Abstract
Objective
To determine the skin barrier changes during postnatal month one among infants receiving routine mustard oil massage in the humid conditions of rural Nepal.
Study Design
An observational study among 500 live born neonates receiving mustard oil massage. Skin integrity as erythema, rash and, dryness, skin pH, stratum corneum protein concentration, and transepidermal water loss was measured on days 1, 3, 7, 14 and 28.
Results
Erythema and rash increased (worsened) during weeks 1 and 2, then decreased over weeks 3 and 4. Skin pH (6.1±0.5 to 5.0±0.6) and stratum corneum protein (16.6 ±7.9 to 13.5 ±5.9 μg/cm2,) decreased. Transepidermal water loss increased from 33.2±23.5 to 43.0±24.5 g/m2/hr at day 28. Skin pH and stratum corneum protein were higher for early versus late premature infants.
Conclusion
Premature and full term skin condition was generally poor especially during the first two weeks, improving thereafter. Maturational changes were evident.
INTRODUCTION
Globally, 2.7 million neonatal deaths occur annually, with 98% occurring in developing countries 1, and mainly attributable to preterm birth complications, infections (e.g., sepsis, pneumonia, diarrhea), and intrapartum events, such as birth asphyxia 2. In settings with high mortality, 50% are due to infections 3, and half of all deaths are associated with preterm and low birth weight infants 4. Skin care practices have emerged as an important area to reduce global newborn mortality, especially among preterm births; specific intervention approaches demonstrating benefit include chlorhexidine cleansing 5, 6, kangaroo mother care 7 and application of topical emollients 8, 9, 10.
Given the innate immune functions of newborn skin, an improved understanding of neonatal skin barrier integrity in the environments where such interventions might be scaled up is critical. Healthy, full term newborn infant skin is well-formed and fully functional at birth with excellent barrier properties, indicated by a low transepidermal water loss (TEWL) of 4–8 g/m2/hr 11, equal to or lower than adults 12. Adaptive changes occur, particularly in the first month, and continue throughout the first year of life 12, 13. Full term infant skin acidity (apparent pH) is neutral at birth, decreasing considerably during days 1–4 and further during the first three months as acidic component generating enzymes are activated 12, 14. Stratum corneum (SC) acidification enhances its integrity, cohesion, and lipid processing and acidic treatments may assist in reducing inflammation and normalizing SC function 15. Skin pH increases may reduce integrity and enhance susceptibility to mechanical trauma 16. Premature infants have a poor epidermal barrier and are at risk for increased permeability of exogenous materials, water loss, irritant exposure, skin compromise, delayed skin maturation and infection 17, 18. TEWL is significantly higher than in full terms, particularly for extremely premature infants 19. SC acid mantle formation is incomplete and may be as long as 9 weeks postnatal age 20, 21. The literature on the ontogeny of immediate postnatal barrier maturation is severely limited, particularly for premature infants and for those born in low resource communities of South Asia and/or sub Saharan Africa.
Reductions in nosocomial infections and mortality among preterm infants receiving topical applications of sunflower seed oil have been attributed to improved skin barrier function, as evidenced by reduced SC dryness 8, 9, 10. However, these studies were conducted in controlled hospital settings and compared massaged to non-massaged infants. As such, the results may not directly apply to communities, such as rural south Asia, where daily massage of infants with oils is routinely practiced and humidity and temperature are markedly higher than in previous reports on infant skin development. Little is known about the skin barrier integrity during the critical first weeks of life in these settings.
We describe the skin barrier integrity changes during the first postnatal month in rural Nepal among infants in continuously humid conditions, and exposed to routine traditional massage with mustard seed oil. We used a set of established methods to collectively measure skin barrier integrity and maturation, specifically visual skin condition (rash, erythema, and dryness) and instrumental skin surface pH acidity (apparent pH), TEWL, and SC protein concentration.
SUBJECTS AND METHODS
Setting and Parent Trial
This observational study was nested within a larger cluster-randomized community-based trial (ClinicalTrials.gov, NCT01177111) of the impact of topical skin emollient application on newborn mortality and morbidity in rural Nepal. That trial, which will be completed in 2017, targets live born infants in Sarlahi, a district in the low-lying plains region (‘terai”). Communities (clusters) are randomized to promotion of topical applications to newborn skin (“newborn massage”) with sunflower seed oil (intervention) or the traditional mustard seed oil (comparison), which is used almost universally there as a standard neonatal care practice 22. Pregnant women are identified through regular monitoring by locally-resident female project workers; married women aged 15–35 are visited at home every five weeks, queried about menstruation, and offered pregnancy tests. Pregnant women who provide consent are visited at approximately 28–32 weeks gestation for promotion of newborn massage with their cluster-allocated oil, provided with an initial (100ml) supply of locally-purchased high quality oil. After delivery, the local worker visits the women daily to monitor usage of project-provided oil, and to encourage its use as part of their routine massage practices; they also notify a supervising data collector, who initiates visits on days 1, 3, 7, 10, 14, 21, and 28. At each visit, the infant is examined for vital status and signs of illness, and mother/caretaker reports signs of morbidity, care-seeking, and newborn care practices. Mothers are re-supplied with oil (500ml) on days 1, 10, and 21. Allocation status of clusters and mother/baby dyads was not masked.
