Abstract
Objective
The aim of this study was to investigate the prognostic factors and to evaluate the impact of adjuvant therapy on clinical outcome for early-stage cervical cancer.
Methods
The clinical-pathological data of all 1,335 patients with the International Federation of Gynecology and Obstetrics (FIGO) Ib–IIa cervical cancer treated with primary radical surgery at the Chinese National Cancer Center between May 2007 and Dec 2013 were retrospectively reviewed. The median follow-up was 70 months.
Results
Of all the patients, 61.6% of the cases received adjuvant therapy, with 5-year disease-free survival (DFS) of 92.1% and 5-year overall survival (OS) of 95.0%. In multivariate analysis, differentiation of G3 (P<0.05), lymph node metastasis (LNM, P<0.05) and lymphovascular space invasion (LVSI, P<0.05) were independent predictors for OS, while LNM (P<0.05), deep stroma invasion (DSI, P<0.05) and LVSI (P<0.05) were independent factors for DFS. The samples were stratified by histologic type, and cervical squamous cell carcinoma (SCC) was found to share the same independent factors except for differentiation of OS. As to patients with cervical adenocarcinoma/adenosquamous carcinoma (AC/ASC), differentiation was the independent predictor of OS (P<0.05); and LVSI of DFS (P<0.05). Of 236 patients with high-risk factors, there was no significant difference in survival between concurrent chemoradiotherapy (CCRT, n=195), radiotherapy (RT, n=24), and chemotherapy (CT, n=17). Among the 190 patients with LNM who underwent CCRT, 124 cases showed improved DFS after sequential CT (P=0.118), with a recurrence rate decrease of 14%, though the difference was not statistically significant. Patients with single intermediate-risk factors like DSI or LVSI were found to partially benefit from adjuvant therapy, but the difference was not statistically significant.
Conclusions
LNM, LVSI, DSI and differentiation were found to be independent prognostic factors for operable cervical cancer. Aggressive postoperative adjuvant therapy based on single risk factors in Chinese National Cancer Center could benefit survival. CCRT+CT outperformed CCRT in high-risk patients. For patients with single non-high-risk factor, the role of adjuvant therapy needs to be further discussed.
Keywords: Cervical neoplasm, adjuvant therapy, prognostic factors, DFS, OS
Introduction
Cervical cancer is the second most common cancer affecting women in China (1). According to the latest statistics released in 2015, China ranks second in the world in annual new cases of cervical cancer (2). Due to the widespread implementation of cytology screening, the majority of patients were diagnosed at an early-stage and at younger ages. Though equal cure rates may be obtained with radical radiation therapy and radical hysterectomy plus pelvic lymphadenectomy (RH-PLND) for early-stage cervical cancer, most premenopausal patients prefer radical surgical treatment, mainly for avoiding the radiation injury of the in situ ovary function (3). The 5-year survival rate of early-stage cervical cancer [the International Federation of Gynecology and Obstetrics (FIGO) I–II] can generally reach or exceed 80% (4). Indications for postoperative adjuvant therapy have been determined by evaluating the prognostic risk factors based on surgical pathology report for recurrence (5). Pathological findings identify pelvic lymph node metastasis (LNM), parametrial involvement (PI), and positive results in the margin of the vagina as high-risk factors, while lymphovascular space invasion (LVSI), deep stromal invasion (DSI), and tumor size over 4 cm have been identified as intermediate-risk factors for prognosis (6,7).
Numerous studies have investigated the 5-year recurrence-free survival rates of women with cervical cancer subjected to various types of postoperative adjuvant therapy (8-10), but less effort has been made to evaluate the Chinese experience with prognostic evaluation of adjuvant therapy on operable patients in the long term. In addition, the relationship of pathologic risk factors to the clinical outcomes of adjuvant therapy needs to be discussed in more detail. There are still controversies over which therapeutic regimen is most beneficial to patients with high-risk factors, or whether single intermediate-risk factor is required for adjuvant therapy, or whether there are other indications that should be suggest treatment with adjuvant therapy. In this present study, we aimed to conduct a single-center retrospective study to identify the prognostic factors related to 10-year overall and recurrence-free survival and to evaluate the impact of postoperative adjuvant therapy on survival of cervical cancer (FIGO stages Ib–IIa) following conventional radical surgery based on our experience.
Materials and methods
Study population
Patients with a diagnosis of stage Ib–IIa cervical cancer as defined by the FIGO cervical cancer who received RH-PLND as primary radical treatment at National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences from May 2007 to December 2013 were included in this retrospective study. There were 1,335 cases with complete clinical data, across all histological subtypes. We excluded 35 patients who were lost to follow-up. Until the endpoint at May 2017, the median follow-up of survivors was 70 (range: 2–119) months. The protocol of this study was approved by the institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences. Medical records were reviewed to collect patient’s demographics, surgical-pathologic data, and survival results.
Baseline and pathological characteristics
Basic characteristics of the study population at initial diagnosis include age, body mass index (BMI), FIGO stage, histological type, tumor size and treatment administered, such as neo-adjuvant chemotherapy (NACT), radical surgery and postoperative adjuvant therapy. All patients underwent Piver type III conventional radical hysterectomy, of whom, 94 cases underwent laparoscopy. Of all the patients, 25.9% received NACT mainly due to bulky tumor. NACT consisted of paclitaxel plus cisplatin/carboplatin every three week for median 2 cycles, except for 45 cases in preoperative brachytherapy only. Postoperative management was individually established according to pathological findings reported on surgical specimens for each one, including the status of differentiation, LVSI, DSI, LNM, PI and vaginal margins.
