Abstract
Nonalcoholic fatty liver disease (NAFLD) can manifest as nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH). NASH is often associated with progressive fibrosis which can lead to cirrhosis and hepatocellular cancer (HCC). NASH is increasing as an etiology for end-stage liver disease as well as HCC. There are currently no approved therapies for NASH. A major barrier to development of therapeutics for NASH is the lack of preclinical models of disease that are appropriately validated to represent the biology and outcomes of human disease. There are many in vitro and animal models that have been developed. In vitro models do not fully capture the hepatic and extrahepatic mileu of human NASH and large animal models are expensive and logistically difficult to use. There is therefore considerable interest in the development and validation of mouse models for NAFLD including NASH. Several models based on varying genetic or dietary manipulations have been developed. The majority of models do not develop steatohepatitis as defined strictly by the presence of hepatocellular ballooning with or without Mallory-Denk bodies and accompanying inflammation in the presence of macrovesicular steatosis. Others lack validation against human disease. In this review, we describe the best practices in development of mouse models of NASH. We further review existing models and the literature supporting their use as a surrogate for human disease. Finally, data on models to evaluate protective genes are discussed. It is hoped these will provide guidance in the interpretation of data derived from mouse models and also in the development and validation of newer models.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, fibrosis, NAFLD activity score, transcriptome, mouse models, preclinical models
INTRODUCTION
Nonalcoholic fatty liver disease has emerged as a major cause of chronic liver disease in many parts of the world 1. It has two principal clinical-histological phenotypes i.e. nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFLD is associated with increased cardiovascular, cancer- and liver-related mortality 2. Liver-related outcomes are mainly due to the progression of the disease to cirrhosis 3. It is anticipated that with ongoing levels of obesity and increases in type 2 diabetes, the two principal risk factors for NAFLD, the burden of NASH is expected to increase over the next two decades 4. With aging of the population and increased duration of exposure to the disease, it is of particular concern that the prevalence of cirrhosis and end-stage liver disease is very likely to increase unless public health measures to check the burden of disease are put in place. There is not only a lack of widely implemented primary prevention strategies for NASH and its associated co-morbidities but there is also no approved drug therapy for NASH. These underscore the importance of developing pathways to accelerate development of preventive and therapeutic strategies for NASH.
Many compounds are currently being tested for NASH. Unfortunately, none of the trials reported to date have demonstrated a dramatic improvement in disease status and many compounds have failed to show improvement. A potential method to improve the probability of success in clinical trials is to develop robust preclinical models of the disease that are translatable to human disease. Such models would allow the testing of many compounds to look for evidence of efficacy before going in to clinical trials. They could also be used to identify novel targets and test the impact of engaging specific combinations of molecular targets to improve the outcomes of NASH. In this review, we will focus on preclinical animal models of NAFLD with a specific focus on their translatability to the human disease.
1. Properties of an ideal animal model of NAFLD
The ideal characteristics of an animal model of NAFLD depends on what it will be used for. If the objective is to enhance the likelihood that a drug that improves NAFLD in the animal also improves the disease in humans, then it is imperative that the model studied should mimic human disease as closely as possible (Table 1).
Table 1.
Considerations in assessment of validity of preclinical models of NASH
| Characteristic | Comment |
|---|---|
|
| |
Genetics:
|
Need to relate to gene knock out or gain in function in humans and if it involves all humans This important to understand differences between strains and also with human disease given the same environmental manipulation |
|
| |
Diet:
|
Forced feeding induces stress and needs to be considered. Total calories consumed should be within relevant boundaries |
| Should broadly resemble that in human obesity Humans do not generally exceed 0.1–0.2% | |
|
| |
Physiological requirements:
|
The extrahepatic features are very relevant to human disease by setting up increased free fatty acid delivery and increased systemic inflammatory cytokine exposure. |
|
| |
Histological requirements:
|
Most papers on mouse models do not provide unequivocal evidence of hepatocellular ballooning the hallmark lesion of NASH. This is critical to determine if the model truly develops steatohepatitis. It is not possible to show NAS or SAF in these models without clear cut evidence of steatohepatitis. Progression to cirrhosis is ideal and a key endpoint in preclinical models. |
|
| |
Cell signaling requirements:
|
These are key pathways in human disease and preclinical models must be validated to demonstrate activation of these pathways amongst others. The relationship of these pathway activation to histological course of the disease must be defined. |
|
| |
Transcriptomic validation:
|
This further strengthens the evidence that the disease in the model resembles human disease |
|
| |
Metabolomic validation:
|
Provides physiological read out of similarity of disease to human disease. |
It is obvious that a non-human species will never be identical to humans. Animal models should however mimic human disease with respect to its development by diet-induced obesity the most common risk factor for the disease in humans5. Importantly, the dietary composition should broadly resemble human diets in terms of their macronutrient composition and not contain unnatural toxins such as very high levels of cholesterol or di-ethylnitrosamine. It should develop obesity, insulin resistance and features of systemic inflammation seen in humans with insulin resistance 6. It should also recapitulate the systemic metabolic and inflammatory milieu by development of dyslipidemia and increase in inflammatory cytokines such as TNF-α, IL-6 and a decrease in adiponectin. Furthermore, it should develop a hepatic phenotype that resembles human disease by having predominantly macrovesicular steatosis, lobular inflammation, hepatocellular ballooning (ideally with Mallory-Denk bodies) and hepatic fibrosis7. The lesions should be mostly centrilobular or panacinar and the fibrosis should be perisinusoidal in nature and start in zone III as seen in humans and then progress through portal and sinusoidal fibrosis to bridging fibrosis and nodule formation. Finally, once advanced fibrosis develops, the model should have an increased propensity for development of hepatocellular cancer.