Design of Current Study
In this observational study, a specialized team of field workers measured skin integrity among a subset of infants on days 1, 3, 7, 14, and 28. Biological measures were visual skin condition (erythema, rash, dryness), skin pH, TEWL and protein concentration (SC cohesion). Erythema (abnormal redness from capillary dilation and increased blood flow) indicates inflammation, irritation or infection 23. Rash is discrete areas of irritation, often around a hair follicle, and may indicate fluid filled vesicles (miliaria crystallina) or papules with deeper involvement (e.g., miliaria rubra) that may be infected.
Initially (July 2012), seven Village Development Committees (a geographically defined administrative unit within districts) were selected for inclusion given their proximity to our main field office, thus ensuring our ability to maintain a quality cold chain for collected specimens; two more were added by January 2013. Among all infants born alive, we further defined eligibility based on gestational age and timing of first postnatal visit. Specifically, all preterm (based on difference between last menstrual period and date of birth) and a randomly selected 20% subset of term infants were eligible. This proportion was chosen to achieve ~1:1 ratio of term/preterm infants, given our hypothesis that outcome measures may be more important in preterm infants due to skin immaturity. Eligible infants were enrolled if the infant was met prior to reaching 48 hours of age, and caretakers provided consent for the measures.
Skin Measurements
Skin condition was measured as percent area of involvement and severity using a validated scoring method as erythema (0–3), rash (0–3) and dryness (0–5) with 0.5 grade increments presented separately as they indicate different variations in skin irritation. After four days of training, field workers’ scoring of varying skin conditions (photographs) were compared with those of an expert (MV) with a kappa of 0.88. During implementation, workers’ assessments were periodically compared to an investigator’s (AS) scores during supervised visits. Workers recorded skin scores for the chest (using a standardized 4cm × 4cm template), left arm, and right leg.
TEWL (g/m2/hr), relative humidity (RH) and temperature were measured with a closed chamber device (VapoMeter, Delfin, Technologies, Ltd, Finland) 24, as recommended for non-clinical conditions 25. Skin pH was measured with a flat electrode (SkincheckTM, Hanna Instruments, UK) calibrated daily to pH 4 and 7 26. The average of three measurements made at the mid-chest nipple line (easily accessible, a massaged area, reasonably flat) was analyzed.
SC protein content was determined from skin surface samples collected with 380 mm2 D-Squame adhesive discs (CuDerm, TX) applied to the chest for two minutes. Disc placement sites differed at each visit to avoid previous adhesive exposure. After transfer to field headquarters in cold boxes (2–8°C), the protein concentration was determined from optical absorption with a daily-calibrated spectrophotometer SquameScanTM 850A (Heiland electronic, Wetzlar, Germany) and quantified as 27:
Statistical Analysis
Sample size was determined based on logistical constraints (i.e. limited instruments, minimize field staff, and distance from field headquarters/laboratory), and to achieve adequate precision of estimates overall and by term status. A sample size of 500 infants exposed to mustard oil massage was selected with approximately equal numbers of pre/full term infants. Analyses were conducted using STATA v14 (College Station, TX). Biologically implausible outliers were removed before analyses as being due to a confounding biological process (e.g. perspiration rather than TEWL). Skin pH values >8 and <3 (Marty Visscher, personal communication) and TEWL values >100 g/m2/hr. (Aki Immonen, personal communication) were removed.
Newborn characteristics were estimated for three GA groups (<34 weeks, 34–36 weeks, and ≥37 weeks) and included: sex, initial visit weight, small-for-gestational age (SGA) status, time since most recent massage, and average number of massages through age 7 days. The subjects were grouped by gestational age because SC barrier integrity is inversely related to GA and, therefore expected to be poorer for the youngest cohort 19. Visit-specific temperature and RH were converted to heat index. Means and standard deviations of all skin measures were calculated for days 1, 3, 7, 14, and 28. Locally weighted scatterplot smoothing (lowess) curves were created to show changes of skin barrier integrity measures over time for each GA group. Skin measures were compared between GA groups using a bivariate random-effects model, accounting for repeated measures. Infants who missed visits were not included in the analyses for that visit, but were included in analyses if met on future visits.
Approval
The Ethical Review Committee of the Institute of Medicine, Tribhuvan University (Kathmandu, Nepal) and the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) approved the study.
RESULTS
Subjects
There were 707 (20% of 3531) term and 551 preterm eligible live births between July 23, 2012 and May 18, 2014. Of these 5 (0.4%) infants died, 253 (20%) infants were >48 hours old when the field worker arrived, and mothers refused participation for 5 (0.4%) infants. A total of 995 infants were enrolled, 500 of whom were born in clusters receiving routine mustard oil massage and included in this analysis. Field workers completed 2,434 visits to the homes of these 500 infants; 451 (90%) infants contributed at least 4 of 5 visits, and 394 (79%) completed follow-up through 28 days; less than full follow-up was due to death (n=10, 2%) and mother/infant moving before day 28 (n=96, 19%).