Criteria and regimen for postoperative adjuvant therapy
Postoperative therapeutic strategies were selected based on the recommendations given in the National Comprehensive Cancer Network (NCCN) guideline. In Cancer Hospital, Chinese Academy of Medical Sciences, adjuvant therapy was also administered with single intermediate-risk factor (DSI, LVSI) or low-grade differentiation. Patients with high- or intermediate-risk factors generally received concurrent chemoradiotherapy (CCRT), radiotherapy (RT), or chemotherapy (CT). RT consisted of conventional external beam (EBRT) to the pelvis (6eM X-ray median 45 Gy) in fractions of 1.8–2.0 Gy for 25 d. Six patients received only vaginal brachytherapy in fractions of 7.0 Gy for a total median dose of 21.0 Gy. In the CCRT group, additional cisplatin (40 mg/m2) or nedaplatin (40 mg/m2) was administered by intravenous infusion every week. In the CT group, patients received paclitaxel (175 mg/m2) with carboplatin [area under the curve (AUC) 4–5 mg/mL/min] every 3 weeks for 3–6 cycles.
Follow-up
After completion of the initial treatment, all patients were required to check every 3 months for 2 years; and every 6 months for the next 3 years to patients without recurrence; patients with recurrence-free survival lasting more than 5 years required annual follow-up. A total of 1,221 (91.5%) patients obeyed the periodical follow-up. Regular follow-up consisted of a physical examination, thinprep cytology test (TCT) of vaginal stump, serum squamous cell carcinoma antigen monitoring, and pelvic-abdominal ultrasonography scan each time. The patients underwent chest X-ray annually, with computed tomography or magnetic resonance imaging if necessary. Survival information was available for all recruited patients. The diagnostic criteria for recurrence include the elevation of serum tumor markers accompanied by positron emission tomography-computed tomography (PET-CT) or CT examinations of metastatic sites along with cytological biopsy positive.
Statistical analysis
Descriptive statistics were used to present patients’ baseline and pathologic characteristics. Disease-free survival (DFS) was calculated as the period from primary surgical treatment to the date of first confirmed recurrence. Overall survival (OS) was calculated as the time of the initial diagnosis of cervical cancer until the date of disease-related death or last follow-up. Survival curves were generated using the Kaplan-Meier method and differences between survival curves were compared using the log-rank test. The univariate analysis used log-rank test, and multivariate Cox model with forward Wald method was used to determine independent prognostic factors for DFS and OS. Data were analyzed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA), and statistical significance was set at P<0.05.
Results
Baseline characteristics
The clinical-pathological characteristics of all the 1,335 patients who met the inclusion criteria are summarized in Table 1. These include age, BMI, FIGO stage, histology, NACT and adjuvant therapy status, surgical procedure, differentiation, LNM, parametrial infiltration, condition of the vaginal margin, DSI, LVSI and tumor size.
1.
Clinicopathological characteristics of study population (N=1,335)
| Variables | n (%) |
| Age at diagnosis (year) | |
| ≥45 | 664 (49.7) |
| <45 | 671 (50.3) |
| BMI (kg/m2) | |
| ≥24 | 600 (44.9) |
| <24 | 735 (55.1) |
| FIGO stage | |
| Ib1 | 743 (55.7) |
| Ib2 | 348 (26.1) |
| IIa1 | 175 (13.1) |
| IIa2 | 69 (5.2) |
| Histology | |
| Squamous | 1,199 (89.8) |
| Adenomatous | 98 (7.3) |
| Adenosquamous | 28 (2.1) |
| Others | 10 (0.7) |
| NACT | |
| Yes | 346 (25.9) |
| No | 989 (74.1) |
| AT | |
| RT | 96 (7.2) |
| CT | 147 (11.0) |
| CCRT | 579 (43.4) |
| Observation | 513 (38.4) |
| Surgical procedure | |
| Laparotomy | 1,241 (93.0) |
| Laparoscopy | 94 (7.0) |
| Differentiation | |
| G1 | 557 (41.7) |
| G2 | 611 (45.8) |
| G3 | 167 (12.5) |
| LNM | |
| Positive | 228 (17.1) |
| Negative | 1,107 (82.9) |
| Parametrial infiltration | |
| Positive | 11 (0.8) |
| Negative | 1,324 (99.2) |
| Vaginal margin | |
| Positive | 2 (0.1) |
| Negative | 1,333 (99.9) |
| Table 1 (continued ) |
Survival analysis of patients with operable cervical cancer
Data on OS and DFS for each stage and different histologic type are shown in Table 2. Of all the patients, 61.6% received adjuvant therapy. The 5-year DFS for all is 92.1%, and those for each stage are 93.1%, 86.7%, 92.5% and 88.7%, corresponding to Ib1, Ib2, IIa1 and IIa2. The 5-year OS for all is 95.0%, and those for each stage are 97.6%, 88.4%, 93.2% and 87.9%, respectively. As to different histologic type, the 5-year OS of cervical squamous cell carcinoma (SCC) is 96.2%, which is higher than the 92.4% for cervical adenocarcinoma/adenosquamous carcinoma (AC/ASC). Similarly, the 5-year DFS of SCC is 92.9%, also higher than 89.5% for AC/ASC.
2.