Relevant models of NAFLD should not only recapitulate the diet, systemic milieu and histological spectrum of the disease but also demonstrate activation of the key cellular pathways known to be associated with human disease such as activation of de novo lipogenesis and unfolded protein response7. In addition, other pathogenic elements such as oxidative stress, apoptosis and fibrogenic pathways that are relevant in human disease should also be activated. Finally, transcriptomic analyses should be able to demonstrate that there is a broad concordance between the human and mouse transcriptomic signature at various phases of disease development and these should be further reflected in the metabolome.
In addition to these criteria, the models should be reproducible and the data in the model repeatable. The robustness of the model in terms of sensitivity to light-dark cycles, housing conditions, ambient temperature should all be further considered when selecting a specific model 8. Below, we will critically review the existing models and identify where they do or do not meet these criteria (Table 2).
2. Dietary Animal Models of NASH
Diet, dietary patterns, and various types of high calorie nutrients are the important regulators and contributors for many diseases including the development, progression, and treatment of NAFLD, metabolic syndrome and cancers9. The animal models of NASH are mainly based on various types of diet such as high fat, high glucose, sucrose, fructose, methionine and choline deficient (MCD) diet, choline-deficient L-amino-defined (CDAA) diet, high cholesterol diet (HCD), cholesterol and cholate etc. Even in the genetic animal models of NASH, diet is used as a means of secondary trigger for disease progression 10, 7, 11, 12. These diets are provided individually or in combination of one or more in order to induce simple steatosis and steatohepatitis.
Methionine and choline deficient (MCD) diet
MCD is one of the very commonly used diets which produces the most severe phenotype of NASH in the shortest time. This diet with high sucrose (40%) and 10% fat but deficient in methionine and choline has been used for over 40 years and is known to very quickly induce measurable hallmarks of NAFLD such as hepatic steatosis (mainly macrovesicular) in mice and rats by 2–4 weeks and this progresses to inflammation and fibrosis shortly thereafter 13, 14. In addition, the MCD diet alters glucose metabolism with no insulin resistance, affects hepatic lipid metabolism with a significant increase in fatty acid uptake and reduction in VLDL secretion and induces significant fibrosis compared to other dietary animal models 15,16,17. Although the MCD model replicates human NASH histological phenotype in relatively short period, the associated weight loss and lack of systemic insulin resistance makes it quite different from human NAFLD. Thus the use of MCD model is limited by its disparity with the metabolic parameters of human NASH. Importantly, there is poor concordance between differentially expressed genes in this model and human NASH 18.
Choline-Deficient L-Amino-Defined (CDAA) Diet
The choline-deficient, L-amino acid-defined (CDAA) dietary model is another model that develops steatohepatitis, liver fibrosis and hepatocarcinogenesis 19, 20, 21. Similar to MCD diet, the mice fed on CDAA diet increases lipid synthesis, inflammation and causes liver injury. In addition, these mice do not gain weight and do not display insulin resistance 22, 23, 24. Hence the CDAA model displays a metabolic profile different from human NASH and are unsuitable as surrogates for human disease 25,26.
High-cholesterol diet (HCD)
Many foods mainly western diets consumed by humans contain high levels of cholesterol. Recent studies strongly suggest that dietary cholesterol is a critical factor in the progression of steatohepatitis and hepatic inflammation not only in animal models 27,28,29 but also in humans 30. Mice fed a HCD (1%) alone show strikingly increased serum insulin levels with only slight increase in liver weight, triglyceride levels, FFA levels, and serum ALT levels30,31. Several studies have proven that features of NASH are not pronounced with just the use of HCD30,31,32. Importantly, this level of dietary cholesterol is virtually never seen in humans with NAFLD.