Environment
Almost half (45.6%) of visits occurred at RH over 80%. Mean RH from 2,301 visits was 75.1±13.2% and ranged from 10% (n=3) to 100% (n=5). The within-child RH variability across visits was ±8.1%. Mean temperature and heat index were 30.0±4.5°C and 33.6±9.0°C, respectively.
Baseline Characteristics
There were more males (53.8%) than females (46.2%) with imbalance highest in the <34 week group (57.1% vs. 42.9%) (Table 1). Mean weight (initial visit) was 2638±440g and 167 (33.5%) infants were <2500 grams. The average daily number of applications of mustard oil through full-body massage was 4.6±1.4 (first week) and similar for all GA groups.
Table 1.
Baseline newborn characteristics of neonates by gestational age groups, Sarlahi, Nepal
| Gestational Age | ||||
|---|---|---|---|---|
|
| ||||
| <34 Weeks | 34–36 Weeks | ≥37 Weeks | Total | |
|
| ||||
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) |
| Sex | N=56 | N=148 | N=294 | N=500 |
| Male | 32 (57.1) | 73 (49.3) | 162 (55.1) | 269 (53.8) |
| Female | 24 (42.9) | 75 (50.7) | 132 (44.9) | 231 (46.2) |
|
| ||||
| Weight at initial visit (g) | N=54 | N=148 | N=294 | N=498 |
| <1500 | 4 (7.4) | 1 (0.7) | 1 (0.3) | 6 (1.2) |
| 1500–2499 | 20 (37.0) | 58 (39.2) | 83 (28.2) | 161 (32.3) |
| ≥2500 | 30 (55.6) | 89 (60.1) | 210 (71.4) | 331 (66.5) |
|
| ||||
| SGA1 Status | N=54 | N=148 | N=269 | N=471 |
| AGA2 | 51 (94.4) | 128 (86.5) | 133 (49.4) | 312 (66.2) |
| SGA 3–10% | 3 (5.6) | 11 (7.4) | 56 (20.8) | 70 (14.9) |
| SGA 3% | 0 (0) | 9 (6.1) | 80 (29.7) | 89 (18.9) |
|
| ||||
| Time between visit and last massage (minutes) | N=245 | N=696 | N=1343 | N=2293 |
| <30 | 46 (18.9) | 154 (22.1) | 293 (21.8) | 497 (21.7) |
| 30–59 | 36 (14.7) | 139 (20.0) | 237 (17.7) | 413 (18.0) |
| 60–119 | 67 (27.4) | 145 (20.8) | 312 (23.2) | 526 (22.9) |
| 120–179 | 32 (13.1) | 92 (13.2) | 185 (13.8) | 309 (13.5) |
| ≥180 | 64 (26.1) | 166 (23.9) | 316 (23.5) | 548 (23.9) |
|
| ||||
| MEAN (SD) | MEAN (SD) | MEAN (SD) | MEAN (SD) | |
|
| ||||
| Birthweight (g) | 2511.7 (545) | 2527 (434) | 2717 (406) | 2638 (440) |
| Gestational Age (weeks) | 31.3 (2.6) | 35.8 (0.8) | 40.2 (2.0) | 37.9 (3.6) |
| Average # of massages per day during first week of life | 4.6 (1.4) | 4.8 (1.4) | 4.6 (1.4) | 4.6 (1.4) |
Small for Gestational Age
Adequate for Gestational Age
Baseline Skin Condition
On day 1, almost half of infants (44.2%) had chest erythema and 12.2% of infants had chest rash (Table 2), while 0.4% had chest dryness. The arm and leg showed similar patterns. Erythema scores were significantly lower for the chest versus the arm and leg sites (P<0.05). The sites did not differ at baseline for rash or dryness. The mean skin pH, SC protein concentration, and TEWL at the initial visit were 6.09±0.53, 16.6±7.88 μg/cm2, and 33.2±23.5 g/m2/hr, respectively.
Table 2.
