Abstract
IN BRIEF A patient-centered approach to device design can provide important advantages in optimizing diabetes care technology for broadened adoption and improved adherence. Results from two surveys of people with diabetes and the parents of children with diabetes (n = 1,348) regarding continuous glucose monitoring (CGM) devices reveal the importance of the concept of “user burden” in patients’ and caregivers’ evaluations of the acceptability of available devices. Survey respondents’ strongly favorable reactions to a proposed 1-year, fully implanted CGM device with no skin-attached components further confirm that minimizing system obtrusiveness will likely be of significant value in reducing hurdles to CGM device use and adherence.
For insulin-using patients, the increased risk of hypoglycemic episodes that accompanies the pursuit of improved glucose control via intensive therapy (i.e., multiple daily injection [MDI] or continuous subcutaneous insulin infusion [insulin pump] therapy with episodic self-monitoring of blood glucose [SMBG]) is well documented (1–3). Although a continuous glucose monitoring (CGM) device can be effective in helping patients reduce both A1C and the incidence of hypoglycemia and thus provide relief from this dilemma, the benefits of CGM require adherence with continuous or near-continuous use of the device (4–17), a use pattern that has only been achievable in a small minority of people with type 1 diabetes. Recent data from patients with type 1 diabetes treated in specialty clinics show CGM adoption ranging from 8 to 17% (18–21).
To provide significant benefits to patient populations, diabetes technologies must be designed to address the barriers to patient adoption that limit dissemination of innovation because the clinical benefits depend on both broad engagement and individual adherence with consistent use (22). So, why is CGM, a clinically beneficial technology, so little used and remaining effectively in an “early adoption” phase of dissemination (22)? One answer to this question may lie in the “user burden” associated with available CGM systems, which includes a requirement for needle insertion through the skin of an indwelling sensor; the wearing and maintenance of a sensor-attached transmitting device adhered to the skin; one or two daily calibrations by SMBG; reinsertion of a new sensor at a different site every 7–14 days; and the carrying of a receiving device. The opinions of patients about the user burden of medical devices play an important role in their decisions regarding utilization (22). Examining such opinions can improve understanding of the extent to which these factors may be limiting utilization and continuous use, thereby aiding efforts to develop new and more user-acceptable CGM system configurations.
Designing technological systems based on the human factors of lifestyle and functional needs is a worthy goal and one that is necessary for broad market adoption (23). For people with diabetes, the promotion of psychological well-being and the optimization of medical outcomes are both essential for improving motivation and ability to perform self-care (24). Without the consideration of human factors in the design of diabetes care devices, those devices may not be widely adopted.
To address these factors with the goal of easing user burden, we have developed and are currently performing clinical trials of a system comprising a long-lived, fully implanted CGM device with no requirement for body-attached elements. The design was based on assessment of the system features most desired by patients to promote a less fettered life while using CGM technology. We report here the results of two separate surveys of people with diabetes and caregivers undertaken to inform the design process. The surveys provide important information regarding patients’ attitudes toward features and functionality of conventional through-the-skin CGM systems and about a new, fully implanted system currently in clinical development (the GlySens® Eclipse® ICGM® System; GlySens, Inc., San Francisco, Calif.).
Methods
We performed two separate, Internet-based surveys of people with diabetes or their caregivers in the United States.
Surveys
Survey 1 was performed in April 2014 by dQ&A Market Research, Inc., of San Francisco, Calif., and enrolled adult participants with type 1 or type 2 diabetes who were using an MDI regimen or an insulin pump. Invitations were sent to 1,093 selected subjects from dQ&A’s Patient Panel; 701 qualified responders were paid $10 each to complete an online questionnaire focused on their impressions of a proposed long-term, fully implanted CGM system.
Survey 2 was performed by the T1D Exchange type 1 diabetes registry and research network and enrolled 533 adults with type 1 diabetes and 114 parents of children (<18 years of age) with type 1 diabetes. Participants were recruited in January and February 2016 from the Glu social media network of about 16,000 individuals and were paid $25 each to participate. In addition to documenting participants’ impressions of the proposed long-term, fully implanted CGM system, Survey 2 also queried participants about their attitudes and use patterns regarding commercially available CGM systems.
