Abstract
OBJECTIVES
This multi-center pragmatic investigation assessed outcomes following clinical implementation of CYP2C19 genotype-guided antiplatelet therapy after PCI.
BACKGROUND
CYP2C19 loss-of-function alleles impair clopidogrel effectiveness after percutaneous coronary intervention (PCI).
METHODS
After clinical genotyping, each institution recommended alternative antiplatelet therapy (prasugrel, ticagrelor) in PCI patients with a loss-of-function allele. Major adverse cardiovascular events (MACE, defined as myocardial infarction, stroke, or death) within 12 months of PCI were compared between patients with a loss-of-function allele prescribed clopidogrel versus alternative therapy. Risk was also compared between patients without a loss-of-function allele and loss-of-function allele carriers prescribed alternative therapy. Cox regression was performed, adjusting for group differences with inverse probability of treatment weights.
RESULTS
Among 1,815 patients, 572 (31.5%) had a loss-of-function allele. The risk for MACE was significantly higher in patients with a loss-of-function allele prescribed clopidogrel versus alternative therapy [23.4 vs. 8.7 per 100 patient-years; adjusted hazard ratio (HR): 2.26; 95% confidence interval (CI): 1.18 to 4.32; p=0.013]. Similar results were observed among 1,210 patients with an acute coronary syndrome at the time of PCI (adjusted HR: 2.87; 95% CI: 1.35 to 6.09, p=0.013). There was no difference in MACE between patients without a loss-of-function allele and loss-of-function allele carriers prescribed alternative therapy (adjusted HR: 1.14, 95% CI: 0.69 to 1.88, p=0.60).
CONCLUSIONS
These data from real-world observations demonstrate a higher risk for cardiovascular events in patients with a CYP2C19 loss-of-function allele if clopidogrel versus alternative therapy is prescribed. A future randomized study of genotype-guided antiplatelet therapy may be of value.
Keywords: clopidogrel, CYP2C19, pharmacogenomics, percutaneous coronary intervention, cardiovascular events, anti-platelet therapy
INTRODUCTION
Treatment with a P2Y12 inhibitor plus aspirin is the standard of care following percutaneous coronary intervention (PCI) (1,2). The P2Y12 inhibitor clopidogrel is a prodrug requiring bioactivation by cytochrome P450 (CYP) 2C19. CYP2C19 loss-of-function (LOF) alleles lead to reduced or absent CYP2C19 activity, lower plasma concentrations of the clopidogrel active metabolite and reduced inhibition of platelet aggregation during clopidogrel therapy (3,4). Retrospective analyses from randomized clinical trials and patient registries have demonstrated a higher risk for major adverse cardiovascular events (MACE) in clopidogrel-treated patients with versus without a CYP2C19 LOF allele, particularly after PCI (3,5–7).
Prasugrel and ticagrelor are alternative P2Y12 inhibitors, shown to be superior to clopidogrel in preventing MACE in patients with an acute coronary syndrome (ACS) in the TRITON-TIMI 38 and PLATO trials, respectively (8,9). Post hoc genetic analyses from these trials showed no effect of CYP2C19 genotype on outcomes with either prasugrel or ticagrelor (7,10). However, both drugs are more expensive than clopidogrel, which is available generically, and are associated with an increased bleeding risk. Ticagrelor is also associated with more frequent discontinuation due to side effects compared to clopidogrel (8).
While several institutions have implemented clinical CYP2C19 genotyping to guide antiplatelet therapy selection after PCI (11–14), the impact of this strategy on clinical outcomes is not well defined. Therefore, among patients who underwent a PCI and clinical CYP2C19 genotyping, we compared the risk for MACE between patients with a CYP2C19 LOF allele prescribed clopidogrel 75 mg/day and those with a CYP2C19 LOF allele prescribed alternative antiplatelet therapy. We also compared MACE risk between those with a LOF allele prescribed alternative therapy and those without a CYP2C19 LOF allele treated with any P2Y12 inhibitor.
METHODS
STUDY DESIGN
This was a multi-center investigation of clinical CYP2C19 genotype-guided antiplatelet therapy post-PCI. The study design was pragmatic based on delivery of the genotype intervention as part of clinical care, the ultimate decision to order genetic testing and choice of drug therapy left to the discretion of the physician, unobtrusive collection of data from the electronic health record (EHR), and the focus on an objectively measured and clinically meaningful outcome (15–17). Seven institutions (University of Florida; University of North Carolina, Chapel Hill; University of Maryland, Baltimore; University of Alabama, Birmingham; University of Illinois, Chicago; University of Pittsburgh; and Indiana University) implemented clinical CYP2C19 genotyping with results returned via the EHR for consideration during antiplatelet therapy prescribing. All sites participated in the NIH-funded Implementing Genomics in Practice (IGNITE) Network Pharmacogenetics Working Group and contributed data (18). All patients from each site ≥18 years of age who underwent PCI and CYP2C19 genotyping (per the strategy described in ONLINE TABLE 1) and received a P2Y12 inhibitor after PCI were included, regardless of length of follow-up. A total of 1,815 patients across the seven institutions met these criteria and were included in the analysis.
CYP2C19 GENOTYPING AND PHENOTYPING
Genotyping was performed at each institution in a Clinical Laboratory Improvement Amendments (CLIA)-licensed laboratory, with the test ordered prior to or at the time of PCI. All sites genotyped for the LOF CYP2C19*2 and *3 alleles, with additional rare alleles genotyped at 5 institutions (ONLINE TABLE 1). CYP2C19 LOF allele status was defined by presence of at least one LOF allele.
CYP2C19 phenotype was assigned similarly across sites based on standardized definitions (19). Patients with one or two LOF alleles were assigned the intermediate metabolizer (IM) or poor metabolizer (PM) phenotype, respectively. Alternative antiplatelet therapy, consisting of prasugrel or ticagrelor in the absence of contraindications, was recommended for IMs and PMs, according to Clinical Pharmacogenetics Implementation Consortium guidelines (20). Ultimate antiplatelet therapy selection was left to the discretion of the prescriber.