DFS and OS with percentage of cases received AT for each stage
| Variables | All
(N=1,335) |
Ib1
(n=743) |
Ib2
(n=348) |
IIa1
(n=175) |
IIa2
(n=69) |
SCC
(n=1,199) |
AC/ASC
(n=126) |
| DFS, disease-free survival; OS, overall survival; AT, adjuvant therapy; SCC, squamous cell carcinoma; AC/ASC, adenocarcinoma/adenosquamous carcinoma. | |||||||
| Cases received
AT [n (%)] |
822 (61.6) | 417 (56.1) | 232 (66.7) | 128 (73.1) | 45 (65.2) | 728 (60.7) | 87 (69.0) |
| 2-year DFS (%) | 95.6 | 96.6 | 92.9 | 95.8 | 91.6 | – | – |
| 5-year DFS (%) | 92.1 | 93.1 | 86.7 | 92.5 | 88.7 | 92.9 | 89.5 |
| 5-year OS (%) | 95.0 | 97.6 | 88.4 | 93.2 | 87.9 | 96.2 | 92.4 |
Prognostic factors for operable cervical cancer
In multivariate analysis, the Cox regression model was used for different study population stratified by histology. Of the whole population shown in Tables 3 and 4, differentiation of G3 (P=0.022), LNM positive (P<0.001) and LVSI positive (P=0.001) were independent predictors of worse OS; and LNM positive (P<0.001), DSI (P=0.021) and LVSI positive (P=0.001) were predictors of worse DFS. LNM and LVSI were independent prognostic factors both for OS (PLNM<0.001, PLVSI<0.001, respectively) and DFS (PLNM<0.001, PLVSI=0.007, respectively), while DSI was an independent prognostic factor for DFS (P=0.005) in patients with SCC, shown in Tables 5 and 6. Regarding patients with AC/ASC, differentiation of G3 was the independent predictor of worse OS (P=0.037); and LVSI was an independent predictor of DFS (P=0.013), as shown in Tables 7 and 8.
3.
Univariate and multivariate analysis for factors predictive of DFS (N=1,335)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| DFS, disease-free survival; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous carcinoma; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 0.877 | 0.604–1.273 | 0.488 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 0.941 | 0.648–1.366 | 0.749 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 0.779 | 0.465–1.306 | 0.342 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 0.778 | 0.534–1.134 | 0.190 | – | – | – | |
| Histology (SCC vs.AC vs. ASC) | 0.598 | 0.278–1.258 | 0.182 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.760 | 0.523–1.105 | 0.149 | – | – | – | |
| LNM (+ vs. –) | 3.884 | 2.664–5.661 | 0.000 | 2.911 | 1.944–4.357 | 0.000 | |
| Stromal invasion (≥1/2 vs. <1/2) | 2.117 | 1.455–3.080 | 0.000 | 1.578 | 1.070–2.326 | 0.021 | |
| LVSI (+ vs. –) | 2.804 | 1.919–4.098 | 0.000 | 1.974 | 1.323–2.946 | 0.001 | |
4.
Univariate and multivariate analysis for factors predictive of OS (N=1,335)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| OS, overall survival; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous carcinoma; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 1.034 | 0.576–1.854 | 0.912 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 0.949 | 0.528–1.705 | 0.861 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 1.057 | 0.509–2.195 | 0.882 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 0.890 | 0.490–1.618 | 0.703 | – | – | – | |
| Histology (SCC vs.AC vs. ASC) | 1.165 | 0.460–2.952 | 0.747 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.317 | 0.169–0.596 | 0.000 | 0.469 | 0.245–0.896 | 0.022 | |
| LNM (+ vs. –) | 7.809 | 4.300–14.181 | 0.000 | 5.117 | 2.725–9.610 | 0.000 | |
| Stromal invasion (≥1/2 vs. <1/2) | 1.760 | 0.969–3.199 | 0.060 | – | – | – | |
| LVSI (+ vs. –) | 5.622 | 3.111–10.162 | 0.000 | 3.054 | 1.620–5.755 | 0.001 | |
5.
Univariate and multivariate analysis of prognostic factors associated with DFS for cervical SCC (n=1,199)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| DFS, disease-free survival; SCC, squamous cell carcinoma; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 0.812 | 0.540–1.222 | 0.317 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 0.949 | 0.631–1.426 | 0.800 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 0.816 | 0.469–1.418 | 0.469 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 0.810 | 0.536–1.223 | 0.315 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.881 | 0.584–1.328 | 0.544 | – | – | – | |
| LNM (+ vs. –) | 4.074 | 2.701–6.144 | 0.000 | 3.014 | 1.942–4.678 | 0.000 | |
| Stromal invasion (≥1/2 vs. <1/2) | 2.443 | 1.625–3.674 | 0.000 | 1.831 | 1.201–2.792 | 0.005 | |
| LVSI (+ vs. –) | 2.695 | 1.779–4.083 | 0.000 | 1.828 | 1.100–2.835 | 0.007 | |
6.
Univariate and multivariate analysis of prognostic factors associated with OS for cervical SCC (n=1,199)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| OS, overall survival; SCC, squamous cell carcinoma; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 0.982 | 0.511–1.887 | 0.956 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 1.017 | 0.527–1.962 | 0.961 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 1.002 | 0.439–2.289 | 0.996 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 1.075 | 0.544–2.124 | 0.835 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.397 | 0.201–0.784 | 0.006 | 0.584 | 0.291–1.173 | 0.134 | |
| LNM (+ vs. –) | 8.365 | 4.279–16.354 | 0.000 | 5.804 | 2.869–11.741 | 0.000 | |
| Stromal invasion (≥1/2 vs. <1/2) | 2.118 | 1.097–4.091 | 0.022 | 1.310 | 0.666–2.576 | 0.465 | |
| LVSI (+ vs. –) | 5.692 | 2.911–11.046 | 0.000 | 3.462 | 1.725–6.950 | 0.000 | |
7.