Both commissioned surveys were crafted by survey opinion professionals at the respective organizations. Subjects were grouped as either “current CGM users,” “past CGM users,” or “never used CGM.” In Survey 1, never used CGM subjects were restricted to respondents reporting that they perform SMBG ≥3 times/day.
Data Reporting and Statistical Analysis
Results from the two surveys were tabulated separately and expressed as means and SDs or percentages based on the number of subjects responding to a question. In Survey 1, the z test for proportions-independent groups was used for comparisons of multiple groups, and significance was set at 90%. In Survey 2, Fisher’s exact test was used for contingency table comparisons, Student’s t test was used for group data comparison, and Mann-Whitney or Kruskal-Wallis test was used for testing frequency; significance was set at 95%. Statistical analyses were exploratory; no correction for multiple comparisons was applied.
Results
Demographics are shown in Table 1. Both surveys appeared to have a similar selection bias for technology-savvy participants based on the nature of individuals who participate in long-term clinical databases. Both surveys included a high percentage of insulin pump users and participants who either currently used or had quit using CGM. Two-thirds of the adult respondents in each survey were women.
TABLE 1.
Survey Demographics
| Characteristic | Value/Prevalance | ||
|---|---|---|---|
| Survey 1 | Survey 2 | ||
| Adults | Children | ||
| Number | 701 | 533 | 114 |
| Age (years; mean ± SD) | 48.3 ± 13.4 | 45.3 ± 14.8 | 10.7 ± 3.8 |
| Age at diagnosis (years; mean ± SD) | 25.8 ± 14.4 | 20.9 ± 14.0 | 6.9 ± 3.7 |
| Male/female (%) | 36/64 | 32/64 | 49/51 |
| College degree (%) | 62 | 76 | 71* |
| Income >$75,000 (%) | 35 | 55 | 70* |
| Private insurance (%) | 71 | 85 | 93 |
| Current CGM users (%) | 40 | 72 | 70 |
| Past CGM users (%) | 30 | 14 | 8 |
| Never used CGM (%) | 30 | 14 | 22 |
| Insulin pump users (%) | 75 | 84 | 77 |
| MDI users (%) | 25 | 16 | 23 |
| Type 1 diabetes (%) | 80 | 100 | 100 |
| Type 2 diabetes (%) | 20 | 0 | 0 |
Data shown are for children’s parents.
SMBG Frequency (Surveys 1 and 2)
In Survey 1, 90% of all participants reported performing SMBG ≥4 times/day, and there was a tendency for current CGM users to test less than nonusers of CGM (P = 0.081). In Survey 2, 78% of all participants reported performing SMBG ≥3 times/day (current CGM users 5.4 ± 2.6 times/day; past CGM users and nonusers of CGM 6.0 ± 2.5 times/day [P <0.02]).
CGM Use Patterns (Survey 2 Only)
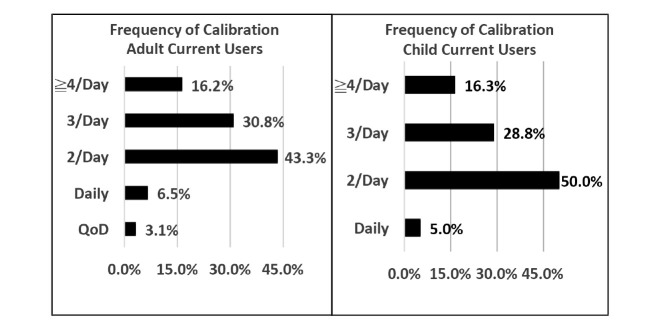
A high percentage of current CGM users reported using CGM 4 weeks/month (87% in adults and 80% in children). Figure 1 shows current CGM users’ frequency of calibration, with 47% of adults and 45% of parents reporting that they calibrate more than the recommended 2 times/day. The average number of reported calibrations per day was 2.4 for Dexcom CGM users and 3.1 for Medtronic CGM users (P <0.05).
