Abstract
Objective
CareHPV is a human papillomavirus (HPV) DNA test for low-resource settings (LRS). This study assesses optimum triage strategies for careHPV-positive women in LRS.
Methods
A total of 2,530 Chinese women were concurrently screened for cervical cancer with visual inspection with acetic acid (VIA), liquid-based cytology and HPV testing by physician- and self-collected careHPV, and physician-collected Hybrid Capture 2 (HC2). Screen-positive women were referred to colposcopy with biopsy and endocervical curettage as necessary. HPV-positivity was defined as ≥1.0 relative light units/cutoff (RLU/CO) for both careHPV and HC2. Primary physician-HC2, physician-careHPV and self-careHPV and in sequential screening with cytology, VIA, or increased HPV test-positivity performance, stratified by age, were assessed for cervical intraepithelial neoplasia (CIN) grade 2/3 or worse (CIN2/3+) detection.
Results
The sensitivities and specificities of primary HPV testing for CIN2+ were: 83.8%, 88.1% for physician-careHPV; 72.1%, 88.2% for self-careHPV; and 97.1%, 86.0% for HC2. Physician-careHPV test-positive women with VIA triage had a sensitivity of 30.9% for CIN2+ versus 80.9% with cytology triage. Self-careHPV test-positive women with VIA triage was 26.5% versus 66.2% with cytology triage. The sensitivity of HC2 test-positive women with VIA triage was 38.2% versus 92.6% with cytology triage. The sensitivity of physician-careHPV testing for CIN2+ decreased from 83.8% at ≥1.0 RLU/CO to 72.1% at ≥10.00 RLU/CO, while the sensitivity of self-careHPV testing decreased from 72.1% at ≥1.0 RLU/CO to 32.4% at ≥10.00 RLU/CO; similar trends were seen with age-stratification.
Conclusions
VIA and cytology triage improved specificity for CIN2/3 than no triage. Sensitivity with VIA triage was unsuitable for a mass-screening program. VIA provider training might improve this strategy. Cytology triage could be feasible where a high-quality cytology program exists. Triage of HPV test-positive women by increased test positivity cutoff adds another LRS triage option.
Keywords: Cervical cancer, human papillomavirus, careHPV testing , screening, triage
Introduction
Cervical cancer incidence and mortality have declined in countries where cytology-based screening has been implemented (1). Cervical cancer remains the fourth most common female cancer in the world (2), however, because of the disease-burden disparity between high and low-income countries, which do not have effective national cervical cancer screening programs (3). Because of the complexity, need for trained personnel, and frequent follow-up (4,5), it is difficult to establish cytology-based cervical cancer screening programs in low-resource settings (LRS), where there are no sufficient health infrastructure, personnel and equipment.
Alternative screening methods have emerged as options for LRS, including visual inspection with acetic acid (VIA) and human papillomavirus (HPV) DNA tests. The sensitivity of VIA is variable, ranging from 31% to 95% for detection of cervical intraepithelial neoplasia (CIN) grade 2 or worse (CIN2+) (6). Another option is DNA testing for carcinogenic HPV. HPV testing has been shown to reduce CIN2+ prevalence more than VIA (7) and to be more effective than VIA or cytology in reducing cervical cancer mortality with a single round of screening (8). The United States Food and Drug Administration’s first-approved HPV DNA test, Hybrid Capture 2 (HC2; Qiagen, Gaithersburg, MD, USA) has been widely used in studies of primary HPV testing. HC2 has been approved for diagnostic use at a positivity cutoff of 1.0 relative light units/cutoff (RLU/CO) or higher. CareHPV (Qiagen, Gaithersburg, MD) is a more affordable, simplified HPV DNA test developed for use in low-income countries. CareHPV has been approved for diagnostic use at a cutoff of 1.0 RLU/CO or higher by China Food and Drug Administration (CFDA). The careHPV test has been shown to have comparable performance to HC2 in screening for CIN2+ using cervical samples, takes 2.5 h to run, and can be conducted by technicians in varied working conditions (9).
HPV DNA testing offers the opportunity of using self-collected vaginal samples for primary cervical cancer screening. Self-collection can be done by a woman herself without a pelvic exam, health professionals, or a visit to a health clinic, and thus presents a viable option for primary screening in LRS. A pooled analysis of 13,000 women from China showed that, with HC2 DNA testing, self-HPV was as sensitive as liquid-based cytology (LBC) and superior to VIA in detection for high-grade CIN and that physician-HPV testing was more sensitive but similarly specific than self-HPV testing (10). A multi-country analysis found that self-careHPV and physician-careHPV testing had higher sensitivities than cytology or VIA (11).
Primary HPV testing is characteristic of a high average sensitivity, albeit a low average specificity for CIN2+ detection in comparison to VIA or cytology (12), and as such, HPV-positive women with transient or non-progressing high-risk (hr) HPV infections are at risk for unnecessary detection or overtreatment. Therefore, triage of hrHPV-positive women is appropriate for clinical management (13). However, few studies discussed the performance of careHPV test by triage methods and across test positivity cutoffs stratifed by age. This study evaluates the clinical performances of primary self-careHPV triage and primary physician-careHPV triage by VIA, cytology, and test positivity cutoff with age stratification for high-grade CIN detection, compared with primary physician-HC2 triage, VIA and cytology, in order to explore new approaches to current screening algorithms in LRS. Both HPV tests are approved for use at a cutoff of 1.0 RLU/CO or higher, and so we sought to explore the utility in triage by increasing test positivity cutoff for research investigation only, without changing the primary screening cutoffs. This study contributes data to the future discussions around the possible off-label use of raising HPV DNA testing positivity to increase test specificity in areas where other triage methods are not viable.
Materials and methods
Study population and ethics approval
Women aged 30 to 54 years were recruited to undergo cervical cancer screening from May 10th to June 15th, 2007 from two communes in each of Wuxiang and Xiangyuan counties, Shanxi Province, China. The four communes were selected through a simple randomized sampling method from the list of all communes in these two counties. Then all eligible women in these four communes were invited to attend the screening. Women were study-eligible if they had no history of CIN, pelvic radiation, hysterectomy, were not currently pregnant and were able to provide informed consent. This study was approved by the Institutional Review Board of Cancer Institute/Hospital, Chinese Academy of Medical Sciences (CICAMS) and the Human Subjects Protection Committee of Program for Appropriate Technology in Health (PATH).
Screening procedures
Cervical cancer screenings were carried out at Women and Children’s Hospitals in Wuxiang and Xiangyuan. A trained health care worker provided each woman with informed consent and administered a questionnaire regarding socio-demographic, reproductive, and behavioral information in a confidential interview.
Under the instruction of a nurse in clinic, each woman provided a vaginal-brush specimen for the self-careHPV test, and two vaginal nylon-swab specimens for storage. A physician then performed a speculum exam and collected cervical specimens. One cervical specimen was collected for the physician-careHPV test, one cervical specimen was collected for liquid-based cytology classification (SurePath, Becton Dickinson, Franklin Lakes, NJ, USA; LBC) and HC2 testing, and two cervical specimens using nylon swabs were collected for storage. All women had VIA followed by a digital colposcopy (Goldway, Shenzhen, China).
As described in previous studies from our lab (9), if women had an abnormal colposcopy exam, directed cervical biopsies were taken where lesions were visiable. Endocervical curettage (ECC) was performed if women had an unsatisfactory colposcopy exam (the squamocolumnar junction was not completely visable), if the lesion extended into the endocervical canal and if the lesions were inaccessible to biopsy. Cytological slides were classified according to the Bethesda system. Women with negative colposcopy results but positive LBC results, defined as atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesions or worse (ASC-H+); unsatisfactory cytology reading; a positive HC2 test; or a positive careHPV test, were called back to undergo a second colposcopy exam, where four-quadrant biopsies of the cervical squamocolumnar junction and ECC were taken. Women found to have CIN2+ were offered free treatment according to local clinical guidelines. The study methods have been previously published (9).
Screening tests
VIA
VIA positivity was defined as observing a distinct, dense, non-moveable acetowhite areas in the transformation zone near the squamocolumnar junction of the cervix, visible 1 min after application of 3% to 5% acetic acid. Visual inspection was performed by nurse midwives.