DATA ABSTRACTION
Data abstraction procedures were approved by the Institutional Review Board at each institution. Clinical data at baseline and for up to 12 months following the index PCI, defined as the PCI performed in association with genotyping, were manually abstracted from the EHR through review of patient encounters, including the index PCI hospitalization and subsequent hospitalizations and outpatient visits, using a common data collection form (18). The occurrence of clinical outcomes of interest, including death, myocardial infarction (ST-segment or non-ST-segment elevation myocardial infarction), ischemic stroke, stent thrombosis, and unstable angina occurring over the 12-month period after the index PCI was determined. The date range for data abstraction was June 2012 through April 2016.
PRIMARY AND SECONDARY OUTCOMES
The primary outcome was the composite of MACE, defined as first occurrence of myocardial infarction, ischemic stroke, or death within 12 months following the index PCI (5). Secondary outcomes were the composite of MACE plus stent thrombosis and unstable angina and individual components of MACE. Outcomes were identified based on physician-reported diagnoses abstracted from the cardiac catheterization laboratory report, hospital discharge summary notes, or clinical notes in the event of death. Antiplatelet therapy was assessed at the time of event or last follow-up in which P2Y12 inhibitor treatment was documented. The number of days between the index PCI and initiation of alternative antiplatelet therapy was determined in patients with a LOF allele and was available for all but four patients.
STATISTICAL ANALYSIS
Data were curated and aggregated at the University of Florida. The time of index PCI was considered time zero. Patients who did not experience MACE during the 12 months post-PCI were censored at the time of the last EHR-documented follow-up in which treatment with a P2Y12 inhibitor was documented. Event rates were calculated as number of events divided by follow-up time (time-to-event or censoring) and expressed as events per 100 patient-years.
Patient characteristics and PCI features at the time of the index PCI were compared between patients with a LOF allele prescribed either clopidogrel 75 mg/day (LOF-clopidogrel group) or alternative antiplatelet therapy (LOF-alternative group) by the Student’s unpaired t-test, Chi-square analysis, or Fisher’s exact test as appropriate. Additional comparisons were made between non-LOF patients prescribed clopidogrel or alternative antiplatelet therapy (non-LOF group) and LOF-alternative patients and between clopidogrel-treated versus alternatively treated-patients in the non-LOF group. All patients were included in the MACE outcome analyses. A pre-specified secondary analysis limited to patients with an ACS indication (ST-segment elevation or non-ST-segment elevation myocardial infarction or unstable angina) at the index PCI was also conducted.
Kaplan Meier plots were generated to estimate the cumulative risk of an event comparing patients in the LOF-clopidogrel versus LOF-alternative groups, and also patients in the non-LOF versus LOF-alternative groups. To adjust for differences between groups, we used logistic regression to estimate the probability (propensity score) of receiving clopidogrel versus alternative antiplatelet therapy using previously reported risk factors for cardiovascular events, and study site (2). Propensity scores were estimated separately for each comparison. Stabilized inverse probability of treatment weights (IPTW) were calculated using the estimated propensity score (21). Covariate balance between groups was assessed by examining the magnitude of any residual differences between groups after applying the weights. Differences were quantified as the weighted standardized differences, where a threshold of 10% was used to signify a meaningful difference in covariates (21). We also examined the weights themselves to ensure that no observations were overly influential. To compare risk for primary and secondary outcomes, we constructed cause-specific Cox proportional hazard models, weighted by IPTW. As a sensitivity analysis, we also estimated cluster robust standard errors by accounting for clustering by study site. All statistical analyses were performed in SAS (version 9.4, SAS Institute, Cary, North Carolina).
A total sample size of 1,815 patients, with at least 30% having an LOF allele and 60% of LOF allele carriers receiving alternative therapy, provided over 90% power with an alpha level of 0.05 to detect a hazard ratio of 2.0 for MACE between the LOF-clopidogrel and LOF-alternative groups.
RESULTS
PATIENT CHARACTERISTICS
Patient characteristics are summarized in Table 1. Among 1,815 patients, 1,210 (66.7%) presented with an ACS, and 1,793 (98.8%) had a stent placed at the time of PCI. The majority of patients received a drug eluting stent (83.6%), and were prescribed aspirin (98.2%) in addition to a P2Y12 inhibitor.
TABLE 1.