FIGURE 1.
Frequency of CGM calibration reported in Survey 2 for adults and children currently using CGM. Among adult participants, average calibration frequency for insulin pump users (2.54 times/day) was not different from the frequency reported by those using an MDI regimen (2.27 times/day).
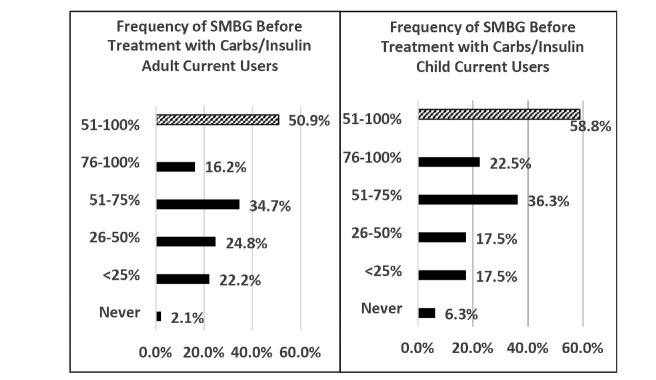
Figure 2 shows the percentage of time participants reported using SMBG to confirm a CGM reading before treating a glucose level with insulin or carbohydrate. About half of current CGM users reported checking SMBG at least half of the time before intervention.
FIGURE 2.
Frequency of SMBG before treatment with carbohydrates or insulin reported in Survey 2. Participants were asked what percentage of time in quartiles they use SMBG to confirm a result before treating (solid bars). About half reported checking SMBG before treatment at least half of the time (hatched bar).
Reasons for CGM Use (Survey 2 Only)
Adult and parent participants respectively rated factors as extremely or very important for starting CGM use as follows: better control, 83 and 83%; lower A1C, 64 and 54%; avoiding hypoglycemia or hypoglycemia unawareness 59 and 53%; and clinician recommendation, 42 and 27%. Other factors were much less frequently rated as important.
Important Features in CGM Use and Nonuse (Survey 2 Only)
Among participants who had never used CGM, by far the most important impediments selected as reasons for not trying CGM were cost, having a device attached to the body, and expectations of discomfort in wearing (Table 2). Men and women ranked these impediments in the same order of importance.
TABLE 2.
Reasons Given in Survey 2 for Not Trying CGM
| Participants (%)* | |
|---|---|
| Too expensive | 55.3 |
| Not covered by insurance | 39.5 |
| Likely uncomfortable | 35.5 |
| Device attached to body | 27.6 |
| Satisfied with SMBG | 13.2 |
| Not as accurate as SMBG | 10.5 |
| Too painful to wear | 9.2 |
| Possibility of infection | 9.2 |
| Not familiar with CGM | 9.2 |
| FDA: adjunctive use only | 5.3 |
Percentage of adult subjects indicating that a reason for not trying CGM was “important.”
