Abstract
Rationale
Extracellular vesicles (EVs) are tiny membrane-enclosed droplets released by cells through membrane budding or exocytosis. The myocardial reparative abilities of EVs derived from induced pluripotent stem cells (iPSCs) have not been directly compared with the source iPSCs.
Objective
To examine whether iPSC-derived EVs can influence the biological functions of cardiac cells in vitro; and compare the safety and efficacy of iPSC-EVs and iPSCs for cardiac repair in vivo.
Methods and Results
Murine iPSCs were generated and EVs isolated from culture supernatants by sequential centrifugation. Atomic force microscopy, high-resolution flow cytometry, real-time qRT-PCR, and mass spectrometry were used to characterize EV morphology and contents. iPSC-EVs were enriched in miRNAs and proteins with proangiogenic and cytoprotective properties. iPSC-EVs enhanced angiogenic, migratory, and antiapoptotic properties of murine cardiac endothelial cells in vitro. To compare the cardiac reparative capacities in vivo, vehicle, iPSCs, and iPSC-EVs were injected intramyocardially at 48 h after a reperfused myocardial infarction in mice. Compared with vehicle-injected mice, both iPSC- and iPSC-EV-treated mice exhibited improved LV function at 35 d after MI, albeit iPSC-EVs rendered greater improvement. iPSC-EV injection also resulted in reduction in LV mass and superior perfusion in the infarct zone. Both iPSCs and iPSC-EVs preserved viable myocardium in the infarct zone, while reduction in apoptosis was significant with iPSC-EVs. iPSC injection resulted in teratoma formation, while iPSC-EV injection was safe.
Conclusions
iPSC-derived EVs impart cytoprotective properties to cardiac cells in vitro and induce superior cardiac repair in vivo with regard to LV function, vascularization, and amelioration of apoptosis and hypertrophy. Due to their acellular nature, iPSC-EVs represent a safer alternative for potential therapeutic applications in patients with ischemic myocardial damage.
Keywords: Extracellular vesicles, induced pluripotent stem cells, myocardial infarction, remodeling, angiogenesis, apoptosis, stem cell, cellular transplantation
Subject Terms: Cell Therapy, Cellular Reprogramming, Myocardial Infarction
INTRODUCTION
Since the adult mammalian heart possesses limited regenerative capacity following injury, cell therapy has emerged as a potential approach for repair of the infarcted heart. A number of adult stem and progenitor cells from various sources have already been tested in patients, albeit with modest benefits.[1] Although induced pluripotent stem cells (iPSC) offer exciting opportunities for tissue restoration, recent evidence indicates that in addition to cellular replacement, paracrine factors released by injected cells are also important for cell-based heart repair.[2] These paracrine factors may be secreted directly from the transplanted cells or released as cargo in tiny membrane-enclosed structures termed extracellular vesicles (EVs).[2,3] Given the potential of both iPSCs and iPSC-derived EVs (iPSC-EVs) for myocardial repair, there is a strong rationale to directly compare the safety and reparative efficacy of these two approaches.
EVs represent small (30–1000 nm) vesicles released by cells either by outward budding from the plasma membrane or via exocytosis.[4] Upon shedding, EVs may fuse with the recipient cells directly or after endocytosis followed by the release of contents, or interact with target cells by receptor-ligand interactions.[5,6] Since EVs carry proteins, mRNAs, and miRNAs from their parental cells, they represent an important component of paracrine signaling and cell-to-cell communication.[7] While the regenerative abilities of EVs harvested from mesenchymal stem cells, and endothelial and cardiac progenitors have been reported,[8–10] the biological basis of EV actions needs further elucidation. Thus, another major rationale for this study was to characterize the molecular contents of EVs with regard to potential paracrine activities relevant for myocardial repair. Finally, although iPSCs have been known to produce teratomas,[11] the safety of iPSC-derived EVs remains unknown.
Accordingly, we meticulously characterized the morphology and contents of iPSC-EVs with a multi-instrumental approach, examined their biological function in vitro, and compared head-to-head, for the first time, the safety and efficacy of iPSC-EVs with their parental iPSCs for cardiac repair in vivo. Our results show that iPSC-EVs carry unique sets of proteins and regulatory miRNAs and impact by angiogenic capacity, migratory activity, and survival of cardiac endothelial cells in cytotoxic environment. In vivo, injection of iPSC-EVs after acute myocardial infarction (MI) improves left ventricular (LV) function and remodeling. Importantly, we provide evidence that although injection of iPSCs leads to tumor formation, the use of EVs derived from these cells is safe.
METHODS
An expanded methods section is available in the Online Data Supplement.
The authors declare that all data that support the findings of this study are available within the article and its online supplementary files.
The present study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Kansas Medical Center and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 86-23); and also in accordance with the Polish and European legislation following approval by the First Local Ethical Committee on Animal Testing at the Jagiellonian University. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
iPSC generation
Murine fibroblasts were transfected with lentivirus expressing Oct4, Sox2 and Klf4. iPSC colonies were picked, further expanded and adapted to serum-free conditions. Colonies with high contents of pluripotency-associated transcripts (Online Figure I) were chosen for further studies. iPSCs were phenotyped by flow cytometry and immunocytochemistry following standard protocols.
Extracellular vesicle purification
EVs were prepared from the culture supernatant of iPSCs using a protocol optimized in our laboratory (Online Figure II).[12] Nanoparticle tracking analysis, atomic force microscopy, high resolution flow cytometry and qRT-PCR were used to characterize EVs. MicroRNA expression profiling was performed using qRT-PCR.
EV protein analysis by LC-MS/MS
Following lysis, samples from iPSCs and iPSC-EVs for LC-MS/MS analysis were prepared using the FASP method.[13] Peptide fractions were analyzed using Q Exactive high-resolution mass spectrometer coupled with nanoHPLC. The data were analyzed using Proteome Discoverer 1.4 and a MASCOT server against the Swissprot_201509 database. Search result validation was performed using Percolator algorithm.[14] Gene Ontology was analyzed using FatiGO software.
Assessment of EV function in vitro
Murine cardiac endothelial cells (CECs) and fluorescent EVs were used for cellular uptake studies. Capillary tube formation assay was performed on Matrigel. The movement of CECs was time-lapse recorded, cell trajectories were constructed and analyzed. CEC viability and apoptosis were analyzed by flow cytometry.