Univariate and multivariate analysis of prognostic factors associated with DFS for cervical AC/ASC (n=126)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| DFS, disease-free survival; AC/ASC, adenocarcinoma/adenosquamous carcinoma; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 1.274 | 0.491–3.303 | 0.617 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 1.068 | 0.412–2.770 | 0.891 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 0.721 | 0.165–3.157 | 0.662 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 0.513 | 0.197–1.335 | 0.163 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.363 | 0.134–0.981 | 0.037 | 0.460 | 0.163–1.299 | 0.135 | |
| LNM (+ vs. –) | 2.480 | 0.915–6.727 | 0.065 | – | – | – | |
| Stromal invasion (≥1/2 vs. <1/2) | 0.692 | 0.226–2.124 | 0.517 | – | – | – | |
| LVSI (+ vs. –) | 3.411 | 1.297–8.969 | 0.008 | 3.411 | 1.297–8.969 | 0.013 | |
8.
Univariate and multivariate analysis of prognostic factors associated with OS for cervical AC/ASC (n=126)
| Prognostic factors | Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| OS, overall survival; AC/ASC, adenocarcinoma/adenosquamous carcinoma; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LVSI, lymphovascular space invasion; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||
| Age at diagnosis (year) (≥45 vs. <45) | 1.106 | 0.276–4.425 | 0.887 | – | – | – | |
| BMI (kg/m2) (≥24 vs. <24) | 0.988 | 0.247–3.951 | 0.986 | – | – | – | |
| FIGO stage (Ib vs. IIa) | 1.763 | 0.354–8.777 | 0.483 | – | – | – | |
| Tumor size (cm) (≤4 vs.>4) | 0.297 | 0.070–1.258 | 0.081 | – | – | – | |
| Differentiation (G1–G2 vs. G3) | 0.100 | 0.012–0.817 | 0.008 | 0.107 | 0.013–0.871 | 0.037 | |
| LNM (+ vs. –) | 4.113 | 1.024–16.525 | 0.031 | 3.716 | 0.926–14.908 | 0.064 | |
| Stromal invasion (≥1/2 vs. <1/2) | 0.343 | 0.042–2.797 | 0.295 | – | – | – | |
| LVSI (+ vs. –) | 4.640 | 1.160–18.564 | 0.017 | 2.361 | 0.546–10.217 | 0.240 | |
Evaluation of adjuvant therapy for patients with high-risk prognostic factors
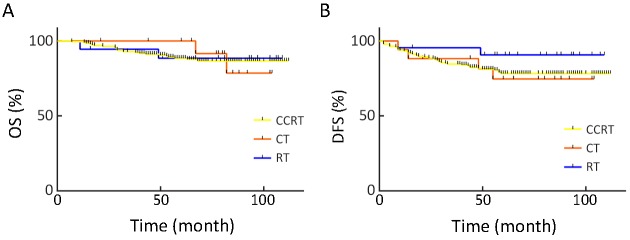
Out of all the patients, 236 who had high-risk prognostic factors (meet one of LNM+, PI+ or vagina margin+) underwent adjuvant therapy, among whom 195 received CCRT, 24 received RT alone, and 17 received CT alone. There was no significant difference in OS or DFS between the three therapeutic regimens, as shown in Figure 1.
1.
Comparison of various regimens of adjuvant therapy [concurrent chemoradiotherapy (CCRT), chemotherapy (CT) and radiotherapy (RT)] for patients with high-risk factors. (A) Overall survival (OS) (P=0.982); (B) Disease-free survival (DFS) (P=0.412).
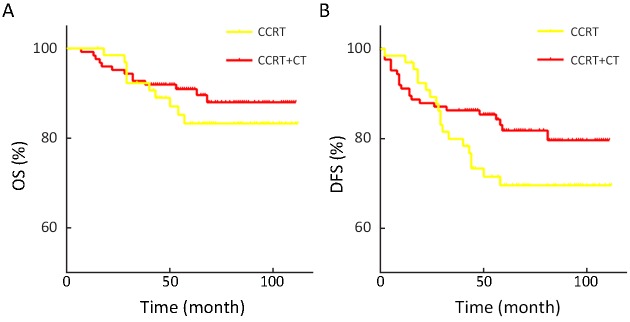
Among the 190 patients with LNM who received CCRT, 124 cases also had systemic CT which consists of paclitaxel plus carboplatin in median 3 cycles. CCRT+CT showed a better prognosis not only for DFS (P=0.118) but also for OS (P=0.374), though the difference did not reached statistical significance, as shown in Figure 2. CCRT+CT decreased the recurrence rate of 14%.
2.
Comparison of concurrent chemoradiotherapy (CCRT) vs. CCRT sequentially with systemic chemotherapy (CT) for patients with lymph node metastasis. (A) Overall survival (OS) (P=0.374); (B) Disease-free survival (DFS) (P=0.118).