Cholesterol and Cholate
Cholesterol and Cholate are known for their atherogenic (1.25% cholesterol and 0.5% cholate) properties. Cholic acid is a primary bile acid and is chemically known as 3α, 7α, 12α-trihydroxy-5β-cholan-24-oic acid and salt form of this acid is called as cholate. As noted above, this level of dietary cholesterol is not seen in most humans with obesity. Cholesterol and Cholate together induces progressive development of steatosis, inflammation, and fibrosis including hepatocellular ballooning which are important features of human NASH in a time-dependent manner over 6–24 weeks. Along with the cholesterol and cholate diet, addition of 60% fat (cocoa butter) resulted in accelerated development of NASH features and formation of hepatocellular ballooning around 12 weeks27. This diet also induces dyslipidemia, lipid peroxidation and oxidative stress leading to liver injury. HCD combined with high fat and high cholate are known to display more pronounced features of NASH but the major drawback of this diet combination is that mice were systematically insulin sensitive and lost 9% body weight with small epididymal fat pads and low plasma triglyceride levels compared to control mice33. Therefore even though this diet replicate human disease pathology, the diet is not relevant to the human state and the metabolic status differs from human NASH.
High Fat Diet (HFD)
A high fat diet composed of 71% fat, 11% carbohydrates, and 18% proteins fed to rats for 3 weeks is known to develop insulin resistance with marked panlobular steatosis, inflammation and induce fibrosis 7,10,11,12. Whereas the mice fed with HFD showed similar results around 16 weeks. Thus, the key feature of this model is that the results vary with rodent strain and the composition of the diet 34. The model displays NASH features similar to human NASH but the pathological outcome is not as severe and limits the use of the model for the study. The C57Bl6/J mice are more insulin resistant and thus more likely to be relevant than the C57Bl6/N mice 35.
High-fructose diet
Fructose rich foods are being highly consumed by humans and this has been associated with the development of obesity and NASH 36. Findings from various studies with C57BL/6 mice fed an HFD or high-fat, high-fructose (HFHF) diet suggest that fructose consumption is necessary for the development and progression of liver fat deposition to fibrogenesis (NAFL to NASH). It is interesting to note that when compared to mice fed with high fat diet; the HFHF diet fed mice had increased hepatic inflammation, oxidative stress and fibrosis 36,37. The use of high fat, fructose and cholesterol (FFC) diet by Charlton et al., composed of 40% fat, 42 g/l final concentration fructose and 0.2% cholesterol recapitulated the features of insulin resistance, steatosis, inflammation with hepatocellular ballooning and progressive fibrosis 38. The FFC model further mimicked the human NASH displaying the interrelationship between inflammation in both liver and adipose tissue 38,11. Thus the addition of fructose to a high fat diet promotes the development of hepatocellular ballooning with increased inflammasome activation and fibrosis in the mouse models of NASH. However, in a C57Bl6/J mouse, a high fat diet with fructose does not consistently progress to advanced fibrosis or hepatocellular cancer. Of note, a high fructose diet alone when administered ad libitum does not produce a hepatic phenotype of NASH 38,39.
The Streptozotocin high fat diet model
In this model, C57Bl6/J mice were give streptozotocin (200 µg) two days after birth. Surviving mice were then started on a high fat diet 40. These mice develop steatohepatitis and fibrosis and hepatocellular cancers in approximately 20 weeks 40. This model is recapitulates several important histological aspects of human NAFLD and is also associated with oxidative stress 41 but differs from the human state in recreating beta cell function loss with streptozotocin rather than a systemic inflammatory insulin resistant mileu. However, in a similar model where mice were given streptozotocin followed by a high fat diet, investigators failed to demonstrate concordance with the respect to differentially expressed genes in the mouse compared to humans 42.