Experimental protocol for the in vivo studies
A total of 43 wild-type (WT, C57BL6/J) mice were used for the in vivo studies. The experimental protocol is summarized in Online Figure III. All mice underwent a 30-min coronary occlusion followed by reperfusion. After 48h, chest was reopened and mice received intramyocardial injection of vehicle (group I), iPSCs (group II), or iPSC-EVs (group III). Echocardiographic studies were performed 4d prior to coronary occlusion/reperfusion, at 48h after cell injection, and 35d after MI using a Vevo 2100 Ultrasound System.[15]
Morphometry and immunohistochemistry
After 35d, mice were euthanized and hearts harvested and formalin-fixed. All morphometric and immunohistochemical analyses were performed on 4-µm thick sections using standard methods.[15,16] Capillary density was quantitated in isolectin-B4-stained sections. Interstitial collagen was quantified on images taken from the nonischemic myocardium of picrosirius red-stained sections using ImagePro Plus. Myocyte apoptosis was quantitated using TUNEL assay.[15]
Statistical analysis
Data are expressed as mean ± SEM or SD as indicated. The impact of iPSC-derived EVs on the formation of CECs into capillary-like structures was evaluated using the Mann-Whitney U-test. The movement parameters for CECs were compared using the Student’s t-test. Morphometric and histologic data were analyzed using one-way analysis of variance (ANOVA), whereas serial echocardiographic parameters were analyzed using two-way (time and group) ANOVA followed by Student’s t-tests with the Bonferroni correction. Comparisons between 2 groups were performed by unpaired Student’s t-test. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using the GraphPad Prism (version 4.03; GraphPad Software Inc, La Jolla, CA) and SPSS software version 22.0 (IBM, Armonk, NY).
RESULTS
In vitro studies
iPSCs expanded in optimized serum-free and feeder-free conditions maintained pluripotency as a source of purified EVs
To obtain pure iPSC-EV specimens without contamination of nanoparticles that originated from other cells or from serum present in the culture system,[17] all iPSC clones were expanded in optimized culture conditions in serum-free media without feeder layer cells. Using a multi-instrumental approach, we confirmed the presence of a stable pluripotent phenotype in the selected iPSC lines (Online Figure I). iPSCs expressed pluripotency-related markers, including Oct3/4A, Nanog, Sox2, and Rex-1 at the mRNA and protein levels, as well as placental alkaline phosphatase (Figure 1A–C; Online Figure I). iPSCs also expressed adhesion molecules and growth factor receptors considered important for stem cell activities, including CD49e, CD29, CD105 and CD117, which may be potentially transferred to iPSC-EVs (Figure 1D). These results provide evidence that pluripotent iPSCs can be effectively cultured in feeder-fee and serum-free conditions for uncontaminated harvest of EVs.
Figure 1. iPSC characterization.
A. Representative dot-plots showing the expression of intracellular markers of pluripotency in iPSCs. B–C. Representative images showing the morphology of iPSC colonies in serum- and feeder-free culture and fluorescence staining for fetal alkaline phosphatase (PALP) activity (B); and expression of pluripotency markers Oct3/4, Nanog, Sox2 and SSEA-1 (C). Scale bar: 200 µm. D. Representative histograms showing the antigenic phenotype of iPSCs by flow cytometry. The red-colored histograms represent samples stained for specific surface antigens, while gray-colored ones correspond to respective unstained control samples. Data represent mean ± SD from three independent experiments.
Next, iPSC-EVs were successfully isolated from the iPSC conditioned media by a sequential centrifugation procedure, including ultracentrifugation, resulting in EV specimens containing both microvesicles and exosome fractions (Figure 2A). Importantly, we confirmed the presence of iPSC-EVs according to the current recommendations[18] by examining their: i) size distribution by NTA platform; ii) high-resolution morphology and membrane elasticity by AFM; and iii) expression of EV-related antigens (e.g. CD9 and CD81 tetraspanins) by Western and high-resolution flow cytometry (Figure 2B–E). Moreover, we confirmed the iPSC origin by detecting transcripts for pluripotent transcription factors Oct3/4, Nanog and Rex1 (Figure 2F). These data confirm that the iPSC-EV specimens used in further studies represent homogenous vesicular fractions that exhibit common EV morphology and antigen expression, along with the presence of iPSC-derived markers.
Figure 2. Characterization of iPSC-EVs.
A. Representative iPSC-EV size distribution histogram by nanoparticle tracking analysis (NTA). The cumulative D50 parameter indicates that 50% of the population of vesicles is smaller than 143 nm in diameter. B. Left: representative atomic force microscopy (AFM) image of iPSC-derived EVs. Scale bar, 50 nm. Right: 3D topography of individual vesicles. Scan area: 250×250 nm. C. The Young’s modulus [kPa] and adhesion [pN] values by AFM, reflecting mechanical properties of iPSC-EVs. D. High resolution flow cytometry. Left: histogram showing the size distribution of mixed-size synthetic beads (PS, fluorescently labeled polystyrene calibration beads detected in FITC channel; Si, unlabeled silicone calibration beads). Right: representative dot-plots show the medium angle light scatter (MALS) related to EV size vs. fluorescence intensity indicating expression of selected EV-specific (CD81), and iPSC-specific (SSEA-1) antigens on iPSC-EVs. E. Representative Western immunoblot confirming the presence of typical exosomal marker CD9 in iPSC-EV specimens. F. Presence of mRNA transcripts for pluripotency-related markers in iPSCs and iPSC-EVs by real-time RT-PCR. Data are shown as CT Mean values. G. Venn diagram showing the number of miRNAs common and specific for iPSCs and iPSC-EVs. H. Scatter plot of miRNA expression in iPSCs (x axis) and iPSC-EVs (y axis); each dot represents one transcript. Data represent mean ± SD from three independent experiments.
iPSC-EVs contained iPSC-specific molecules and were enriched in a distinctive set of miRNAs and proteins
To identify the molecular contents, which may be responsible for the biological activity and regenerative capacity of EVs, we next performed global miRNA and proteomic profiling of iPSC-EV specimens. Among 282 miRNAs detected in iPSCs, 199 were also present in iPSC-EVs (Figure 2G), indicating efficient transfer of these regulatory transcripts from the parental iPSCs to EVs. Importantly, miRNAs belonging to the miR-290-295 cluster regulating pluripotency,[19] including miR-292, -293, -294 and miR-295, were abundant not only in the parental cells, but also in the iPSC-EVs (Online Tables I,II). Moreover, among the miRNAs present in both iPSCs and iPSC-EVs, we found miR-19b, miR-20a, miR-126-3p, miR-130a-3p, miR-210-3p and the 'longevimir' cluster miR-17-92, reportedly involved in the promotion of angiogenesis, adaptation to hypoxic stress, regulation of cell cycle, mammalian development, and aging.[20]
Moreover, certain miRNAs were differentially expressed in iPSCs and iPSC-EVs (Figure 2G; Online Tables I,II). Indeed, 33 miRNAs were detected only in iPSC-EVs by qPCR-based method (Figure 2G; Online Table III), which suggests their high enrichment during EV production and release by iPSCs, a phenomenon previously suggested.[21] MicroRNAs enriched in EVs, including let-7, miR-145, miR-17-92 cluster and ESC-specific miR-302a-5p, have been suggested to play a role in late developmental timing, regulation of cell proliferation, differentiation, apoptosis and maintenance of self-renewal and pluripotency,[19,20,22] and may exert possible beneficial effects on myocardial tissue. Moreover, the heatmap analysis indicated other signaling pathways potentially regulated by miRNA contents of iPSC-EVs, including Wnt, PI3K-Akt, and MAPK pathways, which are involved in the regulation of actin cytoskeleton, focal adhesion and ECM-receptor interactions (Online Figures IV,V). Moreover, additional examination by Ingenuity Pathway Analysis tool (IPA, Qiagen) identified several miRNAs abundant in iPSC-EVs, including miR-294, miR-16, miR-34 and miR-20, which may regulate VEGFA signaling, thereby potentially enhancing angiogenesis (Online Figure VI).
