Abstract
Objective
To study the impact of implementing a protocol to standardize the duration of observation in preterm infants with apnea/bradycardia/desaturation spells before hospital discharge on readmission rates and length of stay (LOS).
Study design
A protocol to standardize the duration of in-hospital observation for preterm infants with apnea, bradycardia and desaturation spells who were otherwise ready for discharge was implemented in December 2013. We evaluated the impact of this protocol on the LOS and readmission rates of very low birth weight infants (VLBW). Data on readmission for apnea and apparent life-threatening event (ALTE) within 30 days of discharge were collected. The pre-implementation epoch (2011-2013) was compared to the post-implementation period (2014-2016).
Results
There were 426 and 368 VLBW discharges before and after initiation of the protocol during 2011-2013 and 2014-2016 respectively. The LOS did not change with protocol implementation (66±42 vs. 64±42 days before and after implementation of the protocol respectively). Inter-provider variability on the duration of observation for apneic spells (F-8.8, p=0.04) and bradycardia spells (F-17.4, p<0.001) decreased after implementation of the protocol. The readmission rate for apnea/ALTE after protocol decreased from 12.1% to 3.4% (p=0.01).
Conclusions
Implementing an institutional protocol for VLBW infants to determine the duration of apnea/ bradycardia/ desaturation spell-free observation period as recommended by American Academy of Pediatrics clinical report did not prolong the LOS but effectively reduced inter-provider variability and readmission rates.
Keywords: Apnea, length of stay, apparent life-threatening event (ALTE), readmission, bronchopulmonary dysplasia (BPD)
Introduction
Apnea of prematurity is a common problem in the neonatal intensive care unit (NICU). It is most widely defined as cessation of breathing for more than 20 seconds or a shorter respiratory pause associated with oxygen desaturation and/or bradycardia in infants who are younger than 37 weeks’ postmenstrual age (PMA) at birth 1. Apnea occurs in almost all babies born less than 28 weeks’ PMA, and the frequency of symptoms are inversely proportional to PMA 2, 3. The management strategies currently available for these infants include respiratory support, caffeine and continuous monitoring of heart rate, respiratory rate, and oxygen saturation 4, 5. Management practices surrounding discharge decisions for infants with spells vary widely among neonatologists 6, 7. Most physicians require infants to be apnea/bradycardia event free and off caffeine for a variable period before discharge. The Clinical Report from the American Academy of Pediatrics (AAP) Committee on Fetus and Newborn (COFN) 1 states that a clinically significant apnea event-free period before discharge of 5 to 7 days is commonly used. This report also suggests that individual NICUs should develop their own protocols for management of preterm infants with such spells before discharge. Darnall et al. 6 reported that cessation of apnea was an important factor that determined discharge. These authors reported physician variability and concluded that the margin of safety period for apneic spell-free period before discharge to be eight days (in the absence of other comorbidities). Another large study involving 1403 infants by Lorch et al. reported the effect of observing the infants for different apnea/bradycardia spell free periods before discharge and their success rates for not having additional spells. The success rate in this large cohort of preterm infants who were observed for a 7-day apnea/bradycardia free period was 95% 8. In our institution, our period of observation before discharge varied widely among physician providers. We developed a standard protocol to determine the duration of observation in the hospital based on the nature and severity of apnea, bradycardia and/or desaturation. We report the impact of implementing this protocol on the length of stay (LOS), inter-provider variance in the duration of observation and readmission rates at our regional perinatal center.
Methods
Definitions
For the purpose of this protocol, the following definitions were established. Apnea was defined as cessation of breathing for a duration of ≥ 15 seconds (following a 5-second delay in alarm triggering). Bradycardia was defined as a drop in heart rate of < 80/minute and desaturations as a drop in oxygen saturation < 85%. The duration of isolated bradycardia and desaturation was ≥ 10 seconds but this period varied based on associated events such as color change and rapidity of decline in heart rate and oxygen saturation.
Observation
Our current NICU is an open 6-8 bed pods and the infants at the time of observation of spells before discharge are either in an open crib or bassinet. The parents are encouraged to feed their babies and room in, especially if they are being discharged on oxygen or on an apnea monitor. A nurse is always at the bedside or in the room, and only the observed events were recorded. We did not use telemetry waveforms to review the events later. Infants in our NICU were monitored on both cardiorespiratory monitor and pulse oximetry until hospital discharge. The monitors we use in our NICU are Phillips IntelliVue MX800 (N.V. USA). It provides continuous oxycardiogram (OxyCRG) with beat-to-beat heart rate (btbHR), an oxygenation measurement trend (SpO2) and compressed respiration wave. Electrocardiogram output uses an impedance of <2.5KΩ±20%. Our cardiorespiratory monitor immediately alarms for bradycardia and desaturation as soon as the values declines below the defined cut-off. The alarm has a 5-second delay for apnea. So if the baby was apneic for 10 seconds after the alarm, the total duration was 15 seconds (10+5 seconds). The bradycardia detection is beat-to-beat according to the monitor specification for neonates, and the alarm goes on displaying heart rate averaged over 3 seconds.