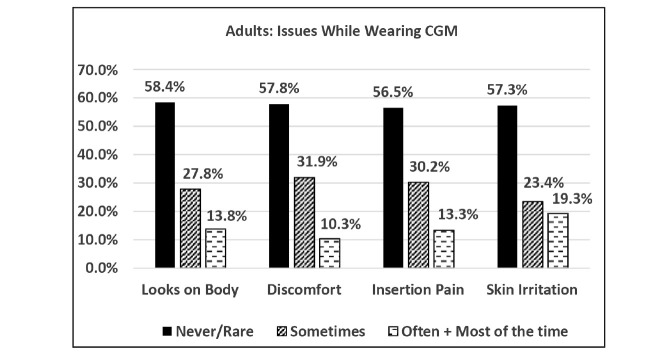
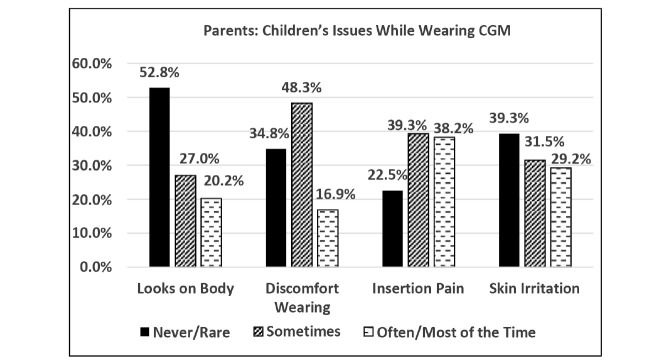
We queried adult and parent current and past CGM users regarding their satisfaction with the following features of CGM devices: alarm customization, battery life, sensor insertion, frequency of calibration, menu navigation, range, receiver size, sensor life span, and transmitter/sensor size. “Satisfied” or “very satisfied” ratings for each of these features were reported by 65–90% of participants. However, current and past CGM users reported having problems sometimes or often with the appearance of CGM on the body, discomfort while wearing, insertion pain, and skin irritation; parents reported problems occurring with children more frequently than was reported by adults (Figures 3 and 4). The reported frequencies of body image and discomfort issues were significantly greater in adult women than in men (P <0.002). Adult past users of CGM were asked to rate the importance of 21 possible reasons for stopping; the most frequent involved obtrusiveness (i.e., discomfort, skin irritation, sensor change requirements, and adhesive failure), cost, and too-frequent alarms (Table 3). Of these, only pain and sensor change requirements were significantly more important reasons for stopping among women than men.
FIGURE 3.
Frequency of reported problems among adult current and past CGM users in Survey 2.
FIGURE 4.
Frequency of reported problems among parents of children who were current or past CGM users in Survey 2.
TABLE 3.
Reasons Given for Stopping CGM Use by Adult Past Users of CGM in Survey 2
| Participants Rating “Very” or “Extremely” Important (%)* | |
|---|---|
| Not as accurate as SMBG | 53 |
| Not covered by insurance | 52 |
| Uncomfortable to wear | 47 |
| Too many false alarms | 46 |
| Too expensive | 45 |
| Skin irritated by adhesive | 41 |
| Need for SMBG before treatment | 40 |
| Adhesive did not hold | 38 |
| Frequency of sensor change | 37 |
| Alarmed too frequently | 36 |
| Sensor application painful | 31 |
| Too frequent calibration | 30 |
| Not enough advice on use | 30 |
| Difficulty applying sensor | 28 |
| Too invasive | 23 |
| Interfered with activities | 22 |
| How sensor looked on body | 18 |
| Too complicated to use | 17 |
| Too complicated | 13 |
| Concern about infection | 10 |
| Not approved for dosing | 9 |
Subjects selected from five levels of importance for each reason: extremely, very, somewhat, slightly, or not at all important. The percentage of patients reporting extremely or very important is shown.
Attitudes Toward Features of a Fully Implanted CGM System (Surveys 1 and 2)
Without identifying the manufacturer/sponsor, participants were presented with a description of a fully implanted CGM system with features representative of the intended characteristics of the system currently in commercial development by GlySens, Inc. The full text of the survey description language is provided in the Supplementary Appendix. Briefly, the system was described as comprising a fully implanted sensor (i.e., no on-the-skin element) and an external receiver/display unit. The implant site was described as being under the skin below the bathing suit line, with a 15-minute insertion procedure accomplished in a doctor’s office. The sensor was described as functioning for 1 year, after which it could be removed and replaced in a repeat procedure, and the system was expected to require calibration once or twice per month. Both surveys provided sensor dimensions and presented the photograph shown in Figure 5. No assessment of accuracy was provided.
FIGURE 5.
Photograph of the self-contained, implanted CGM model shown to participants in Surveys 1 and 2.
After receiving the description of the fully implanted system, participants in Survey 1 were asked: “If this implanted-sensor CGM system was approved by the FDA [U.S. Food and Drug Administration], suggested by your doctor, and covered by your insurance, how likely would you be to get and use the implanted glucose monitor?” Those in Survey 2 were asked the same question with “get and use” revised to “switch to” for current CGM users, “resume use with” for past CGM users, and “start using” for those who had never used CGM. Participants were given four answer choices: definitely yes, likely, unlikely, and definitely not.