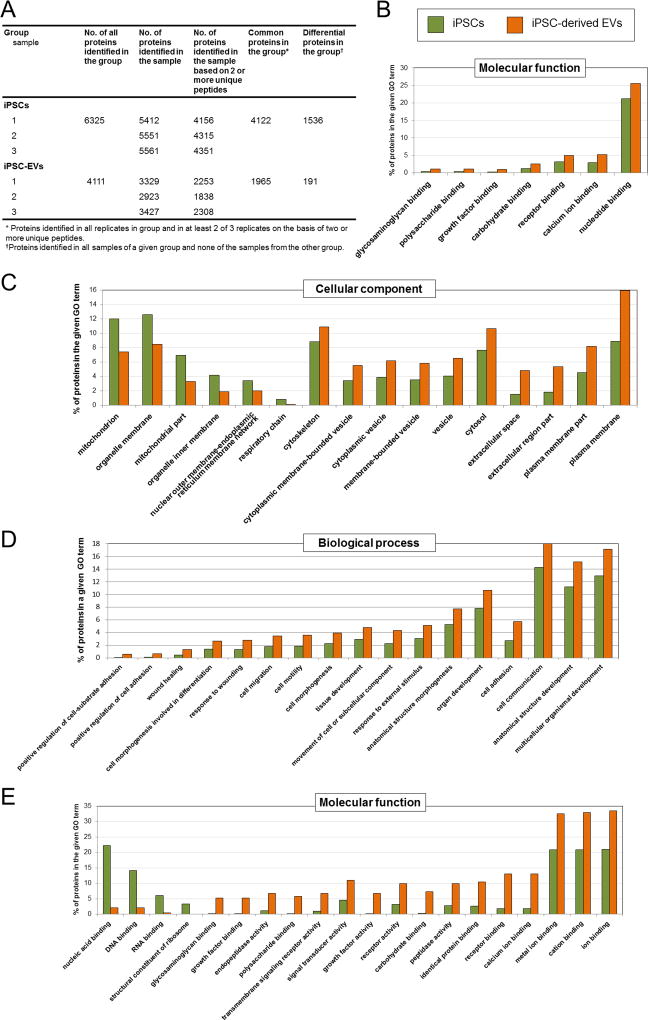
Next, we performed high-throughput global proteomic analysis of iPSCs and iPSC-EVs using mass spectrometry. Importantly, the numbers of proteins identified in challenging iPSC-EV samples and also in iPSC samples were highly reproducible and consistent across all replicates, confirming the high quality of samples and proteomic procedures (Figure 3A). As reliably identified common proteins within the each group (iPSCs or iPSC-EVs) where assigned the proteins identified i) in each replicate of the group and ii) in at least 2 out of 3 replicates, on the basis of two or more unique peptides. These strict analytical criteria were fulfilled by 4,122 and 1,965 proteins detected in iPSCs and iPSC-EVs, respectively (Figure 3A). Due to the high number of common proteins detected in both groups, confirming again the effective transfer of major molecular contents from the parental iPSCs to EVs, we focused our analysis on comparing the unique proteins identified in all samples of a given group and none of the samples from the other groups. A total of 1,536 differential proteins were found to be present in iPSCs and 191 in iPSC-EVs (Figure 3A).
Figure 3. Global proteomic contents of iPSCs and iPSC-EVs by mass spectrometry.
A. The number of proteins identified in iPSCs and their EVs. The absolute values are shown individually for each replicate (n=3) and as average for each examined group. B–D. Gene Ontology (GO) analysis, including common proteins detected in both iPSCs and iPSC-EVs, with focus on molecular function, cellular components, and biological processes. E. GO enrichment analysis for differential expression of proteins in iPSCs and iPSC-EVs, with focus on molecular function. GO terms related to the most apparent differences between iPSCs and iPSC-EVs are shown.
Gene ontology (GO) analysis for molecular function, cellular component and biological processes related to the most apparent differences between parental iPSCs and iPSC-EVs are shown in Figure 3B–E and Online Figure VII. As expected, the proteins identified in iPSC-EVs were the most strongly represented (by GO terms) in plasma membrane, cytosol and cytoskeleton, but not in other organelle compartments not present in EV structure (Figure 3C). Importantly, the GO analysis of biological processes revealed an enrichment of differential proteins involved in response to wound healing, regulation of cell differentiation, as well as organ, anatomical structure and multicellular organism development (Figure 3D; Online Figure VIIA). Importantly, several proteins involved in angiogenesis signaling pathways (VEGF-C, BMP-4, PDGFα, TDGF1, CTGF, thrombospondin-1) were found within GO group guiding multicellular organism development (Online Figure VIIIA). Moreover, proteins stimulating cardiomyogenesis (BMP-4, FGFs); promoting cardiac, endothelial and smooth muscle cell proliferation (PDGFs, IGF-2, FGFs); and protecting cells against oxidative damage (Hpx [Hemopexin]) were identified within GO group involved in signal transduction (Online Figure VIIIB). Thus, proteomic data indicated that the majority of iPSC-specific proteins is transferred from the parental iPSCs to iPSC-EVs and may suggest the impact of such protein contents on several biological processes in target cells, including the ones associated with tissue repair.