Record of events
Spells have been routinely recorded in the electronic medical record since 2011. For each event, a ‘yes’ or ‘no’ is documented for apnea, bradycardia, and desaturation. In addition to the duration of the event in seconds, the absolute lowest heart rate (beats per minute) and lowest saturation (percentage) are also recorded. All available events had a heart rate and saturation recorded. If apnea was present, this duration was recorded. The EMR system would not allow recording an event without this information. The spells recorded were based on nursing documentation and not based on reviewing telemetry 9, 10.
Protocol development
Available data from previous studies and consensus statements were reviewed 6-8, 11.
Protocol (figure 1)
Figure 1.
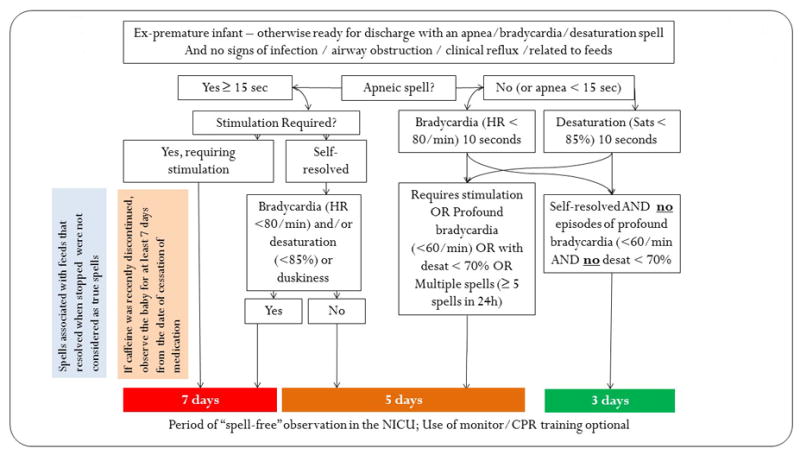
The Protocol for determining the duration of “spell-free” observation period for a premature infant who is otherwise ready for discharge (on full enteral feeds, gaining weight and maintaining the temperature in a crib).
Based on the above review of literature and consensus among the physicians, mid-level providers and nurses, we implemented the protocol shown in figure 1. This figure depicts the period of observation before hospital discharge for spells of varying severity. Spells associated with feeds that resolved when the nipple was taken out of the mouth were not considered significant 12, 13.
Implementation
The protocol was incorporated into our institutional policy following approval by neonatal physicians and providers. The protocol was loaded on the digital documentation software, Neodata (Isoprime, Chicago, IL) which could be accessed by all neonatal providers. The protocol was implemented in late December 2013 as our standard of care following three months of nursing education. In assessing compliance with the protocol, the events were reviewed during daily morning rounds with the bedside nurse and resident/practitioner. The nursing manager and leader had frequent interactions with the nurses to review compliance.
Data collection
After approval by the Institutional Review Board (University at Buffalo), premature infants <1500 grams who were admitted &discharged from the NICU during the study period were included. The study period was divided into two epochs 2011-2013 and 2014-2016. Demographic information including birth weight, gestational age, sex, inborn/outborn status, growth and Apgar scores at 5 minutes was collected. The number of infants admitted in a particular year and discharged each year (from January 1 – December 31) were collected separately. The incidence of bronchopulmonary dysplasia (BPD - defined as oxygen requirement at 36 weeks PMA), severe IVH (≥ grade 3), and mortality before discharge were recorded. The number of infants discharged home with apnea monitors was collected. The infants who required home oxygen were discharged with an apnea monitor. Also, parents who specifically requested apnea monitors were counseled on the literature (or lack thereof) supporting the use of monitors for apnea of prematurity 14. If parents continued to insist, these infants were sent home on apnea monitors. The overall LOS for all infants discharged between 2011 and 2016 was collected. The duration of in-hospital observation for VLBW infants whose discharges were delayed due to spells was compared between the two epochs. We screened all patients presenting to the ED and/or admitted for any reason within 30 days of discharge and evaluated all those with complaints of apnea and/or apparent life-threatening event (ALTE) for this assessment. The readmission rates were calculated as a percentage of VLBW infants discharged from the NICU in that particular year. Women and Children’s Hospital of Buffalo is a regional referral center of Western New York and is the only provider of pediatric inpatient/critical care in the region. Any pediatric patient presenting to an emergency department or urgent care facility with complaints of apnea/ALTE is transferred to our institution.