Evaluation of adjuvant therapy for patients with single intermediate-risk factor or low-grade differentiation as a potential risk factor
In the stratification analysis, patients with single intermediate-risk factor defined as DSI or LVSI were screened separately. Survival analysis showed that adjuvant therapy could be partially beneficial, though the difference was not statistically significant. This might be due to the small number of cases in the observation group, as shown in Table 9. Because low-grade differentiation is an independent predictor of worse OS in our study, adjuvant therapy tended to improve prognosis. Unfortunately, cases in whom G3 was the sole potential risk factor did not here benefit from adjuvant therapy either with OS (P=0.799) or DFS (P=0.750).
9.
Survival analysis of patients with single IRF or no risk factors receiving AT
| Variables | All (n) | AT [n (%)] | Observation [n (%)] | P | |
| OS | DFS | ||||
| IRF, intermediate-risk factor; AT, adjuvant therapy; LVSI, lymphovascular space invasion ; DSI, deep stromal invasion; OS, overall survival; DFS, disease-free survival. | |||||
| Single IRF | 473 | 288 (60.9) | 185 (39.1) | 0.273 | 0.197 |
| LVSI only | 100 | 91 (91.0) | 9 (9.0) | 0.146 | 0.785 |
| DSI only | 132 | 116 (87.9) | 16 (12.1) | 0.708 | 0.866 |
| G3 only | 172 | 97 (56.4) | 75 (43.6) | 0.799 | 0.750 |
Discussion
Early-stage cervical cancer has a relatively favorable prognosis, with a survival rate over 80% (4). The treatment for most patients with early-stage cervical cancer involves radical hysterectomy and pelvic lymph node dissection, and indications for postoperative adjuvant therapy have been determined by evaluating the prognostic risk factors among surgical-pathologic parameters for recurrence. Because the existing five guidelines for adjuvant therapy, which are from different institutions and regions, are not exactly the same, postoperative adjuvant therapy is applied in different ways in practice. In general, patients with two or more intermediate-risk factors receiving radiotherapy alone have similar cure effect with patients with at least one high-risk factor underwent concurrent chemoradiotherapy (11,12), but these guidelines have slightly different criteria. For one thing, according to the guidelines of the United States National Cancer Institute and Germany’s Working Group of Gynecological Oncology (AGO), patients with only one intermediate-risk factor are indicated for RT or CRT as adjuvant therapy (13). Additionally, AGO guidelines do not classify prognostic factors as intermediate-risk or high-risk ones, so patients with even one risk factor are believed to have indications for adjuvant therapy (13). The standards of Cancer Hospital, Chinese Academy of Medical Sciences are similar to those of the AGO, recommending postoperative adjuvant therapy for patients with single risk factors, including DSI and LVSI. We have had these standards for the past 10 years. We have observed the 5-year survival of early-stage cervical cancer to be around 95% in our data, which is significantly higher than previously reported. This is mainly attributable to timely, aggressive adjuvant therapy.
Risk factors for early-stage cervical cancer after radical surgery have been evaluated for recurrence in many studies. According to the prognostic significance of each factor, they have been defined as intermediate-risk and high-risk groups. In the present study, we identified LNM, LVSI and differentiation as independent prognostic factors for OS, while LNM, LVSI and DSI for DFS regardless of histologic type of cervical cancer. In the stratification analysis, SCC has the same prognostic factors as the whole population except that differentiation is not independent of OS. Regarding AC/ASC, differentiation is the only factor affecting OS; LVSI is the only factor affecting DFS. Histologic grade was thought to be a prognostic factor. However, few studies previously supported that poorly differentiation could predict unfavorable prognosis (5,14,15) so that there is no indication for adjuvant therapy. Our study showed that patients with only G3 cannot benefit from adjuvant therapy. Although G3 could predict poor prognosis for AC/ASC based on our data, it is not clear whether patients with poorly differentiated adenocarcinoma of the cervix but no other risk factors can benefit from adjuvant therapy; this needs to be verified with a larger sample size.
Pelvic LNM is a widely accepted high-risk factor for cervical cancer. Patients with LNM have a recurrence rate of up to 40% higher than those without this factor (16,17). The anatomic level of positive nodal was also shown to be related to the risk of recurrence, with higher recurrence rates when common iliac or para-aortic nodal involvement was observed than when the involved nodes were confined to pelvis (18). Duo the greater rate of distant failure with LNM, postoperative adjuvant therapy can prevent distant metastasis, for which chemotherapy plays an important role as the systemic therapy (19). The combination of additional cycles of chemotherapy following RT or CCRT might reduce distant metastases. In our study, 65.3% of the cases with LNM received CCRT following with CT. Compared to CCRT alone, the former practice showed a better prognostic tendency, especially for DFS (P=0.118), even though the difference was not statistically significant. No statistically significant difference was observed among adjuvant therapy/CT alone or in combined therapy in patients with high-risk factors. A randomized phase III trial (20) compared paclitaxel and carboplatin followed by RT versus RT concurrent with cisplatin in patients at stage IB–IIB after surgery. The research team concluded that sequential CT and RT did not show any survival benefit. It has been proposed that systemic CT alone could have a survival benefit even without RT. But unfortunately, a randomized trial (17) designed to evaluate the efficacy of CT alone compared with external pelvic radiotherapy for patients with high-risk factors after surgery was stopped because the number of patients was far below expectations, which left the results with low statistical power. In addition, another randomized phase III trial (21) comparing the clinical efficacy of adjuvant CT alone versus CT plus RT for patients with high-risk factors failed to establish CT plus RT as a superior adjuvant therapy. Besides the prospective trials, several retrospective studies shared several results. Takekuma et al. reviewed 393 high-risk patients and reported chemotherapy after surgery to have similar efficacy but less toxicity than CCRT (22). Another retrospective study compared postoperative pelvic radiotherapy alone (n=253), chemotherapy alone (n=319), and CCRT (n=502) and found that systemic CT may be as effective as radiation-based therapy in node-positive high-risk stage IB–IIB cervical cancer, but CT alone was likely insufficient for local control. For this reason, additional treatment with radiation is recommended (23). Confronting with such a variety of adjuvant therapy policies, prospective clinical trials to assess the efficacy or non-inferiority of single or combined or sequential postoperative therapy with clear constraints are required.