HPV testing
HPV infection was assessed using the HC2 assay (QIAGEN, Gaithersburg, Maryland) and careHPV test (QIAGEN, Gaithersburg, Maryland). HC2 assay was conducted using the residual LBC medium after processing. The HC2 assay detects a pool of 13 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). The careHPV assay is broadly based on the HC2 assay with some important modifications for LRS, including faster assay time, adaptability to a wide range of conditions, and the targeting of 14 high-risk HPV types (HPV66 in addition to the 13 high-risk HPV genotypes detected by HC2 assay). Both HC2 and careHPV positivities were calculated at 1.0 RLU/CO (approximately equal to 1.0 pg DNA per mL) according to the manufacturer’s recommendation. Trained local technicians performed careHPV testing at the field sites in Shanxi. The HC2 assay was performed by technicians at CICAMS, Beijing.
Cytology and histology
Preparation and reading of cytological and histological slides were done at CICAMS by a cyto-pathologist and a pathologist. The Bethesda and the CIN classification systems were used for cytology and histology, respectively. All abnormal slides and 10% of normal histological and cytological slides were randomly selected to be read by an external pathologist in Canada, who was blinded to the CICAMS diagnoses. If the diagnoses were not in agreement, the final diagnosis was based on the Canadian pathologist’s reading with discussion of any discordant readings with the Chinese pathologist. The final diagnosis for each woman was based on the highest reading across all histological findings, including directed and four-quadrant biopsies and ECC. If a biopsy had not been indicated or if the histology finding was negative for a woman, then she was assessed as negative for CIN.
Statistical analysis
Analyses were focused on the clinical performances of self-careHPV and physician-careHPV as primary screening methods. HPV DNA test-positive women were triaged with either VIA or cytology if CIN2+ and CIN grade 3 or worse (CIN3+) were detected, in comparison to VIA and cytology as primary screening, or HC2 followed by VIA or cytology triage. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden’s index and area under the curve (AUC) of the receiver operating characteristics (ROC) of each strategy were calculated, as well as the number of CIN2+ or CIN3+ women detected per strategy and the number of colposcopies examined per CIN2+ or CIN3+ detection. McNemar’s test was used to evaluate the differences in sensitivities and specificities between strategies. Chi-square test was used to compare the differences in PPV and NPV. The z-test was used to evaluate for differences in AUC. The clinical performance of self-careHPV, physician-careHPV and physician-HC2 primary screening was also calculated at different cutoffs of test positivity (1.0, 2.0, 5.0 and 10.0 RLU/CO).
The mean menopausal age of the study population was 46.7 years old, and so the study population was stratified into two age groups, women aged 30 to 44 years and 45 to 54 years. The above analyses were performed among the stratified age groups.
When performing the pairwise comparison among different strategies, a two-sided α error level of 0.05 was adjusted to the value that equaled to 0.05 divided by the number of comparison on basis of the Bonferroni correction. Otherwise, a P value less than or equal to 0.05 (two-sided) was considered statistically significant. Analyses were done using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA).
Results
Study participants
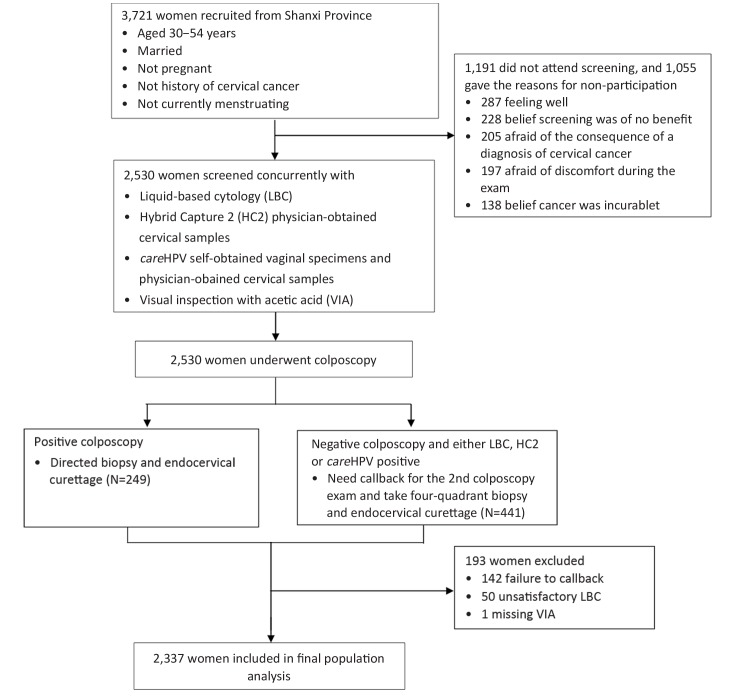
Of the 3,721 eligible women invited to participate, 2,530 (68.0%) came for cervical cancer screening. One hundred and forty-two women did not return for callback examinations. Besides these 142 women, 50 women with unsatisfactory cytology and 1 woman with missing VIA were also excluded from the analysis. Thus, a total of 2,337 women with complete results were included in the analysis (Figure 1).
1.
Study flowchart.
Among women included in the final population analysis, the mean age was 43.4±6.2 years old, and there were 1,350 women (57.8%, 1,350/2,337) aged at 30–44 years and 987 (42.2%, 987/2,337) aged at 45–54 years, respectively. The mean age of sexual debut was 20.5±2.4 years, and the mean age of menopause was 46.7±4.2 years. Only 4 women (0.2%, 4/2,337) were currently using hormonal contraceptives, and 81.3% (1,900/2,337) had been sterilized. The mean number of live-births was 2.7±1.0. Nearly all of women were currently married and had never smoked. The HPV positive rates were 14.0% (328/2,337) of physician-careHPV, 13.6% (317/2,337) of self-careHPV and 16.4% (384/2,337) of physician-HC2, respectively. Six percent of women (140/2,337) had an abnormal VIA result and 5.4% (127/2,337) were cytology grade ASC-H+. Finally, 2.4% (56/2,337) were diagnosed as CIN1, 1.9% (45/2,337) as CIN2 and 1.0% (23/2,337) as CIN3+.
Primary screening performance of physician- and self-collected careHPV compared to other screening methods for CIN2+ and CIN3+ detection
When comparing the sensitivity of primary screening methods, physician-careHPV testing for CIN2+ was significantly higher than VIA (83.8% vs. 39.7%, P<0.0001), but lower than that of HC2 testing (97.1%, P=0.004), and comparable to cytology (85.3%, P=1.000) (Table 1). In terms of the specificity of primary screening methods, physician-careHPV for CIN2+ was significantly lower than cytology and VIA (88.1% vs. 97.0% and 95.0%, all P<0.0001) and higher than HC2 (86.0%, P<0.0001). No significant differences of sensitivity and specificity were observed between primary physician-careHPV testing vs. self-careHPV testing (sensitivity: 83.8% vs. 72.1%, P=0.057; specificity: 88.1% vs. 88.2%, P=0.890). Youden’s index for CIN2+ ranged from 83.0% for HC2 testing to 34.7% for VIA, and was 71.9% for physician-careHPV and 60.2% for self-careHPV. However, the AUCs were not significantly different between physician-careHPV and self-careHPV (0.859 vs. 0.801, P=0.075) (Figure 2). A similar pattern was observed for CIN3+ detection (Table 2, Figure 3).
1.
