Abstract
Objective
Data on the clinical activity of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer (NSCLC) and uncommon EGFR mutations remain insufficient. This study aimed to investigate the effect of first-line EGFR-TKIs or platinum-based chemotherapy in NSCLC patients with uncommon EGFR mutations.
Methods
We retrospectively enrolled 504 patients with EGFR-mutant NSCLC. The clinical characteristics and treatment outcomes were collected and compared between patients with common and uncommon EGFR-mutant NSCLC.
Results
Seventy patients (13.9%) harboring uncommon EGFR mutations were included. Thirty of these patients received EGFR-TKIs and 40 received platinum-based chemotherapy as first-line therapy. The objective response rate (ORR) and median progression-free survival (mPFS) of patients treated with TKIs in the uncommon mutation group was significantly inferior to that in the common mutation group (ORR: 23.3% vs. 51.8%, P=0.003; mPFS: 7.1 vs. 10.9 months, P<0.001). In the uncommon group, mPFS was similar between first-line EGFR-TKIs treatment and platinum-based chemotherapy (7.1vs. 6.1 months, P=0.893). In patients with EGFR G719X or L861Q mutations, the mPFS was longer in the first-line EGFR-TKIs treatment group than in the chemotherapy group, but the difference was not statistically significant (G719X: 8.2 vs. 5.8 months, P=0.061; L861Q: 7.6 vs. 4.1 months, P=0.872). Multivariate analyses identified adenocarcinoma (P=0.003) as the independent predictive factor for PFS in patients with uncommon EGFR mutations who were treated with first-line EGFR-TKIs.
Conclusions
The current study demonstrated that the effect of first-line EGFR-TKIs was similar to that of platinum-based chemotherapy in patients with uncommon EGFR-mutant NSCLC. Adenocarcinoma was the independent predictive factor for PFS in uncommon EGFR-mutant NSCLC patients treated with first-line EGFR-TKIs.
Keywords: EGFR, uncommon mutation, tyrosine kinase inhibitors, chemotherapy
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Non-small-cell lung cancer (NSCLC) accounts for about 85% of all lung malignancies (2). Molecular targeted therapy, especially epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), significantly prolong progression-free survival (PFS) and improve the quality of life for patients with advanced NSCLC who harbor EGFR mutations (3,4). Two major types of mutations (common EGFR mutations), in-frame deletion in exon 19 (19DEL) and L858R point mutation in exon 21, constitute approximately 50% to 90% of total cases (5-9) and have been repeatedly confirmed as mutations that are sensitive to EGFR-TKIs. Currently, the four kinds of EGFR-TKIs, gefitinib, erlotinib, afatinib, and icotinib, are commonly recommended as standard first-line therapies for patients harboring sensitizing EGFR mutations because the response rate is around 70% to 80% and PFS reaches approximately 10–12 months (6,10,11).
Other than these two common EGFR mutations, mutations involving exon 20, insertion in exon 20 (20INS), and primary T790M point mutation in exon 20 (T790M) are considered to be the primary resistance to EGFR-TKIs, with an objective response rate (ORR) of approximately 10% and a PFS of approximately 2.5 months (12-18). In addition, there are many categories of uncommon EGFR mutations in exons 18–21, such as G719X, L861Q, S768I, and so on, which are also part of the spectrum of EGFR mutations (7,8). Due to the limited number of cases, no prospective and large-scale clinical trials have investigated the clinical activity of EGFR-TKIs in patients with uncommon EGFR mutations. Data from several retrospective studies remain controversial and insufficient. Hence, we performed this study to evaluate the clinical features and effect of first-line EGFR-TKIs treatment and platinum-based chemotherapy in patients with uncommon EGFR mutations. Mutations such as G719X and L861Q have been analyzed in many institutions (9,19) and also have been reported in small case numbers, and their influences on the effectiveness of first-line EGFR-TKIs or platinum-based chemotherapy have not been fully elucidated. In this study, we also investigated the clinical significance of uncommon mutations and the efficacy of first-line EGFR-TKIs treatment and platinum-based chemotherapy in this selected group of patients to increase understanding of the entire EGFR mutation spectrum.
Materials and methods
Patient inclusion
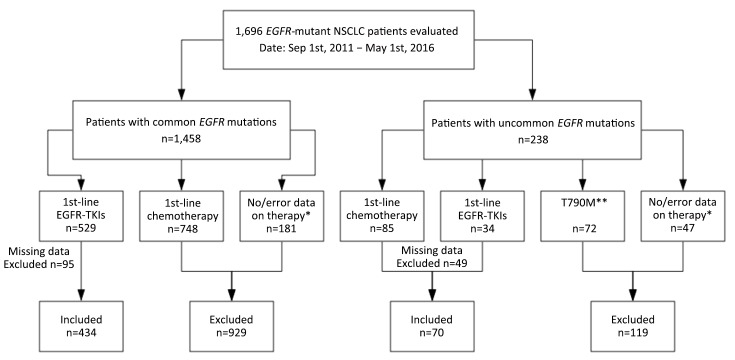
We retrospectively evaluated a cohort of 1,696 EGFR-mutant NSCLC patients from the Department of Medical Oncology at Shanghai Pulmonary Hospital, Tongji University between September 2011 and May 2016. Patients with missing data on baseline clinicopathological characteristics and follow-up were excluded. The study enrolled a total of 504 patients who had been definitively diagnosed with common EGFR-mutant NSCLC and were being treated with first-line EGFR-TKIs or had been definitively diagnosed with uncommon EGFR-mutant NSCLC and were being treated with first-line EGFR-TKIs or platinum-based chemotherapy. For all 504 patients, data on baseline clinicopathological characteristics [including age, sex, smoking history, histology, TNM stage, and Eastern Cooperative Oncology Group performance status (ECOG PS)], treatments, and clinical outcomes were extracted from the electronic medical records. Never smokers were defined as persons who had smoked less than 100 cigarettes during their lifetime. Age and smoking status were documented at the time of diagnosis. This study was approved by the Ethics Committee and Institutional Review Board of Shanghai Pulmonary Hospital. A flowchart of patient inclusion is presented in Supplementary Figure S1.
S1.
A flowchart of patient cohort. NSCLC, non-small cell lung cancer; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; *, patients who: 1) only accepted epidermal growth factor receptor (EGFR) mutation status tests but refused following standard therapies, or 2) transferred to local hospitals for continuous therapies but standardized treatments cannot be guaranteed; **, post-treatment T790M mutations which are not included in our analyses.