iPSC-EVs improved cardiac endothelial cell function by enhancing angiogenic capacity, migration, and survival in vitro
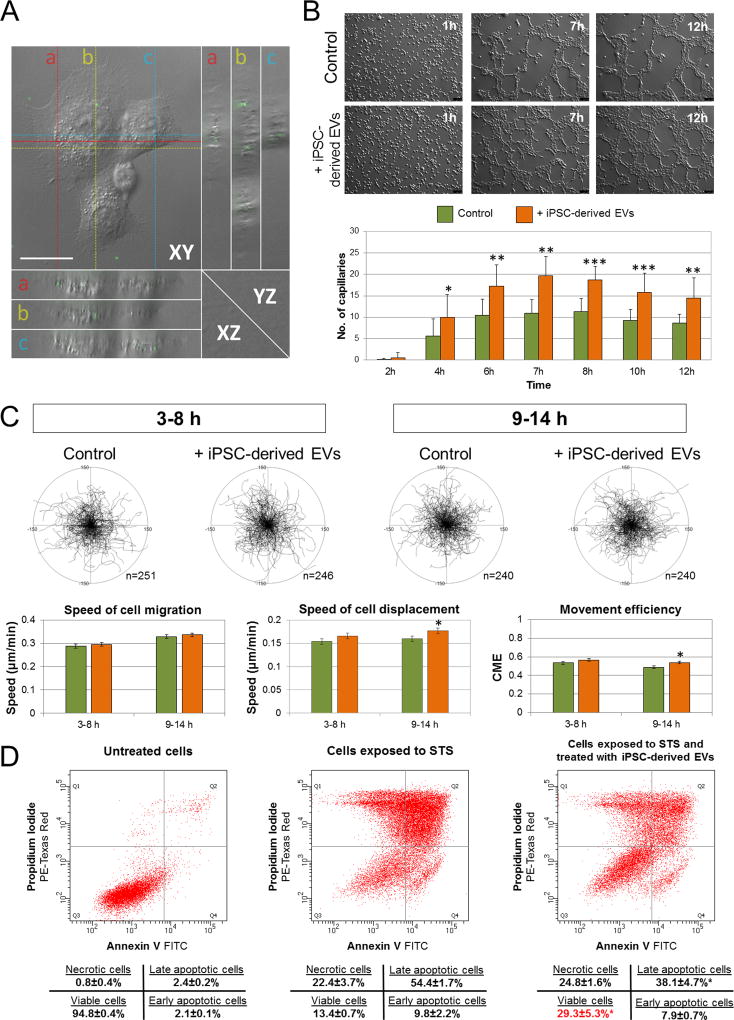
Increasing evidence indicates that angiogenesis is a major mechanism responsible for the improvement in LV function with cell therapy after ischemic myocardial injury. Since our data identified several miRNAs and proteins within the iPSC-EVs, which may regulate target cell function, including proliferation, differentiation, survival, and angiogenesis, we investigated the impact of iPSC-EVs on selected functions of murine cardiac endothelial cells (CECs) in vitro, with emphasis on features potentially important for heart repair. First, we confirmed that iPSC-EVs were internalized by CECs after only 1 h of co-incubation, as shown by optical orthogonal sectioning in XY axis by fluorescent microscopy (Figure 4A). Compared with control untreated cells, CECs treated with iPSC-EVs exhibited greater ability to form endotubules on Matrigel by quantitative analysis (Figure 4B; Online Figure IX; Online Movies I, II). These data suggest that iPSC-EVs are likely to exert proangiogenic effects following myocardial injection in vivo.
Figure 4. Impact of iPSC-EVs on cardiac endothelial cell (CEC) function.
A. Representative Z-stacked image showing uptake of fluorescently labeled iPSC-EVs by CECs. One set of orthogonal slices is shown. Middle, right, and bottom panels represent XY, YZ and XZ planes, respectively. YZ and XZ planes intersect according to the crosshairs. Scale bar: 10 µm. B–D. Impact of iPSC-EVs on CEC functions: (B) angiogenic capacity on Matrigel; scale bar: 100 µm. Each bar represents mean values ± SD from 3 independent experiments (***P<0.001, **P<0.01, *P<0.1); (C) migratory activity of untreated CECs (Control) and CECs treated with iPSC-EVs. Cell trajectories for each period are depicted as circular diagrams (axis scale in µm). Selected migration parameters are shown on the graphs (bottom). Each bar represents mean values from 3 independent experiments (*P<0.05); (D) Representative dot-plots showing the impact of treatment with iPSC-EVs on survival of CECs after exposure to cytotoxic agent staurosporine. Data represent mean ± SD from 3 independent experiments (*P<0.05).
Next, we addressed whether iPSC-EVs influence CEC migratory capacity, which has been reported as a crucial functional property required in wound healing, tissue repair, including myocardial regeneration, as well as during new vessel formation and maturation.[23] The quantitative analysis of recorded CEC trajectories, based on time-lapse monitoring of spontaneous unguided movement of single cells, showed greater speed of cell displacement following iPSC-EV treatment compared with controls (Figure 4C). This phenomenon was more pronounced and statistically significant following prolonged EV treatment, when migration was analyzed between 9–14 h of CEC co-incubation with iPSC-EVs. These data indicate that iPSC-EVs enhance the migratory capacity of cardiac endothelial cells, which may augment new vessel formation and maturation following iPSC-EV transplantation in vivo.
Finally, to examine the impact of iPSC-EVs on cell survival in cytotoxic conditions that often accompany tissue injury, we used staurosporine (STS, 1µM), a cytotoxic agent commonly used to induce apoptosis, to challenge CECs prior to their incubation with iPSC-EVs. We measured cell death processes, including apoptosis and necrosis after 24 h of co-incubation of STS-challenged CECs with iPSC-EVs by flow cytometry. Exposure to iPSC-EVs significantly decreased the incidence of STS-induced apoptosis (38.1±4.7% vs. 54.4±1.7% of cells in late apoptosis among CECs incubated with iPSC-EVs vs. control untreated cells, respectively, P<0.05, Figure 4D). Moreover, the number of viable cells were significantly greater in samples treated with iPSC-EVs (29.3±5.3%) when compared with control CECs treated with STS alone (13.4±0.7%). Importantly, there was no change in the number of cells undergoing necrosis in either iPSC-EV-treated or untreated samples (Figure 4D). These results indicate cytoprotective and antiapoptotic effects of iPSC-EVs on cardiac endothelial cells in vitro, which may play an important role in protecting ischemic myocytes in vivo.
In vivo studies
Exclusions
Four mice died in the perioperative period and 3 mice died at 5, 19 and 29 d after intramyocardial injection. Eight mice were excluded from the study because of cardiac tumor formation in the iPSC-treated group, leaving a total of 9, 7, and 12 mice in Vehicle, iPSCs, and iPSC-EVs groups, respectively (Online Table IV).