Sedlis criteria first met the public eye in 2015 in the NCCN guidelines, defined as an intermediate-risk group by various combinations of three factors, including LVSI, DSI and large tumor size, as follows: 1) positive LVSI, deep and middle third penetration, with tumor size ≥2 cm; 2) positive LVSI, superficial third penetration with tumor size ≥5 cm; and 3) negative LVSI, middle or deep third penetration with clinical tumor size ≥4 cm. Putte et al. successfully validated the Sedlis criteria and showed them to exhibit good performance (24). In another study, Rogers et al. conducted a subgroup analysis of OS using the Sedlis criteria and showed that postoperative adjuvant radiotherapy significantly reduced the mortality of patients with the combination of negative LVSI, DSI and tumor size ≥4 cm (25). The sensitivity of these criteria was only 50%, as reported by Ryu et al. (26), which means that half of the recurrences occurred in patients who did not meet these criteria. Sedlis’s study excluded histology of AC, which has been suggested to have poorer prognosis than SCC. A retrospective study recruited one-quarter of the patients with non-squamous carcinoma showed that AC, tumor size, DSI and LVSI were significantly closely associated with disease recurrence and defined a new four-factor model for the intermediate-risk group of patients with cervical cancer (8). However, the prognostic significance of these factors remains unclear. AC was an independent prognostic indicator of poor survival in early-stage cervical cancer with risk factors (27). According to our data, histology was not considered as an independent prognostic factor probably due to the small proportion of non-squamous cases. However, the 5-year OS of AC/ASC was visibly lower than that of SCC. Moreover, LVSI has been found to have a significant impact on recurrence-free survival and OS by itself. However, as reported previously, only when these factors are combined does the risk of recurrence could increase by 15%–20% (5,24).
Practice patterns of adjuvant therapy for patients with intermediate-risk factor are also the subject of heated discussion. A review suggested that the addition of platinum-based CT to adjuvant RT may improve survival in patients with early-stage (Ia2–IIa) cervical cancer with risk factors (28). In a retrospective study (26) covering patients with two or more intermediate-risk factors, patients were categorized into those who underwent no further treatment (NFT) group (n=34), RT group (n=49), and CCRT group (n=89). The 3-year recurrence-free survival rate increased from 67.5% (NFTs) to 90.5% (RT), and increased further to 97.5% (CCRT). In trial GOG092 (29), the role of RT alone for patients with intermediate-risk factor after radical hysterectomy was investigated. In that work, 137 patients were randomly allocated to receive RT and 140 were kept under observation. Final results showed that the use of adjuvant RT to be associated with a hazard ratio (HR) of 0.54 for recurrence (P=0.007). However, the improvement of OS with RT did not reach statistical significance (HR=0.70, P=0.074). In the GOG0263 trial (30) of 868 patients with intermediate-risk factor, the study concluded with no 5-year survival benefit of CCRT over RT. In our data, nearly 80% of the patients with two or more intermediate-risk factors received CCRT, and 10% of the patients receiving RT alone. Because of the disparity of cases among these two groups, it was not possible for us to draw comparable statistical results. Accordingly, the ability of adjuvant therapy to improve OS and the choice of single or combined therapy are still controversial due to overtreatment concerns, although these treatments have been found to reduce recurrence. Further improvement for adjuvant therapy should focus on the definition of prognostic risk factors, refined patient selection, and concretization of both local and systematic therapies.
The weakness of this study is its retrospective nature. However, it does have sufficient sample size and 10-year follow-up.