Primary and triage screening performance of physician- and self-collected careHPV at ≥1.0 RLU/CO for CIN2+ detection among 2,337 women, compared to VIA and cytology primary screening, and physician-HC2 triage strategies
| Primary Screening | Triage strategy | CIN2+ (N=68) | % (95% CI) | Colposcopy per CIN2+ (N) | ||||
| Sensitivity | Specificity | PPV | NPV | Youden’s index | ||||
| HPV, human papillomavirus; RLU/CO, relative light units/cutoff; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; VIA, visual inspection with acetic acid; HC2, Hybrid Capture 2; ASC-H+, atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesions or worse; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value; ASC-US+, atypical squamous cells of undetermined significance or worse. | ||||||||
| Physician- careHPV ≥1.0 RLU/CO | None | 57 | 83.8
(73.3–90.7) |
88.1
(86.7–89.3) |
17.4
(13.7–21.9) |
99.5
(99.0–99.7) |
71.9
(70.1–73.7) |
5.8 |
| ASC-US+ | 55 | 80.9
(70.0–88.5) |
95.0
(94.0–95.8) |
32.5
(25.9–39.9) |
99.4
(99.0–99.6) |
75.9
(74.2–77.6) |
3.1 | |
| VIA | 21 | 30.9
(21.2–42.6) |
99.2
(98.7–99.5) |
52.5
(37.5–67.1) |
98.0
(97.3–98.5) |
30.0
(28.1–31.9) |
1.9 | |
| Self-careHPV ≥1.0 RLU/CO | None | 49 | 72.1
(60.4–81.3) |
88.2
(86.8–89.5) |
15.5
(11.9–19.8) |
99.1
(98.5–99.4) |
60.2
(58.2–62.2) |
6.5 |
| ASC-US+ | 45 | 66.2
(54.3–76.3) |
96.4
(95.5–97.1) |
35.4
(27.7–44.1) |
99.0
(98.4–99.3) |
62.6
(60.6–64.6) |
2.8 | |
| VIA | 18 | 26.5
(17.5–38.0) |
99.2
(98.7–99.5) |
50.0
(34.5–65.5) |
97.8
(97.1–98.3) |
25.7
(23.9–27.5) |
2.0 | |
| Physician-HC2 ≥1.0 RLU/CO | None | 66 | 97.1
(89.9–99.2) |
86.0
(84.5–87.4) |
17.2
(13.7–21.3) |
99.9
(99.6–100) |
83.0
(81.5–84.5) |
5.8 |
| ASC-US+ | 63 | 92.6
(83.9–96.8) |
94.4
(93.3–95.2) |
33.0
(26.7–39.9) |
99.8
(99.5–99.9) |
87.0
(85.6–88.4) |
3.0 | |
| VIA | 26 | 38.2
(27.6–50.1) |
99.0
(98.5–99.0) |
54.2
(40.3–67.4) |
98.2
(97.5–98.6) |
37.3
(35.3–39.3) |
1.8 | |
| Cytology (ASC-H+) | None | 58 | 85.3
(75.0–91.8) |
97.0
(96.2–97.6) |
45.7
(37.3–54.3) |
99.5
(99.2–99.8) |
82.3
(80.8–83.8) |
2.2 |
| VIA | None | 27 | 39.7
(28.9–51.6) |
95.0
(94.0–95.8) |
19.3
(13.6–26.6) |
98.1
(97.5–98.6) |
34.7
(32.8–36.6) |
5.2 |
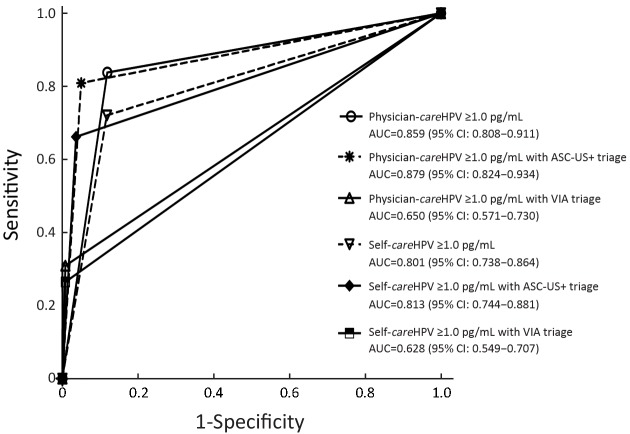
2.
Receiver operating characteristics (ROC) curves of physician-careHPV and self-careHPV using atypical squamous cells of undetermined significance or worse (ASC-US+) or visual inspection with acetic acid (VIA) triage at positivity cutoff ≥1.0 relative light units/cutoff (RLU/CO), for cervical intraepithelial neoplasia (CIN) grade 2 or worse (CIN2+) detection among 2,337 women. AUC, area under the ROC curve.
2.
Primary and triage screening performance of physician- and self-collected careHPV at ≥1.0 RLU/CO for CIN3+ detection among 2,337 women, compared to VIA and cytology primary screening, and physician-HC2 triage strategies
| Primary Screening | Triage strategy | CIN3+ (N=23) | % (95% CI) | Colposcopy per CIN3+ (N) | ||||
| Sensitivity | Specificity | PPV | NPV | Youden’s index | ||||
| HPV, human papillomavirus; RLU/CO, relative light units/cutoff; CIN3+, cervical intraepithelial neoplasia grade 3 or worse; VIA, visual inspection with acetic acid; HC2, Hybrid Capture 2; ASC-H+, atypical squamous cells- cannot exclude high-grade squamous intraepithelial lesions or worse; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value; ASC-US+, atypical squamous cells of undetermined significance or worse. | ||||||||
| Physician- careHPV ≥1.0 RLU/CO | None | 20 | 87.0
(67.9–95.5) |
86.7
(85.2–88.0) |
6.1
(4.0–9.2) |
99.9
(99.6–99.9) |
73.6
(71.8–75.4) |
16.4 |
| ASC-US+ | 19 | 82.6
(62.9–93.0) |
93.5
(92.4–94.5) |
11.2
(7.3–16.9) |
99.8
(99.5–99.9) |
76.1
(74.4–77.8) |
8.9 | |
| VIA | 12 | 52.2
(33.0–70.8) |
98.8
(98.3–99.2) |
30.0
(18.1–45.4) |
99.5
(99.1–99.7) |
51.0
(49.0–53.0) |
3.3 | |
| Self-careHPV ≥1.0 RLU/CO | None | 18 | 78.3
(58.1–90.3) |
87.1
(85.7–88.4) |
5.7
(3.6–8.8) |
99.8
(99.4–99.9) |
65.3
(63.4–67.2) |
17.6 |
| ASC-US+ | 16 | 69.6
(49.1–84.4) |
95.2
(94.3–96.0) |
12.6
(7.9–19.5) |
99.7
(99.3–99.8) |
64.8
(62.9–66.7) |
7.9 | |
| VIA | 10 | 43.5
(25.6–63.2) |
98.9
(98.4–99.2) |
27.8
(15.8–44.0) |
99.4
(99.0–99.7) |
42.4
(40.4–44.4) |
3.6 | |
| Physician- HC2 ≥1.0 RLU/CO | None | 22 | 95.7
(79.0–99.2) |
84.4
(82.8–85.8) |
5.7
(3.8–8.5) |
99.9
(99.7–100) |
80.0
(78.4–81.6) |
17.5 |
| ASC-US+ | 21 | 91.3
(73.2–97.6) |
92.7
(91.5–93.6) |
11.0
(7.3–16.2) |
99.9
(99.7–100) |
84.0
(82.5–85.5) |
9.1 | |
| VIA | 13 | 56.5
(36.8–74.4) |
98.5
(97.9–98.9) |
27.1
(16.6–41.0) |
99.6
(99.2–99.8) |
55.0
(53.0–57.0) |
3.7 | |
| Cytology (ASC-H+) | None | 20 | 87.0
(67.9–95.5) |
95.4
(94.4–96.2) |
15.7
(10.4–23.1) |
99.9
(99.6–100) |
82.4
(80.9–83.9) |
6.4 |
| VIA | None | 13 | 56.5
(36.8–74.4) |
94.5
(93.5–95.4) |
9.3
(5.5–15.2) |
99.5
(99.2–99.8) |
51.0
(49.0–53.0) |
10.8 |
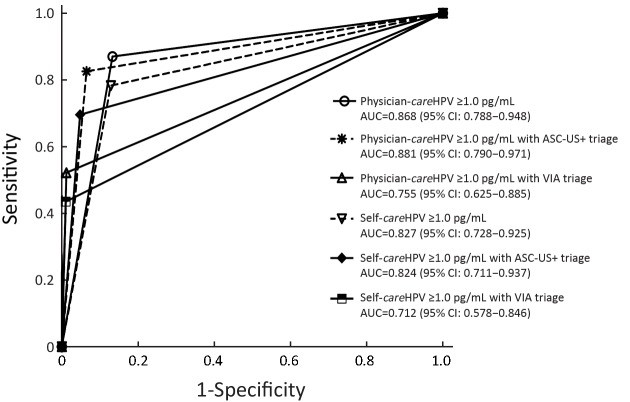
3.