Patient Characteristics at the Time of Index PCI
| Characteristic | All patients (n=1815) | LOF- Alternative (n=346) | LOF- Clopidogrel (n=226) | Non-LOF (n=1243) |
|---|---|---|---|---|
| Age, years | 62.7 ± 11.8 | 61.4 ± 11.4 | 64.3 ± 11.7* | 62.8 ± 11.8* |
| Male sex | 1224 (67.4) | 245 (70.8) | 150 (66.4) | 829 (66.7) |
| Race | ||||
| White | 1416 (78.0) | 267 (77.2) | 172 (76.1) | 977 (78.6) |
| Black | 285 (15.7) | 54 (15.6) | 39 (17.3) | 192 (15.4) |
| Other | 114 (6.3) | 25 (7.2) | 15 (6.6) | 74 (6.0) |
| BMI, kg/m2 | 30.0 ± 6.2 | 30.1 ± 7.0 | 30.0 ± 6.5 | 29.8 ± 5.9 |
| Current smoker | 548 (30.2) | 96 (27.7) | 80 (35.4) | 372 (29.9) |
| PCI indication | ||||
| ACS | 1210 (66.7) | 237 (68.5) | 145 (64.2) | 828 (66.6) |
| STEMI | 350 (19.3) | 75 (21.7) | 33 (14.6) | 242 (19.5) |
| Non-STEMI | 513 (28.3) | 96 (27.7) | 72 (31.8) | 345 (27.7) |
| Unstable angina | 347 (19.1) | 66 (19.1) | 40 (17.7) | 241 (19.4) |
| Stable coronary disease | 553 (30.5) | 99 (28.6) | 70 (31.0) | 384 (30.9) |
| Other/unknown | 52 (2.8) | 10 (2.9) | 11 (4.9) | 31 (2.5) |
| Pre-PCI P2Y12 inhibitor† | ||||
| Clopidogrel | 1204 (66.3) | 202 (58.4) | 178 (78.8) | 824 (66.3) |
| Prasugrel | 235 (12.9) | 68 (19.7) | 6 (2.7) | 161 (13.0) |
| Ticagrelor | 218 (12.0) | 47 (13.6) | 23 (10.2) | 148 (11.9) |
| Not available | 158 (8.7) | 29 (8.4) | 19 (8.4) | 110 (8.8) |
| PCI type‡ | ||||
| Drug eluting stent | 1518 (83.6) | 293 (84.7) | 182 (80.5) | 1043 (83.9) |
| Bare metal stent | 275 (15.2) | 46 (13.3) | 40 (17.7) | 189 (15.2) |
| Balloon angioplasty | 21 (1.2) | 7 (2.0) | 3 (1.3) | 11 (0.9) |
| Medical history | ||||
| Hypertension | 1449 (79.8) | 260 (75.1) | 183 (81.0) | 1006 (80.9)* |
| Diabetes | 691 (38.1) | 110 (31.8) | 93 (41.2)* | 488 (39.3)* |
| Dyslipidemia | 1229 (67.7) | 236 (68.2) | 154 (68.1) | 839 (67.5) |
| Chronic Kidney Disease§ | 538 (29.6) | 106 (30.6) | 77 (34.1) | 355 (28.6) |
| Myocardial infarction | 470 (25.9) | 86 (24.9) | 68 (30.1) | 316 (25.4) |
| Revascularization | 779 (42.9) | 137 (39.6) | 103 (45.6) | 539 (43.4) |
| Coronary artery bypass graft | 313 (17.3) | 61 (17.7) | 43 (19.0) | 209 (16.9) |
| Heart failure | 252 (13.9) | 42 (12.1) | 37 (16.4) | 173 (13.9) |
| Left ventricular EF (%)? | 51.7 ± 11.9 | 52.4 ± 11.4 | 50.2 ± 12.8 | 51.8 ± 11.9 |
| Stroke or TIA | 183 (10.1) | 24 (6.9) | 36 (15.9)* | 123 (9.9) |
| Peripheral vascular disease | 155 (8.5) | 24 (6.9) | 28 (12.4)* | 103 (8.3) |
| Atrial fibrillation/flutter | 159 (8.8) | 27 (7.8) | 25 (11.1) | 107 (8.6) |
| Gastrointestinal bleed | 54 (3.0) | 14 (4.1) | 10 (4.4) | 30 (2.4) |
| Discharge medication | ||||
| Aspirin | 1782 (98.2) | 342 (98.8) | 220 (97.4) | 1220 (98.2) |
| Proton pump inhibitor | 586 (32.3) | 110 (31.8) | 72 (31.9) | 404 (32.5) |
| Anticoagulant | 152 (8.4) | 22 (6.4) | 26 (11.5)* | 104 (8.4) |
| Statin | 1686 (92.9) | 329 (95.1) | 210 (92.9) | 1147 (92.3) |
| ACE inhibitor or ARB | 1202 (66.2) | 238 (68.8) | 152 (67.3) | 812 (65.3) |
| B-blocker | 1539 (84.8) | 285 (82.4) | 181 (80.1) | 1073 (86.3) |
No. (%) or mean ± SD
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; EF, ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack
LOF-Clopidogrel patients were those with at least 1 loss-of-function allele (i.e. *2, *3…) treated with clopidogrel
LOF-Alternative patients were those with at least 1 loss-of-function allele (i.e. *2, *3…) treated with prasugrel, ticagrelor, or high dose clopidogrel
Non-LOF patients were those with no loss-of-function allele: *1/*1, *1/*17, or *17/*17 genotype.
p<0.05 compared to LOF-Alternative group
Pre-PCI P2Y12 inhibitor defined as drug used for loading or, if the drug used for loading was not reported, P2Y12 inhibitor on admission was used.
One patient had a stent placed but no data were provided on type of stent
Chronic kidney disease defined as an estimated creatinine clearance (based on the Cockcroft-Gault formula) <60 ml/min
Left ventricular ejection fraction, as measured during cardiac catheterization, was available for 1,274 patients
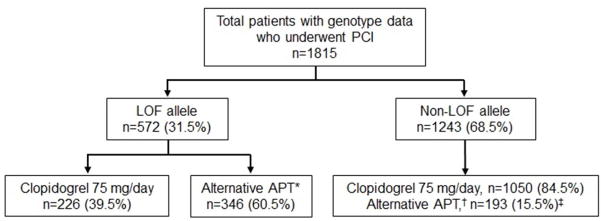
Genotypes and associated phenotypes are shown in Online Table 2. The median time from index PCI to available genotype result was one day (interquartile range, IQR, one to three days). A LOF allele was present in 572 patients (31.5%; Figure 1, Online Table 2); 518 patients (28.5%) were IMs, and 54 patients (3%) were PMs. Alternative antiplatelet therapy was prescribed to a higher proportion of patients with a LOF allele (60.5%) compared to patients without a LOF allele (15.5%, p<0.0001, Figure 1). Fifty-eight percent of IMs and 87% of PMs were prescribed alternative antiplatelet therapy (Online Figure 1). Among patients with a LOF allele, the median time from genotype result to initiation of alternative antiplatelet therapy was one day (IQR: one to six days).
FIGURE 1. Study Population by CYP2C19 Genotype Group and Antiplatelet Therapy.