Skin barrier integrity measures of neonates at five time points during the neonatal period, Sarlahi, Nepal
| Skin Barrier Integrity Measure | Visit Number
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | 28 | ||||||
|
| ||||||||||
| N | Mean (SD1) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Erythema Score, Chest
|
500 | 0.46 (0.61) | 480 | 0.73 (0.63) | 474 | 0.68 (0.59) | 464 | 0.74 (0.63) | 394 | 0.49 (0.54) |
| Erythema Score, Arm
|
500 | 0.55 (0.67) | 480 | 0.77 (0.63) | 474 | 0.80 (0.58) | 464 | 0.90 (0.63) | 394 | 0.61 (0.53) |
| Erythema Score, Leg
|
500 | 0.57 (0.68) | 480 | 0.74 (0.65) | 474 | 0.78 (0.61) | 464 | 0.82 (0.62) | 394 | 0.63 (0.59) |
| Rash Score, Chest
|
500 | 0.13 (0.39) | 480 | 0.67 (0.76) | 474 | 0.72 (0.77) | 464 | 0.97 (0.79) | 394 | 0.66 (0.70) |
| Rash Score, Arm
|
500 | 0.17 (0.47) | 480 | 0.65 (0.76) | 474 | 0.93 (0.84) | 464 | 1.18 (0.87) | 394 | 0.74 (0.72) |
| Rash Score, Leg
|
500 | 0.15 (0.44) | 480 | 0.52 (0.70) | 474 | 0.71 (0.78) | 464 | 0.98 (0.78) | 394 | 0.74 (0.74) |
| Dryness Score, Chest
|
500 | 0.002 (0.03) | 480 | 0.010 (0.16) | 474 | 0.017 (0.20) | 464 | 0.050 (0.37) | 394 | 0 |
| Dryness Score, Arm
|
500 | 0.004 (0.07) | 480 | 0.067 (0.40) | 474 | 0.16 (0.65) | 464 | 0.15 (0.64) | 394 | 0.020 (0.20) |
| Dryness Score, Leg
|
500 | 0.01 (0.15) | 480 | 0.027 (0.23) | 474 | 0.12 (0.56) | 464 | 0.039 (0.31) | 394 | 0.024 (0.26) |
| Skin pH
|
498 | 6.09 (0.53) | 480 | 5.72 (0.53) | 472 | 5.33 (0.58) | 459 | 5.20 (0.57) | 389 | 5.00 (0.61) |
| TEWL2 (g/m2/hr)
|
499 | 33.2 (23.5) | 480 | 36.1 (24.7) | 468 | 37.7 (24.5) | 458 | 40.7 (24.3) | 386 | 43.0 (24.5) |
| Protein concentration (μg/cm2) | 497 | 16.6 (7.88) | N/A | N/A | 473 | 13.3 (6.52) | 460 | 13.4 (6.96) | 391 | 13.5 (5.89) |
Standard deviation;
Transepidermal water loss
Skin Changes Over Time
Visual Skin Condition
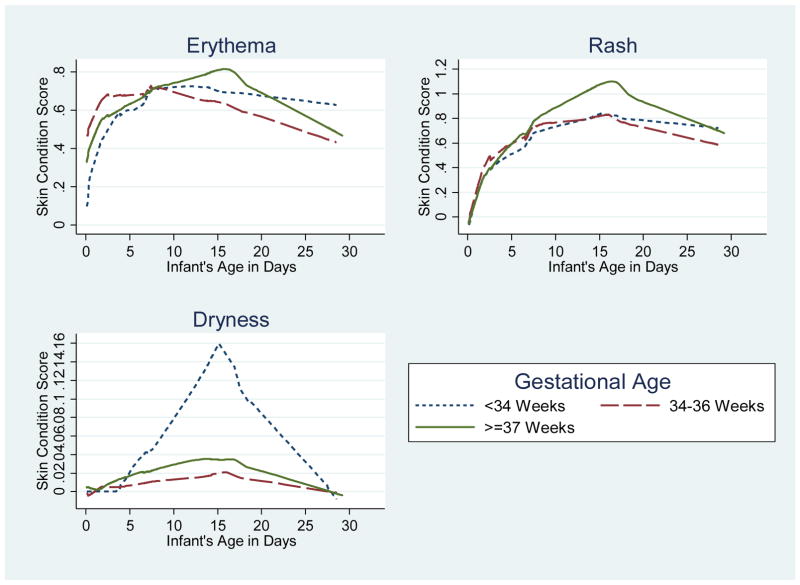
Skin condition scores for erythema and rash increased throughout the first two weeks, peaked at day 14 and decreased in weeks 3 and 4, indicating an initial worsening of the skin barrier followed by some relative improvement (Figure 1, Table 2). Dryness was much less frequently observed, but followed a similar pattern. Arm and leg scores showed similar patterns as the chest (Table 2). Scores were not significantly different among infants <34 weeks compared with late preterm or term infants for erythema, rash, or dryness over 28 days (Table 3).
Figure 1.
Lowess curves of the change in chest skin condition throughout the neonatal period by gestational age groups, Sarlahi, Nepal
Table 3.
Bivariate random-effects model comparing skin integrity measures throughout the entire study period by gestational age group, Sarlahi, Nepal
| Skin Barrier Integrity Measure | Gestational Age1 | |||
|---|---|---|---|---|
| 34–36 Weeks | ≥37 Weeks | |||
| β | 95%CI2 | β | 95%CI | |
| Erythema, Chest | 0.03 | −0.09–0.15 | −0.01 | −0.11–0.12 |
| Erythema, Arm | 0.04 | −0.09–0.17 | 0.04 | −0.08–0.16 |
| Erythema, Leg | 0.03 | −0.10–0.16 | −0.002 | −0.13–0.12 |
| Rash, Chest | 0.03 | −0.10–0.16 | 0.07 | −0.06–0.19 |
| Rash, Arm | 0.05 | −0.10–0.19 | 0.11 | −0.02–0.25 |
| Rash, Leg | −0.02 | −0.14–0.11 | 0.05 | −0.07–0.17 |
| Dryness, Chest | −0.03 | −0.05–0.004 | −0.01 | −0.04–0.01 |
| Dryness, Arm | −0.02 | −0.09–0.05 | −0.01 | −0.07–0.06 |
| Dryness, Leg | 0.01 | −0.04–0.06 | 0.02 | −0.03–0.06 |
| Skin pH | −0.19 | −0.32–−0.06 | −0.08 | −0.20–0.04 |
| TEWL3 (g/m2/hr) | 2.13 | −3.39–7.66 | 2.63 | −3.10–7.22 |
| Protein concentration (μg/cm2) | −1.33 | −2.60–−0.06 | −0.89 | −2.07–0.30 |
Reference Group: Gestational age <34 weeks;
Confidence Interval;
Transepidermal water loss
Skin pH
Skin pH decreased throughout the neonatal period for each GA category, indicating barrier maturation and acid mantle development (Figure 2, Table 2). Infants <34 weeks GA had a significantly higher skin pH over the entire study versus infants 34–36 weeks GA (P=0.004) (Table 3). On day 28, skin pH did not differ significantly among infants in the three groups.