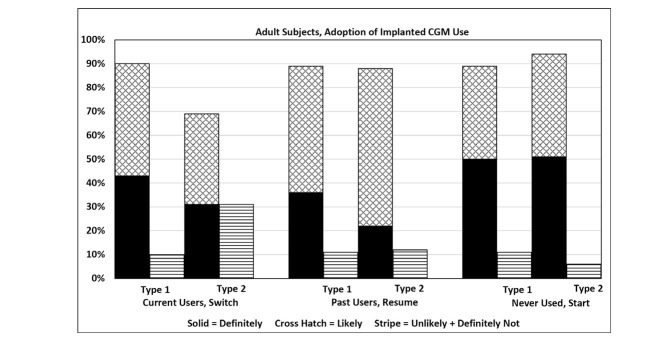
Survey 1 participants’ attitudes about potential use of a fully implanted CGM system are shown in Figure 6. A large majority of participants indicated that they would be likely to or definitely would adopt the described system. Survey 1 and Survey 2 participants’ attitudes are summarized in Table 4; the results from the two surveys for participants with type 1 diabetes were nearly identical. Results were equivalent in all age-groups and for both men and women (data not shown). The majority of patients with type 2 diabetes in Survey 1 also indicated likely or definite adoption of the proposed CGM system.
FIGURE 6.
Likelihood of adoption of the described fully implanted CGM device among adult participants in Survey 1.
TABLE 4.
Participants’ Reported Likelihood of Adopting and Using the Described Implanted CGM Device in Surveys 1 and 2
| Percentage Identifying | |||||
|---|---|---|---|---|---|
| Definitely Yes | Likely | Definitely + Likely | Unlikely | Definitely Not | |
| Survey 1: Adults with type 1 diabetes | |||||
| Current user/switching (n = 266) | 43 | 47 | 90 | 8 | 1 |
| Past user/resuming (n = 174) | 36 | 53 | 89 | 10 | 1 |
| Nonuser/starting (n = 122) | 50 | 39 | 89 | 8 | 2 |
| Survey 1: Adults with type 2 diabetes | |||||
| Current user/switching (n = 16) | 31 | 38 | 69 | 31 | 0 |
| Past user/resuming (n = 32) | 22 | 66 | 88 | 6 | 6 |
| Nonuser/starting (n = 91) | 51 | 43 | 94 | 5 | 1 |
| Survey 2: Adults with type 1 diabetes | |||||
| Current user/switching (n = 383) | 44 | 42 | 86 | 11 | 3 |
| Past user/resuming (n = 76) | 63 | 30 | 93 | 7 | 0 |
| Nonuser/starting (n = 74) | 49 | 35 | 84 | 12 | 4 |
| Survey 2: Parents of children with type 1 diabetes | |||||
| Current user/switching (n = 80) | 30 | 49 | 79 | 21 | 0 |
| Past user/resuming (n = 9) | 22 | 56 | 78 | 22 | 0 |
| Nonuser/starting (n = 25) | 36 | 44 | 80 | 16 | 4 |
Subjects who had never used CGM were also provided with a description of currently marketed percutaneous CGM devices and were queried about which system they preferred (conventional CGM vs. the described fully implanted system). The fully implanted system was preferred by 92% of participants in Survey 1 and 86% of those in Survey 2. In Survey 2, past CGM users were asked which they would prefer if they resumed use; 93% preferred the fully implanted system, whereas 7% preferred the currently available systems. Among CGM past users, combined reported definite and likely adoption of the proposed system was greater among insulin pump users than among those with MDI regimens (93 vs. 73%, P <0.05).
In Survey 1 among subjects reporting likely or definite adoption of the fully implanted system, ranking of the “most appealing” feature was: 12-month operation (46%), nothing worn on the body (32%), and calibration frequency (12%). In Survey 2, subjects ranked the fully implanted sensor’s features from 1 (liked best) to 5 (liked least). The ranking of the first two most-liked features was the same as in Survey 1.