Receiver operating characteristics (ROC) curves of physician-careHPV and self-careHPV using atypical squamous cells of undetermined significance or worse (ASC-US+) or visual inspection with acetic acid (VIA) triage at positivity cutoff ≥1.0 relative light units/cutoff (RLU/CO), for cervical intraepithelial neoplasia (CIN) grade 3 or worse (CIN3+) detection among 2,337 women. AUC, area under the ROC curve.
Comparision of primary HPV testing with VIA and cytology triage for CIN2+ and CIN3+ detection
The sensitivity of primary physician-careHPV for CIN2+ detection was 83.8%, which was comparable with cytology triage (80.9%, P=0.500) and lower with VIA triage (30.9%, P<0.0001) (Table 1). The specificity of primary physician-careHPV for CIN2+ was 88.1% and increased with cytology triage (95.0%, P<0.0001) and VIA triage (99.2%, P<0.0001). The number of colposcopies per CIN2+ decreased from 5.8 for primary screening using physician-careHPV to 1.9 with VIA triage. AUC was comparable between primary physician-careHPV screening and cytology triage (0.859 vs. 0.879, P=0.354), but decreased to 0.650 for VIA triage (P<0.001) (Figure 2). Youden’s index showed the same trend. PPV increased from 17.4% for primary physician-careHPV screening to 52.5% with VIA triage (all P<0.0001), while NPV was comparable. Similar trends were observed for self-careHPV based strategies and HC2 based strategies (Table 1, Figure 2).
The sensitivity of physician-careHPV with cytology triage for CIN2+ detection was 80.9%, higher than self-careHPV with cytology triage of 66.2% (P=0.006) (Table 1). For careHPV with VIA triage, there was no statistically significant difference in sensitivity between physician-careHPV and self-careHPV (30.9% vs. 26.5%, P>0.05). Of note, the sensitivity of HC2 with cytology triage was the highest for CIN2+ detection (92.6%, P<0.001). Meanwhile, there were no statistically significant differences of specificity, PPV, NPV and the number of colposcopy per CIN2+ between primary physician-careHPV and self-careHPV with cytology triage and VIA triage (all P>0.05) (Table 1).
The clinical performance of the above strategies for CIN3+ detection followed similar trends (Table 2, Figure 3).
There were no statistically significant differences for CIN2+ or CIN3+ detection for HPV DNA testing and cytology triage between 30 to 44 and 45 to 54 years old, but among women aged 45 to 54 years, HPV DNA testing with VIA triage was less sensitive for CIN2+/CIN3+ detection than among women aged 30 to 44 years (Supplementary Tables S1, S2).
S1.
Clinical performance of physician- and self-collected careHPV testing in primary and triage screening strategies, stratified by age, for CIN2+ detection among 2,337 women
| Primary screening | Triage strategy | Age (year) | CIN2+ (N=68) | % (95% CI) | Colposcopy per CIN2+ (N) | ||||
| Sensitivity | Specificity | PPV | NPV | Youden’s index | |||||
| HPV, human papillomavirus; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; RLU/CO, relative light units/cutoff; HC2, Hybrid Capture 2; ASC-US+, atypical squamous cells of undetermined significance or worse; VIA, visual inspection with acetic acid; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value. | |||||||||
| Physician-careHPV ≥1.0 RLU/CO | ASC-US+ | 30 to 44 | 32 | 82.1
(67.3–91.0) |
96.1
(94.9–97.0) |
38.6
(28.8–49.3) |
99.4
(98.9–99.7) |
78.2 | 2.6 |
| 45 to 54 | 23 | 79.3
(61.6–90.2) |
93.4
(91.7–94.8) |
26.7
(18.5–36.9) |
99.3
(98.6–99.7) |
72.7 | 3.7 | ||
| VIA | 30 to 44 | 13 | 33.3
(20.6–49.0) |
99.0
(98.3–99.4) |
50.0
(32.1–67.9) |
98.0
(97.1–98.7) |
32.3 | 2.0 | |
| 45 to 54 | 8 | 27.6
(14.7–45.7) |
99.4
(98.6–99.7) |
57.1
(32.6–78.6) |
97.8
(96.7–98.6) |
27.0 | 1.8 | ||
| Self-careHPV ≥1.0 RLU/CO | ASC-US+ | 30 to 44 | 29 | 74.4
(58.9–85.4) |
96.9
(95.8–97.7) |
41.4
(30.6–53.1) |
99.2
(98.6–99.6) |
71.2 | 2.4 |
| 45 to 54 | 16 | 55.2
(37.5–71.6) |
95.7
(94.2–96.8) |
28.1
(18.1–40.8) |
98.6
(97.6–99.2) |
50.9 | 3.6 | ||
| VIA | 30 to 44 | 13 | 33.3
(20.6–49.0) |
99.0
(98.3–99.4) |
50.0
(32.1–67.9) |
98.0
(97.1–98.7) |
32.3 | 2.0 | |
| 45 to 54 | 5 | 17.2
(7.6–34.5) |
99.5
(99.6–100) |
50.0
(23.7–76.3) |
97.5
(96.4–98.3) |
16.7 | 2.0 | ||
| Physician-HC2 ≥1.0 RLU/CO | AS-CUS+ | 30 to 44 | 37 | 94.9
(83.1–98.6) |
95.2
(93.9–96.2) |
37.0
(28.2–46.8) |
99.8
(99.4–100) |
90.1 | 2.7 |
| 45 to 54 | 26 | 89.7
(73.6–96.4) |
93.2
(91.4–94.6) |
28.6
(20.3–38.6) |
99.7
(99.0–99.9) |
82.9 | 3.5 | ||
| VIA | 30 to 44 | 16 | 41.0
(27.1–56.6) |
98.9
(98.1–99.3) |
51.6
(34.8–68.0) |
98.3
(97.4–98.8) |
39.9 | 1.9 | |
| 45 to 54 | 10 | 34.5
(19.9–52.7) |
99.3
(98.5–99.6) |
58.8
(36.0–78.4) |
98.0
(97.0–98.4) |
33.8 | 1.7 | ||
S2.