Myocardial infarct size
The infarct area fraction denotes the average area of scar tissue, expressed as a percentage of the LV area in three LV sections 0.5–1.0 mm apart. The average infarct area fraction did not differ significantly among the three groups (Online Figure X).
Intramyocardial injection of iPSC-EVs induced greater attenuation of LV dysfunction
Before coronary occlusion (baseline), all parameters of LV function, were similar in groups I-III (Figure 5). At 48 h after cell or EV transplantation (4 d after MI), the degree of LV dysfunction was also similar among the 3 groups (Figure 5B,C), indicating that the injuries sustained during ischemia/reperfusion and intramyocardial injection were similar in all groups. In vehicle-treated (group I) mice, there was further LV functional deterioration between 4 d and 35 d after reperfusion. In contrast, both iPSC- and iPSC-EV-treated mice (groups II and III, respectively) exhibited significantly improved global (Figure 5B–E) LV function compared with group I at 35 d after MI. Mice in groups II and III also showed enhanced regional myocardial function in the infarct zone, as evidenced by a 10% (P<0.05) and 19% (P<0.01) greater systolic infarct wall thickness (Figure 5A,F), respectively, compared with group I. The preservation of LV function was significantly greater in hearts injected with iPSC-EVs compared with iPSC-injected hearts (Figure 5B,C). In addition, mice in group III also exhibited smaller LV end-systolic volume compared with vehicle-treated mice (Figure 5E).
Figure 5. Assessment of LV systolic function.
A. Representative M-mode images from vehicle-treated, iPSC-treated, and iPSC-EV-treated mice at 35 d after coronary occlusion/reperfusion. Compared with the vehicle-treated heart, both iPSC-treated and iPSC-EV-treated hearts exhibit improved wall motion, smaller LV cavity (D,E), and thicker infarct wall (F). Transplantation of iPSC-EVs resulted in greater improvement in LV systolic function (B,C). Data are mean ± SEM. n=7–12 mice per group. *P<0.05 vs. Vehicle at 35 d; #P<0.05 vs. iPSC group at 35 d. BSL, baseline; d, days; LV, left ventricular.
Intramyocardial delivery of iPSCs and iPSC-EVs halted LV remodeling and hypertrophy
The echocardiographic measurements of LV remodeling at 35 d mirrored the observations regarding LV function. The LV end-diastolic diameter (LVEDD) was significantly smaller in groups II and III compared with group I (Figure 6B). Also, compared with group I, the infarct wall thickness in diastole was significantly greater in both group II and III (Figure 6C), indicating superior infarct repair. The posterior LV wall thickness was smaller in groups II and III compared with group I (Figure 6D), indicating less hypertrophy of the viable myocardium. Although the improvements in LVEDD and infarct wall thickness were numerically greater in iPSC-EV-treated hearts compared with iPSC-treated hearts, the differences were not statistically significant.
Figure 6. Assessment of LV remodeling and hypertrophy.
A. Representative Masson’s trichrome-stained myocardial sections at 35 d after MI show improved remodeling in iPSC and iPSC-EV-treated hearts. Scar tissue and viable myocardium are identified in blue and red, respectively. Scale bar = 500 µm. Echocardiographically estimated LV end-diastolic diameter (B) was smaller in both iPSC- and iPSC-EV-treated groups compared with the vehicle-treated group. LV infarct wall thickness in diastole (C) was greater and posterior wall thickness (D) was smaller in both groups. E–F. Interstitial fibrosis in the viable myocardium was quantitated in picrosirius red-stained myocardial sections (E) at 35 d after MI and quantified (F). Scale bar = 50 µm. G. Echocardiographically estimated LV mass was smaller in iPSC-EV-treated hearts compared with vehicle-treated hearts. Data are mean ± SEM. n=7–12 mice per group. *P<0.05 vs. Vehicle at 35 d.
Intramyocardial delivery of iPSC-EVs attenuated LV hypertrophy
To assess the impact of therapy on the myocardium, interstitial fibrosis was carefully quantitated. At 35 d after MI, interstitial fibrosis was similar in vehicle-treated and iPSC-treated hearts (Figure 6E,F). Although not statistically significant, in mice treated with iPSC-EVs, interstitial fibrosis was 11% less compared with vehicle-treated hearts (Figure 6E,F). At 35 d after MI, the echocardiographically estimated LV mass increased significantly in all groups compared with baseline values. Compared with group I, the LV mass was 16% (P<0.05) smaller in iPSC-EVs-treated mice (Figure 6G). Taken together, these data indicate that injection of iPSC-EVs attenuates the adverse adaptations in surviving LV myocardium following MI.
Effects of iPSC and iPSC-EV therapy on myocardial capillary density
Since myocardial vascularity plays an important role in cardiac repair post-MI, we assessed capillary density in the infarct zone, borderzone and nonischemic zone separately. At 35 d after MI, compared with vehicle-treated hearts, capillary numbers in nonischemic zone as well as infarct borderzone were greater in both iPSC-treated and iPSC-EV-treated hearts (Figure 7). In the infarct zone, compared with vehicle treatment, iPSC-EV injection, but not iPSC injection, resulted in significantly greater capillary numbers. These data indicate that intromyocardial delivery of both iPSCs and iPSC-EVs promote angiogenesis in infarct border zone and nonischemic zone, while iPSC-EV therapy extends neovascularization in the infarct zone as well.
Figure 7. Impact on myocardial capillary density.
Panel A shows representative images of capillary profiles in the infarct zone, border zone, and nonischemic zone. Panel B shows quantitative myocardial capillary density. Data represent mean ± SEM. *P<0.05 vs. Vehicle. IZ, infarct zone; BZ, infarct border zone; NZ, nonischemic zone. Scale bar = 50 µm.
Effects on viable myocardium within the infarct zone and myocyte apoptosis
The effects of reparative therapies with regard to potential cellular replacement and myocyte salvage were assessed quantitatively. Myocytes constituted 45.1±1.2%, 51.4±2.7%, and 53.1±1.6% of the infarct zone in groups I, II, and III, respectively (Figure 8A–D); therefore, the amount of viable myocardium in the infarct zone was, on average, 14% and 18% greater in iPSC- and iPSC-EV-treated mice compared with vehicle-treated mice, respectively (both P<0.05 vs. Vehicle-treated group). However, there was no significant difference between iPSCs and iPSC-EVs groups.
Figure 8. Impact on cardiomyocyte salvage and neoplastic growth.