In Survey 1 among subjects who were unlikely to or would definitely not use the fully implanted system, ranking of least appealing feature was surgery required for implant (60%), out-of-pocket costs might be high (15%), and sensor size and possible problem requiring removal (12%). The ranking of greatest concerns was similar in Survey 2.
Discussion
Survey 1 was commissioned by GlySens, Inc., to inform the development process for a long-term, fully implanted CGM system by assessing the attitudes about its proposed features and potential for adoption of the described system. Survey 2 was commissioned to confirm the findings of Survey 1 in a different participant population and to provide additional details regarding attitudes about current CGM systems. Both surveys enrolled participants likely to have an above-average level of technology savviness, a known selection bias.
What Did the Surveys Find About Attitudes Toward, and Reported Clinical Use Patterns of, CGM in This Population?
As expected, adult subjects and parents reported trying CGM to improve glycemic control and avoid hypoglycemia. However, clinician recommendation was cited by only 42% of adult subjects and 27% of parents as an important reason to try CGM. Clinicians may be reluctant to recommend a device that a majority of patients are unwilling to use and for which insurance reimbursement may not be adequate to support the time and effort involved (7,16,18–20).
Survey participants reported extensive nonadjunctive use of CGM to dose insulin or carbohydrate (Figure 2); this use was contrary to manufacturer labeling at the time these surveys were fielded. It is difficult to predict the impact of the recent FDA approval for labeling CGM for insulin dosing on utilization and reimbursement.
Frequency of reported calibration for both adults and children significantly exceeded FDA-approved label instructions (Figure 1). Some users may have developed an inherent distrust of the calibration stability and calibrated at increased frequency, or perhaps some subjects maintained a general attitude of “the more calibrations, the better.” Some of the reported calibrations likely derived from fingerstick confirmation of a CGM reading; if there was a significant difference between the two, subjects could have been motivated to recalibrate. Unfortunately, SMBG measurements taken for CGM confirmation before intervention may often be made during a period of rapidly changing glucose. CGM-SMBG differences may be increased during such episodes due to time lags inherent with CGM and could result in less accurate calibrations. Some of the attitudes about CGM inaccuracy compared to SMBG may have been influenced by the effects of CGM time lag; it would be of interest to have more specific information to clarify this issue (25). Our results are compatible with published findings indicating that reduced frequency of SMBG is not a strong motivation for CGM use (6,14,18).
What Attitudes of These Technology-Savvy Participants Might Affect CGM Use Patterns?
Subjects reported obtrusiveness (i.e., skin-attached components, pain, discomfort, and skin irritation), impact on body image, and cost as principal reasons for not trying CGM or for stopping its use. Smaller, less obtrusive, skin-applied CGM sensors currently in development might reduce the significance of these impediments. However, our survey implies that complete elimination of these factors could potentially increase use to nearly 90%, at least among these technology-savvy respondents. Current and past CGM users reported relatively high satisfaction with operational features, including sensor insertion. However, when they were queried about the frequency of problems, their responses indicated a significantly negative device tolerance profile (Figures 3 and 4), similar to previous reports (13,16,17,20,26). One possible interpretation is that people with diabetes are willing (at least initially) to tolerate otherwise obtrusive features of CGM because of their tremendous motivation to improve their health. It could be interesting to query past users about whether a lack of improvement in glycemic control was a reason for stopping CGM use. Our surveys did not include this question.
Recent improvements in CGM accuracy, including the availability of devices labeled for limited, nonadjunctive use, might increase the use of body-adhered CGM devices and better enable glycemic control. However, the surveys do not provide sufficient information to make such predictions. Recent results from another survey of adult participants from the T1D Exchange found attitudes about current CGM systems similar to those reported here; its major conclusion was that “efforts to increase device use need to target physical barriers to wearing devices” (27). Our findings confirm that physical barriers (i.e., obtrusiveness) are critical impediments to CGM use for both adults and children. Diffusion of Innovation Theory (DIT) indicates that CGM is in the “early-adoption” phase, and the process of adoption depends strongly on population attitudes and experiences more than on potential health benefits; decisions regarding adoption of use are highly dependent on subjects’ impressions and human-use factors (22). Because the clinical benefit of CGM systems as a class of devices has been reasonably well established, a system that reduces obtrusive physical barriers would be expected to foster more widespread adoption.