*Alternative therapy in patients with a LOF allele consisted of prasugrel (n=222), ticagrelor (n=116), or high dose clopidogrel (150 mg/day, n=2; 225 mg/day, n=6).
†Alternative therapy in the non-LOF group consisted of prasugrel (n=125) or ticagrelor (n=68).
‡p<0.001 for use of alternative therapy in the non-LOF group compared to the LOF group.
APT, antiplatelet therapy; LOF, loss-of-function; PCI, percutaneous coronary intervention
There were differences between the LOF-clopidogrel and LOF-alternative groups in age, prevalence of diabetes, stroke, peripheral vascular disease, and use of oral anticoagulation (Table 1). Age and prevalence of hypertension and diabetes differed between the non-LOF and LOF-alternative groups. These imbalances were negligible after adjustment with propensity score-derived IPTW (Table 2).
TABLE 2.
Patient Characteristics after Adjustment with Inverse Probability of Treatment Weights
| Characteristic | LOF- Clopidogrel (n=226) | LOF- Alternative (n=346) | Standardized differences* | Non-LOF (n=1243) | LOF- Alternative (n=345) | Standardized differences* |
|---|---|---|---|---|---|---|
| Age | 62.7 ± 11.7 | 62.5 ± 11.4 | 0.02 | 62.5 ± 11.8 | 62.3 ± 11.6 | 0.01 |
| Male | 150 (67.5) | 237 (68.9) | 0.03 | 836 (67.6) | 229 (67.0) | 0.01 |
| Race | ||||||
| White | 166 (75.2) | 263 (76.4) | 0.03 | 968 (78.2) | 266 (77.7) | 0.01 |
| Black | 39 (17.6) | 55 (15.9) | 0.05 | 193 (15.6) | 53 (15.6) | 0.00 |
| BMI | 30.2 ± 6.3 | 30.1 ± 7.3 | 0.01 | 29.8 ± 5.9 | 29.8 ± 6.9 | 0.00 |
| Current smoker | 69 (31.4) | 107 (31.2) | 0.00 | 363 (29.4) | 97 (28.5) | 0.02 |
| PCI indication | ||||||
| Stable angina | 65 (28.2) | 100 (29.4) | 0.03 | 376 (30.4) | 101 (29.6) | 0.02 |
| ACS | 148 (67.0) | 232 (67.3) | 0.01 | 828 (66.9) | 233 (67.9) | 0.02 |
| Stent type | ||||||
| Drug eluding | 185 (84.3) | 290 (83.8) | 0.01 | 1040 (84.1) | 290 (84.8) | 0.02 |
| Bare metal | 34 (15.0) | 48 (14.1) | 0.03 | 182 (14.7) | 48 (14.0) | 0.02 |
| Medical history | ||||||
| Hypertension | 168 (76.0) | 273 (76.4) | 0.01 | 986 (79.7) | 271 (79.2) | 0.01 |
| Diabetes | 81 (36.8) | 121 (35.3) | 0.03 | 466 (37.6) | 124 (36.2) | 0.03 |
| Dyslipidemia | 153 (69.1) | 236 (68.5) | 0.01 | 837 (67.6) | 232 (67.7) | 0.00 |
| GFR† | 74.0 ± 27.7 | 74.7 ± 31.5 | 0.02 | 74.5 ± 28.1 | 75.2 ± 31.6 | 0.02 |
| MI | 60 (27.1) | 92 (26.8) | 0.01 | 313 (25.4) | 88 (25.8) | 0.01 |
| Revascularization | 95 (43.2) | 147 (42.9) | 0.05 | 143 (41.8) | 525 (42.4) | 0.01 |
| Heart failure | 32 (14.8) | 51 (14.8) | 0.00 | 166 (13.5) | 45 (13.2) | 0.01 |
| Stroke or TIA | 23 (10.7) | 33 (9.7) | 0.03 | 114 (9.2) | 28 (8.3) | 0.03 |
| PVD | 21 (9.3) | 31 (9.0) | 0.01 | 99 (8.0) | 26 (7.6) | 0.01 |
| Medication use | ||||||
| Aspirin | 217 (98.3) | 339 (98.4) | 0.01 | 1216 (98.2) | 336 (98.2) | 0.00 |
| PPI | 69 (31.4) | 109 (31.7) | 0.01 | 398 (32.2) | 106 (30.9) | 0.05 |
| Anticoagulant | 19 (8.9) | 28 (8.2) | 0.03 | 98 (8.0) | 24 (7.3) | 0.03 |
| Statin | 209 (94.3) | 325 (94.5) | 0.01 | 1149 (92.8) | 319 (93.3) | 0.02 |
| ACEi/ARB | 149 (67.4) | 231 (67.3) | 0.00 | 817 (66.0) | 224 (65.4) | 0.01 |
| Beta blocker | 180 (81.5) | 282 (81.8) | 0.01 | 1055 (85.3) | 291 (85.0) | 0.01 |
No. (%) or mean ± SD
ACEi, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; GFR, glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PVD, peripheral vascular disease; TIA, transient ischemic attack
Weighted absolute standardized differences are calculated using stabilized inverse probability of treatment weighting. All values are less than 0.1, which indicate elimination of imbalance between the two groups.