Clinical performance of physician- and self-collected careHPV testing in primary and triage screening strategies, stratified by age, for CIN3+ detection among 2,337 women
| Primary screening | Triage strategy | Age (year) | CIN3+ (N=23) | % (95% CI) | Colposcopy per CIN3+ (N) | ||||
| Sensitivity | Specificity | PPV | NPV | Youden’s index | |||||
| HPV, human papillomavirus; CIN3+, cervical intraepithelial neoplasia grade 3 or worse; RLU/CO, relative light units/cutoff; HC2, Hybrid Capture 2; ASC-US+, atypical squamous cells of undetermined significance or worse; VIA, visual inspection with acetic acid; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value. | |||||||||
| Physician-careHPV ≥1.0 RLU/CO | ASC-US+ | 30 to 44 | 10 | 83.3
(55.2–95.3) |
94.5
(93.2–95.6) |
12.0
(6.7–20.8) |
99.8
(99.4–100) |
77.9 | 8.3 |
| 45 to 54 | 9 | 81.8
(52.3–94.9) |
92.1
(90.2–93.6) |
10.5
(5.6–18.7) |
99.8
(99.2–99.9) |
73.9 | 9.6 | ||
| VIA | 30 to 44 | 7 | 58.3
(32.0–80.7) |
98.6
(97.8–99.1) |
26.9
(13.7–46.1) |
99.6
(99.1–99.8) |
56.9 | 3.7 | |
| 45 to 54 | 5 | 45.5
(28.0–78.7) |
99.1
(98.3–99.5) |
35.7
(16.3–61.2) |
99.4
(98.7–99.7) |
44.5 | 2.8 | ||
| Self-careHPV ≥1.0 RLU/CO | ASC-US+ | 30 to 44 | 9 | 75.0
(46.8–91.1) |
95.4
(94.2–96.4) |
12.9
(6.9–22.7) |
99.8
(99.3–99.9) |
70.4 | 7.8 |
| 45 to 54 | 7 | 63.6
(35.4–84.8) |
94.9
(93.3–96.1) |
12.3
(6.1–23.2) |
99.6
(98.9–99.8) |
58.5 | 8.1 | ||
| VIA | 30 to 44 | 7 | 58.3
(32.0–80.7) |
98.6
(97.8–99.1) |
26.9
(13.7–46.1) |
99.6
(99.1–99.8) |
56.9 | 3.7 | |
| 45 to 54 | 3 | 27.3 (9.7–56.6) | 99.3 (98.4–99.6) | 30.0 (10.8–60.3) | 99.2 (98.4–99.6) | 26.6 | 3.3 | ||
| Physician-HC2 ≥1.0 RLU/CO | ASC-US+ | 30 to 44 | 10 | 83.3
(55.2–95.3) |
93.3
(91.8–94.5) |
10.0
(5.5–17.4) |
99.8
(99.6–100) |
76.6 | 10.0 |
| 45 to 54 | 11 | 100
(74.1–100) |
91.8
(89.9–93.4) |
12.1
(6.9–20.4) |
100
(99.4–100) |
91.8 | 8.3 | ||
| VIA | 30 to 44 | 7 | 58.3
(32.0–80.7) |
98.2
(97.3–98.8) |
22.6
(11.4–39.8) |
99.6
(99.1–99.8) |
56.5 | 4.4 | |
| 45 to 54 | 6 | 54.5
(28.0–78.7) |
98.9
(98.0–99.4) |
35.3
(17.3–58.7) |
99.5
(98.0–99.4) |
53.4 | 2.8 | ||
Performance of HPV testing in age-specific primary screening at different positive cutoffs for CIN2+ and CIN3+ detection
For CIN2+ detection using primary careHPV testing, triaging physician-careHPV test-positive women by increasing HPV test-positivity cutoff increased specificity (from 88.1% at ≥1.0 RLU/CO to 93.5% at ≥10.0 RLU/CO) and PPV (from 17.4% at ≥1.0 RLU/CO to 25.0% at ≥10.0 RLU/CO), but decreased referral rate (from 14.0% at ≥1.0 RLU/CO to 8.4% at ≥10.0 RLU/CO), sensitivity (from 83.8% at ≥1.0 RLU/CO to 72.1% at ≥10.0 RLU/CO) and the number of colposcopy per CIN2+ (from 5.8 at ≥1.0 RLU/CO to 4.0 at ≥10.0 RLU/CO) (Table 3). NPVs were ≥97% for all RLU/CO cutoffs. For CIN2+ detection using self-careHPV testing, a similar trend was observed, but in comparision to primary care-HPV testing, the sensitivity declined more rapidly with increasing HPV test-positivity cutoffs (from 72.1% at ≥1.0 RLU/CO to 32.4% at ≥10.0 RLU/CO) and more colposcopies were needed per one CIN2+ detection (from 6.5 at ≥1.0 RLU/CO to 6.9 at ≥10.0 RLU/CO). For CIN2+ detecting using physician-HC2 testing, the sensitivity declined with increasing HPV test-positivity cutoffs but remained ≥82.0% even at ≥10.0 RLU/CO. Similar trends were observed for CIN3+ detection (Table 4), and there was no statistical significance in overall sensitivities between women aged 30 to 44 years, and women aged 44 to 54 years (Table 3, 4).
3.
Performance of physician- and self-collected careHPV in age-specific primary screening, stratified by RLU/CO cutoffs, for CIN2+ detection among 2,337 women, compared to physician-HC2 testing
| Age (year) | Primary screening | Positivity cutoff (RLU/CO) | % (95% CI) | Colposcopy per CIN2+ (N) | ||||
| Referral rate | Sensitivity | Specificity | PPV | NPV | ||||
| 30 to 54 | Physician-
careHPV |
1.00 | 14.0
(12.7–15.5) |
83.8
(73.3–90.7) |
88.1
(86.7–89.3) |
17.4
(13.7–21.9) |
99.5
(99.0–99.7) |
5.8 |
| 2.00 | 11.8
(10.5–13.1) |
80.9
(70.0–88.5) |
90.3
(89.0–91.5) |
20.0
(15.7–25.1) |
99.4
(98.9–99.6) |
5.0 | ||
| 5.00 | 10.1
(8.9–11.3) |
76.5
(65.1–85.0) |
91.9
(90.7–93.0) |
22.1
(17.3–27.9) |
99.2
(98.8–99.5) |
4.5 | ||
| 10.00 | 8.4
(7.3–9.6) |
72.1
(60.4–81.3) |
93.5
(92.4–94.5) |
25.0
(19.5–31.5) |
99.1
(98.6–99.4) |
4.0 | ||
| Self-careHPV | 1.00 | 13.6
(12.2–15.0) |
72.1
(60.4–81.3) |
88.2
(86.8–89.5) |
15.5
(11.9–19.8) |
99.1
(98.5–99.4) |
6.5 | |
| 2.00 | 10.9
(9.7–12.2) |
63.2
(51.4–73.7) |
90.7
(89.4–91.8) |
16.9
(12.8–22.0) |
98.8
(98.2–99.2) |
5.9 | ||
| 5.00 | 8.3
(7.3–9.5) |
47.1
(35.7–58.8) |
92.8
(91.7–93.8) |
16.4
(11.9–22.2) |
98.3
(97.7–98.8) |
6.1 | ||
| 10.00 | 6.5
(5.5–7.5) |
32.4
(22.4–44.2) |
94.3
(93.3–95.2) |
14.6
(9.8–21.1) |
97.9
(97.2–98.4) |
6.9 | ||
| Physician-HC2 | 1.00 | 16.4
(15.0–18.0) |
97.1
(89.9–99.2) |
86.0
(84.5–87.4) |
17.2
(13.7–21.3) |
99.9
(99.6–100) |
5.8 | |
| 2.00 | 14.3
(12.9–15.8) |
95.6
(87.8–98.5) |
88.1
(86.8–89.4) |
19.5
(15.6–24.0) |
99.9
(99.6–100) |
5.1 | ||
| 5.00 | 12.7
(11.4–14.1) |
91.2
(82.1–95.9) |
89.6
(88.3–90.8) |
20.9
(16.6–25.9) |
99.7
(99.4–99.9) |
4.8 | ||
| 10.00 | 11.5
(10.3–12.9) |
86.8
(76.7–92.9) |
90.7
(89.5–91.9) |
21.9
(17.4–27.3) |
99.6
(99.2–99.8) |
4.6 | ||
| 30 to 44 | Physician-
careHPV |
1.00 | 12.1
(10.4–13.9) |
84.6
(70.3–92.8) |
90.1
(88.3–91.6) |
20.2
(14.8–27.1) |
99.5
(98.9–99.8) |
4.9 |
| 2.00 | 10.3
(8.8–12.0) |
84.6
(70.3–92.8) |
91.9
(90.3–93.3) |
23.7
(17.4–31.5) |
99.5
(98.9–99.8) |
4.2 | ||
| 5.00 | 8.7
(7.3–10.3) |
76.9
(61.7–87.4) |
93.4
(91.9–94.6) |
25.6
(18.6–34.2) |
99.3
(98.6–99.6) |
3.9 | ||
| 10.00 | 7.6
(6.3–9.1) |
74.4
(58.9–85.4) |
94.4
(93.1–95.5) |
28.4
(20.6–37.8) |
99.2
(98.5–99.6) |
3.5 | ||
| Self-careHPV | 1.00 | 12.7
(11.0–14.6) |
79.5
(64.5–89.2) |
89.3
(87.5–90.9) |
18.1
(13.1–24.6) |
99.3
(98.7–99.7) |
5.5 | |
| 2.00 | 9.9
(8.4–11.6) |
64.1
(48.4–77.3) |
91.8
(90.1–93.1) |
18.8
(13.1–26.3) |
98.8
(98.1–99.3) |
5.3 | ||
| 5.00 | 8.1
(6.8–9.7) |
48.7
(33.9–63.8) |
93.1
(91.6–94.3) |
17.3
(11.3–25.4) |
98.4
(97.5–99.0) |
5.8 | ||
| 10.00 | 6.1
(5.0–7.6) |
28.2
(16.5–43.8) |
94.5
(93.1– 95.6 ) |
13.3
(7.6–22.2 ) |
97.8
(96.8–98.5) |
7.5 | ||
| Physician-HC2 | 1.00 | 14.6
(12.8–16.6) |
97.4
(86.8–99.5) |
87.9
(86.0–89.5) |
19.3
(14.4–25.4) |
99.9
(99.5–100) |
5.2 | |
| 2.00 | 12.7
(11.1–14.6) |
94.9
(83.1–98.6) |
89.7
(87.9–91.2) |
21.5
(16.0–28.2) |
99.8
(99.4–100) |
4.6 | ||
| 5.00 | 11.1
(9.5–12.9) |
92.3
(79.7–97.3) |
91.3
(89.7–92.7) |
24.0
(17.9–31.4) |
99.8
(99.3–99.9) |
4.2 | ||
| 10.00 | 10.0
(8.5–11.7) |
89.7
(76.4–95.9) |
92.4
(90.8–93.7) |
25.9
(19.3–33.9) |
99.7
(99.2–99.9) |
3.9 | ||
| Table 3 (continued ) | ||||||||
4.