Statistics
Categorical variables are presented as numbers and percentage, while continuous variables are presented as a mean and standard deviation (S.D) or median and interquartile range (IQR) as applicable. The Chi-square test analyzed categorical variables. For parametric and nonparametric continuous variables, the analysis was performed by unpaired t-test and Mann-Whitney U test respectively. Multivariate analysis for interactions with LOS and ANOVA with post hoc analysis was conducted to compare groups. Variance between the spell-free observation periods before and after implementing the protocol was compared and analyzed by the F test. A probability of less than five percent was considered significant.
Results
Admissions
There were 752 VLBW infants admitted to the NICU at Women and Children’s Hospital of Buffalo during 2011 and 2016. Table 1 shows the admission characteristics of these infants. There were no significant differences in any of the baseline characteristics during this period.
Table 1.
Admission Characteristics of VLBW infants
| Parameters | 2011 (n=137) | 2012 (n=141) | 2013 (n=124) | 2014 (n=117) | 2015 (n=122) | 2016 (n=111) |
|---|---|---|---|---|---|---|
| Gestational age (weeks)(mean ± S.D) | 27.9±3.0 | 27.8±3.2 | 28.3±3.9 | 28.8±3.1 | 28.4±2.9 | 28.0±3.2 |
| Birth weight (kg)(mean ± S.D) | 1.05±0.29 | 1.04±0.29 | 1.08±0.29 | 1.10±0.27 | 1.08±0.26 | 1.03±0.29 |
| Birth weight ≤1000 g n (%) | 57 (42%) | 60 (43%) | 49 (40%) | 41 (35%) | 45 (37%) | 51 (47%) |
| Female n (%) | 57 (42%) | 64 (45%) | 70 (56%) | 58 (50%) | 58 (48%) | 52 (47%) |
| Inborn n (%) | 107 (78%) | 111 (79%) | 102 (82%) | 96 (82%) | 94 (77%) | 91 (82%) |
| AGA n (%) | 107 (78%) | 114 (81%) | 92 (74%) | 86 (74%) | 96 (79%) | 79 (72%) |
| Apgar at 5 min median (IQR) | 8 (7,9) | 8 (7,9) | 8 (7,9) | 8 (7,9) | 8 (7,9) | 8 (7,9) |
AGA – appropriate for gestational age, IQR – interquartile range, VLBW – very low birth weight
Discharges
731 VLBW infants were discharged during this period. Table 2 shows the discharge characteristics. There were 426 and 368 discharges before and after initiation of the protocol respectively. The PMA at discharge did not differ in the two epochs (37.4±5.6 vs. 37.3±5.8 weeks). There was no difference in the number of infants who were discharged home on an apnea monitor. The discharges were more than the admissions secondary to the infants admitted in the previous year being discharged the next year.
Table 2.
Discharge Characteristics of VLBW infants
| Parameters | 2011 (n=146) | 2012 (n=145) | 2013 (n=135) | 2014 (n=131) | 2015 (n=118) | 2016 (n=119) |
|---|---|---|---|---|---|---|
| GA at discharge (weeks) (mean ± S.D) | 37.6±6.0 | 36.9±5.2 | 37.8±5.5 | 38.0±5.6 | 37.5±5.8 | 37.1±6.2 |
| Discharge weight (kg) (mean ± S.D) | 2.6±1.1 | 2.6±0.8 | 2.7±0.8 | 2.7±0.9 | 2.7±1.1 | 2.5±0.9 |
| Caffeine use (days) (median) (IQR)¶ | 1 (1-8) | 2 (1-6) | 3 (1-7) | 2 (1-5) | 3 (1-7) | 3 (1-5) |
| Apnea monitor n (%) | 4 (3%) | 4 (3%) | 3 (2%) | 2 (2%) | 3 (3%) | 4 (4%) |
| Monitor for BPD n (%) | 12 (9%) | 12 (10%) | 14 (10%) | 13 (11%) | 13 (12%) | 12 (12%) |
| BPD n (%) | 32 (23%) | 30 (25%) | 30 (22%) | 23 (19%) | 27 (24%) | 36 (36%) |
| IVH grade 3 & 4 n (%) | 10 (7%) | 8 (7%) | 9 (7%) | 7 (6%) | 7 (6%) | 8 (8%) |
| Mortality n (%) | 21 (15%) | 16 (13%) | 7 (5%)* | 8 (7%)* | 12 (11%) | 11 (11%) |
p<0.05 by ANOVA compared to other groups.