Estimated creatinine clearance using Cockcroft-Gault formula
CLINICAL OUTCOMES
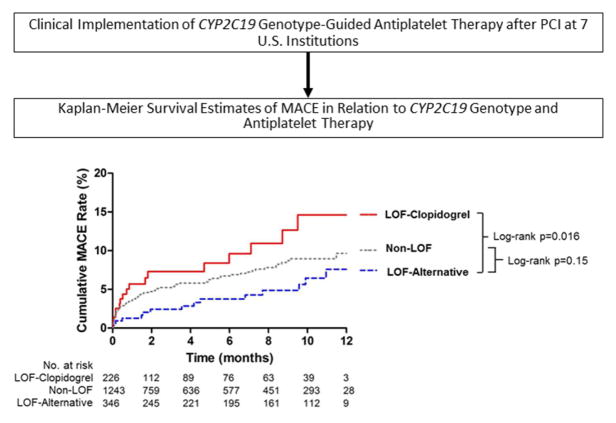
The median follow-up from index PCI to MACE or censoring was 4.8 months (IQR: 0.6 to 9.9 months). MACE was documented in 108 patients (5.95%) over the follow-up period (event rate of 13.5 per 100 patient-years). There was a higher rate of MACE in the LOF-clopidogrel group (n=18 events; event rate of 23.4 per 100 patient-years) compared to the LOF-alternative group (n=16 events; 8.7 per 100 patient-years; log rank p=0.016, Table 3, Figure 2). After propensity score adjustment, risk for MACE remained significantly higher in the LOF-clopidogrel versus LOF-alternative group (adjusted HR: 2.26; 95% CI: 1.18 to 4.32, p=0.013, Table 3). There was no difference in event rates between the non-LOF and LOF-alternative groups (13.7 versus 8.7 per 100 patient-years; log rank p=0.15; propensity score adjusted HR: 1.14; 95% CI: 0.69 to 1.88, p=0.60, Table 3). Similarly, within the non-LOF group, there was no difference in MACE rates between non-LOF patients treated with clopidogrel versus non-LOF patients treated with alternative therapy after adjusting for clinical differences between groups (adjusted HR: 1.01; 95% CI: 0.52 to 1.94, p=0.98, Online Tables 3 and 4). Accounting for within site clustering did not change the estimates for the primary outcome (Online Table 5).
TABLE 3.
Primary and Secondary Outcomes by CYP2C19 Genotype and by Antiplatelet Therapy
| Outcome | LOF-Clopidogrel (n=226) | LOF-Alternative (n=346) | Non-LOF (n=1243) | Adjusted HR (95% CI) for LOF- Clopidogrel vs LOF- Alternative | Adjusted HR (95% CI) for Non- LOF vs LOF- Alternative | |||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Event rate* | No. (%) | Event rate* | No. (%) | Event rate* | |||
| MACE | 18 (7.96) | 23.4 | 16 (4.62) | 8.7 | 74 (5.95) | 13.7 | 2.26 (1.18–4.32) | 1.14 (0.69–1.88) |
| Death | 8 (3.54) | 10.4 | 6 (1.73) | 3.3 | 36 (2.90) | 6.6 | 3.76 (1.37–10.35) | 1.56 (0.68–3.56) |
| MI | 11 (4.87) | 14.3 | 9 (2.60) | 4.9 | 38 (3.06) | 7.0 | 1.85 (0.77–4.45) | 1.00 (0.52–1.92) |
| Ischemic stroke | 3 (1.33) | 3.9 | 2 (0.58) | 1.1 | 13 (1.05) | 2.4 | 2.81 (0.43–18.03) | 2.52 (0.49–12.91) |
| MACE plus other ischemic events† | 25 (11.06) | 32.5 | 28 (8.09) | 15.2 | 106 (8.53) | 19.6 | 1.82 (1.07–3.12) | 1.09 (0.72–1.63) |
| Stent Thrombosis | 4 (1.77) | 5.2 | 4 (1.16) | 2.2 | 13 (1.05) | 2.4 | 1.68 (0.37–7.53) | 1.01 (0.32–3.15) |
| Unstable angina | 7 (3.10) | 9.1 | 12 (3.47) | 6.5 | 31 (2.49) | 5.7 | 1.41 (0.55–3.64) | 0.87 (0.44–1.70) |
Unadjusted proportion of patients experiencing an event during follow-up (%), event rate (per 100-patient years), adjusted hazard ratio (HR) and 95% confidence interval (CI). The hazard ratio was adjusted with inverse probability weights derived from exposure propensity scores. Refer to Table 2 for variables included in the propensity score.
Event rates were calculated the number of events divided by follow-up time from index PCI to MACE or censoring in patient-years and are presented as medians.
The median length of follow-up was 7.3 (IQR 1.5–10.9), 1.8 (0.2–8.2), and 4.5 (0.5 to 9.7) months in the LOF-alternative, LOF-clopidogrel, and non-LOF groups, respectively (p<0.001).
LOF-Clopidogrel patients were those with at least 1 loss-of-function allele (i.e. (*2, *3…) treated with clopidogrel
LOF-Alternative patients were those with at least 1 loss-of-function allele (i.e. (*2, *3…) treated with prasugrel (n=222), ticagrelor (n=116), or high dose clopidogrel (n=8)
Non-LOF patients were those with no loss-of-function allele: *1/*1, *1/*17, or *17/*17 genotypes.
Median event rate expressed as events per 100 patient-years
Composite of MACE (defined as first occurrence of myocardial infarction, ischemic stroke, or death) plus stent thrombosis and unstable angina
FIGURE 2, CENTRAL ILLUSTRATION. Outcomes with Clinical Implementation of CYP2C19-Guided Antiplatelet Therapy after PCI.
Data are shown for patients with a CYP2C19 loss-of-function (LOF) allele treated with clopidogrel (LOF-Clopidogrel), patients with a LOF allele treated with alternative antiplatelet drug therapy (LOF-Alternative), and patients without an LOF allele treated with either clopidogrel or alternative therapy (non-LOF). The unadjusted log-rank p-values for the LOF-Clopidogrel group compared to LOF-Alternative group and for the Non-LOF group compared to the LOF-Alternative group are provided. LOF, loss-of-function; MACE, major adverse cardiovascular events
In the 518 patients with the IM phenotype only, the risk for MACE was significantly higher with clopidogrel versus alternative antiplatelet therapy (log rank p=0.003, Online Figure 2), with event rates of 24.0 versus 6.7 per 100 patient-years, respectively. Only 7 of 54 PMs (13%) were treated with clopidogrel, precluding analysis of outcomes in this group.