Performance of physician- and self-collected careHPV in age-specific primary screening, stratified by RLU/CO cutoffs, for CIN3+ detection among 2,337 women, compared to physician-HC2 testing
| Age (year) | Primary screening | Positivity cutoff (RLU/CO) | % (95% CI) | Colposcopy per CIN3+ (N) | ||||
| Referral rate | Sensitivity | Specificity | PPV | NPV | ||||
| 30 to 54 | Physician-
careHPV |
1.00 | 14.0
(12.7–15.5) |
87.0
(67.9–95.5) |
86.7
(85.2–88.0) |
6.1
(4.0–9.2) |
99.9
(99.6–99.9) |
16.4 |
| 2.00 | 11.8
(10.5–13.1) |
87.0
(67.9–95.5) |
89.0
(87.6–90.2) |
7.3
(4.8–11.0) |
99.9
(99.6–100) |
13.8 | ||
| 5.00 | 10.1
(8.9–11.3) |
82.6
(62.9–93.0) |
90.7
(89.4–91.8) |
8.1
(5.2–12.3) |
99.8
(99.5–100) |
12.4 | ||
| 10.00 | 8.4
(7.3–9.6) |
78.3
(58.1–90.3) |
92.3
(91.2–93.3) |
9.2
(5.9–14.0) |
99.8
(99.5–99.9) |
10.9 | ||
| Self-careHPV | 1.00 | 13.6
(12.2–15.0) |
78.3
(58.1–90.3) |
87.1
(85.7–88.4) |
5.7
(3.6–8.8) |
99.8
(99.4–99.9) |
17.6 | |
| 2.00 | 10.9
(9.7–12.2) |
69.6
(49.1–84.4) |
89.7
(88.4–90.9) |
6.3
(3.9–10.0) |
99.7
(99.3–99.8) |
15.9 | ||
| 5.00 | 8.3
(7.3–9.5) |
56.5
(36.8–74.4) |
92.1
(91.0–93.2) |
6.7
(4.2–11.8) |
99.5
(99.1–99.7) |
15.0 | ||
| 10.00 | 6.5
(5.5–7.5) |
39.1
(22.2–59.2) |
93.9
(92.8–94.8) |
6.0
(3.2–10.9) |
99.4
(98.9–99.6) |
16.8 | ||
| Physician-HC2 | 1.00 | 16.4
(15.0–18.0) |
95.7
(79.0–99.2) |
84.4
(82.8–85.8) |
5.7
(3.8–8.5) |
99.9
(99.7–100) |
17.5 | |
| 2.00 | 14.3
(12.9–15.8) |
95.7
(79.0–99.2) |
86.5
(85.1–87.9) |
6.6
(4.4–9.8) |
100
(99.7–100) |
15.2 | ||
| 5.00 | 12.7
(11.4–14.1) |
95.7
(79.0–99.2) |
88.1
(86.7–89.4) |
7.4
(4.9–11.0) |
100
(99.7–100) |
13.5 | ||
| 10.00 | 11.5
(10.3–12.9) |
91.3
(73.2–97.6) |
89.3
(88.0–90.5) |
7.8
(5.2–11.6) |
99.9
(99.6–100) |
12.8 | ||
| 30 to 44 | Physician-
careHPV |
1.00 | 12.1
(10.4–13.9) |
91.67
(64.6–98.6) |
88.6
(86.8–90.2) |
6.8
(3.8–11.7) |
99.9
(99.5–100) |
14.8 |
| 2.00 | 10.3
(8.8–12.0) |
91.67
(64.6–98.5) |
90.4
(88.7–91.9) |
7.9
(4.5–13.6) |
99.9
(99.5–100) |
12.6 | ||
| 5.00 | 8.7
(7.3–10.3) |
83.33
(55.2–95.3) |
92.0
(90.4–93.3) |
8.6
(4.7–15.0) |
99.8
(99.4–100) |
11.7 | ||
| 10.00 | 7.6
(6.3–9.1) |
83.33
(55.2–95.3) |
93.1
(91.6–94.4) |
9.8
(5.4–17.1) |
99.8
(99.4–100) |
10.2 | ||
| Self-careHPV | 1.00 | 12.7
(11.0–14.6) |
91.7
(64.6–98.5) |
88.0
(86.2–89.7) |
6.4
(3.6–11.1) |
99.9
(99.5–100) |
15.5 | |
| 2.00 | 9.9
(8.4–11.6) |
75.0
(46.8–91.1) |
90.7
(89.1–92.2) |
6.8
(3.6–12.4) |
99.8
(99.3–100) |
14.8 | ||
| 5.00 | 8.1
(6.8–9.7) |
58.3
(32.0–80.7) |
92.3
(90.7–93.6) |
6.4
(3.1–12.6) |
99.6
(99.1–99.8) |
15.7 | ||
| 10.00 | 6.1
(5.0–7.6) |
33.3
(13.8–60.9) |
94.1
(92.7–95.2) |
4.8
(1.9–11.7) |
99.4
(98.8–99.7) |
20.8 | ||
| Physician-HC2 | 1.00 | 14.6
(12.8–16.6) |
91.7
(64.6–98.5) |
86.1
(84.1–87.8) |
5.6
(3.1–9.7) |
99.9
(99.5–100) |
17.9 | |
| 2.00 | 12.7
(11.1–14.6) |
91.7
(64.6–98.5) |
88.0
(86.1–89.6) |
6.4
(3.6–11.1) |
99.9
(99.5–100) |
15.6 | ||
| 5.00 | 11.1
(9.5–12.9) |
91.7
(64.6–98.5) |
89.6
(87.9–91.1) |
7.3
(4.1–12.7) |
99.9
(99.5–100) |
13.6 | ||
| 10.00 | 10.0
(8.5–11.7) |
91.7
(64.6–98.5) |
90.7
(89.1–92.2) |
8.2
(4.6–14.0) |
99.9
(99.5–100) |
12.3 | ||
| Table 4 (continued ) | ||||||||
Discussion
In over 2,300 screened women from a low-income rural setting in China, we evaluated multiple triage strategies for both self-careHPV and physician-careHPV testing in order to expand the available options for secondary prevention of cervical cancer through screening in LRS. In LRS where screening is limited, the goal is to explore new screening options while keeping in mind that there is no single strategy that is suitable across all environments. Instead, it is up to each country to adapt a program that best suits the needs of the country. Across the HPV-test positive triage strategies we tested to detect CIN2/3, we found that both VIA and cytology improve specificity than no triage at all. However, VIA drops lowered sensitivity to a level unsuitable for a mass-screening program. We found that triage of HPV-test positive women by increasing test positivity cutoff could improve specificity, but at a loss of sensitivity.