The diet-induced animal model of NAFLD (DIAMOND)
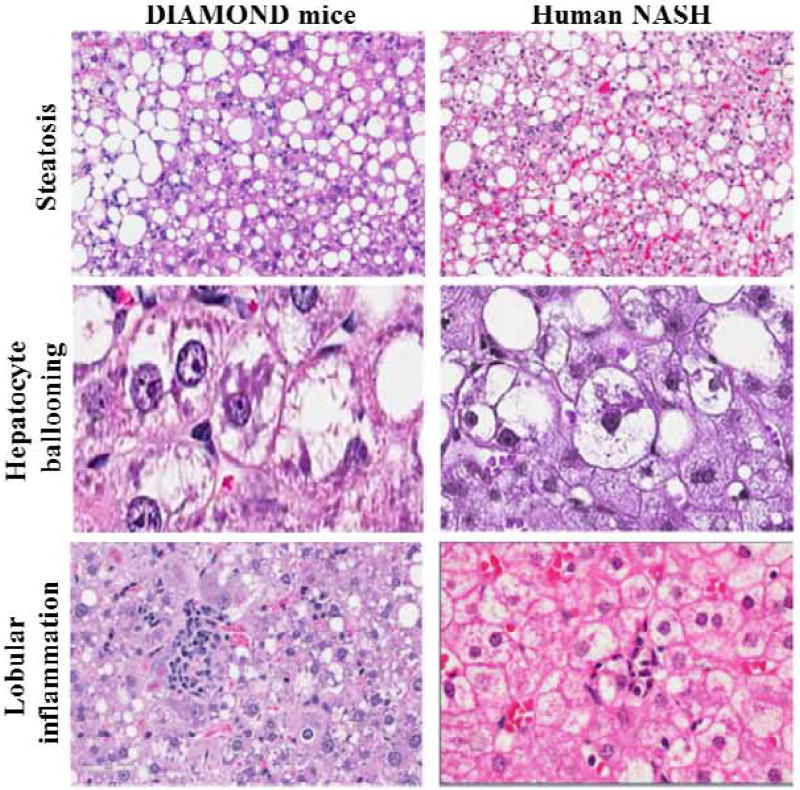
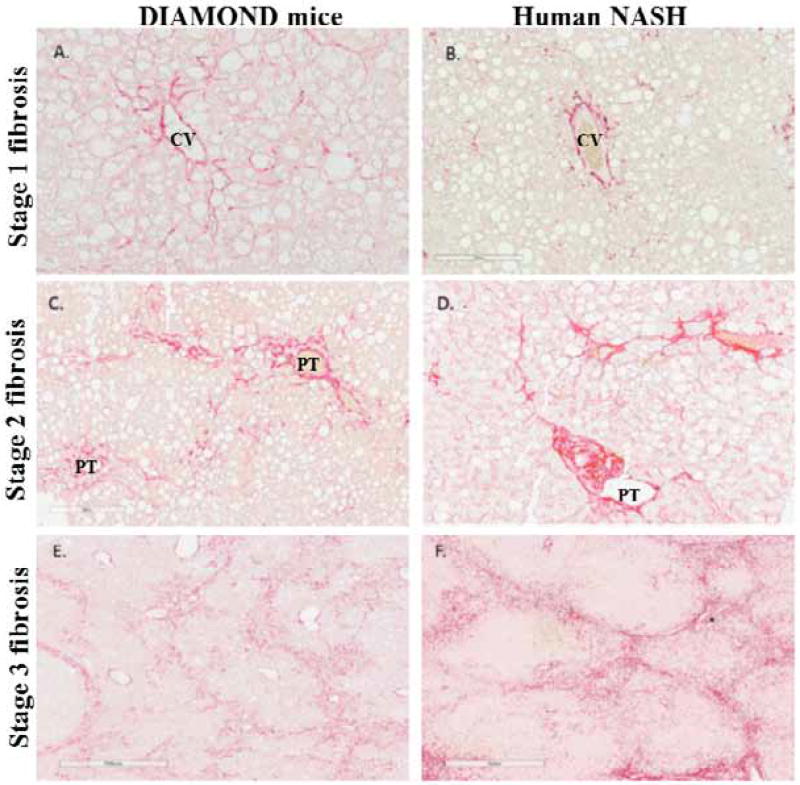
The limitations of existing models led us develop a mouse model of NAFLD that meets many criteria for a relevant NASH model outlined above 7. It is based on an inbred isogenic strain of a C57Bl6/J and S129S1/svlmJ mice where approximately 60% of genes are from the C57Bl6/J background based on a panel of SNPs from Jackson labs. Importantly, the isogenic nature of the mice have been confirmed across multiple generations and the phenotype of the disease has been maintained by careful attention to the breeding protocol over 20 generations. Under conditions of a chow diet, the mice retain normal weight and have a normal life span. However, upon starting a high fat, high carbohydrate diet (Western Diet, WD) with 42% Kcal from fat and containing 0.1% cholesterol with ad lib administration of glucose/fructose (SW, 23.1g/L d-fructose + 18.9 g/L d-glucose) in drinking water, the mice faithfully recapitulates human NAFLD by development of obesity, insulin resistance, dyslipidemia and sequentially develop fatty liver, then steatohepatitis followed by stage 1 fibrosis which progresses to early nodule formation (stage 3- early4 fibrosis) and hepatocellular cancer when advanced fibrosis is present. Importantly, there is true steatohepatitis with hepatocellular ballooning and Mallory-Denk bodies and clearing of cytoplasmic CK18 and not simply steatosis and inflammation (Figure 1) 7,43. The fibrosis also progresses in a manner similar to that in humans (Figure 2). It also develops adipose tissue inflammation and hypoadiponectinemia similar to that seen in humans. Furthermore, the key pathways known to be activated in human NAFLD are also activated along with oxidative stress, activation of unfolded protein response, activation of inflammatory, apoptotic and fibrogenic pathways 7,44. In contrast to data from the MCD model, high fat diet with streptozotocin administration and the PTEN genetic models, there is a strong concordance with the human NAFLD transcriptome throughout the various stages of disease development and this data have been deposited in the NIH data base 7. This model is thus similar to human disease with respect to many of the key features noted above.