Analysis of EGFR mutation
All mutation analyses were performed at the Thoracic Cancer Institute of the Tongji University School of Medicine in Shanghai, China. Briefly, DNA from tissue was extracted using the DNeasy Blood and Tissue Kit or the QIAamp DNA FFPE Tissue Kit (both from Qiagen, Hilden, Germany). EGFR mutations were detected by an amplification refractory mutation system as described in our previous studies (AmoyDx Co. Ltd., Xiamen, China) (20-23). All assays are designed to identify deletions or insertions in EGFR exons 19 and 20 and hot spot mutations in EGFR exons 18 through 21. For analytical purposes, 19DEL and L858R point mutations in exon 21 are referred to as common EGFR mutations. The post-treatment T790M mutations are not included in our analyses, nor are common EGFR polymorphisms.
Treatment and outcomes
All patients harboring common mutations and some patients harboring uncommon mutations were treated with EGFR-TKIs, including gefitinib (250 mg daily), erlotinib (150 mg daily), afatinib (40 mg daily), and icotinib (125 mg three times daily). The remaining patients harboring uncommon mutations were treated with platinum-based chemotherapy. Not until tumor progression, death, significant uncontrolled toxicity, or patient refusal was treatment stopped. Chest computed tomography scans were taken after the first month of treatment and every 4–8 weeks subsequently as a routine clinical procedure and to confirm the treatment response and assess disease progression. Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1) (24). ORR was reported as the proportion of patients with complete or partial response, and the disease control rate (DCR) was calculated as the proportion of patients with an objective response or stable disease (for at least 6 weeks). PFS was calculated as the time from the first day of treatment until progression of disease or date of death (from any cause). Patients who were alive at the cutoff date (November 20, 2016) or who were lost to follow-up were censored at the last date of follow-up.
Statistical analysis
Statistical analyses of categorical variables were performed with Pearson’s χ2 test or Fisher’s exact test, where appropriate. The Kaplan-Meier method was used for survival analyses, and the log-rank test was used to test for significance. Cox regression was used to analyze independent factors. P<0.05 was considered statistically significant, and 95% confidence intervals (95% CIs) were calculated. Statistical analyses were conducted using SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics
A total of 504 patients were enrolled in this study. Among them, 70 (13.9%) harbored uncommon EGFR mutations. The median age at diagnosis was 60 (range, 22–85) years. Two hundred and twenty-one (43.8%) patients were male, and 128 (25.4%) were current or former smokers. Most patients (455/504, 90.3%) were evaluated as initial stage IIIb/IV, and 275 (54.6%) were diagnosed with no less than one distant metastasis. The majority of histological types were adenocarcinoma (471/504, 93.5%). From the perspective of ECOG PS, 469 (93.1%) patients were evaluated as 0–1 at diagnosis, and 35 (6.9%) were evaluated as more than 2.
A total of 518 mutations were identified in the 504 patients. The most frequent mutation was 19DEL (43.2%), followed by L858R (41.7%), G719X (5.2%), 20INS (4.1%), L861Q (3.3%), S768I (1.7%), and T790M (0.8%). The frequency of uncommon mutations was 15.1% (78/518). Uncommon EGFR mutations were associated with male sex (P=0.003) and current/former smokers (P<0.001). The detailed clinicopathological characteristics of the 504 patients and the overall frequency ofEGFR mutations are presented in Table 1.
1.
Clinicopathological characteristics of 504 patients
| Variables | All | EGFR mutation status [n (%)] | P | |
| Common (N=434) | Uncommon (N=70) | |||
| EGFR, epidermal growth factor receptor; NE, not evaluable; ADC, adenocarcinoma; SQC, squamous carcinoma; NSCLC-NOS, non-small cell lung cancer-not otherwise specified; ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor; 19DEL, in-frame deletion in exon 19; 20INS, insertion in exon 20; a, P-value based on Kruskal-Wallis test, otherwise P-value based on Chi-square test; b, data based on cohort harboring common or uncommon mutations treated by first-line EGFR-TKIs; c, overall mutation frequency: 518 mutations in 504 patients. | ||||
| Age (year) | 0.974a | |||
| Median | 60 | 60 | 61 | |
| Mean | 59.3 | 59.4 | 58.7 | |
| Range | 22–85 | 25–85 | 22–81 | |
| Sex | 0.003 | |||
| Male | 221 | 179 (41.2) | 42 (60.0) | |
| Female | 283 | 255 (58.8) | 28 (40.0) | |
| Status | 0.320 | |||
| Recurred | 46 | 38 (8.8) | 8 (11.4) | |
| Initial IIIb/IV | 455 | 394 (90.8) | 61 (87.1) | |
| NE | 3 | 2 (0.5) | 1 (1.4) | |
| Distant metastases | 0.508 | |||
| No | 225 | 192 (44.2) | 33 (47.1) | |
| Yes | 275 | 239 (55.1) | 36 (51.4) | |
| NE | 4 | 3 (0.7) | 1 (1.4) | |
| Smoking | <0.001 | |||
| Never | 376 | 341 (78.6) | 35 (50.0) | |
| Current/former | 128 | 93 (21.4) | 35 (50.0) | |
| Pathology | 0.907 | |||