Viable myocyte area fraction in the infarct zone. Panels A–C illustrate representative examples of the infarct scar area in Masson’s trichrome-stained vehicle-treated (A), iPSC-treated (B), and iPSC-EV-treated (C) hearts. Scale bar = 50 µm. Quantitative data are presented in (D). Data are mean ± SEM. n=7–12 mice per group. *P<0.05 vs. Vehicle. Myocyte apoptosis. Panel E shows representative images from the infarct borderzone after TUNEL staining at 35 d after MI. Apoptotic nuclei (white arrows) are visualized by the green fluorescence. DAPI staining identifies nuclei in blue. Cardiac myocytes are positive for α-sarcomeric actin (red). Scale bar = 50 µm. Quantitative data are presented in panel F. Data represent mean ± SEM. *P<0.05 vs. Vehicle; #P<0.05 vs. iPSCs. IZ, infarct zone; BZ, borderzone; NZ, nonischemic zone. Panel G shows representative gross morphologies of tumors from two iPSC-injected hearts (upper images); photomicrographs from Masson’s trichrome-stained myocardial sections showing the variegated tissue composition of tumors (middle images, scale bar = 200 µm); and differentiation into ectodermal (neuroectoderm with pigment granules [arrows], lower left image), mesodermal (cartilage, lower middle image) and endodermal (respiratory epithelium with cilia [arrows], lower right image) lineages in H & E-stained sections from hearts harboring tumors. Scale bar = 50 µm.
In the infarct zone, the percentage of TUNEL-positive apoptotic cardiomyocyte nuclei was similar in groups I and II, while iPSC-EV treatment reduced apoptosis modestly yet significantly (Figure 8E,F). In the borderzone and nonischemic zone, compared with vehicle injection, iPSC injection reduced the percentage of apoptotic myocytes minimally, while iPSC-EV injection was associated with significant reduction in myocyte apoptosis. These data indicate that intromyocardial delivery of iPSC-EVs protects against myocyte apoptosis during post-infarct remodeling.
Intramyocardial delivery of iPSCs induced teratoma formation
Development of cardiac tumor was noted in a large number of mice (8/15, 53%) in the iPSC-treated group. Gross morphological features, variegated tissue composition, and histological characteristics suggesting differentiation into derivatives of all 3 germ layers identified these tumors as teratomas (Figure 8G; Online Figure XI). No such tumor was noted in any mouse in iPSC-EV-injected or vehicle-injected groups.
DISCUSSION
Although numerous strategies for cell-based cardiac repair have been tested in patients with ischemic heart disease, the benefits of such therapies are still only modest in clinical trials.[1,24] Growing evidence indicates that paracrine effects of EVs released by various cells can protect the endogenous myocardial tissue from cell death and other adverse effects, leading to improved remodeling and preserved function.[8,25] The current results provide novel information in this regard.
Salient findings
We performed an extensive multidimensional evaluation of the molecular contents, biological effects, and regenerative capacity of iPSC-derived EVs, consisting of both ectosomal and exosomal fractions, on cardiac cell function in vitro. For the first time, the safety and cardiac reparative efficacy of iPSC-EVs were directly compared with those of iPSCs in a model of reperfused MI in vivo. Our results show that: (1) iPSCs release EVs that carry significant amounts of bioactive contents of the parent cell, along with a distinct set of miRNAs and proteins; (2) iPSC-EVs enhance angiogenic capacity, migratory properties, and survival of heart-derived cells; (3) intramyocardial transplantation of both iPSCs and iPSC-EVs after a reperfused MI improves LV function, albeit iPSC-EVs render superior benefits; (4) the in vitro effects of iPSC-EVs are translated in vivo with reduced myocyte apoptosis and enhanced angiogenesis; and (5) iPSC injection is associated with tumor formation, while iPSC-EVs appear safe. Together, these results indicate that injection of bioactive, cell-free, iPSC-derived EV specimens represent an effective and safe approach for cardiac repair after ischemic injury.
Paracrine activities of transplanted cells have been widely implicated in tissue regeneration and heart repair. Indeed, our previous work suggest that adult bone marrow-derived cells improve heart function by increasing new vessel formation and myocardial perfusion due to growth factors released by injected cells.[26,27] Recent evidence also indicates that tiny vesicles termed EVs carry various bioactive molecules that may ultimately mediate the paracrine effects of stem cells. Although the composition of EV cargo depends on the specific cell type of origin, and influences the EV functional effects on target cells,[28] iPSC-derived EVs have not been characterized in sufficient detail. Our current data constitute the first comprehensive multi-instrumental analysis of cargo in murine iPSC-derived EVs, and show that iPSC-EVs are enriched in numerous mRNA, miRNA, and proteins that originate from parental iPSCs, and also possess a unique set of factors, which may be transferred to cardiac cells. The miRNAs and proteins in iPSC-EVs were related not only to pluripotency, but also to activation of cell proliferation, differentiation, and angiogenic activity.
Importantly, we cultured iPSCs in feeder-free and serum-free conditions in composition-defined medium, which are crucial factors for the quality and purity of iPSC-EVs. It has been shown that the presence of any serum in EV donor cell culture significantly increased the number of serum-derived particles co-isolated with the EV specimens, which may influence the results and data interpretation.[29] Moreover, several laboratories, including ours, have reported major impacts of different expansion media on the molecular composition and biological properties of EVs released by the same type of stem cell, suggesting the critical need for appropriate EV preparation and characterization prior to their administration.[30,31] Thus, we selected serum-free, fully defined medium for iPSC culture prior to iPSC-EV harvest, which simulated closely the GMP standards for cellular expansion for future application in humans. Our results confirm that similar to iPSCs cultured in presence of serum,[32] iPSCs expanded under our defined serum-free conditions preserved pluripotent characteristics (Figure 1) and released iPSC-EVs with normal morphology and antigenic phenotype (Figure 2).
By performing global miRNA analysis, we found more than 200 regulatory miRNAs in iPSC-EV cargo, including 33 that were enriched in the vesicles when compared with the donor iPSCs (Online Table I). The miRNAs enriched in iPSC-EVs included the miR-145, let-7 family and miR-302a-5p that are known to regulate cell proliferation, differentiation as well as self-renewal and pluripotency.[19] Moreover, the iPSC-EV-enriched miRNAs were accompanied with other transcripts common for iPSCs, including miR-290-295 embryonic cluster regulating pluripotency,[19,33] as well as miR-19b, miR-20a, miR-126-3p, miR-130a-3p, miR-210-3p and embryonic 'longevimir' cluster miR-17-92, which promote angiogenesis, adaptation to hypoxic stress, regulation of cell cycle, mammalian development, and aging.[20] These data suggest potential regulatory effects of iPSC-EVs on survival, neovascurization, and pro-regenerative properties following the transfer of such miRNAs into cardiac cells.