Secondary outcomes are shown in Table 3. The risk for MACE plus other ischemic events (stent thrombosis and unstable angina) was higher in the LOF-clopidogrel versus LOF-alternative group (adjusted HR: 1.82; 95% CI: 1.07 to 3.12, p=0.027). There was no difference in MACE plus ischemic events between non-LOF and LOF-alternative groups (adjusted HR: 1.09; 95% CI: 0.72 to 1.63, p=0.69, Table 3) or between non-LOF patients treated with clopidogrel versus alternative therapy (adjusted HR: 1.02; 95% CI: 0.59 to 1.78, Online Table 3). Patients presenting with ACS at the time of index PCI (1,210 of 1,815 patients) contributed the majority of events to the analysis, including 86 of 108 (80%) MACE and 48 of 58 (83%) myocardial infarctions. Consistent with the analysis of the overall study population, among patients with ACS, the LOF-clopidogrel group had a higher rate of MACE compared to the LOF-alternative group (39.0 versus 8.9 per 100 patient-years, respectively; adjusted HR: 2.87; 95% CI: 1.35 to 6.09, p=0.013, Online Figure 3, Online Table 6). The risk of MACE plus other ischemic events (adjusted HR: 2.10; 95% CI: 1.12 to 3.90, p=0.019) was also significantly elevated. No differences in outcomes were observed between non-LOF and LOF-alternative groups in the ACS subset. Though not an outcome of the study, moderate and severe/life-threatening bleeding events, defined according to the GUSTO (Global Utilization of t-PA and Streptokinase for Occluded Coronary Arteries) criteria, were observed in 2.3% of patients in the overall study population and were similar across groups (22).
Based on the prevalence of patients with a LOF allele (31.5%), the number needed to genotype to identify a LOF allele carrier for whom alternative therapy would be recommended is 3.2. With an absolute difference in the proportion of patients who had a MACE in the LOF-clopidogrel (8.0%) and LOF-alternative (4.6%) groups (Table 3), the number of patients with a LOF allele needed to treat with alternative antiplatelet therapy to prevent one event is 29 (1/0.034). Therefore, the number of patients needed to genotype, with alternative antiplatelet therapy prescribed for all patients with a LOF allele, to prevent one cardiovascular event is 93 (29 times 3.2).
DISCUSSION
Our study is the first large multi-center study to examine outcomes after clinical implementation of CYP2C19 genotype-guided antiplatelet therapy. We demonstrate the feasibility of genotype-guided antiplatelet therapy after PCI across multiple institutions, with efficient return of genotype results and high uptake of alternative antiplatelet therapy in patients with a LOF allele. More importantly, our results show a higher risk for MACE in patients with a CYP2C19 LOF allele who are treated with clopidogrel versus alternative antiplatelet therapy. Most events occurred in patients with an ACS indication at the index PCI, in whom the risk for MACE was higher in LOF-clopidogrel versus LOF-alternative patients.
Retrospective genetic substudies of large clinical trials suggested worse outcomes in patients with a CYP2C19 LOF allele treated with clopidogrel (3,5–7), but there is a paucity of data from large prospective clinical trials on outcomes with CYP2C19 genotype-guided antiplatelet therapy. This has hindered inclusion of CYP2C19 genotyping in clinical practice and PCI practice guidelines. Guidelines state that routine genetic testing is not recommended, but might be considered in high-risk patients (Class IIb, Level of Evidence C) (1,2). A randomized controlled trial assessing the efficacy of genotype-guided antiplatelet therapy in a target population of over 5,000 patients began in 2013, but is not expected to be completed until 2020 (ClinicalTrials.gov Identifier NCT01742117). Given the magnitude, time, and expense of conducting traditional randomized controlled trials, other approaches are needed to generate an evidence base quantifying clinical outcomes related to pharmacogenetic-tailored therapy. Our pragmatic approach to assessing outcomes following clinical implementation of CYP2C19 genotyping with real-world evidence is one such approach.
Our findings are in line with recent data from the Netherlands and Spain (23,24). The Netherlands study focused on patients undergoing elective PCI, who were clinically genotyped, with prasugrel recommended for PMs (23). Fewer adverse cardiovascular events were observed in PMs treated with prasugrel versus clopidogrel. The Spanish study included patients undergoing elective or emergent PCI, and recommendations for alternative therapy were made for both PMs and IMs (24). Compared to historic controls without genotyping, fewer adverse events were observed in patients receiving genotype-guided therapy. Our data are also consistent with those from two trials of Chinese patients undergoing PCI and randomized to either clopidogrel 75 mg/day or genotype-guided antiplatelet therapy, consisting of high dose clopidogrel (150 mg/day) for IMs and high dose clopidogrel plus cilostazol for PMs in one trial and ticagrelor for PMs in the other (25,26). Both studies observed a significant reduction in MACE in the genotype-guided arm.
The FDA-approved clopidogrel label warns of reduced clopidogrel effectiveness in PMs, and recommends consideration of alternative antiplatelet therapy in these patients, but is silent regarding risk and recommendations in IMs (27). In our study conducted in the context of routine clinical care, the majority of PMs (87%) were treated with alternative antiplatelet therapy, consistent with labeling recommendations, but a lower proportion of IMs were treated with alternative antiplatelet therapy (58%). This observation suggests that an IM test result is weighed less heavily than a PM result in the post-PCI antiplatelet therapy prescribing decision. However, when limiting our analysis to IMs, we observed a significantly higher rate of MACE with clopidogrel versus alternative antiplatelet therapy. These data indicate that IMs, like PMs, are at higher risk of adverse cardiovascular outcomes if treated with clopidogrel, and suggest that alternative antiplatelet therapy should be considered in both IMs and PMs.