| ADC | 471 | 404 (93.1) | 67 (95.7) | |
| SQC | 6 | 5 (1.2) | 1 (1.4) | |
| ADC-SQC | 5 | 5 (1.2) | 0 (0) | |
| NSCLC-NOS | 22 | 20 (4.6) | 2 (2.9) | |
| ECOG PS | 0.232 | |||
| 0–1 | 469 | 401 (92.4) | 68 (97.1) | |
| 2–4 | 35 | 33 (7.6) | 2 (2.9) | |
| TKIsb | 0.203 | |||
| Gefitinib | 312 | 295 (68.0) | 17 (56.7) | |
| Erlotinib | 46 | 40 (9.2) | 6 (20.0) | |
| Icotinib | 105 | 98 (22.6) | 7 (23.3) | |
| Afatinib | 1 | 1 (0.2) | 0 (0) | |
| Mutation subtypesc | 518 | 440 (84.9) | 78 (15.1) | |
| 19DEL | 224 (43.2) | – | ||
| L858R | 216 (41.7) | – | ||
| G719X | – | 27 (5.2) | ||
| 20INS | – | 21 (4.1) | ||
| S768I | – | 9 (1.7) | ||
| T790M | – | 4 (0.8) | ||
| L861Q | – | 17 (3.3) | ||
Outcomes of first-line EGFR-TKIs treatment in patients with common and uncommon mutations
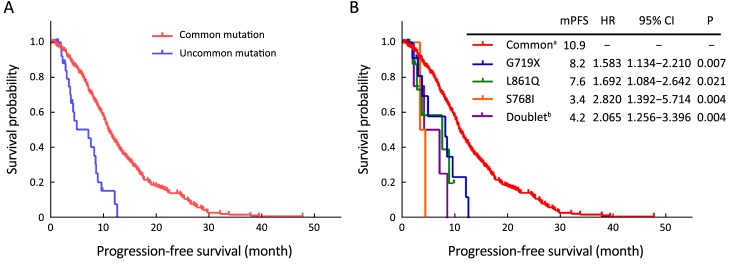
Four hundred and sixty-four patients (common, 434/434; uncommon, 30/70) received EGFR-TKIs as first-line treatment. Of these patients, 312 (67.2%) received gefitinib, 46 (9.9%) received erlotinib, 105 (22.6%) received icotinib, and 1 (0.2%) received afatinib. By the cutoff date, first-line EGFR-TKIs treatment was still ongoing in 121 patients, and the rest had developed disease progression. Statistically significantly inferior ORR and median PFS (mPFS) with first-line EGFR-TKIs were observed in patients with uncommon mutations (ORR: 23.3% vs. 51.8%, P=0.003; mPFS: 7.1 vs. 10.9 months, P<0.001). However, no difference was found between the two groups in terms of DCR (93.3% in uncommonvs. 95.6% in common, P=0.897). Subgroup analysis results showed that the mPFS of patients with G719X, L861Q, S768I, and doublet uncommon EGFR mutations were 8.2 months, 7.6 months, 3.4 months, and 4.2 months, respectively. When compared to the common EGFR mutation group, the efficacy was statistically significantly different (P=0.007, 0.021, 0.004, and 0.004, respectively). These data are summarized in Figure 1, Table 2, and Supplementary Table S1.
1.
Common epidermal growth factor receptor (EGFR) mutation is associated with better outcome in EGFR-mutated non-small cell lung cancer (NSCLC) patients treated with first-line epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). (A) Progression-free survival (PFS) of first-line EGFR-TKIs treatment in patients with common and uncommon mutations; (B) PFS of first-line EGFR-TKIs treatment in patients with common and different subtypes of single uncommon mutations and doublet uncommon mutations. mPFS, median PFS; HR, hazard ratio; 95% CI, 95% confidence interval; a, common EGFR mutation; b, doublet uncommon EGFR mutations.
2.
Summary of responses to first-line treatment in NSCLC patients harboring common and uncommon mutations
| Variables | Responses to first-line EGFR-TKIs in NSCLC patients harboring common and uncommon mutations | Responses to different treatments in NSCLC patients harboring uncommon mutations | |||||
| EGFR mutation status | P | Treatment subgroup | P | ||||
| Common | Uncommon | Chemotherapy | TKIs | ||||
| NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate. | |||||||
| No. of patients | 434 | 30 | 40 | 30 | |||
| Response | |||||||
| CR | – | – | – | – | |||
| PR | 225 | 7 | 11 | 7 | |||
| SD | 190 | 21 | 22 | 21 | |||
| PD | 19 | 2 | 7 | 2 | |||
| ORR [n (%)] | 225 (51.8) | 7 (23.3) | 0.003 | 11 (27.5) | 7 (23.3) | 0.693 | |
| DCR [n (%)] | 415 (95.6) | 28 (93.3) | 0.897 | 33 (82.5) | 28 (93.3) | 0.328 | |
S1.
Details of uncommon mutations and response to first-line EGFR-TKIs
| Case | Sex | Age
(year) |
Smoking | Pathology | Status | Distant
metastases |
ECOG
PS |
TKI | Response | PFS
(month) |
EGFR
mutation |
Co-alteration |
| EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival; M, male; F, female; ADC, adenocarcinoma; SD, stable disease; PR, partial response; PD, progression disease. | ||||||||||||
| 1 | M | 51 | Smoker | ADC | IV | No | 1 | Icotinib | SD | 8.6 | G719X | S768I |
| 2 | F | 55 | Never | ADC | IV | Yes | 2 | Gefitinib | SD | 4.2 | G719X | S768I |
| 3 | F | 62 | Never | ADC | Recurred | Yes | 1 | Gefitinib | SD | 1.0+ | G719X | S768I |
| 4 | M | 67 | Smoker | ADC | IV | No | 1 | Erlotinib | PR | 7.1 | G719X | L861Q |
| 5 | M | 55 | Smoker | ADC | IV | Yes | 0 | Icotinib | SD | 2.2 | G719X | L861Q |
| 6 | F | 71 | Never | ADC | IV | No | 1 | Gefitinib | SD | 12.6 | G719X | |
| 7 | F | 77 | Never | ADC | NE | NE | 1 | Gefitinib | SD | 12.1 | G719X | |
| 8 | M | 74 | Smoker | ADC | IV | No | 1 | Gefitinib | SD | 9.6 | G719X | |
| 9 | F | 70 | Never | ADC | IV | No | 1 | Gefitinib | SD | 8.5 | G719X | |
| 10 | M | 62 | Smoker | ADC | IV | Yes | 1 | Gefitinib | PR | 8.2 | G719X | |
| 11 | M | 63 | Smoker | ADC | IV | No | 1 | Erlotinib | SD | 4.9 | G719X | |
| 12 | M | 62 | Smoker | ADC | IV | Yes | 2 | Gefitinib | SD | 3.8 | G719X | |
| 13 | M | 68 | Smoker | ADC | IV | No | 1 | Erlotinib | SD | 3.0+ | G719X | |
| 14 | M | 61 | Smoker | ADC | IV | Yes | 1 | Gefitinib | SD | 3.0 | G719X | |
| 15 | F | 81 | Never | ADC | IV | Yes | 1 | Gefitinib | PR | 2.7+ | G719X | |