The results of the present retrospective analysis appear to suggest that aggressive postoperative adjuvant therapy is beneficial for FIGO stage Ib–IIa cervical cancer with risk factors and increases the survival rate. CCRT is the main choice of postoperative adjuvant therapy in Cancer Hospital, Chinese Academy of Medical Sciences, though it seems to have no advantage over RT or CT alone in patients with high-risk factors. CCRT sequential with CT could reduce the recurrence rate by 14% compared to CCRT for patients with LNM. Perspective studies in larger populations are needed to verify the unsolved questions such as whether patients with single intermediate-risk factors could benefit from adjuvant therapy and the preferred patterns. LVSI is an independent prognostic factor both for OS and DFS, and it should be highly valued as an indication for adjuvant therapy, even when found alone.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11. <DOI: 10.3978/j.issn.1000-9604.2016.02.08> <PMID: 27041922>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. <DOI: 10.3322/caac.21262> <PMID:25651787>] [DOI] [PubMed] [Google Scholar]
- 3.Swailes AL, Gockley A, Phaëton R, et al. The Wertheim hysterectomy: Development, modifications, and impact in the present day. Gynecol Oncol. 2017;145:3–8. doi: 10.1016/j.ygyno.2017.01.011. [Swailes AL, Gockley A, Phaëton R, et al. The Wertheim hysterectomy: Development, modifications, and impact in the present day. Gynecol Oncol 2017;145:3-8. <DOI: 10.1016/j.ygyno.2017.01.011> <PMID: 28094020>] [DOI] [PubMed] [Google Scholar]
- 4.Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S43–103. doi: 10.1016/S0020-7292(06)60030-1. [Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S43-103. <DOI: 10.1016/S0020-7292(06)60030-1> <PMID: 17161167>] [DOI] [PubMed] [Google Scholar]
- 5.Ryu SY, Kim MH, Nam BH, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer. 2014;110:278–85. doi: 10.1038/bjc.2013.716. [Ryu SY, Kim MH, Nam BH, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer 2014;110:278-85. <DOI: 10.1038/bjc.2013.716> <PMID: 24357798>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obrzut B, Semczuk A, Naróg M, et al. Prognostic parameters for patients with cervical cancer FIGO stages IA2-IIB: A long-term follow-up. Oncology. 2017;93:106–14. doi: 10.1159/000471766. [Obrzut B, Semczuk A, Naróg M, et al. Prognostic parameters for patients with cervical cancer FIGO stages IA2-IIB: A long-term follow-up. Oncology 2017;93:106-14. <DOI: 10.1159/000471766 > <PMID: 28463843>] [DOI] [PubMed] [Google Scholar]
- 7.Ho CM, Chien TY, Huang SH, et al. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol. 2004;93:458–64. doi: 10.1016/j.ygyno.2004.01.026. [Ho CM, Chien TY, Huang SH, et al. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol 2004;93:458-64. <DOI: 10.1016/j.ygyno.2004.01.026> <PMID: 15099962 >] [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K, Kitahara Y, Satoh T, et al. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC Study) World J Surg Oncol. 2016;14:173. doi: 10.1186/s12957-016-0931-4. [Nakamura K, Kitahara Y, Satoh T, et al. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC Study). World J Surg Oncol 2016;14:173. <DOI: 10.1186/s12957-016-0931-4> <PMID: 27356862>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takekuma M, Kasamatsu Y, Kado N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I-II cervical cancer: A review. J Obstet Gynaecol Res. 2017;43:617–26. doi: 10.1111/jog.13282. [Takekuma M, Kasamatsu Y, Kado N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I-II cervical cancer: A review. J Obstet Gynaecol Res 2017;43:617-26. <DOI: 10.1111/jog.13282> <PMID: 28190285>] [DOI] [PubMed] [Google Scholar]
- 10.Twu NF, Ou YC, Liao CI, et al. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: A Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol. 2016;25:229–35. doi: 10.1016/j.suronc.2016.05.028. [Twu NF, Ou YC, Liao CI, et al. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: A Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol 2016;25:229-35. <DOI: 10.1016/j.suronc.2016.05.028> <PMID: 27566027>] [DOI] [PubMed] [Google Scholar]
- 11.Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 suppl_4:iv72–iv83. doi: 10.1093/annonc/mdx220. [Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28 suppl_4:iv72-iv83. <DOI: 10.1093/annonc/mdx220 > <PMID: 28881916>] [DOI] [PubMed] [Google Scholar]
- 12.Ebina Y, Yaegashi N, Katabuchi H, et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2015;20:240–8. doi: 10.1007/s10147-015-0806-7. [Ebina Y, Yaegashi N, Katabuchi H, et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240-8. <DOI: 10.1007/s10147-015-0806-7> <PMID: 25800808>] [DOI] [PubMed] [Google Scholar]
- 13.Beckmann MW, Mallmann P. Interdisciplinary S2k guideline on the diagnosis and treatment of cervical carcinoma. J Cancer Res Clin Oncol. 2009;135:1197–206. doi: 10.1007/s00432-009-0560-1. [Beckmann MW, Mallmann P. Interdisciplinary S2k guideline on the diagnosis and treatment of cervical carcinoma. J Cancer Res Clin Oncol 2009;135:1197-206. <DOI: 10.1007/s00432-009-0560-1> <PMID: 19288271>] [DOI] [PubMed] [Google Scholar]
- 14.Soisson AP, Soper JT, Clarke-Pearson DL, et al. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990;37:390–5. doi: 10.1016/0090-8258(90)90374-t. [Soisson AP, Soper JT, Clarke-Pearson DL, et al. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol 1990;37:390-5. <PMID: 2351324>] [DOI] [PubMed] [Google Scholar]
- 15.Zaino RJ, Ward S, Delgado G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer. 1992;69:1750–8. doi: 10.1002/1097-0142(19920401)69:7<1750::aid-cncr2820690717>3.0.co;2-s. [Zaino RJ, Ward S, Delgado G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer 1992;69:1750-8. <PMID: 1551060>] [DOI] [PubMed] [Google Scholar]