F test used to compare variance.
GA – gestational age, BPD – Bronchopulmonary dysplasia, IVH – Intraventricular hemorrhage, VLBW – very low birth weight
Observation period just before discharge (figure 2)
Figure 2.
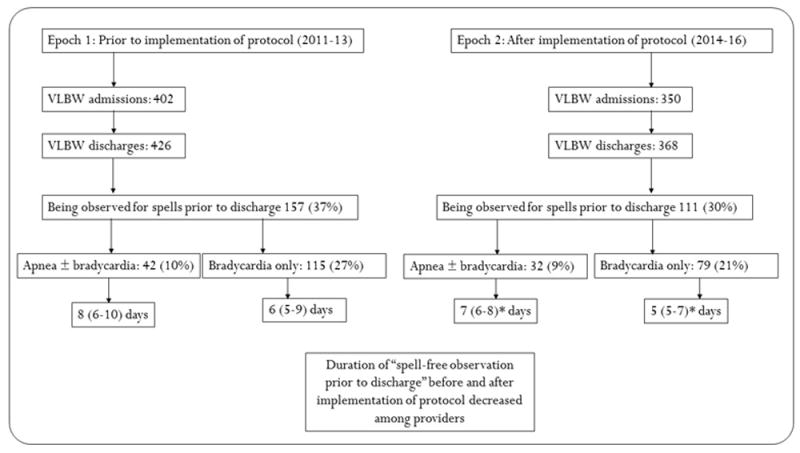
Inter-provider variability before and after implementation of the protocol. *p<0.05 by F-test, between 2 epochs.
Between 2011 and 2016, 268 VLBW infants were observed on a “spell-watch” when they were otherwise ready for discharge. The percentage of infants observed during the two epochs before and after protocol implementation - 157/426 (37%) vs. 111/368 (30%) respectively were not different (p=0.05). The spells were classified into apneic spells (with or without bradycardia) and bradycardic spells without apnea. The number of infants and the delay in discharge due to spells in each of these categories is shown in figure 2.
The implementation of the protocol did significantly change the duration of observation secondary to apneic spells and bradycardia spells following protocol implementation (figure 2).
Apneic spells requiring stimulation: Out of 426 infants discharged between 2011 and 2013, 42 (10%) infants were observed on an apnea (with or without bradycardia) spell count for 8 (6-10) days. During the 2014-16 epoch, 32 out of 368 infants (9%) were observed on an “apnea spell watch” for 7 (6-8) days. The duration of observation for apneic spells significantly decreased following implementation of the protocol (F=8.8, p=0.04).
Self-resolved apnea, without bradycardia (HR<85/m) and without desaturations (<85%) and no color change: Twenty infants (5%) were observed for a 5-day period between 2014-2016. No comparison was made before the implementation of the protocol.
Bradycardic spells: 115 (27%) infants were on an isolated “bradycardia (without apnea) spell watch” for 6 (5-9) days before discharge in the period before implementation of the protocol. 79 (21%) infants had isolated “bradycardia spell watch” observation for 5 (5-7) days before discharge after the implementation of the protocol. The duration for observing preterm infants with isolated bradycardia significantly decreased (F=17.4, p<0.001) after implementation of the protocol.
Isolated bradycardia or isolated desaturations: 17 (5%) infants between 2014-2016 were observed for isolated desaturations for an average of 3 days. We did not have a comparison group before the implementation of the protocol.
The length of stay (LOS) of all infants discharged (figure 3a)
Figure 3.