Figure 2.
Lowess curves of the change in skin pH, SC protein concentration, and TEWL throughout the neonatal period by gestational age groups, Sarlahi, Nepal
Stratum Corneum Protein Concentration
SC protein decreased during week one for each group, increasing for infants <34 weeks at day 14 then decreasing by day 28. For 34–36 week GA infants, protein increased slightly between days 7 and 28. In contrast, for full term infants, protein concentration continually decreased over 28 days (Figure 2, Table 2). Infants <34 weeks GA had significantly higher protein over 28 days versus infants 34–36 weeks (P=0.04) (Table 3). Infants <34 weeks GA had a significantly higher protein on day 14 versus both other groups. The groups did not differ on day 28 (Table 3).
Transepidermal Water Loss
TEWL increased throughout the neonatal period for each group, indicating a worsening skin barrier (Figure 2, Table 2) with no differences among GA groups (Table 3). Infants <34 weeks GA had a lower TEWL on day 28 versus the other groups, but differences did not reach significance
DISCUSSION
Understanding the effects of topical emollients on skin barrier integrity in the target population, i.e., among neonates in hot, humid rural Nepal, is crucial for decision making regarding the implementation of skin-based interventions to reduce neonatal morbidity and mortality. To our knowledge, our study is the first prospective evaluation of postnatal skin barrier integrity and maturation in a realistic (i.e. non-hospital) setting with typical environmental conditions (i.e. consistently high humidity) among infants routinely massaged with vegetable oil. The vigorous oil massage typically occurs several times daily, differing considerably from the more gentle application in previous reports 9. Our findings are generalizable to settings where routine oil massage with locally available vegetable oils occurs because a mature, competent skin barrier may reduce neonatal morbidity and mortality in these populations 28.
Skin pH decreased consistently signifying maturational changes and acid mantle development. The acid mantle is necessary for SC formation and integrity, bacterial homeostasis, skin colonization and inhibition of pathogenic bacteria 16, 29. Application of acidic treatments or treatments that facilitate acid mantle development may assist in reducing inflammation and normalizing SC function 15.
High initial skin pH and decreasing values are consistent with previous studies 12, 14. After birth, skin pH is elevated in full term neonates decreasing most significantly during the first four days, continuing to drop during the first three months as the enzymes needed to generate acidic components are activated, e.g., proteolysis of filaggrin to NMF 12, 14, 30. Consistent with previous reports, younger infants (<34 weeks) had a significantly higher skin pH over 28 days than infants of 34–36 weeks GA. Skin pH decreased but varied with GA for premature infants, remaining higher for longer in younger infants (< 1000g) 31. More neutral skin diminishes the activities of lipid processing and activates serine proteases which damage SC desmosomes, thus compromising skin barrier integrity 32.
The elevated protein concentration over two weeks in the youngest infants (<34 weeks) indicates a less cohesive, i.e., less well formed, stratum corneum 33. In contrast, SC protein among full term infants decreased as expected from previous reports 12. There are no published reports of protein changes over time in premature infants using our methods for comparison.
Skin condition (erythema, rash, and dryness) increased (i.e., worsened), for all infants during the first two weeks, followed by relative improvement (i.e. decreasing scores), during weeks 3 and 4. Fifty-eight percent of infants had skin erythema on day 1. This may indicate the initial stages of erythema toxicum neonatorum (ET), a common transient newborn rash, typically regressing within a week 34. Rash developed, peaked after 2 weeks then regressed somewhat, characteristic of miliaria a common rash caused in part by high temperature and humidity 35. This finding is in contrast to previous reports in controlled conditions (and likely somewhat lower humidity) where rash was either not observed 12, 13, or found at very low (0.5%–8.5%) prevalence. The literature on skin maturation for premature infants during days 1–28 is limited, i.e., no comparative studies without massage are available. To our knowledge, there are no previous reports of neonatal skin barrier development under hot humid conditions, making this the first such report. In the more similar environment of New Delhi, miliaria was the most common non-infectious disorder among 100 newborns with pustular disorders 36. The high levels of miliaria in our study could also be due to occlusion of sweat ducts caused by oil application 37. Additionally, high temperature and humidity promote sweating as a normal cooling mechanism 38. Increased temperature resulted in sweating among infants ≥ 36 weeks on days 4–14 of life and for premature infants (~32 weeks), suggestive of maturation/adaptation. In these studies, miliaria occurred as sweating continued.