| 16 | M | 62 | Smoker | ADC | IV | Yes | 1 | Icotinib | PD | 1.9 | G719X | |
| 17 | F | 73 | Never | ADC | IIIb | No | 1 | Gefitinib | SD | 1.0+ | G719X | |
| 18 | F | 58 | Never | ADC | IV | Yes | 1 | Icotinib | SD | 1.0+ | G719X | |
| 19 | F | 22 | Never | ADC | IV | No | 1 | Gefitinib | PR | 4.4 | S768I | |
| 20 | M | 72 | Smoker | NOS | IV | Yes | 1 | Icotinib | PR | 3.4 | S768I | |
| 21 | F | 65 | Never | ADC | IIIb | No | 1 | Gefitinib | SD | 1.0+ | S768I | |
| 22 | M | 54 | Never | ADC | IV | Yes | 1 | Gefitinib | SD | 9.8+ | L861Q | |
| 23 | F | 49 | Never | ADC | IV | Yes | 1 | Erlotinib | PR | 8.9 | L861Q | |
| 24 | F | 70 | Never | ADC | Recurred | Yes | 0 | Gefitinib | SD | 7.6 | L861Q | |
| 25 | F | 55 | Never | ADC | IV | Yes | 1 | Erlotinib | SD | 4.1+ | L861Q | |
| 26 | M | 45 | Smoker | ADC | IV | Yes | 0 | Gefitinib | PR | 3.7 | L861Q | |
| 27 | F | 38 | Never | ADC | IV | No | 1 | Gefitinib | SD | 2.8 | L861Q | |
| 28 | M | 69 | Smoker | ADC | IV | No | 1 | Icotinib | SD | 2.5+ | L861Q | |
| 29 | M | 70 | Never | ADC | IV | No | 1 | Erlotinib | PD | 2.0 | L861Q | |
| 30 | M | 79 | Smoker | ADC | IV | No | 1 | Icotinib | SD | 1.4+ | L861Q | |
Outcomes in patients with uncommon mutations treated with first-line EGFR-TKIs vs. platinum-based chemotherapy
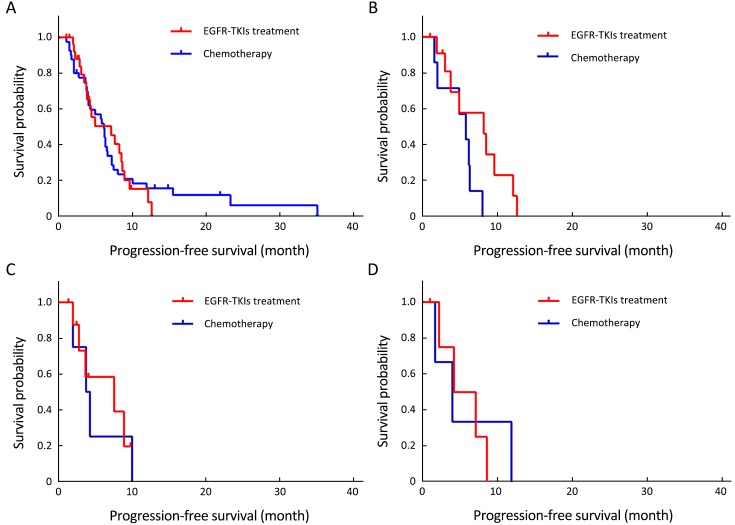
Of the 70 patients with uncommon mutations, 30 (42.9%) received EGFR-TKIs, and 40 (57.1%) received platinum-based chemotherapy as first-line therapy. At the end of follow-up, 56 (80.0%) patients had developed disease progression. Statistical analysis results indicated no difference in response and survival with EGFR-TKIs and chemotherapy as first-line therapy (TKIs vs. chemotherapy: ORR, 23.3% vs. 27.5%, P=0.693; DCR, 93.3% vs. 82.5%, P=0.328; mPFS, 7.1 vs. 6.1 months, P=0.893). Subgroup analysis results showed no statistical difference between first-line TKIs treatment and chemotherapy in patients with G719X, L861Q, or doublet uncommon EGFR mutations, respectively (P=0.061, 0.872, and 0.834, respectively). These data are summarized in Figure 2, Table 2, and Supplementary Table S2.
2.
No statistical difference was shown in progression-free survival (PFS) among uncommon epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) patients treated by first-line epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and platinum-based chemotherapy. (A) PFS of all uncommon EGFR-mutated NSCLC patients treated by first-line EGFR-TKIs and platinum-based chemotherapy; (B) PFS of NSCLC patients harboring G719X mutation treated by first-line EGFR-TKIs and platinum-based chemotherapy; (C) PFS of NSCLC patients harboring L861Q mutation treated by first-line EGFR-TKIs and platinum-based chemotherapy; (D) PFS of NSCLC patients harboring doublet uncommon EGFR mutations treated by first-line EGFR-TKIs and platinum-based chemotherapy.
S2.
Details of uncommon mutations and response to first-line platinum-based chemotherapy
| Case | Sex | Age
(year) |
Smoking | Pathology | Status | Distant
metastases |
ECOG
PS |
Response | PFS
(month) |
EGFR
mutation |
Co-alteration |
| ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival; EGFR, epidermal growth factor receptor; F, female; M, male; ADC, adenocarcinoma; PR, partial response; PD, progression disease; SD, stable disease; NOS, not otherwise specified. | |||||||||||
| 1 | F | 60 | Never | ADC | Recurred | Yes | 1 | PR | 11.9 | G719X | S768I |
| 2 | M | 65 | Smoker | ADC | IV | No | 1 | PD | 1.7 | G719X | L861Q |
| 3 | M | 59 | Smoker | ADC | IV | No | 1 | SD | 8.0 | G719X | |
| 4 | M | 67 | Smoker | ADC | IV | No | 1 | PR | 6.3 | G719X | |
| 5 | M | 57 | Smoker | ADC | IV | Yes | 1 | PR | 6.2 | G719X | |
| 6 | M | 52 | Smoker | ADC | IV | No | 1 | SD | 5.8 | G719X | |
| 7 | F | 69 | Never | ADC | IV | Yes | 1 | SD | 4.9 | G719X | |
| 8 | M | 58 | Smoker | ADC | IV | No | 1 | PD | 2.0 | G719X | |
| 9 | M | 62 | Smoker | ADC | IV | No | 1 | PD | 1.6 | G719X | |
| 10 | F | 66 | Never | ADC | IV | Yes | 1 | PR | 35.1 | 20INS | |
| 11 | M | 63 | Never | ADC | Recurred | Yes | 1 | SD | 23.3 | 20INS | |
| 12 | F | 56 | Never | ADC | IV | Yes | 1 | SD | 21.9+ | 20INS | |
| 13 | F | 41 | Never | ADC | IV | Yes | 1 | SD | 15.5 | 20INS | |
| 14 | M | 58 | Smoker | ADC | IIIb | Yes | 1 | PR | 14.8+ | 20INS | |
| 15 | M | 59 | Smoker | ADC | IV | No | 1 | SD | 13.0+ | 20INS | |
| 16 | F | 61 | Never | ADC | Recurred | No | 1 | SD | 8.9 | 20INS | |
| 17 | F | 64 | Never | ADC | IV | Yes | 1 | PR | 7.4 | 20INS | |
| 18 | M | 61 | Smoker | ADC | IIIb | No | 1 | SD | 7.2 | 20INS | |
| 19 | F | 59 | Never | ADC | IV | Yes | 1 | SD | 7.2 | 20INS | |
| 20 | M | 66 | Never | ADC | Recurred | No | 1 | SD | 6.5 | 20INS | |
| 21 | M | 56 | Smoker | ADC | IV | No | 0 | SD | 6.3 | 20INS | |
| 22 | F | 62 | Never | ADC | IV | Yes | 1 | SD | 5.7 | 20INS | |
| 23 | F | 27 | Never | ADC | IV | No | 1 | PR | 4.0 | 20INS | |
| 24 | M | 57 | Smoker | ADC | IV | No | 1 | SD | 3.9 | 20INS | |
| 25 | F | 67 | Never | ADC | IV | No | 1 | SD | 3.6 | 20INS | |
| 26 | F | 42 | Never | ADC | IV | No | 1 | SD | 2.7 | 20INS | |