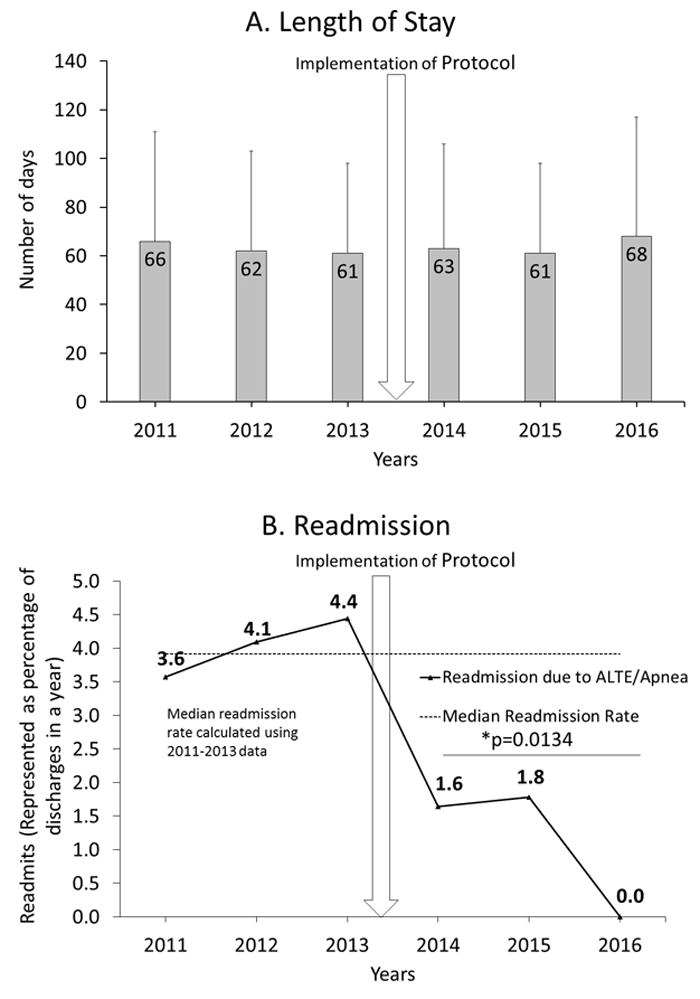
a: Length of stay for all VLBW infants before and after implementation of the protocol (vertical arrow).
b: Readmission rates before and after implementation of the protocol. The median readmission rates were calculated between 2011-2013 (4.9%). The vertical arrow indicates the time of implementation of the protocol. The horizontal line with a *p-value (0.0134) shows the significance in readmission rate before (2011-2013) and after the protocol (2014-2016).
The LOS before and after implementation of the protocol for all VLBW infants was 66±42 vs. 64±42 days. LOS in infants discharged immediately after completion of their spell-count (157 vs. 111 infants before and after implementation of the protocol respectively) was not different (71.3±33.8 vs. 66.4±30.4 days, p=0.22). A multivariate analysis looking at the factors affecting LOS showed gestational age (R squared 0.549), birth weight (R squared 0.493) and comorbidities such as BPD & IVH grade III or above (R squared 0.534) affected the LOS (p<0.001). Further analysis of LOS by 3 categories of birth weight ≤ 750 g (97±56 days), 751-1000g (85±32 days) and >1000g (52±26 days) by ANOVA was significantly different (p<0.006).
Readmission rates
Readmission rates for apnea/ALTE within 30 days of discharge: Readmission as a percentage of discharges is shown in figure 3b. The readmission rates before and after implementation of the protocol were 12.9% vs. 3.4% respectively (p=0.0134). The characteristics of infants readmitted are shown as a table (Supplementary – Table 1). We did not have any readmission for apnea/ALTE for VLBW infants discharged in 2016.
Discussion
To our knowledge, this is one of the largest studies to evaluate the impact of implementing a standardized protocol for the duration of observation in infants with apnea/bradycardia spells before discharge. The recent recommendations by Eichenwald et al. on behalf of the AAP COFN 1 highlight the lack of consistent definitions and consensus in the management of apnea and discuss variations in practice. This clinical report encourages the development of institutional protocols to address the approach to discharge in infants with apnea of prematurity. Our protocol emphasizes the use of standard definitions and observation periods in infants with apnea of prematurity. There are studies to suggest 7, 8 that spell-free observation period before discharge need not be uniform and modified durations can be considered for preterm infants based on gestational age at birth. In a tertiary regional perinatal center with a large practice group consisting of neonatologists, fellows, residents and practitioners the standardized approach implemented in our institution had to be specific but simple. Hence standard duration of observation depending on the type of spell was followed for all preterm infants irrespective of gestational age at birth.
A recent study which looked at the effect of standardizing the definition and management of apnea of prematurity reduced caffeine use and decreased the number of infants discharged on a monitor 15. With the implementation of our standard protocol, we saw a modest reduction in the number of infants placed on a “spell-watch” just before discharge from 40% to 33% (not significant). Our admissions and discharges during this timeframe were fairly consistent. There were no differences in caffeine use and rates of BPD during the period of the study although the use of the high-flow nasal cannula and noninvasive ventilation increased in our NICU 16. We believe this reduction may be secondary to standard definitions of a spell which was easily available for reference to the nurses.