Our real-world data corroborate those from retrospective analyses of clinical trials showing a higher risk for MACE among clopidogrel-treated patients with versus without a LOF allele (3,5–7). In the absence of a LOF allele, data from the TRITON-TIMI 38 trial also suggest that the risk for MACE may be comparable between clopidogrel and prasugrel, with a genetic substudy of the trial showing a relative risk for MACE of 0.98 (95% CI, 0.80–1.20) with prasugrel compared to clopidogrel in patients without a LOF allele. (28) The association between genotype and outcomes with clopidogrel appears to be indication specific, with strong and consistent associations in patients undergoing PCI, but not among lower-risk patients, such as those with atrial fibrillation or ACS managed medically (5,7,29,30). This is most clearly demonstrated in a meta-analysis showing an association between CYP2C19 LOF genotype and risk for MACE when analyzing studies of clopidogrel-treated patients undergoing PCI, but not when analyzing those without PCI (6).
Across institutions in our study, clopidogrel was the most commonly prescribed P2Y12 inhibitor among patients without a CYP2C19 LOF allele. This is consistent with other data suggesting that clopidogrel remains the predominant antiplatelet therapy in the U.S., prescribed 60% to 70% of the time according to published data through the first half of 2013 (31,32). To provide more contemporary use data, we interrogated practice patterns at Vanderbilt University Medical Center in the Southeastern U.S., and Sanford Medical Center, which draws patients from 6 states in the Midwestern U.S. Current practice patterns in these geographic locales demonstrate that clopidogrel remains commonly prescribed following PCI, regardless of clinical context. Specifically, among 10,115 patients who underwent PCI (41% with ACS) at Vanderbilt University Medical Center from 2010 to 2015, clopidogrel use fell minimally, from 93% to 72%, during that time (unpublished data). Similarly, among 1,260 patients from Sanford Medical Center who underwent PCI at 15 catheterization laboratories during the first 9 months of 2016, 53% with an ACS and 77% without ACS were discharged on clopidogrel (unpublished data).
Per PCI guidelines, the use of alternative antiplatelet agents in preference to clopidogrel after ACS and PCI is a Class IIa recommendation based on a moderate quality of evidence (1). In patients started on alternative P2Y12 inhibitors according to PCI guidelines, CYP2C19 genotype may still have an important role in informing therapy, especially after the 30-day post-PCI period when risk for events is highest (1). In patients with a high risk for bleeding or difficulty affording or tolerating newer agents, knowledge that a patient does not carry a LOF allele may give physicians increased confidence when considering switching the patient to the more affordable clopidogrel.
We did not directly assess why physicians chose to start some patients with a LOF allele on alternative therapy but not others. However, some speculation can be made based on prescribing patterns and characteristics of treatment groups. First, the higher use of alternative therapy in PMs versus IMs suggests that physicians may heed the boxed warning in the clopidogrel labeling and place a stronger emphasis on using alternative therapy in PMs, the focus of the boxed warning. Secondly, the higher prevalence of stroke or TIA history and concurrent anticoagulant use in the LOF-clopidogrel group versus the LOF-alternative group suggests that patient bleeding risk influenced preference for antiplatelet therapy.
LIMITATIONS
Our study has several limitations. First, there was no control group who did not undergo genotyping. However, some comparisons can be made with published event rates. In particular, in a genetic sub-study of the TRITON-TIMI 38 trial of patients with ACS and PCI, 8% of clopidogrel-treated patients without a LOF allele and 12% with a LOF allele had a MACE (3). Rates in our study were comparable, with a MACE occurring in 7% of ACS patients without a LOF allele (most of whom received clopidogrel) and 11% of clopidogrel-treated patients with a LOF allele. Secondly, genotype-guided therapy was not randomized, due to our pragmatic design, and antiplatelet therapy selection was left to physician discretion. Thus, propensity scoring methods were used to mitigate the potential confounding effects related to differences across groups, and balance was achieved across comparison groups after weighting with IPTW. However, residual confounding regarding the choice of antiplatelet therapy may remain. Third, because ascertainment of outcomes was confined to the EHR without event adjudication, clinical events, including deaths, may have been missed. Fourth, length of follow-up was variable, as is typical for the clinical setting. As such, event rates were reported. Fifth, bleeding events were not objectively and systematically collected, given known difficulties in accurate bleeding assessment and the focus of data collection for this analysis on ischemic outcomes (33). Finally, because of the limited number of patients who underwent elective PCI, we did not examine outcomes separately in this group.
CONCLUSION
We demonstrate that implementation of clinical CYP2C19 genotyping to guide post-PCI antiplatelet therapy is feasible across multiple institutions. Our data also demonstrate that cardiovascular outcomes were worse when clopidogrel versus alternative antiplatelet therapy was prescribed after PCI in patients with a LOF allele. The higher risk for MACE in LOF carriers prescribed clopidogrel was also evident when analyses were confined to ACS patients and, separately, to IMs (with a single LOF allele). Our data suggest that obtaining genotype data early after PCI allows identification of patients with a CYP2C19 LOF allele in whom alternative antiplatelet therapy would reduce risk for events. A future randomized study of genotype-guided antiplatelet therapy may be of value.
PERSPECTIVES.
WHAT IS KNOWN?
CYP2C19 loss-of-function alleles impair clopidogrel activation and effectiveness after percutaneous coronary intervention. However, the impact of genotype-guided antiplatelet therapy on clinical outcomes is not well defined.
WHAT IS NEW?
This study demonstrates that patients with one or two CYP2C19 loss-of-function alleles prescribed an alternative P2Y12 inhibitor after PCI exhibit lower risk for cardiovascular events compared to patients with one or two CYP2C19 loss-of-function alleles prescribed clopidogrel.
WHAT IS NEXT?
Strategies to more broadly incorporate genotyping into clinical care to inform antiplatelet therapy prescribing decisions after PCI, and to evaluate the impact on health care costs, warrant further investigation.