| 27 | M | 52 | Smoker | ADC | Recurred | Yes | 0 | SD | 2.4+ | 20INS | |
| 28 | M | 41 | Never | ADC | Recurred | Yes | 1 | SD | 1.4 | 20INS | |
| 29 | M | 42 | Smoker | ADC | IV | Yes | 1 | PD | 1.4 | 20INS | |
| 30 | F | 29 | Never | ADC | IV | No | 1 | PD | 1.0 | 20INS | |
| 31 | M | 49 | Smoker | ADC | IV | Yes | 1 | PR | 6.2 | S768I | |
| 32 | M | 71 | Smoker | NOS | IV | Yes | 1 | SD | 3.7 | S768I | |
| 33 | F | 48 | Never | ADC | IV | No | 1 | SD | 4.0 | T790M | L861Q |
| 34 | M | 69 | Smoker | ADC | IV | Yes | 1 | SD | 6.6 | T790M | |
| 35 | M | 72 | Never | ADC | IV | No | 1 | SD | 6.1 | T790M | |
| 36 | M | 52 | Smoker | SQC | IV | Yes | 1 | PD | 2.0 | T790M | |
| 37 | M | 47 | Smoker | ADC | IV | No | 1 | PR | 10.0 | L861Q | |
| 38 | M | 67 | Smoker | ADC | IV | Yes | 1 | PR | 4.3 | L861Q | |
| 39 | M | 44 | Smoker | ADC | IV | Yes | 0 | PR | 3.8 | L861Q | |
| 40 | M | 50 | Never | ADC | IV | Yes | 1 | PD | 2.0 | L861Q | |
Association between clinicopathological factors and effect of first-line EGFR-TKIs
Multivariate analysis demonstrated that pathology [hazard ratio (HR), 0.381; 95% CI, 0.252–0.575; P<0.001] andEGFR mutation status (HR, 3.277; 95% CI, 2.059–5.215; P<0.001) were independent factors predicting the clinical benefit of first-line EGFR-TKIs treatment inEGFR-mutant NSCLC patients. For patients with uncommon EGFR mutations, Cox regression analysis showed that the only independent factor predicting the clinical benefit of different treatments was pathology (HR, 0.260; 95% CI, 0.073–0.923; P=0.037). All of the Cox regression data are detailed in Table 3 and Supplementary Table S3.
3.
Cox regression analyses for PFS in EGFR-mutant NSCLC after first-line EGFR-TKIs treatment
| Variables | Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| PFS, progression-free survival; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor; HR, hazard ratio; 95% CI, 95% confidence interval; ADC, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status. | |||||||
| Age: <60 vs. ≥60 years | 0.807 | 0.652–0.997 | 0.047 | 0.808 | 0.652–1.001 | 0.051 | |
| Sex: female vs. male | 1.025 | 0.825–1.273 | 0.825 | ||||
| Status: recurrence
vs. initial IIIb/IV |
0.809 | 0.571–1.145 | 0.232 | ||||
| Distant metastases: No vs. Yes | 1.101 | 0.889–1.363 | 0.379 | ||||
| Smoking: never
vs. current/former |
1.160 | 0.900–1.497 | 0.252 | ||||
| Pathology: non-ADC
vs. ADC |
0.392 | 0.260–0.593 | <0.001 | 0.381 | 0.252–0.575 | <0.001 | |
|
EGFR mutation status:
common vs. uncommon |
3.068 | 1.934–4.869 | <0.001 | 3.277 | 2.059–5.215 | <0.001 | |
| ECOG PS: 0–1 vs. 2–4 | 0.629 | 0.426–0.929 | 0.020 | 0.675 | 0.457–1.001 | 0.051 | |
S3.
Cox regression analyses for PFS in uncommon mutation patients after first-line EGFR-TKIs treatment or chemotherapy
| Variables | Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| PFS, progression-free survival; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; HR, hazard ratio; 95% CI, 95% confidence interval; ADC, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status. | |||||||
| Age: <60 vs. ≥60 years | 0.945 | 0.553–1.163 | 0.835 | ||||
| Sex: female vs. male | 1.548 | 0.887–2.702 | 0.124 | ||||
| Status: recurrence
vs. initial IIIb/IV |
1.628 | 0.729–3.637 | 0.235 | ||||
| Distant metastases: No
vs. Yes |
0.790 | 0.460–1.358 | 0.394 | ||||
| Smoking: never
vs. current/former |
1.603 | 0.926–2.775 | 0.092 | 1.497 | 0.854–2.626 | 0.159 | |
| Pathology: non-ADC
vs. ADC |
0.216 | 0.062–0.750 | 0.016 | 0.260 | 0.073–0.923 | 0.037 | |
| ECOG PS: 0–1 vs. 2–4 | 2.483 | 0.583–10.585 | 0.219 | ||||
| Therapy: chemotherapy vs. EGFR-TKIs | 1.039 | 0.593–1.819 | 0.894 | ||||
Discussion
With the development of the theory and practice of molecular targeted therapy, the treatment strategy for a subset of patients with NSCLC has been revolutionized in recent years. Therefore, more and more patients benefit from the discovery of tumor driver genes and corresponding molecular targeted agents. EGFR-TKIs in particular have made undeniable contributions. However, considering the toxicities and high costs of these agents, great efforts should be made to determine the individuals who are most likely to respond to and benefit from EGFR-TKIs therapy. We therefore conducted this study in a relatively large-scale cohort of Chinese EGFR-mutant NSCLC patients to recommend a more appropriate first-line therapeutic strategy for patients harboring uncommon EGFR mutations. For patients with NSCLC, we have already reached a consensus that those with common mutations benefit from EGFR-TKIs (4,6,10,25-28). On the contrary, two other mutation types, 20INS and primary T790M, bring about primary resistance to EGFR-TKIs (17). Patients with such mutations were not treated with first-line EGFR-TKIs in Shanghai Pulmonary Hospital, Tongji University, and that is why no patient with these mutations was enrolled in the first-line EGFR-TKIs treatment group. However, other than 20INS and primary T790M, the correlation with response to treatment for the other uncommon mutations is still unclear and controversial.