- 16.Stock RG, Chen AS, Flickinger JC, et al. Node-positive cervical cancer: impact of pelvic irradiation and patterns of failure. Int J Radiat Oncol Biol Phys. 1995;31:31–6. doi: 10.1016/0360-3016(94)00391-W. [Stock RG, Chen AS, Flickinger JC, et al. Node-positive cervical cancer: impact of pelvic irradiation and patterns of failure. Int J Radiat Oncol Biol Phys 1995;31:31-6. <DOI: 10.1016/0360-3016(94)00391-W> <PMID: 7995765>] [DOI] [PubMed] [Google Scholar]
- 17.Lahousen M, Haas J, Pickel H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: A randomized, prospective, multicenter trial. Gynecol Oncol. 1999;73:196–201. doi: 10.1006/gyno.1999.5343. [Lahousen M, Haas J, Pickel H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: A randomized, prospective, multicenter trial. Gynecol Oncol 1999;73:196-201. <DOI: 10.1006/gyno.1999.5343> <PMID: 10329034>] [DOI] [PubMed] [Google Scholar]
- 18.Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96:721–8. doi: 10.1016/j.ygyno.2004.11.007. [Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol 2005;96:721-8. <DOI: 10.1016/j.ygyno.2004.11.007> <PMID: 15721417>] [DOI] [PubMed] [Google Scholar]
- 19.Uno T, Isobe K, Yamamoto S, et al. Postoperative radiation therapy for carcinoma of the uterine cervix. Radiat Med. 2006;24:91–7. doi: 10.1007/BF02493274. [Uno T, Isobe K, Yamamoto S, et al. Postoperative radiation therapy for carcinoma of the uterine cervix. Radiat Med 2006;24:91-7. <PMID: 16715669>] [DOI] [PubMed] [Google Scholar]
- 20.Sehouli J, Runnebaum IB, Fotopoulou C, et al. A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC-R): a NOGGO-AGO Intergroup Study. Ann Oncol. 2012;23:2259–64. doi: 10.1093/annonc/mdr628. [Sehouli J, Runnebaum IB, Fotopoulou C, et al. A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC-R): a NOGGO-AGO Intergroup Study. Ann Oncol 2012;23:2259-64. <DOI: 10.1093/annonc/mdr628> <PMID: 22357252>] [DOI] [PubMed] [Google Scholar]
- 21.Curtin JP, Hoskins WJ, Venkatraman ES, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol. 1996;61:3–10. doi: 10.1006/gyno.1996.0087. [Curtin JP, Hoskins WJ, Venkatraman ES, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol 1996;61:3-10. <PMID: 8626113>] [DOI] [PubMed] [Google Scholar]
- 22.Takekuma M, Kasamatsu Y, Kado N, et al. Adjuvant chemotherapy versus concurrent chemoradiotherapy for high-risk cervical cancer after radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol. 2016;21:741–7. doi: 10.1007/s10147-016-0955-3. [Takekuma M, Kasamatsu Y, Kado N, et al. Adjuvant chemotherapy versus concurrent chemoradiotherapy for high-risk cervical cancer after radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol 2016;21:741-7. <DOI: 10.1007/s10147-016-0955-3> <PMID: 26857458>] [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Shimada M, Aoki Y, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int J Cancer. 2017;141:1042–51. doi: 10.1002/ijc.30793. [Matsuo K, Shimada M, Aoki Y, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int J Cancer 2017;141:1042-51. <DOI: 10.1002/ijc.30793> <PMID: 28524247>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Putte G, Lie AK, Vach W, et al. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol Oncol. 2005;99:106–12. doi: 10.1016/j.ygyno.2005.05.026. [Van de Putte G, Lie AK, Vach W, et al. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol Oncol 2005;99:106-12. <DOI: 10.1016/j.ygyno.2005.05.026> <PMID: 16137752>] [DOI] [PubMed] [Google Scholar]
- 25.Rogers L, Siu SS, Luesley D, et al. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev 2012:CD007583.
- 26.Ryu SY, Park SI, Nam BH, et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int J Radiat Oncol Biol Phys. 2011;79:794–9. doi: 10.1016/j.ijrobp.2009.11.019. [Ryu SY, Park SI, Nam BH, et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int J Radiat Oncol Biol Phys 2011;79:794-9. <DOI: 10.1016/j.ijrobp.2009.11.019> <PMID: 20421158>] [DOI] [PubMed] [Google Scholar]
- 27.Mabuchi S, Okazawa M, Matsuo K, et al. Impact of histological subtype on survival of patients with surgically-treated stage IA2-IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol. 2012;127:114–20. doi: 10.1016/j.ygyno.2012.06.021. [Mabuchi S, Okazawa M, Matsuo K, et al. Impact of histological subtype on survival of patients with surgically-treated stage IA2-IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol 2012;127:114-20. <DOI: 10.1016/j.ygyno.2012.06.021> <PMID: 22728518>] [DOI] [PubMed] [Google Scholar]
- 28.Falcetta FS, Medeiros LR, Edelweiss MI, et al. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2016;11:CD005342. doi: 10.1002/14651858.CD005342.pub4. [Falcetta FS, Medeiros LR, Edelweiss MI, et al. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev 2016;11:CD005342. <DOI: 10.1002/14651858.CD005342.pub4> <PMID: 27873308>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–83. doi: 10.1006/gyno.1999.5387. [Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177-83. <DOI: 10.1006/gyno.1999.5387> <PMID: 10329031>] [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud O, Hathout L, Shaaban SG, et al. Can chemotherapy boost the survival benefit of adjuvant radiotherapy in early stage cervical cancer with intermediate risk factors? A population based study. Gynecol Oncol. 2016;143:539–44. doi: 10.1016/j.ygyno.2016.10.022. [Mahmoud O, Hathout L, Shaaban SG, et al. Can chemotherapy boost the survival benefit of adjuvant radiotherapy in early stage cervical cancer with intermediate risk factors? A population based study. Gynecol Oncol 2016;143:539-44. <DOI: 10.1016/j.ygyno.2016.10.022> <PMID: 27769525>] [DOI] [PubMed] [Google Scholar]