Fig. 1.
The key features of human NAFLD and representative histological features in a mouse model of NAFLD (DIAMOND). Predominantly macro-vesicular steatosis in steatohepatitis (left panel 10x) after 16 weeks of a high fat diet (Harlan Teklad # TD 88137) with ad libitum administration 23.1g/L d-fructose and n18.9 g/L d-glucose) in the DIAMOND mice. The pattern is similar to that seen in humans with NASH (Panels A and B (right panel 10x)). Similarly, Panels C and D demonstrate hepatocellular ballooning in mice and in humans respectively. Panels E and F demonstrate lobular inflammation in mice and in humans. These provide proof of concept that the features of human NASH can be recapitulated in humans (adapted from asgharpour et al., 7).
Fig. 2.
Fibrosis development and progression in a mouse model of steatohepatitis (DIAMOND mouse) and its similarity to human disease. Initially pericellular fibrosis around the central vein is noted followed by both portal and pericellular fibrosis and eventually bridging fibrosis with early nodule formation. Data for mice are shown in the left panels whereas data for humans are shown on the right panel (adapted from asgharpour et al., 7).
The principal differences between the DIAMOND mice and human disease include suppression of cholesterol synthesis and the high frequency of HCC development. The HCC are however similar to human HCC with respect to the molecular gene signatures 7,45. Another limitation is that it takes up to 16 weeks to develop steatohepatitis. Moreover, fibrosis develops and progresses to bridging fibrosis/early cirrhosis over 36 weeks from initiation of the diet. A potential limitation is to breed KO or overexpressing mice into the mixed background of the Diamond mice.
The Ossabaw pig model of NAFLD
This animal has been shown to develop obesity, insulin resistance and hepatic phenotypes similar to that seen in humans along with development of fibrosis 46. The principal limitations of this model are the cost and logistical challenges of using pigs as preclinical models of human NAFLD.
3. Genetic Animal Models of NASH
With the advancement in genetic engineering, it has been possible to create various experimental rodent models. The nature of the specific gene alteration needed renders these mice different from humans who do not have these specific genes altered. However, such models are particularly valuable for the study of specific pathways and how they may alter metabolic homeostasis in the liver including the consequences of such dysregulation. Addition of modified diets are frequently required to induce the histopathological and biochemical changes of NASH in these mice.
Leptin Deficiency (ob/ob Mice)
Leptin is a peptide hormone secreted predominantly by adipocytes of white adipose tissue and plays a vital role in the regulation of energy balance. The ob/ob mice lack functional leptin and they easily develop severe insulin resistance47,48. In ob/ob mice due to the deficiency of this hormone, fat is redistributed from adipose tissue to the liver and other non-adipose tissues. This results in the accumulation of fat in the liver which further induces hepatocyte lipotoxicity and lipoapoptosis. However, ob/ob mice rarely develop NASH, which may be due to alterations in inflammatory responses and other effects 49. Also, leptin deficiency need additional stimulus (LPS, High fat diet, MCD etc.) to induce features of NASH. In addition, it is also known that ob/ob mice are resistant to hepatic fibrosis. Leptin mutations have been reported in humans but clinical studies have shown that serum leptin levels are normal or elevated in NAFL and NASH patients compared to healthy controls 50,51. Thus ob/ob mice model are limited in their ability to be used for the study of NASH.
Leptin Receptor Deficiency (db/db Mice)
Db/db mice or leptin receptor deficient mice carry a point mutation in one of the leptin receptor gene which leads to defective leptin signaling. The phenotype of db/db mice is very much alike to ob/ob mice and although these mice have normal or elevated leptin levels, they confer resistance to the effects of leptin. The db/db mice also have abnormally increased appetite and develop obesity, hyperglycemia, hyperinsulinemia, insulin resistance and fatty liver 11, 52. The db/db mice shows the features of NASH with the additional stimulus such as high calorie diet or MCD 53. Unlike ob/ob mice, db/db mice develop fibrosis when fed with MCD 53, 54, 15,16. Their ability to develop advanced fibrosis and HCC is not well characterized.