Skin dryness scores were very low and visual scaling rare. In contrast, a Bangladesh hospital-based study among infants <33 weeks GA receiving topical emollient therapy found deterioration of skin condition over the entire neonatal period and scores >0 for 59% of infants. Scores were 3 or lower, indicating dry skin (score of 1) or dry skin with scales (scores 2, 3) 39. At lower humidity, skin hydration rapidly decreased on postnatal day one and gradually increased over the first month 12. Skin dryness and low hydration were associated with low levels of water binding amino acids in the upper SC at birth 40. We did not measure skin hydration directly but it is expected to be higher at high RH 41. A potential explanation for rare dryness is the nearly continuous RH between 80% and 95%, a level which is optimum for the proteolysis of filaggrin to form water binding moieties, i.e., natural moisturizing factor (NMF), in the upper SC 42.
The effects of mechanical forces, applied during massage, may impact skin condition. While mechanical massage and occlusion each increased the penetration depth of rigid and non-rigid liposomes into skin via the hair follicles in an ex vivo context 43, the effects of massage on topical oil penetration via the hair follicles have not been reported.
Steady increases in TEWL over the entire neonatal period indicates worsening skin barrier function, possibly exacerbated by oil applications. In a study of 22 preterm infants receiving sunflower oil massage, TEWL increased significantly through age 11 days, and decreased only when application was discontinued 44. Without oil massage, TEWL in full term infants was lower than or equal to adults and remained relatively stable while TEWL in preterm infants decreased quickly during the first few days before leveling off 20, 21.
Our contrasting observation might also be due to the consistently high humidity of 75%, where infant skin may not follow the environmental trajectory of previous reports, i.e., large changes from high humidity in utero to cooler and drier conditions, e.g. 10–40% RH 45. Our environment may be more similar to a study where TEWL decreased more slowly at 75% RH for 28 days than at 50% RH, indicating delayed epidermal barrier maturation 46. While higher TEWL at higher RH suggests that utilizing high RH for very premature infants might delay skin barrier maturation 47, such higher humidity may favor NMF generation and decreased skin pH. The ideal environment for neonatal skin development remains to be determined. TEWL values increase with sweating. Therefore, the increases we observed could be confounded by sweating, i.e. both evaporative and transepidermal water loss contributed to the value.
Our study strengths include the large sample size, oversampling of preterm infants, and repeated measures across the first month. Limitations included TEWL measurements in relatively uncontrolled, but realistic environmental conditions, and use of LMP, rather than ultrasound dating, resulting in some possible misclassification of GA 48,49. In this setting, however, the low-cost and simple LMP method is the best option for estimating GA. The absence of any infants non-exposed to massage precluded determination of the effects of massage with oil. However, such a group holds limited relevance given that application of mustard oil through traditional massage is universal in this population22 and throughout south Asia, and our purpose was to describe skin barrier properties in the context of routine infant care. The generalizability is limited to populations where routine oil massage occurs.
Newborn skin is the first defense against many environmental factors. Among neonates routinely massaged with mustard oil in rural Nepal, we found skin irritation/damage during the first two weeks of life. By the end of the neonatal period, skin pH and SC protein levels indicate a mature skin barrier, perhaps due to high humidity influence on NMF generation. TEWL levels increased, possibly due to high RH, frequent oil application, or a combination of both. Our findings are likely applicable to large populations throughout northern India, Pakistan, northwestern Bangladesh, and Nepal, where numerous cultural, social, economic, and environmental characteristics are shared. Massage practices are culturally entrenched thus the appearance of erythema and rash may be accepted as “normal”. At the individual level, a lack of comparative experiential data (i.e. observation of potentially improved skin condition in the absence of routine massage) likely inhibits changes in community-level behavior norms related to skin care practices. Further research in this population is warranted, e.g., evaluating different massage practices and the effect of massage itself, comparing various oils, and exploring risk factors that may impact beneficial effects. A better understanding of the underlying mechanisms of how emollient therapy can improve health outcomes in low-resource settings is essential to best tailor future interventions aimed at reducing neonatal morbidity and mortality.