The main purpose of implementing this protocol was to standardize the approach to spells before discharge and to reduce inter-provider variability. We achieved this goal with this protocol in our NICU. In a regional perinatal center involving thirteen neonatologists, fifteen practitioners, six fellows with two new incoming fellows every year, there existed considerable variations in our practice. Apneic spells were commonly observed for a spell free period of one week and off caffeine before discharge with some providers monitoring the infants as long as ten days. Isolated bradycardia without apnea is often the last event to resolve among preterm infants and is of uncertain clinical significance 1, 17. There was considerable variation in observing an infant with isolated bradycardia before implementation of the protocol (Figure 2). Following the implementation of the protocol, the variability among providers to monitor for an apneic episode and isolated bradycardic episode significantly decreased. We did not have any specific measures to quantify adherence to the protocol by physicians, practitioners and nurses. With the reduction in inter-provider variability, it is safe to assume that we adhered to the protocol with some variation.
Butler et al. showed that implementation of a standard approach to apnea of prematurity decreased the LOS, cost and reduced readmission rates 18. This study included infants who were between 29 and 34 weeks PMA at birth without continuous positive airway pressure requirement in the delivery room with short pre- and post-standardization epochs (1 year each) compared to our study. In our unit, the overall LOS of all VLBW infants discharged during the two epochs did not change significantly after implementing the standard protocol. The LOS of VLBW infants who were observed for spells immediately before discharge between the two epochs slightly decreased but this reduction was not statistically significant (LOS in infants discharged immediately after completion of their spell-count 157 vs. 111 infants before and after implementation of the protocol respectively slightly decreased was not different (71.3±33.8 vs. 66.4±30.4 days, p=0.22)). Further analysis of our data suggested that gestational age, birth weight, BPD and IVH grade 3 & above had interaction and affected the LOS. This finding is in accordance with the previous studies which have evaluated the prominent role of PMA at birth, birth weight and other co-morbidities in determining LOS 6, 11, 19,20-23. We speculate that continued observation and collecting data from multiple centers may result in a higher ‘n’ and may show a significant reduction in LOS.
Our unit observes infants on cardiorespiratory and pulse oximetry monitors until the time of discharge. Although our protocol included a 3-day observation for isolated desaturations without apnea or bradycardia, these episodes were usually associated with misplaced home oxygen nasal cannula in infants with BPD or with feeds 12, 13, 24, 25. This was not studied in detail as considerable variation existed in the documentation of isolated desaturations in the two epochs. Some NICUs in their step-down unit or convalescent nursery take patients off pulse oximetry monitors before discharge. Such practice would not detect isolated desaturation episodes. The significance of isolated desaturations without apnea or bradycardia remains unclear and requires further evaluation 14, 26. A regional saturation (cerebral oximetry) monitor may give us further information about the isolated desaturations 27.
We observed a significant decrease in readmission rates after implementation of a protocol for apnea/ALTE. In our study, the average age of VLBW infants at discharge was 38 weeks and the number of babies who were sent home on monitors did not differ between the two epochs. Infants who are discharged home on supplemental oxygen from our NICU are always sent home on apnea monitors. The number of infants discharged home on oxygen, and those who were sent home on monitors did not change during the study period (Table 2). Some studies have evaluated multiple factors affecting rehospitalization of preterm neonates. Among them, some of the key factors include insurance and socioeconomic status, BPD, acute respiratory infections and extreme prematurity, and RSV infection 28-33. We primarily looked at the readmission of infants ≤1500g with apnea and or ALTE. We did not have information on the insurance/socioeconomic status, environmental exposure, level of education of the parents, number of siblings at home, etc. All these factors can affect the risk of readmission. With the implementation of the protocol, we encouraged parents when infants were on spell-count to room in and feed these neonates in order to transition to the home environment. Parents were aware of the discharge criteria and the observation for a spell. Although we do not have a clear reason for the decrease in readmissions, we speculate that parental rooming in during the spell-watch educated them on handling these spells and enabled a smooth transition to home environment 34.