Db/db mice supplemented with iron
Along with HFD, high calorie diet and MCD diet, a recent report showed that iron overload in db/db mice also causes progression of simple steatosis to steatohepatitis and fibrosis55. Iron deposition may take place at subcellular location in hepatocytes, sinusoidal lining cells and reticuloendothelial system. Chow diet supplemented with a high iron load also induced major NAFLD features such as hepatocellular ballooning, hepatic inflammatory immune cell activation, increased hepatic oxidative stress and impaired hepatic mitochondrial fatty acid β-oxidation and fibrogenesis in db/db mice 55, 56. Thus it is clear that iron may potentiate the development and progression of NAFLD by various mechanisms such as oxidative stress, altered insulin signaling or lipid metabolism 57. The development of NAFLD with even a chow diet however raises concerns about the translatability of this model.
Foz/Foz mice
Alstrom syndrome 1 or ALMS1 is a protein which in humans is encoded by the ALMS1 gene. Alms1 is a ubiquitous protein which is essential for proper primary cilium function. Although this gene function has not been fully studied and elucidated, it may have a very important role in intracellular transport and appetite regulation. Mice which are mutated or deficient in Alms1 are called foz/foz mice. Similar to ob/ob and db/db mice, these mice are also obese, insulin resistant and display steatosis 58.,59. HFD also promotes the transition of NAFL to NASH with severe fibrosis by aggravating metabolic complications in these mice. However, the effect of diet-induced NAFL to NASH transition in these mice depends on the strain. foz/foz C57BL6/J mice and foz/foz BALB/c mice both gained weight when fed with HFD but NAFLD-related liver fibrosis was more severe in foz/foz C57BL6/J mice and not in foz/foz BALB/c mice 60,61.
The other genetic animal models used in the study of NASH include sterol regulatory elementbinding protein 1 (SREBP1)c transgenic mice, KK-Ay/a mice, peroxisome proliferator-activated receptor α (PPARα) null mice, methionine adenosyl transferase (MAT1A) null mice, phosphatase and tensin homolog (PTEN) null mice and Acyl-coenzyme A oxidase (AOX) null mice11,62–67.
4. Animal models to study the effect of specific genes to protect against NASH
Another potential application of animal models is to study the relevance of specific genes for the development and progression of NASH. Macrophages are a special type of immune cells and are large phagocytic in nature found in almost all types of tissues or as a mobile white blood cells, especially at sites of infection during an innate-immune response. They are the primary mediators of the sterile inflammatory response in NASH. Pro-inflammatory macrophages are recruited to the liver during NAFL to NASH transition from hematopoietic stem cell derived myeloid lineage cells rather than yolk sac-derived resident hepatic macrophages (Kupffer cells or liver macrophages) 68,69. These cells are pro-inflammatory in the obesity, insulin resistance and fatty liver condition and secrete a number of inflammatory cytokines such as monocyte chemotactic protein 1 (MCP-1), TNF-α, IL-10, IL-6 etc. MCP-1, is a major chemokine critical to recruiting myeloid cells to the liver via its receptor, chemokine (C-C motif) receptor 2 (CCR-2). This CCR-2 is known to be highly expressed in hepatic macrophages during NAFL, NASH and HCC68, 70 ,71. There are many animal studies showing the importance of the MCP-1-CCR2 signaling in NASH pathogenesis 68, 70. The CCR-2 (Ccr2−/−) deficient mice are protected from the development of hepatic steatosis, inflammation, macrophage infiltration and fibrosis compared to wild-type mice 70. Mice lacking TLR-4, TLR-9 or myeloid cell differentiation (MyD) 88 all demonstrate reduced hepatic macrophage accumulation. Interestingly, these receptors also depend on MCP-1 and CCR-2 signaling for their function72. Similarly, several toll like receptors (TLRs) have been implicated in NASH and the significance of these receptors is established with the use of apolipoprotein E knockout mouse model and mice deficient in TLR9, interleukin 1 receptor, TLR4 and its co-receptor myeloid differentiation protein 2 (MD-2) 73, 74, 11. In addition, C-jun N-terminal kinase (JNK) knockout mice and inflammasome (Caspase 1, NOD-like receptors (NLR) proteins) deficient mice have demonstrated the significance of inflammatory signaling in the pathogenesis of NASH.
In summary, many advances in the development of preclinical models for NAFLD have been made that have provided valuable insights on disease pathogenesis. However, only a few models recapitulate the key elements needed to be representative of human disease and there are no published data to indicate that if drugs are effective in a given model it consistently translates in to efficacy in humans and conversely that if a drug does not work in humans that it will not work in humans. It is therefore important to be cognizant of the boundaries within which data from animal models must be interpreted to most effectively translate findings from such models to improved therapeutics in humans.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 DK081450 and T3207150-40 to Arun J. Sanyal.