What Are the Attitudes of Technology-Savvy Participants Toward a Fully Implanted CGM Device?
The positive response for likely or definite use of a fully implanted CGM system suggests that the described features were viewed quite favorably. The positive attitude toward the described features is further supported by current CGM users indicating that they would likely or definitely switch to a fully implanted system. It is important to point out that our surveys only assessed attitudes toward the system features as described, which did not include data on accuracy or detailed cost factors; these surveys were not market research instruments aimed at positioning a currently available product. Nonetheless, it appears that a long-lived, fully implanted sensor lacking any skin-attached components may overcome some of the major barriers that have heretofore limited the use of CGM.
What Additional Aspects of the Described Fully Implanted CGM System Might Influence User Attitudes?
Cost was identified as a factor in CGM use (Table 3), and the presence of significant barriers, restrictions, and required efforts to gain approval for insurance coverage of CGM are well documented (13,16,17,20). Our description of the fully implanted CGM system posited that the sensor would be “covered by insurance,” but the extent of insurance coverage was not presented in detail. A number of published studies have estimated considerable cost savings for payers from the use of CGM (28–30), but to date, no prospective, randomized, direct cost outcome study has been reported. Such a study may be needed to more completely address this important barrier.
The surveys identified other concerning features for the parents of children with diabetes. The size of the sensor for younger children and the surgical procedure required for implantation were cited as negatives by the 20–22% of parents who identified as being not likely to use the proposed system, and in written comments, several parents indicated that broadcast range (described in the surveys as 9 feet) from the CGM sensor to the receiver would be a problem for young children “running around the house.” Connectivity with other devices (e.g., for remote monitoring by parents or caregivers and data exchanges with smartphones, insulin pumps, insulin pens, and Cloud-based databases) will undoubtedly be an expected feature in next-generation devices. Additionally, automated insulin delivery is predicted to be widely adopted in the future, and connectivity will be an essential requirement for any CGM component in such systems.
Adherence to near-continuous use of CGM is required to achieve key medical benefits (4,6,9,10,14,16,17,25). A fully implanted CGM sensor functioning continuously with minimal maintenance would offer important advantages to help in achieving 100% adherence, provided a patient has a receiver and uses it. Furthermore, the most-liked feature of the described fully implanted system was its operation for 12 months, suggesting that subjects appreciated the potential of the system to enable adherence to continuous use. Consistent with principles of DIT, participants’ positive attitudes toward the described fully implanted system might be expected to portend broad dissemination of use.
What Are the Limitations of These Surveys?
The technology-savvy selection bias and willingness to try technology among current and past CGM users may have influenced their positive attitudes toward the described fully implanted system. However, the high rates of reporting likely or definite use of such a system by those who have never used CGM suggests that the described features could change their attitudes toward this technology. Accuracy was a concern of both past users and nonusers, and the accuracy of the fully implanted system was not stated because data on this are not yet available. The accuracy of currently available CGM systems has recently improved significantly. Our surveys could not measure how improved accuracy would affect attitudes about current CGM systems. Acceptable accuracy will be important for the adoption of any CGM system.
Conclusion
The surveys we performed confirm that user-obtrusive aspects of current CGM systems, along with cost and accuracy considerations, are significant barriers to the adoption of and adherence to CGM systems even among technology-savvy patients. The features described in our surveys of a long-lived, fully implanted CGM system with no skin-attached elements were perceived as highly desirable by patients and could improve attitudes toward CGM use, provided that out-of-pocket costs are perceived as reasonable and accuracy is sufficient.