Because HPV-positive women often have transient or non-progressing hrHPV and are at risk for overtreatment if only screened through HPV testing, sequential testing of HPV-positive women with a second screening test could raise the specificity of the screening strategy. The decision to triage careHPV positive women depends on the resources required and possible harms (false positives and unnecessary colposcopies) of the triage method, versus its clinical performance in detecting cervical cancer precursors. One triage strategy of HPV-test positive women would be to increase test positivity cutoff. This would be the easiest secondary screening test to implement in LRS, as this method does not require additional screening infrastructure, simply an additional test on already collected careHPV samples and is a semi-quantitative estimate of viral load, which has been shown to predict cervical precancerous lesions (14,15). Our data show that as the test cutoff increased, sensitivity decreased and specificity increased for primary self-careHPV and physician-careHPV testing. For CIN2+ detection among women aged 30 to 54 years, physician-careHPV testing dropped slightly from a sensitivity of 83.8% at 1.0 RLU/CO to 72.1% at 10.00 RLU/CO, while the sensitivity of self-careHPV testing dropped drastically from 72.1% at 1.0 RLU/CO to 32.4% at 10.00 RLU/CO. Our results are in agreement with a cross-sectional study from China which showed that the sensitivity of self-careHPV testing decreased much more significantly with increasing test positivity cutoffs than that of physician-careHPV testing (10). The most practical triage point for physician-careHPV would be between 1.0 to 2.0 RLU/CO for CIN2+ and 1.0 to 5.0 RLU/CO for CIN3+ if setting 80% of sensitivity as the bottom limit. However, because the sensitivity of primary self-careHPV testing at 1.0 RLU/CO as positivity cutoff for both CIN2+ and CIN3+ detection was below 80%, it is not good to triage HPV-positive women by increasing self-careHPV positive cutoff. Moreover, these results also indicate that more specific biomarkers with acceptable sensitivity should be developed
In our study, the screening strategy sensitivities of primary physician-careHPV testing and self-careHPV testing, with further triage by increasing cutoff points, were comparable among women aged 30 to 44 years and women aged 45 to 54 years. This is in contrast to a pooled study which showed that the optimal cutoff points and clinical performance of physician-careHPV testing and self-careHPV testing depend on the age of the targeted population to be screened (16). Perhaps the conclusions from our study are limited by a smaller sample size compared to the pooled analyses, and further studies should be conducted to clarify the effect of age on HPV testing performance.
The performance of a screening strategy that triages HPV-positive women with a second screening test whose performance depends on provider experience will have varied outcomes. VIA, an affordable, easy-to-perform test that is suitable for LRS (17), is subjective and depends on provider experience (18). The sensitivity of primary VIA screening in this study 39.7% for CIN2+ detection was lower than that of the SPOCCS I study in China (71%) (19), and that of VIA in controlled study settings (50%) and in a pooled analysis of studies in China (54.6%) (6,20). While SPOCCS I had well-trained gynecologists providing VIA examinations, this and other studies utilized local physicians. Our data show that VIA triage of either primary self-careHPV or physician-careHPV testing decreased sensitivity too low for population-based screening programs and suggest that in areas where VIA performance is poor, other biomarkers for cervical precancerous disease should be explored. VIA performance has been shown to improve with provider training (21), and so in areas where VIA performance is currently low, further provider training can be utilized to make the screening strategy of HPV-positive women followed by VIA triage a feasible cervical cancer screening option. Meanwhile, the sensitivity of VIA is also related with the age, which decreases significantly in postmenopausal women compared to premenopausal performance (22). Our results consist with this phenomenon and show that HPV DNA testing with VIA triage was less sensitive among women 45 to 54 years old than women 30 to 44 years old. Thus, HPV DNA testing with VIA triage is not suitable for older women or postmenopausal women.
Another subjective test, cytology-based screening, has been effective in decreasing cervical cancer incidence in developed countries but has not achieved the same level of success in LRS because laboratory infrastructure and trained personnel are lacking (5,23). In other studies, the sensitivity of one smear to detect high-grade CIN ranged from 50% to 70% (24,25), due to variation in provider experience. In our study, the performance of cytology in primary screening (sensitivity of 85.3% for CIN2+ detection) was better than that of other studies, because the slides were reviewed by well-trained cytopathologists from CICAMS with international quality control as opposed to local physicians, who are often under-trained. Therefore, our data showing that triage of self-careHPV or physician-careHPV positive women by cytology offers acceptable parameters of sensitivity and specificity, should be taken with caution. Our results illustrate that high-quality cytology would be a good triage method for HPV-positive women. However not all programs have established cytology infrastructure and well-trained cytopathologists. Further training in reading cervical cytology slides can produce greater accuracy (26), but the cost of the infrastructure needed for cytology may prohibit cytology as a secondary screening test in LRS. Again, adopting this strategy for a screening program depends on the available resources weighed against the needs of the program.
In LRS where women are not screened for cervical cancer regularly, careHPV offers an acceptable primary screening method. Self-careHPV is a valuable tool for women without easy access to cervical cancer screening. Further studies are needed to find the suitable cutoffs for careHPV testing that maintain high sensitivity, acceptable specificity and increased PPV. Ideally, HPV DNA test-positive women should be followed up within a year to check for persistent infection or disease progression (27), in order to avoid unnecessary treatment of those with transient or non-progressing HPV infections. However, frequent follow-up of women is often not possible in LRS, which points to the need to triage HPV DNA test-positive women for further referral and colposcopy screening. Regarding HPV test positive triage methods, both VIA and cytology are subjective tests, and as such, can perform poorly in areas where providers are under-trained. Objective tests, such as HPV DNA test positivity cutoffs, can be used reliably even in LRS. Our results also point to the need to develop a novel object biomarker to triage HPV DNA test-positive women in LRS.
This study had several advantages. All woman in this study with a positive screening test received colposcopy and diagnostic biopsy, including four-quadrant punch biopsies when no suspicious lesion was observed in colposcopy, to minimize disease misclassification. Senior CICAMS pathologists read all histological and cytological slides. Furthermore, the population-based design of the study determined the feasibility of conducting self-HPV testing in rural China.
The study does have limitations. Samples from liquid-based cytology and HC2 DNA testing were processed in the CICAMS laboratory in Beijing, China, a national laboratory, and therefore do not reflect how these tests would perform in rural areas of China, which could have lower performance. Furthermore, the performances of triage models were simulated. The accuracy of screening algorithms presented should also be conducted with a greater number of high-grade CIN cases to increase statistical power.
Conclusions
Our study demonstrates that triaging HPV test-positive women by VIA, in areas where this test has poor sensitivity, was unfavorable, unless VIA provider training can be implemented to increase VIA screening performance. In areas where there is a high-quality cytology program, triage by cytology could be a feasible option. Utilizing a suitable HPV DNA test positivity cutoff to triage HPV test-positive women supplies another triage option for LRS. Each country should select a screening strategy based on the resources available and their target population, as no single screening program is a fit for all situations.
Acknowledgements
Thanks for the generous support from the Bill & Melinda Gates Foundation, the National Natural Science Foundation of China (No. 81402748) and Chinese Academy of Medical Sciences Initiative for Innovative Medicine (No. 2017-I2M-3-005). The authors also thank the women who participated in this studies from Wuxiang and Xiangyuan counties, Shanxi Province, as well as the local health workers and staffs in the research team from PATH and CICAMS.
Footnote
Conflicts of Interest: Dr. Jennifer S. Smith has received research grants, supplied donations and consultancies; served on paid advisory boards; and/or been a paid speaker for Arbor Vita, BD Diagnostics, Hologic Corporation and Trovagene in the past 5 years. The other authors have no conflicts of interest to declare.
Contributor Information
Caihong Zhou, Email: caihongzhou2006@126.com.
Fanghui Zhao, Email: zhaofangh@cicams.ac.cn.