Consistent with these findings at the miRNA level, we not only found nearly 2,000 proteins that were common for iPSC-EVs and their parental cells, but also about 200 proteins, which were enriched in iPSC-EVs. The GO analysis identified sets of proteins enriched in iPSC-EVs, which are involved in response to external stimuli, wound healing, regulation of cell differentiation, as well as development of organs and multicellular organisms (Figure 3, Online Figure VIA). Importantly, several proteins involved in angiogenesis and remodeling were identified as node proteins within the network (Online Figure VII). Together, the current proteomic data identify key novel components of the iPSC-EV cargo that may exert reparative effects in vivo.
The above findings also indicate that the contents of iPSCs are efficiently transferred into EVs, thereby enabling these vesicles as conveyors of iPSC molecular properties. The potential role of EVs in transfer of pro-regenerative functions to target cells was confirmed by increased angiogenic capacity, migratory ability, and resistance to apoptosis of CECs following exposure to iPSC-EVs (Figure 4). These data also extend our previous observations regarding the transfer of bioactive cargo from human iPSC-EVs to primary cardiac mesenchymal cells, thereby improving their proliferation, metabolic activity as well as angiogenic and cardiomyogenic differentiation.[3] Furthermore, these molecular findings provide plausible mechanisms underlying the iPSC-EV-induced benefits following injection into the infarcted heart, which include: i) enhanced angiogenesis, which in turn, leads to superior remodeling; ii) reduced apoptosis, which leads to greater preservation of myocytes within the scar region; iii) and reduced fibrosis, which leads to improved remodeling and reduced LV mass. However, based on the size criteria, iPSC-EVs used in this study contained both ectosomes and exosomes, described as distinct EV subpopulations in terms of biogenesis, size, molecular contents and function.[34] The potential differences in bioactive molecular contents between these two fractions and the mechanistic implications thereof with regard to heart repair by each fraction separately remain to be explored.
Although both iPSCs and EVs have been used for cardiac repair, each has unique advantages and disadvantages. Therefore, we felt it was important to directly compare the outcomes of cardiac repair with iPSCs and iPSC-EVs in vivo. Although both improved LV function, the benefits of iPSC-EVs were more pronounced. In addition, the improvement in LV remodeling was superior with iPSC-EVs. This was evident in smaller LV end-systolic volume and LV mass in iPSC-EV-treated mice. Although iPSC-EV injection also reduced interstitial fibrosis by 11%, this did not reach statistical significance. Furthermore, iPSC-EV injection enhanced capillary density in the infarct zone, which may enhance the quality of scar and further improve remodeling during longer follow-up. This effect may directly relate to the transference of pro-angiogenic properties by iPSC-EVs to host myocardial cells.
The reduced incidence of myocyte apoptosis in hearts injected with iPSC-EVs constitutes another important observation. These findings are consistent with the in vitro findings of greater survival of CECs following incubation with iPSC-EVs. Similar cytoprotective effects of iPSC-EVs were noted in a previous study by Wang and colleagues,[35] However, in that study, exosomes were injected immediately after the coronary occlusion and before reperfusion was achieved. The reduction of apoptosis with exosome therapy was noted after 24 h. The authors reported reduced myocardial injury, which was explained predominantly by cytoprotective effects mediated by miR-21 and miR-210 transferred from exosomes to cardiac cells.[35] Our results show, for the first time, that iPSC-EVs confer cytoprotective benefits even when administered after reperfusion injury has set in, and these benefits persist during longer follow-up. Although a number of miRNAs have been implicated in cytoprotection, miRNAs from the miR-17-92 cluster (e.g. miR-19a, miR-19b, miR-20a) noted in high concentration in iPSC-EVs may be responsible for this observation.[36] Moreover, the miR-17-92 cluster also enhances angiogenesis,[37] which may contribute toward improvement in heart function following iPSC-EV injection. Consistently, our proteomic data also indicated the enrichment of several proangiogenic molecules in iPSC-EVs, including BMP-4, PDGFα, TDGF1, thrombospondin-1, and VEGF-C. This enrichment of the molecular contents of iPSC-EVs with proangiogenic and cytoprotective agents may explain the beneficial effects observed in vivo.
Finally, the direct comparison of iPSCs and iPSC-EVs also brings to light a vitally important finding with regard to safety of regenerative therapy. Although the follow-up duration was only 6 wks, 8 out of 15 mice injected with iPSCs developed teratomas within various cardiac structures. No such neoplastic transformation was noted in the other 2 groups. Teratoma formation by iPSCs following injection in vivo is a well-known phenomenon related to the pluripotent state of these cells and has been observed in several prior studies.[11,38,39] This phenomenon is currently one of the major hurdles for therapeutic application of iPSCs for tissue regeneration in humans. Our findings show that even though the injection of parental iPSCs remains hazardous, superior reparative benefits may be achieved safely with the injection of EVs derived from those same cells.
Conclusions
The current findings provide comprehensive evidence that iPSC-derived EVs represent bioactive specimens that carry unique payloads of molecules released by donor iPSCs, which may be transferred to cardiac cells resulting in enhancement of biological functions. Further, the first systematic comparison of iPSCs and iPSC-EVs in vivo reveals superior efficacy of iPSC-EVs for cytoprotection, vascularization, and cardiac repair. Finally, the absence of tumor formation in the iPSC-EV group advances the potential candidacy of the same for safe and effective heart repair after ischemic myocardial injury in humans.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Induced pluripotent stem cells (iPSCs) differentiate into numerous cell types, and therefore, hold great promise for heart regeneration.
Exatracellular vesicles (EVs), including the fraction of exosomes derived from various stem cells have been shown to ameliorate myocardial ischemia/reperfusion injury and promote cardiac repair.
The comparative safety and efficacy of iPSCs and iPSC-derived EVs for infarct repair have not been examined.
What New Information Does This Article Contribute?
The molecular cargo within iPSC-derived EVs includes numerous miRNAs that are present in iPSCs, and several additional miRNAs that are enriched specifically in EVs. These miRNAs are known to promote cellular proliferation and differentiation; enhance angiogenesis; and prevent apoptosis.
The proteomic analysis of EVs revealed molecules that promote cardiac, endothelial and smooth muscle cell proliferation and protect against oxidative damage.
The iPSC-derived EVs induced angiogenic, migratory, and antiapoptotic properties in cardiac endothelial cells in vitro; and induced superior infarct repair in vivo compared with iPSCs. iPSC injection resulted in teratoma formation, while EV injection did not produce any tumor.
