SN, Bae S. Involvement of microRNAs in epigallocatechin gallate-mediated UVB protection in human dermal fibroblasts. Oncol Rep. 2013;29:253–259. doi: 10.3892/or.2012.2083. [DOI] [PubMed] [Google Scholar]
- 51.Lee KO, Kim SN, Kim YC. Anti-wrinkle Effects of Water Extracts of Teas in Hairless Mouse. Toxicol Res. 2014;30:283–289. doi: 10.5487/TR.2014.30.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaudhary SC, Singh T, Talwelkar SS, Srivastava RK, Arumugam A, Weng Z, Elmets CA, Afaq F, Kopelovich L, Athar M. Erb-041, an estrogen receptor-β agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by downregulating the WNT signaling pathway. Cancer Prev Res (Phila) 2014;7:186–198. doi: 10.1158/1940-6207.CAPR-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Ji L, Chen J, Zhang W, Ye Z. Wnt/β-catenin signaling pathway in skin carcinogenesis and therapy. Biomed Res Int. 2015;2015:964842. doi: 10.1155/2015/964842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh T, Katiyar SK. Green tea polyphenol, (−)-epigallocatechin-3-gallate, induces toxicity in human skin cancer cells by targeting beta-catenin signaling. Toxicol Appl Pharmacol. 2013;273:418–424. doi: 10.1016/j.taap.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 56.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. (1995) [DOI] [PubMed] [Google Scholar]
- 57.Lu YP, Lou YR, Liao J, Xie JG, Peng QY, Yang CS, Conney AH. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26:1465–1472. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- 58.Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, Huang MT, Conney AH. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99:12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mnich CD, Hoek KS, Virkki LV, Farkas A, Dudli C, Laine E, Urosevic M, Dummer R. Green tea extract reduces induction of p53 and apoptosis in UVB-irradiated human skin independent of transcriptional controls. Exp Dermatol. 2009;18:69–77. doi: 10.1111/j.1600-0625.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 60.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1256. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 61.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Katiyar SK, Singh T, Prasad R, Sun Q, Vaid M. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets. Photochem Photobiol. 2012;88:1066–1074. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murao K, Kubo Y, Ohtani N, Hara E, Arase S. Epigenetic abnormalities in cutaneous squamous cell carcinomas: frequent inactivation of the RB1/p16 and p53 pathways. Br J Dermatol. 2006;155:999–1005. doi: 10.1111/j.1365-2133.2006.07487.x. [DOI] [PubMed] [Google Scholar]
- 65.Nandakumar V, Vaid M, Tollefsbol TO, Katiyar SK. Aberrant DNA hypermethylation patterns lead to transcriptional silencing of tumor suppressor genes in UVB-exposed skin and UVB-induced skin tumors of mice. Carcinogenesis. 2011;32:597–604. doi: 10.1093/carcin/bgq282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Grönniger E, Weber B, Heil O, Peters N, Stäb F, Wenck H, Korn B, Winnefeld M, Lyko F. Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genet. 2010;6:e1000971. doi: 10.1371/journal.pgen.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 68.Plass C, Soloway PD. DNA methylation, imprinting and cancer. Eur J Hum Genet. 2002;10:6–16. doi: 10.1038/sj.ejhg.5200768. [DOI] [PubMed] [Google Scholar]
- 69.Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358–5360. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- 70.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majumdar S, Buckles E, Estrada J, Koochekpour S. Aberrant DNA methylation and prostate cancer. Curr Genomics. 2011;12:486–505. doi: 10.2174/138920211797904061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sproul D, Meehan RR. Genomic insights into cancer-associated aberrant CpG island hypermethylation. Brief Funct Genomics. 2013;12:174–190. doi: 10.1093/bfgp/els063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Micevic G, Theodosakis N, Bosenberg M. Aberrant DNA methylation in melanoma: biomarker and therapeutic opportunities. Clin Epigenetics. 2017;9:34. doi: 10.1186/s13148-017-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller A, Florek M. 5-Azacytidine/Azacitidine. Recent Results Cancer Res. 2010;184:159–170. doi: 10.1007/978-3-642-01222-8_11. [DOI] [PubMed] [Google Scholar]
- 75.Momparler RL, Côté S, Momparler LF. Epigenetic action of decitabine (5-aza-2′-deoxycytidine) is more effective against acute myeloid leukemia than cytotoxic action of cytarabine (ARA-C) Leuk Res. 2013;37:980–984. doi: 10.1016/j.leukres.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Shukla S, Meeran SM, Katiyar SK. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014;355:9–17. doi: 10.1016/j.canlet.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mittal A, Piyathilake C, Hara Y, Katiyar SK. Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5:555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31:496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 79.Choudhury SR, Balasubramanian S, Chew YC, Han B, Marquez VE, Eckert RL. (−)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis. 2011;32:1525–1532. doi: 10.1093/carcin/bgr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kato K, Long NK, Makita H, Toida M, Yamashita T, Hatakeyama D, Hara A, Mori H, Shibata T. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Br J Cancer. 2008;99:647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Jiang N, Liu Q, Gao A, Zhou X, Liang B, Li R, Li Z, Zhu H. Topical treatment of green tea polyphenols emulsified in carboxymethyl cellulose protects against acute ultraviolet light B-induced photodamage in hairless mice. Photochem Photobiol Sci. 2016;15:1264–1271. doi: 10.1039/c6pp00073h. [DOI] [PubMed] [Google Scholar]
- 82.Scalia S, Marchetti N, Bianchi A. Comparative evaluation of different co-antioxidants on the photochemical- and functional-stability of epigallocatechin-3-gallate in topical creams exposed to simulated sunlight. Molecules. 2013;18:574–587. doi: 10.3390/molecules18010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong YH, Jung EY, Shin KS, Kim TY, Yu KW, Chang UJ, Suh HJ. Photoprotective effects of a formulation containing tannase-converted green tea extract against UVB-induced oxidative stress in hairless mice. Appl Biochem Biotechnol. 2012;166:165–175. doi: 10.1007/s12010-011-9413-x. [DOI] [PubMed] [Google Scholar]
- 84.Yoshino S, Mitoma T, Tsuruta K, Todo H, Sugibayashi K. Effect of emulsification on the skin permeation and UV protection of catechin. Pharm Dev Technol. 2014;19:395–400. doi: 10.3109/10837450.2013.788512. [DOI] [PubMed] [Google Scholar]
- 85.Hu C, Gu C, Fang Q, Wang Q, Xia Q. Transdermal solid delivery of epigallocatechin-3-gallate using self-double-emulsifying drug delivery system as vehicle: Formulation, evaluation and vesicle-skin interaction. J Biomater Appl. 2016;30:1080–1091. doi: 10.1177/0885328215617891. [DOI] [PubMed] [Google Scholar]
- 86.Avadhani KS, Manikkath J, Tiwari M, Chandrasekhar M, Godavarthi A, Vidya SM, Hariharapura RC, Kalthur G, Udupa N, Mutalik S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24:61–74. doi: 10.1080/10717544.2016.1228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee EJ, Lee JH, Jin L, Jin OS, Shin YC, Sang JO, Lee J, Hyon SH, Han DW. Hyaluronic acid/poly(lactic-co-glycolic acid) core/shell fiber meshes loaded with epigallocatechin-3-O-gallate as skin tissue engineering scaffolds. J Nanosci Nanotechnol. 2014;14:8458–8463. doi: 10.1166/jnn.2014.9922. [DOI] [PubMed] [Google Scholar]