In the current study, 504 EGFR-mutant NSCLC patients were included. Seventy patients (13.9%) with uncommon EGFR mutations were identified. Previous studies on the incidence of uncommon EGFR mutations among EGFR-mutant NSCLC patients reported incidence rates that varied between 5.9% and 20.4% (7,13,29-33), and this is in accordance with our study. Moreover, our results confirmed that uncommon EGFR mutations constitute a distinct part of the whole group of EGFR mutations from the aspects of heterogeneous composition and various responses to EGFR-TKIs. Wu et al. (9) and Keam et al. (31) reported that the mutations of amino acid substitutions at G719 and L861 were the two major uncommon mutation groups and were associated with favorable efficacy of EGFR-TKIs. Chiu et al. (34) and Zhang et al. (35) reported that after EGFR-TKIs therapy, PFS could be achieved for approximately 7.6 months in patients with such uncommon mutations. Consistent with these findings, our results showed that G719X and L861Q were also major uncommon mutations; the ORR was 20.8%, and mPFS was 7.6 months (95% CI, 2.6–12.6).
Obviously, a significantly inferior ORR and PFS were exhibited compared to common EGFR mutations. Based on the great efforts of Eck’s team, the mechanism of the inferior efficacy of TKIs in G719X may be explained largely by the weaker affinity between TKIs and ATP of G719X, approximately one-seventeenth as potent as that of L858R (36). As for L861Q, a study conducted in Taiwan, China, suggested that gefitinib, a first-generation EGFR-TKIs, may not be a good choice because two patients identified with L861Q experienced frustrating PFS (1.9 and 2.0 months, respectively) (37). However, a series of other studies demonstrated that first-generation EGFR-TKIs did work in L861Q-mutant patients, with a PFS of 8.9 months and an OS close to 22.0 months (38-40). Therefore, L861Q mutation was referred to as one of the TKI-sensitive mutations in the National Comprehensive Cancer Network Guidelines. Similarly, the role of S768I in the efficacy of EGFR-TKIs therapy remains confusing. The S768I mutation is contained in exon 20, which was thought broadly to be associated with a lack of sensitivity to EGFR-TKIs because of T790M and 20INS (18,41). Interestingly, several recent studies suggested that patients harboring S768I mutations might be responsive to EGFR-TKIs therapy (16,42,43). Zhu et al. (44) demonstrated through a retrospective analysis that patients harboring only the S768I mutation appeared to be more sensitive to EGFR-TKIs than those with EGFR wild type. The aforementioned studies of uncommon EGFR mutations share the problem of insufficient numbers of cases and greatly varied responses. Therefore, more large-scale cohort studies and data in this field should be welcomed to help uncover the truth of the correlation between uncommon EGFR mutations and responses to EGFR-TKIs.
Keam et al. (31) also reported the correlation between treatment efficacy and complex mutations: complex mutations with common mutations showed treatment efficacy with EGFR-TKIs similar to that of common mutations alone. In our cohort, we detected 11 patients with doublet EGFR mutations in the EGFR-TKIs treatment group. Unfortunately, none of the 11 patients belongs to the common + uncommon cluster (G719X+S768I, n=3; G719X+L861Q, n=2; 19DEL+L858R, n=6). The mPFS of the doublet common and uncommon EGFR mutations groups was statistically significantly different (mPFS: 9.7 vs. 5.7 months, P=0.041).
The highlight of our study is that we directly compared the survival data between EGFR-TKIs and chemotherapy as first-line therapy among patients with uncommon EGFR mutations, data which are quite scarce in published papers. In our study, the mPFS after first-line EGFR-TKIs was poorer in uncommon mutations (mPFS: 10.9 vs. 7.1 months, P<0.001); when comparing the mPFS with first-line EGFR-TKIs treatment and platinum-based chemotherapy among the uncommon mutation cohort, we found no statistically significant difference (mPFS: 7.1 months with EGFR-TKIsvs. 6.1 months with chemotherapy, P=0.893). Arrieta et al. (29) reported similar results with a response rate to platinum-based chemotherapy of 49.6% and an mPFS with chemotherapy of 6.0 months (95% CI, 5.1–6.6) in NSCLC patients harboring uncommon EGFR mutations. They suggested that only patients with uncommon EGFR mutations should receive platinum-based chemotherapy as first-line treatment. Accordingly, we proposed that NSCLC patients harboring uncommon EGFR mutations could obtain almost equal survival through EGFR-TKIs treatment or platinum-based chemotherapy as first line. Certainly, to make this deduction more convincing, sharing of more data from multicenter studies, especially those restricted to first-line therapy, should be encouraged for clinical practice.
This study has several limitations. The major limitation is the study’s retrospective nature because bias cannot be excluded. Furthermore, considering the high costs of mutation testing and molecular targeted agents, some patients refused to undergo mutation testing, and this would lead to bias in the prevalence of uncommon mutations. Moreover, a considerable part of the patients’ information on treatment-related toxicity could not be retrieved from the medical records. In addition, although the number of patients with uncommon EGFR mutations in this study is relatively high compared with other studies, more clinical experience in the treatment of patients with uncommon EGFR mutations is needed.