References
- 1.IARC. Cervix Cancer Screening. Handbook of Cancer Prevention Volume 10. Available online: http://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/
- 2.GLOBOCAN Cancer Fact Sheets: Cervical cancer. Available online: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp
- 3.IARC. Globocan 2012: Estimated cervical cancer incidence, mortality and prevalence worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- 4.Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 Suppl 3:S3/63–70. doi: 10.1016/j.vaccine.2006.05.113. [Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine 2006;24 Suppl 3:S3/63-70. <DOI:10.1016/j.vaccine.2006.05.113> <PMID: 16950019>] [DOI] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79:954–62. [Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ 2001;79:954-62. <PMID: 11693978>] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Nessa A, Esmy PO, et al. Visual inspection methods for cervical cancer prevention. Best Pract Res Obstet Gynaecol. 2012;26:221–32. doi: 10.1016/j.bpobgyn.2011.08.003. [Sankaranarayanan R, Nessa A, Esmy PO, et al. Visual inspection methods for cervical cancer prevention. Best Pract Res Obstet Gynaecol 2012;26:221-32. <DOI:10.1016/j.bpobgyn.2011.08.003> <PMID: 22075441>] [DOI] [PubMed] [Google Scholar]
- 7.Denny L, Kuhn L, Hu CC, et al. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–67. doi: 10.1093/jnci/djq342. [Denny L, Kuhn L, Hu CC, et al. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst 2010;102:1557-67. <DOI:10.1093/jnci/djq342> <PMID: 20884893>] [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV Screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV Screening for cervical cancer in rural India. N Engl J Med 2009;360:1385-94. <DOI:10.1056/NEJMoa0808516> <PMID: 19339719>] [DOI] [PubMed] [Google Scholar]
- 9.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol 2008;9:929-36. <DOI:10.1016/S1470-2045(08)70210-9> <PMID: 18805733>] [DOI] [PubMed] [Google Scholar]
- 10.Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J Natl Cancer Inst. 2012;104:178–88. doi: 10.1093/jnci/djr532. [Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J Natl Cancer Inst 2012;104:178-88. <DOI:10.1093/jnci/djr532> <PMID: 22271765>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeronimo J, Bansil P, Lim J, et al. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer . Int J Gynecol Cancer. 2014;24:576–85. doi: 10.1097/IGC.0000000000000084. [Jeronimo J, Bansil P, Lim J, et al. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer 2014;24:576-85. <DOI:10.1097/IGC.0000000000000084> <PMID: 24557438> ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26 Suppl 10:K29–41. doi: 10.1016/j.vaccine.2008.06.019. [Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008;26 Suppl 10:K29-41. <DOI:10.1016/j.vaccine.2008.06.019> <PMID: 18847555>] [DOI] [PubMed] [Google Scholar]
- 13.Sherris J, Wittet S, Kleine A, et al. Evidence-based, alternative cervical cancer screening approaches in low-resource settings. Int Perspect Sex Reprod Health. 2009;35:147–52. doi: 10.1363/ifpp.35.147.09. [Sherris J, Wittet S, Kleine A, et al. Evidence-based, alternative cervical cancer screening approaches in low-resource settings. Int Perspect Sex Reprod Health 2009;35:147-52. <DOI: 10.1363/ifpp.35.147.09> <PMID: 19805020>] [DOI] [PubMed] [Google Scholar]
- 14.Thomsen LT, Frederiksen K, Munk C, et al. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer. 2015;137:193–203. doi: 10.1002/ijc.29374. [Thomsen LT, Frederiksen K, Munk C, et al. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer 2015;137:193-203. <DOI:10.1002/ijc.29374> <PMID: 25471319>] [DOI] [PubMed] [Google Scholar]
- 15.Fu Xi L, Schiffman M, Ke Y, et al. Type-dependent association between risk of cervical intraepithelial neoplasia and viral load of oncogenic human papillomavirus types other than types 16 and 18. Int J Cancer. 2017;140:1747–56. doi: 10.1002/ijc.30594. [Fu Xi L, Schiffman M, Ke Y, et al. Type-dependent association between risk of cervical intraepithelial neoplasia and viral load of oncogenic human papillomavirus types other than types 16 and 18. Int J Cancer 2017;140:1747-56. <DOI:10.1002/ijc.30594> <PMID: 28052328>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang LN, Jeronimo J, Qiao YL, et al. Optimal positive cutoff points for careHPV testing of clinician- and self-collected specimens in primary cervical cancer screening: an analysis from rural China . J Clin Microbiol. 2014;52:1954–61. doi: 10.1128/JCM.03432-13. [Kang LN, Jeronimo J, Qiao YL, et al. Optimal positive cutoff points for careHPV testing of clinician- and self-collected specimens in primary cervical cancer screening: an analysis from rural China. J Clin Microbiol 2014;52:1954-61. <DOI:10.1128/JCM.03432-13> <PMID: 24671789> ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Comprehensive cervical cancer control: a guide to essential practice, Second edition. Geneva: WHO Press, 2014. Available online: http://apps.who.int/iris/bitstream/10665/144785/1/9789241548953_eng.pdf?ua=1
- 18.Vedantham H, Silver MI, Kalpana B, et al. Determinants of VIA (visual inspection of the cervix after acetic acid application) positivity in cervical cancer screening of women in a peri-urban area in Andhra Pradesh, India. Cancer Epidemiol Biomarkers Prev. 2010;19:1373–80. doi: 10.1158/1055-9965.EPI-09-1282. [Vedantham H, Silver MI, Kalpana B, et al. Determinants of VIA (visual inspection of the cervix after acetic acid application) positivity in cervical cancer screening of women in a peri-urban area in Andhra Pradesh, India. Cancer Epidemiol Biomarkers Prev 2010;19:1373-80. <DOI:10.1158/1055-9965.EPI-09-1282> <PMID: 20447927>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belinson J, Qiao YL, Pretorius R, et al. Shanxi province cervical cancer screening study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–44. doi: 10.1006/gyno.2001.6370. [Belinson J, Qiao YL, Pretorius R, et al. Shanxi province cervical cancer screening study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001;83:439-44. <DOI:10.1006/gyno.2001.6370> <PMID: 11606114>] [DOI] [PubMed] [Google Scholar]
- 20.Zhao FH, Hu SY, Zhang Q, et al. Risk assessment to guide cervical screening strategies in a large Chinese population. Int J Cancer. 2016;138:2639–47. doi: 10.1002/ijc.30012. [Zhao FH, Hu SY, Zhang Q, et al. Risk assessment to guide cervical screening strategies in a large Chinese population. Int J Cancer 2016;138:2639-47. <DOI:10.1002/ijc.30012> <PMID: 26800481>] [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal PD, Lauterbach M, Sellors JW, et al. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynecol Obstet. 2005;89 Suppl 2:S30–7. doi: 10.1016/j.ijgo.2005.01.012. [Blumenthal PD, Lauterbach M, Sellors JW, et al. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynecol Obstet 2005;89 Suppl 2:S30-7. <DOI:10.1016/j.ijgo.2005.01.012> <PMID: 15823264>] [DOI] [PubMed] [Google Scholar]
- 22.Holt HK, Zhang L, Zhao FH, et al. Evaluation of multiple primary and combination screening strategies in postmenopausal women for detection of cervical cancer in China. Int J Cancer. 2017;140:544–54. doi: 10.1002/ijc.30468. [Holt HK, Zhang L, Zhao FH, et al. Evaluation of multiple primary and combination screening strategies in postmenopausal women for detection of cervical cancer in China. Int J Cancer 2017;140:544-54. <DOI: 10.1002/ijc.30468> <PMID: 27727464>] [DOI] [PubMed] [Google Scholar]
- 23.Catarino R, Petignat P, Dongui G, et al. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol. 2015;6:281–90. doi: 10.5306/wjco.v6.i6.281. [Catarino R, Petignat P, Dongui G, et al. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol 2015;6:281-90. <DOI:10.5306/wjco.v6.i6.281> <PMID: 26677441>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095-101. <DOI:10.1002/ijc.21955> <PMID: 16586444>] [DOI] [PubMed] [Google Scholar]
- 25.Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–60. doi: 10.1002/ijc.23489. [Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer 2008;123:153-60. <DOI:10.1002/ijc.23489> <PMID: 18404671>] [DOI] [PubMed] [Google Scholar]
- 26.Denton KJ. Liquid based cytology in cervical cancer screening. BMJ. 2007;335:1–2. doi: 10.1136/bmj.39262.506528.47. [Denton KJ. Liquid based cytology in cervical cancer screening. BMJ 2007;335:1-2. <DOI:10.1136/bmj.39262.506528.47> <PMID: 17615183>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–82. doi: 10.1016/j.ygyno.2014.12.022. [Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol 2015;136:178-82. <DOI: 10.1016/j.ygyno.2014.12.022> <PMID: 25579107>] [DOI] [PubMed] [Google Scholar]