Acknowledgments
This study was supported by the National Institute for Child Health and Development (HD060712) and the Bill & Melinda Gates Foundation (OPP1084399)
Funding Source: NIH, Bill & Melinda Gates Foundation
Abbreviations
- AGA
Adequate for gestational age
- CI
Confidence Interval
- ET
erythema toxicum neonatorum
- GA
gestational age
- LMP
Last menstrual period
- NMF
natural moisturizing factor
- RH
relative humidity
- SC
stratum corneum
- SD
Standard deviation
- SGA
small-for-gestational age
- TEWL
transepidermal water loss
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
References
- 1.UNICEF, WHO, Bank TW, Division UNP. Report 2015. New York, USA: UNICEF; 2015. Levels and Trends in Child Mortality. [Google Scholar]
- 2.Global regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 4.Belizan JM, McClure EM, Goudar SS, Pasha O, Esamai F, Patel A, et al. Neonatal death in low- to middle-income countries: a global network study. Am J Perinatol. 2012;29(8):649–656. doi: 10.1055/s-0032-1314885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullany LC, Khatry SK, Sherchand JB, LeClerq SC, Darmstadt GL, Katz J, et al. A randomized controlled trial of the impact of chlorhexidine skin cleansing on bacterial colonization of hospital-born infants in Nepal. Pediatr Infect Dis J. 2008;27(6):505–511. doi: 10.1097/INF.0b013e31816791a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC, et al. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119(2):e330–340. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Sys Rev. 2016;(8):Cd002771. doi: 10.1002/14651858.CD002771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darmstadt GL, Badrawi N, Law PA, Ahmed S, Bashir M, Iskander I, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatr Infect Dis J. 2004;23(8):719–725. doi: 10.1097/01.inf.0000133047.50836.6f. [DOI] [PubMed] [Google Scholar]
- 9.Darmstadt GL, Saha SK, Ahmed AS, Ahmed S, Chowdhury MA, Law PA, et al. Effect of skin barrier therapy on neonatal mortality rates in preterm infants in Bangladesh: a randomized, controlled, clinical trial. Pediatrics. 2008;121(3):522–529. doi: 10.1542/peds.2007-0213. [DOI] [PubMed] [Google Scholar]
- 10.Darmstadt GL, Saha SK, Ahmed AS, Chowdhury MA, Law PA, Ahmed S, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet. 2005;365(9464):1039–1045. doi: 10.1016/S0140-6736(05)71140-5. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher MM, O’Carroll M, Gallagher A, Murray DM, Dunn Galvin A, Irvine AD, et al. Newborn transepidermal water loss values: a reference dataset. Pediatr Dermatol. 2013;30(6):712–716. doi: 10.1111/pde.12106. [DOI] [PubMed] [Google Scholar]
- 12.Visscher MO, Chatterjee R, Munson KA, Pickens WL, Hoath SB. Changes in diapered and nondiapered infant skin over the first month of life. Pediatr Dermatol. 2000;17(1):45–51. doi: 10.1046/j.1525-1470.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 13.Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008;128(7):1728–1736. doi: 10.1038/sj.jid.5701239. [DOI] [PubMed] [Google Scholar]
- 14.Hoeger PH, Enzmann CC. Skin physiology of the neonate and young infant: a prospective study of functional skin parameters during early infancy. Pediatr Dermatol. 2002;19(3):256–262. doi: 10.1046/j.1525-1470.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Hachem JP, Roelandt T, Schurer N, Pu X, Fluhr J, Giddelo C, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2010;130(2):500–510. doi: 10.1038/jid.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117(1):44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 17.Evans NJ, Rutter N. Development of the epidermis in the newborn. Biol Neonate. 1986;49(2):74–80. doi: 10.1159/000242513. [DOI] [PubMed] [Google Scholar]
- 18.Rutter N. Clinical consequences of an immature barrier. Semin Neonatol. 2000;5(4):281–287. doi: 10.1053/siny.2000.0014. [DOI] [PubMed] [Google Scholar]
- 19.Sedin G, Hammarlund K, Stromberg B. Transepidermal water loss in full-term and pre-term infants. Acta Paediatr Scand Suppl. 1983;305:27–31. doi: 10.1111/j.1651-2227.1983.tb09855.x. [DOI] [PubMed] [Google Scholar]
- 20.Agren J, Sjors G, Sedin G. Transepidermal water loss in infants born at 24 and 25 weeks of gestation. Acta Paediatr. 1998;87(11):1185–1190. doi: 10.1080/080352598750031194. [DOI] [PubMed] [Google Scholar]
- 21.Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol. 1998;111(2):320–326. doi: 10.1046/j.1523-1747.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Mullany LC, Darmstadt GL, Khatry SK, Tielsch JM. Traditional massage of newborns in Nepal: implications for trials of improved practice. J Trop Pediatr. 2005;51(2):82–86. doi: 10.1093/tropej/fmh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visscher M. A practical method for rapid measurement of skin condition. Newborn Infant Nurs Rev. 2014;14:147–152. [Google Scholar]
- 24.De Paepe K, Houben E, Adam R, Wiesemann F, Rogiers V. Validation of the VapoMeter, a closed unventilated chamber system to assess transepidermal water loss vs. the open chamber Tewameter. Skin Res Technol. 2005;11(1):61–69. doi: 10.1111/j.1600-0846.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- 25.du Plessis J, Stefaniak A, Eloff F, John S, Agner T, Chou TC, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265–278. doi: 10.1111/srt.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parra JL, Paye M. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003;16(3):188–202. doi: 10.1159/000069756. [DOI] [PubMed] [Google Scholar]