Conclusions
To summarize, in this cohort of Chinese EGFR-mutant NSCLC patients, the prevalence and genotype distribution of uncommon EGFR mutations was in accordance with previously published studies. The outcome of first-line EGFR-TKIs treatment was poorer in patients with uncommon EGFR mutations compared to common mutations but was not statistically significantly different from that of first-line platinum-based chemotherapy among patients with uncommon mutations. Our report will provide clinicians with our treatment experience when making clinical decisions. Prospective studies of patients with uncommon EGFR mutations are warranted to hopefully enhance our comprehension of the EGFR spectrum and improve the prognosis of these patients. Furthermore, further exploration of the mechanism of the correlation between efficacy of EGFR-TKIs and these uncommon EGFR mutations is needed.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81372392 and 81402486).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Shengxiang Ren, Email: harry_ren@126.com.
Caicun Zhou, Email: caicunzhou_dr@163.com.
References
- 1.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604. [Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–46. doi: 10.1038/nrc3775. [Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. <DOI: 10.1038/nrc3775> <PMID: 25056707>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer . J Thorac Oncol. 2017;12:612–23. doi: 10.1016/j.jtho.2016.12.014. [Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol 2017;12:612-23. <DOI: 10.1016/j.jtho.2016.12.014> <PMID: 28017789> ] [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. <DOI: 10.1016/S1470-2045(11)70184-X> <PMID: 21783417>] [DOI] [PubMed] [Google Scholar]
- 5.Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer — search and destroy. Eur J Cancer. 2006;42:17–23. doi: 10.1016/j.ejca.2005.07.031. [Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer — search and destroy. Eur J Cancer 2006;42:17-23. <DOI: 10.1016/j.ejca.2005.07.031> <PMID: 16364841>] [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. <DOI: 10.1056/NEJMoa0810699> <PMID: 19692680>] [DOI] [PubMed] [Google Scholar]
- 7.Pallis AG, Voutsina A, Kalikaki A, et al. ‘Classical’ but not ‘other’ mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer. 2007;97:1560–6. doi: 10.1038/sj.bjc.6604068. [Pallis AG, Voutsina A, Kalikaki A, et al. ‘Classical’ but not ‘other’ mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer 2007;97:1560-6. <DOI: 10.1038/sj.bjc.6604068> <PMID: 18000506>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. <DOI: 10.1093/jnci/dji055> <PMID: 15741570>] [DOI] [PubMed] [Google Scholar]
- 9.Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on " uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812–21. doi: 10.1158/1078-0432.CCR-10-3408. [Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on " uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. <DOI: 10.1158/1078-0432.CCR-10-3408> <PMID: 21531810>] [DOI] [PubMed] [Google Scholar]
- 10.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. <DOI: 10.1056/NEJMoa0909530> <PMID: 20573926>] [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. <DOI: 10.1016/S1470-2045(09)70364-X> <PMID: 20022809>] [DOI] [PubMed] [Google Scholar]
- 12.Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics . Mol Cancer Ther. 2013;12:220–9. doi: 10.1158/1535-7163.MCT-12-0620. [Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013;12:220-9. <DOI: 10.1158/1535-7163.MCT-12-0620> <PMID: 23371856> ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network . Ann Oncol. 2014;25:126–31. doi: 10.1093/annonc/mdt418. [Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. <DOI: 10.1093/annonc/mdt418> <PMID: 24285021> ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions . J Thorac Oncol. 2013;8:179–84. doi: 10.1097/JTO.0b013e3182779d18. [Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179-84. <DOI: 10.1097/JTO.0b013e3182779d18> <PMID: 23328547> ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki H, Endo K, Takada M, et al. EGFR exon 20 insertion mutation in Japanese lung cancer . Lung Cancer. 2007;58:324–8. doi: 10.1016/j.lungcan.2007.06.024. [Sasaki H, Endo K, Takada M, et al. EGFR exon 20 insertion mutation in Japanese lung cancer. Lung Cancer 2007;58:324-8. <DOI: 10.1016/j.lungcan.2007.06.024> <PMID: 17686547> ] [DOI] [PubMed] [Google Scholar]
- 16.Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–82. doi: 10.1158/1078-0432.CCR-07-5123. [Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 2008;14:4877-82. <DOI: 10.1158/1078-0432.CCR-07-5123> <PMID: 18676761>] [DOI] [PubMed] [Google Scholar]
- 17.Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–8. doi: 10.1016/S1470-2045(15)00026-1. [Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. <DOI: 10.1016/S1470-2045(15)00026-1> <PMID: 26051236>] [DOI] [PubMed] [Google Scholar]
- 18.Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. doi: 10.1126/scitranslmed.3007205. [Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. <DOI: 10.1126/scitranslmed.3007205> <PMID: 24353160>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol. 2014;9:189–94. doi: 10.1097/JTO.0000000000000048. [Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014;9:189-94. <DOI: 10.1097/JTO.0000000000000048> <PMID: 24419415>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11:1718–28. doi: 10.1016/j.jtho.2016.05.013. [Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 2016;11:1718-28. <DOI: 10.1016/j.jtho.2016.05.013> <PMID: 27237825>] [DOI] [PubMed] [Google Scholar]
- 21.Li X, Ren R, Ren S, et al. Peripheral blood for epidermal growth factor receptor mutation detection in non-small cell lung cancer patients. Transl Oncol. 2014;7:341–8. doi: 10.1016/j.tranon.2014.04.006. [Li X, Ren R, Ren S, et al. Peripheral blood for epidermal growth factor receptor mutation detection in non-small cell lung cancer patients. Transl Oncol 2014;7:341-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Zhao C, Yang Y, et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol. 2015;10:778–83. doi: 10.1097/JTO.0000000000000487. [Wu C, Zhao C, Yang Y, et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol 2015;10:778-83. <DOI: 10.1097/JTO.0000000000000487> <PMID: 25629635>] [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Zhang Y, Cai W, et al. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer. 2014;120:2299–307. doi: 10.1002/cncr.28725. [Zhao M, Zhang Y, Cai W, et al. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer 2014;120:2299-307. <DOI: 10.1002/cncr.28725> <PMID: 24737648>] [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. <DOI: 10.1016/j.ejca.2008.10.026> <PMID: 19097774>] [DOI] [PubMed] [Google Scholar]