Induced pluripotent stem cells (iPSCs), which give rise to numerous cell types, have shown great promise for heart regeneration. Extracellular vesicles (EVs), minute membrane-enclosed droplets released by mammalian cells, have also been shown to ameliorate myocardial ischemia/reperfusion (I/R) injury. However, the myocardial reparative abilities of EVs derived from induced pluripotent stem cells (iPSCs) have not been directly compared with the source iPSCs. The results from qRT-PCR and proteomic analyses revealed that EV cargo is enriched with numerous molecules that impart cytoprotective and angiogenic benefits to cardiac cells. Following I/R and transplantation in vivo, both iPSC- and iPSC-EV-treated mice exhibited improved LV function compared with vehicle-treated mice; however, iPSC-EVs induced greater functional benefits, and resulted in superior perfusion in the infarct zone, and prevented apoptosis. iPSC injections resulted in teratoma formation, while iPSC-EV injections were safe. These observations indicate that iPSC-derived EVs confer cytoprotective properties to cardiac cells in vitro and induce superior cardiac repair in vivo without the risk of teratoma formation seen with parental iPSCs. Thus, iPSC-EVs offer a safer alternative for potential therapeutic applications in patients with ischemic myocardial damage.
Acknowledgments
We thank Dr. Grazyna Drabik, MD from the Department of Transplantation, Polish-American Children’s Hospital in Krakow for assistance with pathological analysis of tumors.
SOURCES OF FUNDING
This work was supported in part by National Institutes of Health grant R01 HL-117730 (BD), 2013/10/E/NZ3/00750 and 2015/16/W/NZ4/00071 grants from National Science Center (EZS), and TEAM-2012/9-6 grant from the Foundation for Polish Science (EZS). The Faculty of Biochemistry, Biophysics and Biotechnology at the Jagiellonian University, Krakow, Poland is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education.
Nonstandard Abbreviations and Acronyms
- EF
ejection fraction
- EVs
extracellular vesicles
- FS
fractional shortening
- iPSCs
induced pluripotent stem cells
- iPSC-EVs
iPSC-derived EVs
- I/R
ischemia/reperfusion
- LV
left ventricle
- LVEDD
LV end-diastolic diameter
- LVEDV
LV end-diastolic volume
- LVESV
LV end-systolic volume
- MI
myocardial infarction
Footnotes
DISCLOSURES
None.
References
- 1.Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights From Randomized Controlled Trials. Circ Res. 2015;117:558–575. doi: 10.1161/CIRCRESAHA.114.304792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobis-Wozowicz S, Kmiotek K, Sekula M, Kedracka-Krok S, Kamycka E, Adamiak M, Jankowska U, Madetko-Talowska A, Sarna M, Bik-Multanowski M, Kolcz J, Boruczkowski D, Madeja Z, Dawn B, Zuba-Surma EK. Human Induced Pluripotent Stem Cell-Derived Microvesicles Transmit RNAs and Proteins to Recipient Mature Heart Cells Modulating Cell Fate and Behavior. Stem Cells. 2015;33:2748–2761. doi: 10.1002/stem.2078. [DOI] [PubMed] [Google Scholar]
- 4.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 5.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 9.Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S, Quercia AD, Migliori M, Panichi V, Giovannini L, Bruno S, Tetta C, Biancone L, Camussi G. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant. 2015;30:410–422. doi: 10.1093/ndt/gfu364. [DOI] [PubMed] [Google Scholar]
- 10.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol. 2015;26:2349–2360. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Macia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 13.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 14.Kall L, Canterbury JD, Weston J, Noble WS, MacCoss MJ. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat Methods. 2007;4:923–925. doi: 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Cheng G, Jin R, Afzal MR, Samanta A, Xuan YT, Girgis M, Elias HK, Zhu Y, Davani A, Yang Y, Chen X, Ye S, Wang OL, Chen L, Hauptman J, Vincent RJ, Dawn B. Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Circ Res. 2016;118:1918–1929. doi: 10.1161/CIRCRESAHA.116.308688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelke GV, Lasser C, Gho YS, Lotvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber AJ, Grandy WA, Balwierz PJ, Dimitrova YA, Pachkov M, Ciaudo C, Nimwegen E, Zavolan M. Embryonic stem cell-specific microRNAs contribute to pluripotency by inhibiting regulators of multiple differentiation pathways. Nucleic Acids Res. 2014;42:9313–9326. doi: 10.1093/nar/gku544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahara N, Brush M, Kawakami Y. Cell migration during heart regeneration in zebrafish. Dev Dyn. 2016;245:774–787. doi: 10.1002/dvdy.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, Ratajczak MZ, Dawn B, Bolli R. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011;15:1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuba-Surma EK, Wojakowski W, Ratajczak MZ, Dawn B. Very small embryonic-like stem cells: biology and therapeutic potential for heart repair. Antioxid Redox Signal. 2011;15:1821–1834. doi: 10.1089/ars.2010.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inal JM, Kosgodage U, Azam S, Stratton D, Antwi-Baffour S, Lange S. Blood/plasma secretome and microvesicles. Biochim Biophys Acta. 2013;1834:2317–2325. doi: 10.1016/j.bbapap.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Bobis-Wozowicz S, Kmiotek K, Kania K, Karnas E, Labedz-Maslowska A, Sekula M, Kedracka-Krok S, Kolcz J, Boruczkowski D, Madeja Z, Zuba-Surma EK. Diverse impact of xeno-free conditions on biological and regenerative properties of hUC-MSCs and their extracellular vesicles. J Mol Med (Berl) 2017;95:205–220. doi: 10.1007/s00109-016-1471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Ghoroghi S, Benito-Martin A, Wu H, Unachukwu UJ, Einbond LS, Guariglia S, Peinado H, Redenti S. Characterization of Induced Pluripotent Stem Cell Microvesicle Genesis, Morphology and Pluripotent Content. Sci Rep. 2016;6:19743. doi: 10.1038/srep19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z, Gocza E. The miR-290-295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation. 2011;81:11–24. doi: 10.1016/j.diff.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 37.Chamorro-Jorganes A, Lee MY, Araldi E, Landskroner-Eiger S, Fernandez-Fuertes M, Sahraei M, Quiles Del Rey M, van Solingen C, Yu J, Fernandez-Hernando C, Sessa WC, Suarez Y. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ Res. 2016;118:38–47. doi: 10.1161/CIRCRESAHA.115.307408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Tang Y, Lu S, Zhou J, Du Z, Duan C, Li Z, Wang C. The tumourigenicity of iPS cells and their differentiated derivates. J Cell Mol Med. 2013;17:782–791. doi: 10.1111/jcmm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamada M, Mitsui Y, Matsuo T, Takahashi T. Reversible transformation and de-differentiation of human cells derived from induced pluripotent stem cell teratomas. Hum Cell. 2016;29:1–9. doi: 10.1007/s13577-015-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.