This study reflects the overall impact of implementing a standard protocol for observing infants with spells. The strength of the study is a large number of infants who were observed during the two epochs and the significant reduction in inter-provider variability and readmission rates after protocol implementation, especially in a large practice group. We have several limitations: The adherence to the protocol and variation in special scenarios such as isolated desaturation associated with color change were not studied in detail due to documentation variability among nurses. Although we had criteria for defining isolated bradycardia and desaturations, the duration of these episodes was based on consensus and not on scientific evidence. We also did not study the effects of blood transfusion 35. Nursing documentation of the desaturation episodes was variable and associated events such as a dislodged nasal cannula, reflux, etc., were not consistently recorded 24, 36. Although a regional perinatal center, it is still possible that few infants could have relocated and we may have lost them to follow up. It is also possible that shorter duration of observation (< 3-5 days) – especially for isolated bradycardia spells and desaturation spells may be adequate and were not investigated in this study. Due to this, we have a revised version of the protocol as a supplemental figure (supplementary figure 1) and we intend to implement it. We will continue to review our practice with the protocols and once we move to our new children’s hospital (Oishei Children’s Hospital, November 2017) with single bed NICU’s, we will study the impact of our protocol and make necessary changes.
Conclusion
We conclude that, as recommended by AAP, implementing an institutional protocol to determine the duration of apnea/ bradycardia/ desaturation spell-free observation period did not prolong the LOS but effectively reduced readmission rates and inter-provider variability. Further large multicenter, prospective trials evaluating these protocols are required before widespread implementation in NICUs.
Supplementary Material
Abbreviations
- ALTE
apparent life-threatening event
- LOS
length of stay
- PMA
post menstrual age
- VLBW
very low birth weight infants
Footnotes
Financial disclosure:
PC – supported by University at Buffalo - Dr. Henry C. and Bertha H. Buswell Grant
MR – Canadian Paediatric Society (Neonatal Resuscitation Program), AMR - Developmental Impact of NICU Exposures (DINE) (UG3 OD023320), Environmental Influences on Child Health Outcomes (ECHO) Program, SL – American Academy of Pediatrics (Neonatal Resuscitation Program) and NICHD RO1HD072929
Conflict of interest – authors report no conflict of interest
References
- 1.Eichenwald EC Committee on F, Newborn AAoP. Apnea of Prematurity. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3757. [DOI] [PubMed] [Google Scholar]
- 2.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 3.Martin R. Pathogenesis, clinical presentation, and diagnosis of apnea of prematurity. Uptodate. 2016 [Google Scholar]
- 4.Miller MJ, Carlo WA, Martin RJ. Continuous positive airway pressure selectively reduces obstructive apnea in preterm infants. J Pediatr. 1985;106:91–4. doi: 10.1016/s0022-3476(85)80475-3. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 6.Darnall RA, Kattwinkel J, Nattie C, Robinson M. Margin of safety for discharge after apnea in preterm infants. Pediatrics. 1997;100:795–801. doi: 10.1542/peds.100.5.795. [DOI] [PubMed] [Google Scholar]
- 7.Zupancic JA, Richardson DK, O’Brien BJ, Eichenwald EC, Weinstein MC. Cost-effectiveness analysis of predischarge monitoring for apnea of prematurity. Pediatrics. 2003;111:146–52. doi: 10.1542/peds.111.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Lorch SA, Srinivasan L, Escobar GJ. Epidemiology of apnea and bradycardia resolution in premature infants. Pediatrics. 2011;128:e366–73. doi: 10.1542/peds.2010-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin SB, Burnell E. Monitoring apnea of prematurity: validity of nursing documentation and bedside cardiorespiratory monitor. Am J Perinatol. 2013;30:643–8. doi: 10.1055/s-0032-1329694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergales BD, Paget-Brown AO, Lee H, Guin LE, Smoot TJ, Rusin CG, et al. Accurate automated apnea analysis in preterm infants. Am J Perinatol. 2014;31:157–62. doi: 10.1055/s-0033-1343769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichenwald EC, Blackwell M, Lloyd JS, Tran T, Wilker RE, Richardson DK. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics. 2001;108:928–33. doi: 10.1542/peds.108.4.928. [DOI] [PubMed] [Google Scholar]