Acknowledgments
Funding:
Work supported by the National Institutes of Health (NIH) grants U01 HG007269 (JAJ, LHC, KWW, AE, CWM, YG, RMC-D, DRN, PS, DMS, DW, MCS), U01 HG007775 (TIP, RBH, LJJ, DN, KP, ARS), U01 HG007253 (JFP, RAW, JCD), and U01 HG007762 (TCS, VMP, RPK), as part of the NIH IGNITE network. Additional support provided by NIH U01 GM074492 and U01 HL105198 (both part of the NIH Pharmacogenomics Research Network), and by substantial institutional support from the University of Florida and its Clinical Translational Science Institute (UL1 TR000064 and UL1 TR001427) for LHC, EA, KW, DRN, MCS, DW, DMS and JAJ; NIH U01 HL105198, and support from the University of Maryland Medical Center and University of Maryland School of Medicine Program for Personalized and Genomic Medicine for ALB, TDA, LJJ, LB, RBH, MDK, MM, DN, REP, KP, TIP, SWR, JS, MRV, RYZ, and ARS; University of Illinois at Chicago Offices of the Vice President for Health Affairs and Vice Chancellor for Research for EAN and JDD; NIH K23 HL112908 for EAN; NIH K23 GM112014 for JDD; American Society of Health System Pharmacists for PEE and JCC, NIH UL1TR0000005 for PEE, and by an Anonymous Donor for PEE, JCC, and JMS; Penn Center for Precision Medicine at the Perelman School of Medicine at the University of Pennsylvania for ST; NIH R01HL092173, 1K24HL133373, University of Alabama, Birmingham Health Service Foundations’ General Endowment Fund, and NIH UL1TR000165 for NAL; Indiana University Health – Indiana University School of Medicine Strategic Research Initiative for RPK and VMP.
ABBREVIATIONS
- ACS
acute coronary syndrome
- CYP
cytochrome P450
- EHR
electronic health record
- IGNITE
Implementing Genomics In Practice
- IM
intermediate metabolizer
- IPTW
inverse probability of treatment weights
- LOF
loss-of-function
- PCI
percutaneous coronary intervention
- PM
poor metabolizer
Footnotes
Disclosures
SEK consults for Pfizer and Bayer, neither related to the content of the study.
RPK has received consultant fees and research funding from Haemonetics, Inc. Reagents provided by Nanospere to the University of Maryland.
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–155. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 4.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorich MJ, Rowland A, McKinnon RA, Wiese MD. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circ Cardiovasc Genet. 2014;7:895–902. doi: 10.1161/CIRCGENETICS.114.000669. [DOI] [PubMed] [Google Scholar]
- 7.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 8.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 10.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 11.Lee JA, Lee CR, Reed BN, et al. Implementation and evaluation of a CYP2C19 genotype-guided antiplatelet therapy algorithm in high-risk coronary artery disease patients. Pharmacogenomics. 2015;16:303–313. doi: 10.2217/pgs.14.180. [DOI] [PubMed] [Google Scholar]
- 12.Shuldiner AR, Palmer K, Pakyz RE, et al. Implementation of pharmacogenetics: the University of Maryland Personalized Anti-platelet Pharmacogenetics Program. Am J Med Genet C Semin Med Genet. 2014;166C:76–84. doi: 10.1002/ajmg.c.31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166C:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson JF, Field JR, Unertl KM, et al. Physician response to implementation of genotype-tailored antiplatelet therapy. Clin Pharmacol Ther. 2016;100:67–74. doi: 10.1002/cpt.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 16.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 17.Sox HC, Lewis RJ. Pragmatic Trials: Practical Answers to “Real World” Questions. JAMA. 2016;316:1205–1206. doi: 10.1001/jama.2016.11409. [DOI] [PubMed] [Google Scholar]
- 18.Cavallari LH, Beitelshees AL, Blake KV, et al. The IGNITE Pharmacogenetics Working Group: An Opportunity for Building Evidence with Pharmacogenetic Implementation in a Real-World Setting. Clin Transl Sci. 2017;10:143–146. doi: 10.1111/cts.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2017;19:215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behav Res. 2011;46:119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 23.Deiman BA, Tonino PA, Kouhestani K, et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Neth Heart J. 2016;24:589–599. doi: 10.1007/s12471-016-0873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Ramos J, Davila-Fajardo CL, Toledo Frias P, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289–295. doi: 10.1016/j.ijcard.2016.09.088. [DOI] [PubMed] [Google Scholar]
- 25.Xie X, Ma YT, Yang YN, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. 2013;168:3736–3740. doi: 10.1016/j.ijcard.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Shen DL, Wang B, Bai J, et al. Clinical Value of CYP2C19 Genetic Testing for Guiding the Antiplatelet Therapy in a Chinese Population. J Cardiovasc Pharmacol. 2016;67:232–236. doi: 10.1097/FJC.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 27.Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost. 2010;8:1678–1684. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 29.Pare G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 30.Doll JA, Neely ML, Roe MT, et al. Impact of CYP2C19 Metabolizer Status on Patients With ACS Treated With Prasugrel Versus Clopidogrel. J Am Coll Cardiol. 2016;67:936–947. doi: 10.1016/j.jacc.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Sherwood MW, Wiviott SD, Peng SA, et al. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2014;3:e000849. doi: 10.1161/JAHA.114.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan W, Plent S, Prats J, Deliargyris EN. Trends in P2Y12 Inhibitor Use in Patients Referred for Invasive Evaluation of Coronary Artery Disease in Contemporary US Practice. Am J Cardiol. 2016;117:1439–1443. doi: 10.1016/j.amjcard.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Serebruany VL, Atar D. Assessment of bleeding events in clinical trials--proposal of a new classification. Am J Cardiol. 2007;99:288–290. doi: 10.1016/j.amjcard.2006.07.091. [DOI] [PubMed] [Google Scholar]