Grant support:
RO1 DK 105961
T32 DK 07150
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
P.K. Santhekadur: None to report
D.P. Kumar: None to report
A.J. Sanyal: None for this project. Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Fibrogen, Jannsen, Gilead, Boehringer, Lilly, Zafgen, Novartis, Pfizer, Immuron, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Novo Nordisk, Affimune, Chemomab, Nordic Bioscience and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate.
References
- 1.Sanyal AJ, Neuschwander-Tetri BA, Tonascia J. End Points Must Be Clinically Meaningful for Drug Development in Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:11–13. doi: 10.1053/j.gastro.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Wattacheril J, Chalasani N. Non-Alcoholic Fatty Liver Disease (NAFLD): Is it really a serious condition? Hepatology. 2012 Oct;56(4):1580–1584. doi: 10.1002/hep.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MH, Husain NE, Almobarak AO. Nonalcoholic Fatty liver disease and risk of diabetes and cardiovascular disease: what is important for primary care physicians? J Family Med Prim Care. 2015 Jan-Mar;4(1):45–52. doi: 10.4103/2249-4863.152252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr Diabetes. 2014 Sep 8;4:e135. doi: 10.1038/nutd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol. 2013 Apr;7(2):206–23. doi: 10.1016/j.molonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D, Jr, Warncke UO, Wijesinghe DS, Sanyal AJ. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016 Sep;65(3):579–88. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Xu MJ, Bertola A, Wang H, Zhou Z, Liangpunsakul S. Animal Models of Alcoholic Liver Disease: Pathogenesis and Clinical Relevance. Gene Expr. 2017 Jul 7;17(3):173–186. doi: 10.3727/105221617X695519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007 Aug;86(2):285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 10.Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS, Lowe C, Roth JD. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol. 2013 Oct 1;305(7):G483–95. doi: 10.1152/ajpgi.00079.2013. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim SH, Hirsova P, Malhi H, Gores GJ. Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig Dis Sci. 2016 May;61(5):1325–1336. doi: 10.1007/s10620-015-3977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan JG, Cao HX. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2013 Dec;28(Suppl 4):81–7. doi: 10.1111/jgh.12244. [DOI] [PubMed] [Google Scholar]
- 13.Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. American journal of physiology. Gastrointestinal and Liver Physiology. 2004;287:G264–73. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- 14.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–53. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 15.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015 May 27;10(5):e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008 May;49(5):1068–76. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce AA, Pickens MK, Siao K, Grenert JP, Maher JJ. Differential hepatotoxicity of dietary and DNL-derived palmitate in the methionine-choline-deficient model of steatohepatitis. BMC Gastroenterol. 2015 Jun 24;15:72. doi: 10.1186/s12876-015-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakae D, Yoshiji H, Maruyama H, Kinugasa T, Denda A, Konishi Y. Production of both 8-hydroxydeoxyguanosine in liver DNA and gamma-glutamyltransferase-positive hepatocellular lesions in rats given a choline-deficient, L-amino acid-defined diet. Jpn. J. Cancer Res. 1990;81:1081–1084. doi: 10.1111/j.1349-7006.1990.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakae D, Yoshiji H, Mizumoto Y, et al. High incidence of hepatocellular carcinomas induced by a choline deficient L-amino acid defined diet in rats. Cancer Res. 1992;52:5042–5045. [PubMed] [Google Scholar]
- 21.Sakaida I, Kubota M, Kayano K, Takenaka K, Mori K, Okita K. Prevention of fibrosis reduces enzyme-altered lesions in the rat liver. Carcinogenesis. 1994;15:2201–2206. doi: 10.1093/carcin/15.10.2201. [DOI] [PubMed] [Google Scholar]
- 22.Denda A, Kitayama W, Kishida H, et al. Expression of inducible nitric oxide (NO) synthase but not prevention by its gene ablation of hepatocarcinogenesis with fibrosis caused by a choline-deficient, l-amino acid-defined diet in rats and mice. Nitric Oxide. 2007;16:164–176. doi: 10.1016/j.niox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, Ito T, Katsume A, Sudoh M. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol. 2013 Apr;94(2):93–103. doi: 10.1111/iep.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamada Y, Matsumoto H, Tamura S, et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J. Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Denda A, Kitayama W, Kishida H, et al. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn. J. Cancer Res. 2002;93:125–132. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodama Y, Kisseleva T, Iwaisako K, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 28.Zheng S, Hoos L, Cook J, et al. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118–124. doi: 10.1016/j.ejphar.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Goodspeed L, Wang S, et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savard C, Tartaglione EV, Kuver R, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol. 2017 Jan;241(1):36–44. doi: 10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J. Biol. Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012 May 21;18(19):2300–2308. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura A, Terauchi Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int J Mol Sci. 2013 Oct 24;14(11):21240–57. doi: 10.3390/ijms141121240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 36.Kohli R, Kirby M, Xanthakos SA, et al. High-fructose, medium chain trans-fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010 Jun;51(6):1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011 Nov;301(5):G825–34. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JS, Jun DW, Kim EK, Jeon HJ, Nam HH, Saeed WK. Histologic and Metabolic Derangement in High-Fat, High-Fructose, and Combination Diet Animal Models. Scientific World Journal. 2015;2015:306326. doi: 10.1155/2015/306326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, Arumugam S, Watanabe K, Ichida T, Asakura H, Yoneyama H. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013 Sep;46(3):141–52. doi: 10.1007/s00795-013-0016-1. [DOI] [PubMed] [Google Scholar]
- 41.Radosavljevic T1, Mladenovic D, Vucevic D, Vukicevic RJ. The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis. Med Pregl. 2010 Nov-Dec;63(11–12):827–32. doi: 10.2298/mpns1012827r. [DOI] [PubMed] [Google Scholar]
- 42.Sadi G, Baloglu MC, Pektas MB. Differential Gene Expression in Liver Tissues of Streptozotocin-Induced Diabetic Rats in Response to Resveratrol Treatment. PLoS One. 2015 Apr 23;10(4):e0124968. doi: 10.1371/journal.pone.0124968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2016 Aug;65(8):1080–6. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008 May;134(6):1641–54. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrisani OM, Studach L, Merle P. Gene signatures in hepatocellular carcinoma (HCC) Semin Cancer Biol. 2011 Feb;21(1):4–9. doi: 10.1016/j.semcancer.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009 Jul;50(1):56–67. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindström P. The physiology of obese-hyperglycemic mice [ob/ob mice] Scientific World Journal. 2007 May 29;7:666–85. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, Ikejima K, Kirikoshi H, Nakajima N, Saito S, Maeyama S, Watanabe S, Wada K, Nakajima A. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012 Jul 3;16(1):44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005 Apr;41(4):753–60. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 50.Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013 Jul;59(1):131–7. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015 Jan;64(1):60–78. doi: 10.1016/j.metabol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 53.Trak-Smayra V, Paradis V, Massart J, Nasser S, Jebara V, Fromenty B. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int J Exp Pathol. 2011 Dec;92(6):413–21. doi: 10.1111/j.1365-2613.2011.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: Role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 55.Handa P, Morgan-Stevenson V, Maliken BD, et al. Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G117–G127. doi: 10.1152/ajpgi.00246.2015. [DOI] [PubMed] [Google Scholar]
- 56.Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol. 2017 Jan;241(1):36–44. doi: 10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007 Jun;102(6):1251–8. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 58.Laurence P, Vanessa L, Geoffrey F, Isabelle L. Role of ciliary dysfunction in a new model of obesity and non-alcoholic steatohepatitis: the foz/foz mice. Arch Public Health. 2014;72(Suppl 1):O7. [Google Scholar]
- 59.Heydet D, Chen LX, Larter CZ, Inglis C, Silverman MA, Farrell GC, Leroux MR. A truncating mutation of Alms1 reduces the number of hypothalamic neuronal cilia in obese mice. Devl Neurobiol. 2012;73:1–13. doi: 10.1002/dneu.22031. [DOI] [PubMed] [Google Scholar]
- 60.Arsov T, Larter CZ, Nolan CJ, Petrovsky N, Goodnow CC, Teoh NC, Yeh MM, Farrell GC. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006 Apr 21;342(4):1152–9. doi: 10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 61.Bell-Anderson KS, Aouad L, Williams H, et al. Coordinated improvement in glucose tolerance, liver steatosis and obesity-associated inflammation by cannabinoid 1 receptor antagonism infat Aussie mice. Int J Obes (Lond) 2011;35:1539–1548. doi: 10.1038/ijo.2011.55. [DOI] [PubMed] [Google Scholar]
- 62.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear srebp-1c in adipose tissue: Model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okumura K, Ikejima K, Kon K, Abe W, Yamashina S, Enomoto N, Takei Y, Sato N. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic kk-a(y) mice. Hepatol Res. 2006;36:217–228. doi: 10.1016/j.hepres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1a. Faseb J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 66.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific pten deficiency results in steatohepatitis and hepatocellular carcinomas. The Journal of clinical investigation. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-coa oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 68.Morinaga H, Mayoral R, Heinrichsdorff J, et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120–1130. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wenfeng Z, Yakun W, Di M, Jianping G, Chuanxin W, Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann Hepatol. 2014 Sep-Oct;13(5):489–95. [PubMed] [Google Scholar]
- 72.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011 Oct 10;11(11):762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One. 2010;5:e12537. doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]