- 12.Wheatley E, Kennedy KA. Cross-over trial of treatment for bradycardia attributed to gastroesophageal reflux in preterm infants. J Pediatr. 2009;155:516–21. doi: 10.1016/j.jpeds.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball AL, Carlton DP. Gastroesophageal reflux medications in the treatment of apnea in premature infants. J Pediatr. 2001;138:355–60. doi: 10.1067/mpd.2001.111324. [DOI] [PubMed] [Google Scholar]
- 14.Silvestri JM. Indications for home apnea monitoring (or not) Clin Perinatol. 2009;36:87–99. doi: 10.1016/j.clp.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Powell MB, Ahlers-Schmidt CR, Engel M, Bloom BT. Clinically significant cardiopulmonary events and the effect of definition standardization on apnea of prematurity management. J Perinatol. 2017;37:88–90. doi: 10.1038/jp.2016.167. [DOI] [PubMed] [Google Scholar]
- 16.Systematic use of the RAM nasal cannula in the Yale–New Haven Children’s Hospital Neonatal Intensive Care Unit: a quality improvement project. The Journal of Maternal-Fetal & Neonatal Medicine. 2015;28:718–21. doi: 10.3109/14767058.2014.929659. [DOI] [PubMed] [Google Scholar]
- 17.Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354–9. doi: 10.1542/peds.100.3.354. [DOI] [PubMed] [Google Scholar]
- 18.Butler TJ, Firestone KS, Grow JL, Kantak AD. Standardizing documentation and the clinical approach to apnea of prematurity reduces length of stay, improves staff satisfaction, and decreases hospital cost. Jt Comm J Qual Patient Saf. 2014;40:263–9. doi: 10.1016/s1553-7250(14)40035-7. [DOI] [PubMed] [Google Scholar]
- 19.Phibbs CS, Schmitt SK. Estimates of the cost and length of stay changes that can be attributed to one-week increases in gestational age for premature infants. Early Hum Dev. 2006;82:85–95. doi: 10.1016/j.earlhumdev.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hintz SR, Bann CM, Ambalavanan N, Cotten CM, Das A, Higgins RD, et al. Predicting time to hospital discharge for extremely preterm infants. Pediatrics. 2010;125:e146–54. doi: 10.1542/peds.2009-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurek Eken M, Tuten A, Ozkaya E, Karatekin G, Karateke A. Major determinants of survival and length of stay in the neonatal intensive care unit of newborns from women with premature preterm rupture of membranes. J Matern Fetal Neonatal Med. 2017;30:1972–5. doi: 10.1080/14767058.2016.1235696. [DOI] [PubMed] [Google Scholar]
- 22.Ringborg A, Berg J, Norman M, Westgren M, Jonsson B. Preterm birth in Sweden: what are the average lengths of hospital stay and the associated inpatient costs? Acta Paediatr. 2006;95:1550–5. doi: 10.1080/08035250600778636. [DOI] [PubMed] [Google Scholar]
- 23.Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 24.Abu Jawdeh EG, Martin RJ. Neonatal apnea and gastroesophageal reflux (GER): is there a problem? Early Hum Dev. 2013;89(Suppl 1):S14–6. doi: 10.1016/S0378-3782(13)70005-7. [DOI] [PubMed] [Google Scholar]
- 25.Martin RJ, Di Fiore JM, Walsh MC. Hypoxic Episodes in Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42:825–38. doi: 10.1016/j.clp.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene MM, Patra K, Khan S, Karst JS, Nelson MN, Silvestri JM. Cardiorespiratory events in extremely low birth weight infants: neurodevelopmental outcome at 1 and 2 years. J Perinatol. 2014;34:562–5. doi: 10.1038/jp.2014.44. [DOI] [PubMed] [Google Scholar]
- 27.Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J Neonatal Perinatal Med. 2014;7:199–206. doi: 10.3233/NPM-14814035. [DOI] [PubMed] [Google Scholar]
- 28.Joffe S, Escobar GJ, Black SB, Armstrong MA, Lieu TA. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104:894–9. doi: 10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen O, Herskind AM, Kamper J, Nielsen JP, Kristensen K. Rehospitalization for respiratory syncytial virus infection in infants with extremely low gestational age or birthweight in Denmark. Acta Paediatr. 2003;92:240–2. doi: 10.1111/j.1651-2227.2003.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinquier D. Respiratory syncytial virus prevention in infants at high risk of rehospitalization. Arch Pediatr. 2005;12(Spec No 3):10–3. [PubMed] [Google Scholar]
- 31.Schiltz NK, Finkelstein Rosenthal B, Crowley MA, Koroukian SM, Nevar A, Meropol SB, et al. Rehospitalization during the first year of life by insurance status. Clin Pediatr (Phila) 2014;53:845–53. doi: 10.1177/0009922814536924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27:614–9. doi: 10.1038/sj.jp.7211801. [DOI] [PubMed] [Google Scholar]
- 34.Berney E. Reducing neonatal readmission rates. Nurs N Z. 2016;22:18–9. [PubMed] [Google Scholar]
- 35.Eichenwald EC. Apnea, intermittent hypoxia and blood transfusions: it works, but now what? J Perinatol. 2014;34:881. doi: 10.1038/jp.2014.145. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43:937–44. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