- 25.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. <DOI: 10.1016/S1470-2045(13)70604-1> <PMID: 24439929>] [DOI] [PubMed] [Google Scholar]
- 26.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–9. doi: 10.1093/annonc/mdv270. [Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. <DOI: 10.1093/annonc/mdv270> <PMID: 26105600>] [DOI] [PubMed] [Google Scholar]
- 27.Li L, Zhang Z, Bie Z, et al. Epidermal growth factor receptor mutation analysis in cytological specimens and responsiveness to gefitinib in advanced non-small cell lung cancer patients. Chin J Cancer Res. 2015;27:294–300. doi: 10.3978/j.issn.1000-9604.2015.05.03. [Li L, Zhang Z, Bie Z, et al. Epidermal growth factor receptor mutation analysis in cytological specimens and responsiveness to gefitinib in advanced non-small cell lung cancer patients. Chin J Cancer Res 2015;27:294-300.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26:1877–83. doi: 10.1093/annonc/mdv276. [Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. <DOI: 10.1093/annonc/mdv276> <PMID: 26141208>] [DOI] [PubMed] [Google Scholar]
- 29.Arrieta O, Cardona AF, Corrales L, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87:169–75. doi: 10.1016/j.lungcan.2014.12.009. [Arrieta O, Cardona AF, Corrales L, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer 2015;87:169-75. <DOI: 10.1016/j.lungcan.2014.12.009> <PMID: 25558790>] [DOI] [PubMed] [Google Scholar]
- 30.De Pas T, Toffalorio F, Manzotti M, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6:1895–901. doi: 10.1097/JTO.0b013e318227e8c6. [De Pas T, Toffalorio F, Manzotti M, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol 2011;6:1895-901. <DOI: 10.1097/JTO.0b013e318227e8c6> <PMID: 21841502>] [DOI] [PubMed] [Google Scholar]
- 31.Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. 2014;19:594–600. doi: 10.1007/s10147-013-0602-1. [Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol 2014;19:594-600. <DOI: 10.1007/s10147-013-0602-1> <PMID: 23912954>] [DOI] [PubMed] [Google Scholar]
- 32.Lohinai Z, Hoda MA, Fabian K, et al. Distinct epidemiology and clinical consequence of classic versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol. 2015;10:738–46. doi: 10.1097/JTO.0000000000000492. [Lohinai Z, Hoda MA, Fabian K, et al. Distinct epidemiology and clinical consequence of classic versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol 2015;10:738-46. <DOI: 10.1097/JTO.0000000000000492> <PMID: 25664625>] [DOI] [PubMed] [Google Scholar]
- 33.Stone E, Allen HA, Saghaie T, et al. High proportion of rare and compound epidermal growth factor receptor mutations in an Australian population of non-squamous non-small-cell lung cancer. Intern Med J. 2014;44:1188–92. doi: 10.1111/imj.12587. [Stone E, Allen HA, Saghaie T, et al. High proportion of rare and compound epidermal growth factor receptor mutations in an Australian population of non-squamous non-small-cell lung cancer. Intern Med J 2014;44:1188-92. <DOI: 10.1111/imj.12587> <PMID: 25228365>] [DOI] [PubMed] [Google Scholar]
- 34.Chiu CH, Yang CT, Shih JY, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol. 2015;10:793–9. doi: 10.1097/JTO.0000000000000504. [Chiu CH, Yang CT, Shih JY, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. <DOI: 10.1097/JTO.0000000000000504> <PMID: 25668120>] [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang Z, Hao X, et al. Clinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutations. Chin J Cancer Res. 2017;29:18–24. doi: 10.21147/j.issn.1000-9604.2017.01.03. [Zhang Y, Wang Z, Hao X, et al. Clinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutations. Chin J Cancer Res 2017;29:18-24. <DOI: 10.21147/j.issn.1000-9604.2017.01.03> <PMID: 28373750>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–27. doi: 10.1016/j.ccr.2006.12.017. [Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007;11:217-27. <DOI: 10.1016/j.ccr.2006.12.017> <PMID: 17349580>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh MH, Fang YF, Chang WC, et al. Complex mutation patterns of epidermal growth factor receptor gene associated with variable responses to gefitinib treatment in patients with non-small cell lung cancer. Lung Cancer. 2006;53:311–22. doi: 10.1016/j.lungcan.2006.06.005. [Hsieh MH, Fang YF, Chang WC, et al. Complex mutation patterns of epidermal growth factor receptor gene associated with variable responses to gefitinib treatment in patients with non-small cell lung cancer. Lung Cancer 2006;53:311-22. <DOI: 10.1016/j.lungcan.2006.06.005> <PMID: 16870303>] [DOI] [PubMed] [Google Scholar]
- 38.Kancha RK, von Bubnoff N, Peschel C, et al. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15:460–7. doi: 10.1158/1078-0432.CCR-08-1757. [Kancha RK, von Bubnoff N, Peschel C, et al. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res 2009;15:460-7. <DOI: 10.1158/1078-0432.CCR-08-1757> <PMID: 19147750>] [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology — mainland China subset analysis of the PIONEER study. PLoS One. 2015;10:e0143515. doi: 10.1371/journal.pone.0143515. [Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology — mainland China subset analysis of the PIONEER study. PLoS One 2015;10:e0143515. <DOI: 10.1371/journal.pone.0143515> <PMID: 26599344>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer. 2016;96:87–92. doi: 10.1016/j.lungcan.2016.01.018. [Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer 2016;96:87-92. <DOI: 10.1016/j.lungcan.2016.01.018> <PMID: 27133756>] [DOI] [PubMed] [Google Scholar]
- 41.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–31. doi: 10.1016/S1470-2045(11)70129-2. [Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. <DOI: 10.1016/S1470-2045(11)70129-2> <PMID: 21764376>] [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:45–51. doi: 10.1097/JTO.0b013e3182781e35. [Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:45-51. <DOI: 10.1097/JTO.0b013e3182781e35> <PMID: 23242437>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol. 2008;26:2745–53. doi: 10.1200/JCO.2007.15.6695. [Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745-53. <DOI: 10.1200/JCO.2007.15.6695> <PMID: 18509184>] [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Bai Q, Lu Y, et al. Response to tyrosine kinase inhibitors in lung adenocarcinoma with the rare epidermal growth factor receptor mutation S768I: a retrospective analysis and literature review. Target Oncol. 2017;12:81–8. doi: 10.1007/s11523-016-0455-4. [Zhu X, Bai Q, Lu Y, et al. Response to tyrosine kinase inhibitors in lung adenocarcinoma with the rare epidermal growth factor receptor mutation S768I: a retrospective analysis and literature review. Target Oncol 2017;12:81-8. <DOI: 10.1007/s11523-016-0455-4> <PMID: 27538584>] [DOI] [PubMed] [Google Scholar]