- 27.Voegeli R, Rawlings AV, Doppler S, Heiland J, Schreier T. Profiling of serine protease activities in human stratum corneum and detection of a stratum corneum tryptase-like enzyme. Int J Cosmet Sci. 2007;29(3):191–200. doi: 10.1111/j.1467-2494.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 28.Darmstadt GL, Mao-Qiang M, Chi E, Saha SK, Ziboh VA, Black RE, et al. Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr. 2002;91(5):546–554. doi: 10.1080/080352502753711678. [DOI] [PubMed] [Google Scholar]
- 29.Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 30.Fluhr JW, Pfisterer S, Gloor M. Direct comparison of skin physiology in children and adults with bioengineering methods. Pediatr Dermatol. 2000;17(6):436–439. doi: 10.1046/j.1525-1470.2000.01815.x. [DOI] [PubMed] [Google Scholar]
- 31.Fox C, Nelson D, Wareham J. The timing of skin acidification in very low birth weight infants. J Perinatol. 1998;18(4):272–275. [PubMed] [Google Scholar]
- 32.Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 33.Voegeli R, Heiland J, Doppler S, Rawlings AV, Schreier T. Efficient and simple quantification of stratum corneum proteins on tape strippings by infrared densitometry. Skin Res Technol. 2007;13(3):242–251. doi: 10.1111/j.1600-0846.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- 34.Haveri FT, Inamadar AC. A cross-sectional prospective study of cutaneous lesions in newborn. ISRN Dermatol. 2014;2014:360590. doi: 10.1155/2014/360590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuniga R, Nguyen T. Skin conditions: common skin rashes in infants. FP Essent. 2013;407:31–41. [PubMed] [Google Scholar]
- 36.Nanda S, Reddy BS, Ramji S, Pandhi D. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19(3):210–215. doi: 10.1046/j.1525-1470.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- 37.Holzle E, Kligman AM. The pathogenesis of miliaria rubra. Role of the resident microflora. Br J Dermatol. 1978;99(2):117–137. doi: 10.1111/j.1365-2133.1978.tb01973.x. [DOI] [PubMed] [Google Scholar]
- 38.Foster KG, Hey EN, Katz G. The response of the sweat glands of the newborn baby to thermal stimuli and to intradermal acetylcholine. J Physiol. 1969;203(1):13–29. doi: 10.1113/jphysiol.1969.sp008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darmstadt GL, Ahmed S, Ahmed AS, Saha SK. Mechanism for prevention of infection in preterm neonates by topical emollients: a randomized, controlled clinical trial. Pediatr Infect Dis J. 2014;33(11):1124–1127. doi: 10.1097/INF.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 40.Visscher MO, Utturkar R, Pickens WL, LaRuffa AA, Robinson M, Wickett RR, et al. Neonatal skin maturation--vernix caseosa and free amino acids. Pediatr Dermatol. 2011;28(2):122–132. doi: 10.1111/j.1525-1470.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 41.Vyumvuhore R, Tfayli A, Duplan H, Delalleau A, Manfait M, Baillet-Guffroy A. Effects of atmospheric relative humidity on Stratum Corneum structure at the molecular level: ex vivo Raman spectroscopy analysis. Analyst. 2013;138(14):4103–4111. doi: 10.1039/c3an00716b. [DOI] [PubMed] [Google Scholar]
- 42.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115(1):84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- 43.Trauer S, Richter H, Kuntsche J, Buttemeyer R, Liebsch M, Linscheid M, et al. Influence of massage and occlusion on the ex vivo skin penetration of rigid liposomes and invasomes. Eur J Pharm Biopharm. 2014;86(2):301–306. doi: 10.1016/j.ejpb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Kanti V, Bonzel A, Stroux A, Proquitte H, Buhrer C, Blume-Peytavi U, et al. Postnatal maturation of skin barrier function in premature infants. Skin Pharmacol Physiol. 2014;27(5):234–241. doi: 10.1159/000354923. [DOI] [PubMed] [Google Scholar]
- 45.Denda M, Sato J, Masuda Y, Tsuchiya T, Koyama J, Kuramoto M, et al. Exposure to a dry environment enhances epidermal permeability barrier function. J Invest Dermatol. 1998;111(5):858–863. doi: 10.1046/j.1523-1747.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 46.Agren J, Sjors G, Sedin G. Ambient humidity influences the rate of skin barrier maturation in extremely preterm infants. J Pediatr. 2006;148(5):613–617. doi: 10.1016/j.jpeds.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Fluhr JW, Darlenski R, Taieb A, Hachem JP, Baudouin C, Msika P, et al. Functional skin adaptation in infancy - almost complete but not fully competent. Exp Dermatol. 2010;19(6):483–492. doi: 10.1111/j.1600-0625.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg RE, Ahmed AS, Ahmed S, Saha SK, Chowdhury MA, Black RE, et al. Determining gestational age in a low-resource setting: validity of last menstrual period. J Health Popul Nutr. 2009;27(3):332–338. doi: 10.3329/jhpn.v27i3.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jehan I, Zaidi S, Rizvi S, Mobeen N, McClure EM, Munoz B, et al. Dating gestational age by last menstrual period, symphysis-fundal height, and ultrasound in urban Pakistan. Int J Gynaecol Obstet. 2010;110(3):231–234. doi: 10.1016/j.ijgo.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]