Supplementary Material
Funding
Survey 1 was performed by dQ&A, and Survey 2 was performed by the T1D Exchange. Both were contracted and paid for by GlySens, Inc.
Duality of Interest
R.E. is on the board of directors of and T.L.R. and J.Y.L. are employees of GlySens, Inc., and all authors have an equity interest or stock options in the company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
All three authors and the survey contractors contributed to the questionnaire design. R.E. wrote the manuscript and analyzed the data. T.L.R. and J.Y.L. edited the manuscript. R.E. is the guarantor of this work and, as such, had full access to the survey data supplied by dQ&A and the T1D Exchange and takes responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
This article contains supplementary material online. (http://clinical.diabetesjournals.org/lookup/suppl/doi:10.2337/cd17-0053/-/DC1)
References
- 1.DCCT Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM; DCCT/EDIC Research Group . The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study at 30 years: overview. Diabetes Care 2014;37:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DCCT/EDIC Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.JDRF Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 5.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.JDRF Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JDRF Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klonoff DC, Buckingham B, Christiansen JS, et al. . Continuous glucose monitoring: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:2968–2979 [DOI] [PubMed] [Google Scholar]
- 9.JDRF Continuous Glucose Monitoring Study Group Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009;32:1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard S, Hanaire H, Baillot-Rudoni S, et al. . Evaluation of the adherence to continuous glucose monitoring in the management of type 1 diabetes patients on sensor-augmented pump therapy: the SENLOCOR study. Diabetes Technol Ther 2016;18:127–135 [DOI] [PubMed] [Google Scholar]
- 11.Battelino T, Conget I, Olsen B, et al. . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.New JP, Ajjan R, Pfeiffer AF, Freckmann G. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med 2015;32:609–617 [DOI] [PubMed] [Google Scholar]
- 13.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care 2005;28:1231–1239 [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain JJ, Dopita D, Gilgen E, Neuman A. Impact of frequent and persistent use of continuous glucose monitoring (CGM) on hypoglycemia fear, frequency of emergency medical treatment, and SMBG frequency after one year. J Diabetes Sci Technol 2015;10:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster NC, Miller KM, Tamborlane WV, Bergenstal RM, Beck RW; T1D Exchange Clinic Network. Continuous glucose monitoring in patients with type 1 diabetes using insulin injections. Diabetes Care 2016;39:e81–e82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016;18(Suppl. 2):S3–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.T1D Exchange Clinic Network Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinical Network. Current state of type 1 diabetes treatment in the U.S: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 20.Anhalt H. Limitations of continuous glucose monitor usage. Diabetes Technol Ther 2016;18:115–117 [DOI] [PubMed] [Google Scholar]
- 21.T1D Exchange Why do some people with T1D stop using a pump and CGM? [Internet] Available from https://t1dexchange.org/pages/why-do-some-people-with-t1d-stop-using-a-pump-and-cgm. Accessed 31 October 2017.
- 22.Gonder-Frederic LA, Shepard JA, Grabman JH, Ritterband LM. Psychology, technology, and diabetes management. Am Psychol 2016;71:577–589 [DOI] [PubMed] [Google Scholar]
- 23.Norman DA. The Design of Everyday Things: Revised and Expanded Edition. Basic Books, New York, N.Y, Perseus Books Group, 2013 [Google Scholar]
- 24.Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariani HS, Layden BT, Aleppo G. Continuous glucose monitoring: a perspective on its past, present, and future applications for diabetes management. Clin Diabetes 2017;35:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivers JP, Mackowiak L, Anhalt H, Zisser H. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol 2013;3:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hook KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.JDRF Continuous Glucose Monitoring Study Group The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care 2010;33:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roze S, Smith-Palmer J, Valentine WJ, et al. . Long-term health economic benefits of sensor-augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: a U.K. perspective. J Med Econ 2016;19:236–242 [DOI] [PubMed] [Google Scholar]
- 30.Vigersky RA. The benefits, limitation, and cost effectiveness of advanced technologies in the management of patients with diabetes mellitus. J Diabetes Sci Technol 2015;9:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.